Abstract
Background:
Guidelines recommend evaluation of cardiac function, valvular and ischemic heart disease, and thyroid, kidney, and liver function on initial diagnosis of atrial fibrillation (AF).
Hypothesis:
We hypothesized that initial workup of patients with newly identified AF would vary by age, sex, and burden of comorbid illness.
Methods:
In a retrospective analysis of a large sample of commercially insured patients 18 to 64 years old (n = 40 245) and a nationally representative 5% cohort of Medicare beneficiaries 65 years or older (n = 204 676), we measured claims for guideline‐recommended services for initial evaluation of AF among patients with a new diagnosis between 2000 and 2008.
Results:
From 30 days before through 90 days after AF diagnosis, basic evaluation, including physician visit, electrocardiogram, and echocardiography, was completed in up to 66.6% of patients. Completion rates for all guideline‐recommended evaluations were 17.4% in the commercially insured sample and 18.5% in the Medicare cohort in 2007. Evaluation rates increased over time. Blood tests assessing thyroid function were documented for approximately one‐third of patients in each cohort. Increasing the observation period to 1 year before through 3 months after the AF diagnosis markedly increased completion rates, but rates of thyroid function testing remained low (50%–60%). There were minor differences in evaluation completeness by sex, race, and geographic region.
Conclusions:
Differences in guideline‐recommended evaluation rates by demographic characteristics after a new diagnosis of AF were of minor clinical importance. Basic evaluation had satisfactory completion rates; however, rates of laboratory testing were low.
The contents of the manuscript are solely the responsibility of the authors and do not necessarily reflect the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the manuscript. Mr. Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
This work was supported by grants R01HL102214, RC1HL101056, R01HL068986, R01HL092577, and T32HL007‐ 902 from the National Heart, Lung, and Blood Institute. Dr. Sinner was supported by the German Heart Foundation.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Supporting Information may be found in the online version of this article.
Introduction
Numerous potentially modifiable conditions increase the risk of atrial fibrillation (AF).1., 2., 3., 4. On first diagnosis of AF, assessment of the presence and severity of these conditions is part of the recommended initial evaluation. Professional guidelines recommend that the initial evaluation include physical examination and history, electrocardiogram, transthoracic echocardiogram, and blood tests for thyroid, renal, and liver function.5., 6., 7. Depending on the clinical context, additional guideline‐recommended procedures include the 6‐minute walk test, exercise stress test, Holter monitoring, transesophageal echocardiography, and chest x‐ray.5., 6., 7. We measured completion rates of guideline‐recommended evaluations among patients with a first diagnosis of AF in a large sample of commercially insured young and middle‐aged patients and a nationally representative cohort of elderly Medicare beneficiaries. We also assessed differences in completion rates by patient demographic characteristics and comorbid conditions and over time.
Methods
MarketScan, a repository of commercial healthcare claims data from more than 100 large employers, health plans, and government agencies, provides detailed information about healthcare delivered to commercially insured individuals in the United States.8 The Commercial Claims and Encounters database includes data for more than 13 million active employees, early retirees, and their dependents insured by employer‐sponsored plans (ie, persons not eligible for Medicare). The MarketScan repository is an established resource for claims‐based analyses and has been previously used for AF research.9., 10. Claims for inpatient and outpatient services are linked to person‐level enrollment information. Each claim contains Current Procedural Terminology codes that describe the nature of the billed services and accompanying International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes, as well as fields for date and place of service. In this analysis, we used data from 2002 through 2007 for beneficiaries aged 18 to 64 years.
We also obtained data for a nationally representative 5% cohort of Medicare beneficiaries from 1998 through 2008 from the Centers for Medicare and Medicaid Services. The inpatient files contain institutional claims for facility costs covered under Medicare Part A, and the outpatient files contain claims from institutional outpatient providers (eg, hospital outpatient departments, ambulatory surgery centers). The carrier files contain noninstitutional provider claims for services covered under Medicare Part B. The denominator files contain beneficiary demographic data and information about program eligibility and enrollment. We restricted the Medicare cohort to fee‐for‐service beneficiaries 65 years or older living in the United States.
We identified patients for whom a diagnosis of AF (ICD‐9‐CM code 427.31) or atrial flutter (427.32) was reported on a single inpatient claim or at least 2 outpatient claims on different days within 365 days. To avoid including patients with prevalent AF that occurred before inclusion in the study, we required at least 2 years of continuous enrollment in either database before adjudicating a new AF diagnosis. We defined the index date as the earlier of (1) the discharge date of the earliest inpatient diagnosis or (2) the service date of the first of the 2 outpatient diagnoses. We included patients with index dates between 2004 and 2007 in the MarketScan sample and between 2000 and 2008 in the Medicare cohort. We used data from previous years to establish the index diagnosis and comorbid conditions at baseline. To avoid counting AF diagnoses made in the context of terminal events, we required patients to be enrolled continuously for at least 90 days after the index date.
Medicare beneficiaries report race at the time of enrollment. We used the reported categories “black” and “white” and combined all others as “other.” We derived rural classification groups from rural‐urban commuting area codes based on zip code of residence.11., 12. We grouped patients into 9 US Census regions by state codes. Race and zip code were not available for patients in the MarketScan database.
Statistical Analysis
We described the cohorts in index years 2004 and 2007 with descriptive statistics. We presented categorical variables as frequencies with percentages and continuous variables as means with standard deviations. We tested for differences by index year using χ 2 tests for categorical variables and Kruskal‐Wallis tests for continuous variables. We identified comorbid conditions using previously validated coding algorithms13., 14. and searched all claims in the 365‐day period preceding the index date for evidence of cancer, cerebrovascular disease, chronic obstructive pulmonary disease, heart failure, dementia, diabetes mellitus, hypertension, ischemic heart disease, peripheral vascular disease, and renal disease (Supporting Appendix 1).
We used Current Procedural Terminology and revenue center codes to identify receipt of guideline‐based evaluations for newly diagnosed AF.6 We searched all claims for evidence of guideline‐based evaluations (ie, physician evaluation and management visit, electrocardiogram, echocardiogram, and blood tests for thyroid function, renal function, and liver function) and elective evaluations (ie, 6‐minute walk test, exercise stress test, Holter monitoring, transesophageal echocardiography, and chest x‐ray [see Supporting Appendix 2]). For each patient we examined claims between 30 days before and 90 days after the index date. For each data source we calculated the age‐ and sex‐adjusted proportions of patients who underwent evaluation in each index year using a direct standardization method.15 We used the full cohort in each data set as the standard population for these adjustments. We used Cochran‐Mantel‐Haenszel tests of nonzero correlation to test for temporal trends in receipt of guideline‐based evaluations between 2004 and 2007 in the MarketScan sample and between 2000 and 2008 in the Medicare cohort. We calculated sex‐adjusted proportions by age, age‐adjusted proportions by sex, and age‐ and sex‐adjusted proportions by race. In addition, we calculated age‐ and sex‐adjusted proportions by rural classification, geographic region, and comorbid conditions. We used χ 2 tests to compare differences in evaluation proportions by subgroup. In a first sensitivity analysis, we searched for evidence of initial evaluation between 90 days before and 90 days after the index date and repeated the analysis. In a second sensitivity analysis, we extended the observation period to cover 365 days before to 90 days after the index date.
We used a significance level of 0.005 for all hypotheses to correct for multiple comparisons. We used SAS software version 9.2 (SAS Institute Inc., Cary, NC) for all analyses. The institutional review board of the Duke University Health System approved the study.
Results
Table 1 shows the baseline characteristics of the study population, including 40245 commercially insured patients in the MarketScan sample and 204676 patients in the Medicare cohort. Comorbid conditions were generally more prevalent in the Medicare cohort. In both groups, approximately half of the patients were diagnosed with AF as inpatients.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | MarketScan Sample | Medicare 5% Cohort | ||||
|---|---|---|---|---|---|---|
| 2004 (n = 9734) | 2007 (n = 8000) | P Value | 2004 (n = 25 087) | 2007 (n = 22 689) | P Value | |
| Age, mean ± SD,y | 55.4 ± 7.9 | 55.1 ± 8.2 | 0.007 | 78.6 ± 7.2 | 78.9 ± 7.3 | <0.001 |
| Age, median (interquartile range), y | 57.0 (52.0–61.0) | 57.0 (51.0–61.0) | 0.04 | 78.0 (73.0–84.0) | 79.0 (73.0–84.0) | <0.001 |
| Age group, no. (%), y | 0.004 | <0.001 | ||||
| 18–44 | 924 (9.5) | 834 (10.4) | — | — | ||
| 45–54 | 2382 (24.5) | 2070 (25.9) | — | — | ||
| 55–64 | 6428 (66.0) | 5096 (63.7) | — | — | ||
| 65–69 | — | — | 2887 (11.5) | 2572 (11.3) | ||
| 70–74 | — | — | 5257 (21.0) | 4576 (20.2) | ||
| 75–79 | — | — | 6086 (24.3) | 5183 (22.8) | ||
| 80–84 | — | — | 5457 (21.8) | 4879 (21.5) | ||
| 85–89 | — | — | 3480 (13.9) | 3576 (15.8) | ||
| ≥90 | — | — | 1920 (7.7) | 1903 (8.4) | ||
| Male, no. (%) | 6205 (63.7) | 5110 (63.9) | 0.86 | 11 290 (45.0) | 10 273 (45.3) | 0.55 |
| Race, no. (%) | <0.001 | |||||
| Black | — | — | 1503 (6.0) | 1196 (5.3) | ||
| White | — | — | 22 903 (91.3) | 20 765 (91.5) | ||
| Other | — | — | 681 (2.7) | 728 (3.2) | ||
| Inpatient diagnosis of atrial fibrillation, no. (%) | 4747 (48.8) | 4125 (51.6) | <0.001 | 13 827 (55.1) | 12 198 (53.8) | 0.003 |
| Comorbid conditions, no. (%) | ||||||
| Cancer | 807 (8.3) | 733 (9.2) | 0.04 | 4616 (18.4) | 4247 (18.7) | 0.37 |
| Cerebrovascular disease | 996 (10.2) | 870 (10.9) | 0.17 | 7442 (29.7) | 7140 (31.5) | <0.001 |
| Chronic obstructive pulmonary disease | 1788 (18.4) | 1522 (19.0) | 0.26 | 9330 (37.2) | 8640 (38.1) | 0.05 |
| Congestive heart failure | 1918 (19.7) | 1550 (19.4) | 0.58 | 10 547 (42.0) | 9201 (40.6) | 0.001 |
| Dementia | 23 (0.2) | 11 (0.1) | 0.13 | 2026 (8.1) | 1821 (8.0) | 0.84 |
| Diabetes mellitus | 2219 (22.8) | 1926 (24.1) | 0.05 | 7978 (31.8) | 7850 (34.6) | <0.001 |
| Hypertension | 4802 (49.3) | 4162 (52.0) | <0.001 | 20 796 (82.9) | 19 569 (86.2) | <0.001 |
| Ischemic heart disease | 3250 (33.4) | 2496 (31.2) | 0.002 | 13 931 (55.5) | 12 281 (54.1) | 0.002 |
| Peripheral vascular disease | 760 (7.8) | 619 (7.7) | 0.86 | 6741 (26.9) | 6758 (29.8) | <0.001 |
| Renal disease | 476 (4.9) | 480 (6.0) | 0.001 | 2631 (10.5) | 3847 (17.0) | <0.001 |
| Rural/urban classification, no. (%) | 0.001 | |||||
| Urban | — | — | 18249 (72.7) | 16 618 (73.2) | ||
| Large rural | — | — | 3383 (13.5) | 2817 (12.4) | ||
| Small or isolated small rural | — | — | 3455 (13.8) | 3254 (14.3) | ||
| US geographic region, no. (%) | <0.001 | <0.001 | ||||
| Northeast | ||||||
| New England | 183 (1.9) | 304 (3.8) | 1311 (5.2) | 1362 (6.0) | ||
| Middle Atlantic | 527 (5.4) | 494 (6.2) | 3538 (14.1) | 3457 (15.2) | ||
| South | ||||||
| South Atlantic | 2441 (25.1) | 2126 (26.6) | 5909 (23.6) | 4661 (20.5) | ||
| East South Central | 1074 (11.0) | 822 (10.3) | 1769 (7.1) | 1586 (7.0) | ||
| West South Central | 701 (7.2) | 538 (6.7) | 2626 (10.5) | 2413 (10.6) | ||
| Midwest | ||||||
| East North Central | 2671 (27.4) | 1691 (21.1) | 4590 (18.3) | 4038 (17.8) | ||
| West North Central | 494 (5.1) | 511 (6.4) | 1807 (7.2) | 1710 (7.5) | ||
| West | ||||||
| Mountain | 477 (4.9) | 358 (4.5) | 1207 (4.8) | 1119 (4.9) | ||
| Pacific | 1166 (12.0) | 1156 (14.5) | 2330 (9.3) | 2343 (10.3) | ||
Abbreviation : SD, standard deviation.
Table 2 and Figure 1 show completion rates of guideline‐recommended and other evaluations (see Supporting Appendix 3 for additional details by age group). A basic evaluation consisting of a physician visit, electrocardiogram, and echocardiogram was completed in 58.3% of patients in 2004 and in 66.6% in 2007 in the MarketScan sample. The corresponding rates in the Medicare cohort were 59.5% in 2004 and 63.7% in 2007. Completion of all guideline‐recommended evaluations increased from 12.4% to 17.4% in MarketScan and from 14.2% to 18.5% in Medicare. Almost all patients were seen by a physician, and 9 out of 10 underwent an electrocardiogram. In 2007, approximately two‐thirds of patients had a transthoracic echocardiogram. Completion rates for additional evaluations also increased over time; however, completion rates for these items were more heterogeneous between patients with different comorbid conditions.
Table 2.
Age‐ and Sex‐Adjusted Rates of Initial Evaluation of Newly Diagnosed Atrial Fibrillation by Data Source and Index Yeara
| Evaluations | MarketScan Sample | Medicare 5% Cohort | ||||
|---|---|---|---|---|---|---|
| 2004 (n = 9734) | 2007 (n = 8000) | P Value | 2004 (n = 25 087) | 2007 (n = 22 689) | P Value | |
| Guideline‐recommended evaluations, no. (%) | ||||||
| Physician evaluation and management visit (history and physical) | 9566 (98.3) | 7921 (99.0) | <0.001 | 24 939 (99.4) | 22 559 (99.4) | 0.01 |
| Any electrocardiogram | 8280 (85.1) | 7158 (89.5) | <0.001 | 22 319 (89.0) | 20 575 (90.7) | 0.005 |
| Echocardiogram | 5973 (61.4) | 5512 (68.9) | <0.001 | 15 204 (60.6) | 14 726 (64.9) | <0.001 |
| Blood test of thyroid function | 2623 (26.9) | 2536 (31.7) | <0.001 | 7700 (30.7) | 7754 (34.2) | <0.001 |
| Blood test of renal function | 4563 (46.9) | 4434 (55.4) | <0.001 | 18 157 (72.4) | 17 284 (76.2) | <0.001 |
| Blood test of hepatic function | 3367 (34.6) | 3348 (41.8) | <0.001 | 12 246 (48.8) | 12 785 (56.4) | <0.001 |
| Completion of all guideline‐recommended evaluations for atrial fibrillation | 1208 (12.4) | 1388 (17.4) | <0.001 | 3561 (14.2) | 4191 (18.5) | <0.001 |
| Additional evaluations, no. (%) | ||||||
| 6‐minute walk test | 15 (0.2) | 23 (0.3) | 0.06 | 72 (0.3) | 128 (0.6) | <0.001 |
| Exercise stress test | 2721 (28.0) | 2388 (29.9) | 0.001 | 5299 (21.1) | 4827 (21.3) | <0.001 |
| Holter monitoring | 1282 (13.2) | 1180 (14.8) | 0.002 | 3535 (14.1) | 3482 (15.3) | 0.04 |
| Transesophageal echocardiography | 881 (9.0) | 919 (11.5) | <0.001 | 1699 (6.8) | 1943 (8.6) | <0.001 |
| Chest x‐ray | 6258 (64.3) | 5526 (69.1) | <0.001 | 18 562 (74.0) | 16 906 (74.5) | <0.001 |
Percentages are standardized to the age and sex distributions of the pooled cohorts of patients with atrial fibrillation in each data source.
Figure 1.
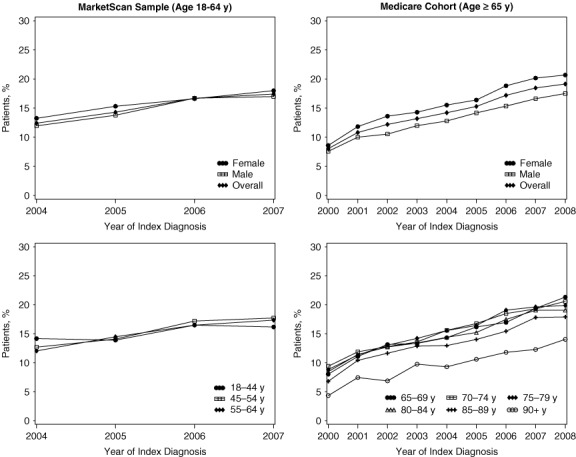
Completion of Guideline‐Recommended Initial Evaluations of Newly Diagnosed Atrial Fibrillation Left‐hand panels show data for commercially insured patients in the MarketScan sample; right‐hand panels show data for patients in the Medicare cohort.
Completeness of evaluation was generally consistent across age groups, except among Medicare beneficiaries older than 90 years, for whom completion rates were lower. Several guideline‐recommended evaluations were distributed differently between men and women (Table 3 and Figure 1). However, absolute differences were small and of limited clinical relevance. The percentage of completed guideline‐recommended evaluations was higher among women in both the MarketScan sample and in the Medicare cohort. We observed similar differences for the additional evaluations.
Table 3.
Age‐Adjusted Rates of Initial Evaluation of Newly Diagnosed Atrial Fibrillation by Data Source and Sexa
| Evaluation | MarketScan Sampleb | Medicare 5% Cohortc | ||||
|---|---|---|---|---|---|---|
| Men (n = 25 826) | Women (n = 14 419) | P Value | Men (n = 92 006) | Women (n = 112 670) | P Value | |
| Guideline‐recommended evaluations, no. (%) | ||||||
| Physician evaluation and management visit (history and physical) | 25 450 (98.5) | 14 233 (98.7) | 0.18 | 91 397 (99.3) | 112 201 (99.6) | <0.001 |
| Any electrocardiogram | 22 644 (87.7) | 12 401 (86.0) | <0.001 | 83 690 (91.0) | 101 509 (90.1) | <0.001 |
| Echocardiogram | 16 992 (65.8) | 9140 (63.4) | <0.001 | 56 916 (61.9) | 70 208 (62.3) | 0.04 |
| Blood test of thyroid function | 7218 (27.9) | 4731 (32.8) | <0.001 | 24 058 (26.1) | 37 716 (33.5) | <0.001 |
| Blood test of renal function | 13 225 (51.2) | 7440 (51.6) | 0.45 | 65 457 (71.1) | 80 185 (71.2) | 0.90 |
| Blood test of hepatic function | 9989 (38.7) | 5578 (38.7) | 0.99 | 44 192 (48.0) | 53 615 (47.6) | 0.04 |
| Completion of all guideline‐recommended evaluations for atrial fibrillation | 3808 (14.7) | 2258 (15.7) | 0.01 | 11 791 (12.8) | 17 253 (15.3) | <0.001 |
| Additional evaluations, no. (%) | ||||||
| 6‐minute walk test | 44 (0.2) | 45 (0.3) | 0.004 | 390 (0.4) | 375 (0.3) | <0.001 |
| Exercise stress test | 7972 (30.9) | 3779 (26.2) | <0.001 | 20 852 (22.7) | 21 746 (19.3) | <0.001 |
| Holter monitoring | 3301 (12.8) | 2109 (14.6) | <0.001 | 13 995 (15.2) | 16 936 (15.0) | 0.26 |
| Transesophageal echocardiography | 2885 (11.2) | 1195 (8.3) | <0.001 | 7857 (8.5) | 6653 (5.9) | <0.001 |
| Chest x‐ray | 17 138 (66.4) | 9563 (66.3) | 0.94 | 69 610 (75.7) | 85 567 (75.9) | 0.13 |
Percentages are standardized to the age distributions of the pooled cohorts of patients with atrial fibrillation in each data source.
Data aggregated from 2004 through 2007.
Data aggregated from 2000 through 2008.
Figure 2 shows differences by race in the Medicare cohort. Race was not available for the MarketScan sample. In general, blood tests were conducted less frequently among black patients than among white patients. Blood tests for thyroid function were performed in 22.0% of black patients, compared with 30.5% of white patients (P < 0.001). Geographic differences by census region and rural vs urban location of residence were minor (data not shown).
Figure 2.

Completion of Guideline‐Recommended Initial Evaluation of Newly Diagnosed Atrial Fibrillation by Race in the Medicare 5% Cohort Bars represent the percentage of completed guideline‐recommended evaluation items by race for patients in the Medicare cohort. Abbreviations: ECG, electrocardiogram; TTE, transthoracic echocardiogram.
To study variations depending on the presence of comorbid conditions, we examined differences between patients with and without heart failure (Table 4). Although almost all patients had a physician visit and an electrocardiogram, more patients with heart failure had an echocardiogram between 30 days before and 90 days after the AF diagnosis (62.8% vs 73.9% in MarketScan, 58.7% vs 66.6% in Medicare). Compared to patients without heart failure, fewer patients with heart failure underwent blood tests for thyroid function, but more underwent blood tests for renal function. Fewer patients with heart failure underwent exercise testing and Holter monitoring, but more patients with heart failure underwent transesophageal echocardiography. Differences in evaluation rates by the presence of other comorbid conditions, such as ischemic heart disease, diabetes mellitus, and chronic obstructive pulmonary disease were similar but less pronounced than those we observed in heart failure (data not shown).
Table 4.
Age‐ and Sex‐Adjusted Rates of Initial Evaluation of Newly Diagnosed Atrial Fibrillation by Data Source and Prior Heart Failure Diagnosisa
| Evaluations | MarketScan Sampleb | Medicare 5% Cohortc | ||||
|---|---|---|---|---|---|---|
| No Heart Failure (n = 32 379) | Heart Failure (n = 7866) | P Value | No Heart Failure (n = 118 368) | Heart Failure (n = 86 308) | P Value | |
| Guideline‐recommended evaluations, no. (%) | ||||||
| Physician evaluation and management visit (history and physical) | 31874 (98.4) | 7808 (99.3) | <0.001 | 117589 (99.3) | 85995 (99.6) | <0.001 |
| Any electrocardiogram | 27975 (86.4) | 7076 (90.0) | <0.001 | 105315 (89.0) | 79845 (92.5) | <0.001 |
| Echocardiogram | 20322 (62.8) | 5817 (73.9) | <0.001 | 69493 (58.7) | 57489 (66.6) | <0.001 |
| Blood test of thyroid function | 10066 (31.1) | 1884 (24.0) | <0.001 | 38544 (32.6) | 22800 (26.4) | <0.001 |
| Blood test of renal function | 16304 (50.4) | 4391 (55.8) | <0.001 | 80768 (68.2) | 64709 (75.0) | <0.001 |
| Blood test of hepatic function | 12441 (38.4) | 3166 (40.3) | 0.003 | 56081 (47.4) | 41473 (48.1) | 0.003 |
| Completion of all guideline‐recommended evaluations for atrial fibrillation | 4943 (15.3) | 1143 (14.5) | 0.10 | 17326 (14.6) | 11479 (13.3) | <0.001 |
| Additional evaluations, no. (%) | ||||||
| 6‐minute walk test | 58 (0.2) | 33 (0.4) | <0.001 | 334 (0.3) | 431 (0.5) | <0.001 |
| Exercise stress test | 9941 (30.7) | 1815 (23.1) | <0.001 | 26991 (22.8) | 15413 (17.9) | <0.001 |
| Holter monitoring | 4647 (14.4) | 763 (9.7) | <0.001 | 19789 (16.7) | 10917 (12.6) | <0.001 |
| Transesophageal echocardiography | 2582 (8.0) | 1520 (19.3) | <0.001 | 6590 (5.6) | 8181 (9.5) | <0.001 |
| Chest x‐ray | 20172 (62.3) | 6512 (82.8) | <0.001 | 81429 (68.8) | 73888 (85.6) | <0.001 |
Percentages are standardized to the age and sex distributions of the pooled atrial fibrillation populations in each data source. bData aggregated from 2004 through 2007. cData aggregated from 2000 through 2008.
In sensitivity analyses, we increased the period for evaluation to 90 days before through 90 days after the index AF diagnosis, then to 1 year before through 90 days after the diagnosis. In the former, we observed a small increase in the completion rate for guideline‐recommended evaluations (0.2 to 9.0 absolute percentage points). Subgroup differences were similar to those in the unstratified sample. In 2007, completion of recommended evaluations increased to 22.1% in MarketScan and 22.8% in Medicare. However, completion rates changed markedly when we extended to a 1‐year observation period before the AF diagnosis. In 2007, the basic evaluation was completed in more than 75% of both MarketScan and Medicare patients. All guideline‐recommended evaluations were performed in 33.9% of patients in the MarketScan sample and in 43.1% of Medicare beneficiaries. Tests of renal and hepatic function had >90% completion rates. In contrast, thyroid function tests were performed in 49.9% of the MarketScan sample and 60.0% of the Medicare cohort.
Discussion
In a large sample of 40245 commercially insured patients derived from MarketScan and a nationally representative cohort of 204676 Medicare beneficiaries, the completion rate for the basic evaluation of AF was >66%. However, the rate for all guideline‐recommended items was relatively low. Even when we extended the timeframe to 1 year before through 90 days after the diagnosis, 40% of Medicare beneficiaries and 50% of MarketScan patients did not undergo thyroid function testing.
Although differences in completion rates between demographic subgroups were statistically significant, the absolute differences generally were of little or no clinical relevance. This lack of variation contrasts with reports that women with cardiovascular conditions tend to be diagnosed later, undergo incomplete evaluations, and receive suboptimal treatment.16., 17. Similarly, black patients with heart failure and AF are 30% less likely than other patients to be discharged from the hospital with warfarin.18 Racial variations have been observed in numerous studies, including studies of patterns of care for prostate cancer,19., 20. kidney transplant,21 and diabetes mellitus.22 Geographic variation in access to and use of healthcare resources also has been widely reported.23., 24., 25., 26.
We also found little difference in initial testing rates with respect to age. Only completion rates in the oldest age group were markedly lower (9.5%). One explanation is that AF in the oldest patients may occur as a complication of other advanced comorbid conditions. Physicians may also be hesitant to burden older patients with numerous diagnostic tests.
Another important finding is the completeness of guideline‐recommended evaluation. Almost all patients were seen by a physician, and 90% had a claim for an electrocardiogram. Approximately two‐thirds of patients had an echocardiogram, and a similar proportion received all 3 basic evaluation items together. These high rates contrast with the relatively low rates for all guideline‐recommended evaluation items. Although evaluation rates increased over time, in 2007 <20% of patients received the complete guideline‐recommended evaluation. Low evaluation rates were due mainly to limited completion rates for laboratory testing, particularly evaluation of thyroid function, which was reported for only one‐third of patients. Adherence rates also rose over time between 2004 and 2007. The reasons for this rise remain unclear. Although both the 2001 and 2006 guidelines for the management of AF recommend similar evaluation items,5., 6. the 2006 guidelines may have reached a broader audience. In addition, it is possible that physicians have generally become more aware of guideline‐based care over time.
We also observed low completion rates among patients with comorbid conditions like heart failure. Although some procedures, such as echocardiogram and tests of renal function, were performed more frequently in patients with heart failure, overall completion of guideline‐recommended AF evaluation in patients with heart failure was lower than in patients without heart failure. In sensitivity analyses, an extended observation period of 90 days before to 90 days after the index AF event would have increased observed evaluation rates to some degree. Completion of additional evaluation varied by comorbid condition. For example, patients with heart failure more often received chest x‐rays. These patients also had more transesophageal echocardiograms, possibly to rule out blood clots in the left atrial appendage before planned external electrical cardioversion, a treatment option considered more frequently for patients with heart failure.6
Many diseases that may contribute to the development of a first AF episode are treatable, if not reversible, and can be identified by laboratory testing.6 For example, abnormal thyroid‐stimulating hormone or thyrotropin values are common among patients with new‐onset AF,27., 28. and persons with high‐normal thyroid function are at considerably elevated risk for AF at the community level.29., 30. Laboratory testing of thyroid function is cost‐effective even in populations without disease.31 Thus, thyroid disorders might be an important underlying differential diagnosis when evaluating new AF, and improvement in routine evaluation of thyroid function would likely be beneficial. Along this line, the results of the sensitivity analyses are interesting. Including results of up to 1 year before through 90 days after the diagnosis revealed that 40% or more of individuals with newly diagnosed AF may not have undergone thyroid testing. Given that hyperthyroidism, particularly in older patients, may be difficult to diagnose without lab testing, and is treatable, our results suggest potential opportunities for clinical improvement.
Although guideline‐recommended evaluation of new AF is relatively straightforward, completion rates within a short timeframe were low. With the exception of thyroid function testing, evaluation rates were higher when the timeframe was greater. At first glance, evaluation performance could be considered a lack of adherence to guideline recommendations. Cabana et al32 found that nonadherence has multiple reasons, which include awareness of guidelines and familiarity and agreement with them. Physicians' active refusal to adhere to guidelines rarely explains nonadherence (16%).33., 34.
Recommendations for initial evaluation of AF reflect the guideline authors' consensus recommendations. Not uncommon in clinical practice guidelines, these recommendations are not classified according to levels of evidence.35 The current scientific basis for some of the recommended components of evaluation is relatively weak. For example, professional guidelines for the management of AF recommend a blood test for liver function but provide no evidence to support the recommendation. A single study reported that elevated liver‐associated enzymes are a common but rarely pathologic finding in patients with newly diagnosed AF.36 Several medications commonly prescribed for AF are metabolized by the liver or carry a potential for liver toxicity. Impaired liver function is considered a risk factor for bleeding complications in patients with AF who are required to use anticoagulants.37., 38. It is unknown whether elevated liver function test results are a risk factor for AF.
Completion rates increased when we extended the observation period to the prior year, suggesting that clinicians may rely on less recent diagnostic information. Guidelines recommend specific evaluation items but do not comment on the appropriate timeframe.6 In an era of concern about healthcare costs, clinicians may believe it is acceptable to use clinical data from 1 year prior, particularly in the absence of intercurrent events. No data are currently available to adjudicate whether this strategy is clinically appropriate. It will merit further study to establish respective standards.
Our study has some limitations. First, the low completion rates for laboratory tests may be related to study design. Laboratory tests are sometimes included in blanket claims for diagnostic procedures, laboratory panels, or diagnosis‐related groups for hospitalized patients, making it difficult to isolate individual components.39., 40. Therefore, we may have underestimated completion rates for some blood tests in this claims‐based analysis. Also, we may have missed other procedures: approximately 10% of patients with newly diagnosed AF did not have a separate claim for an electrocardiogram. Potential reasons include that the provider did not sub mit a claim, or the diagnosis occurred during imaging, transport, or telemetry. However, it is possible that some patients had an antecedent AF episode that was not captured. Second, claims data do not allow assessment of individual clinical decisions. Although we found relatively low evaluation rates, individual decisions may have been clinically justifiable. Third, a highly relevant question is whether completion of initial evaluation leads to improved outcomes in patients with AF. Claims data do not include sufficient clinical data to support such an analysis. Fourth, although we required 2 years of continuous enrollment prior to adjudicating a new AF diagnosis, it is possible that some patients developed AF more than 2 years earlier, without further claims in our blanking period. Also, some of these patients may already have undergone a complete evaluation. In addition, we only examined AF after it was clinically diagnosed; we acknowledge AF may be clinically asymptomatic or unrecognized before being diagnosed. Fifth, information about race was available in the Medicare cohort only, which limited our ability to examine potential racial variations. Finally, the findings may not be generalizable to Medicare beneficiaries in managed care and patients younger than 65 years not covered by commercial carriers represented in MarketScan.
Conclusion
We found minor demographic differences in the completeness of initial evaluation of AF. Two‐thirds of patients received a basic evaluation. Completion of all guideline‐recommended evaluations was low during a circumscribed observation period, and increased when we considered a longer period for capturing evaluation items. Laboratory testing for thyroid function was particularly low and may be the area where most improvement can be achieved. Together, these findings suggest uncertainty about current guidelines regarding evidence‐based justifications of recommendations and the timeframe for performance of evaluation items.
References
- 1. McManus DD, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation. 2010;121:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995;98:476–484. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 5. Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001;38:1231–1266. [DOI] [PubMed] [Google Scholar]
- 6. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 7. European Heart Rhythm Association; European Association for Cardio‐Thoracic Surgery , Camm AJ, Kirchhof P, Lip GY , et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 8. Hansen LG, Chang S. Health research data for the real world: the MarketScan databases. http://info.thomsonhealthcare.com/‐ forms/HealthResearchWPRequest. Accessed October 28, 2011.
- 9. Ghate SR, Biskupiak J, Ye X, et al. All‐cause and bleeding‐related health care costs in warfarin‐treated patients with atrial fibrillation. J Manag Care Pharm. 2011;17:672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ladapo JA, David G, Gunnarsson CL, et al. Healthcare utilization and expenditures in patients with atrial fibrillation treated with catheter ablation. J Cardiovasc Electrophysiol. 2012;23:1–8. [DOI] [PubMed] [Google Scholar]
- 11. Rural‐urban commuting area codes . http://www.ers.usda.gov/‐data/ruralurbancommutingareacodes. Accessed November‐ 30, 2010.
- 12. RUCA data . http://depts.washington.edu/uwruca/ruca‐uses.php. Accessed November 30, 2010.
- 13. Birman‐Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD‐9‐CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. [DOI] [PubMed] [Google Scholar]
- 14. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 15. Brenner H, Arndt V, Gefeller O, et al. An alternative approach to age adjustment of cancer survival rates. Eur J Cancer. 2004;40:2317–2322. [DOI] [PubMed] [Google Scholar]
- 16. Sehgal A, Davies EA. Comparing treatment trends for colorectal cancer in clinical database and cancer registry data: implications for monitoring cancer care. J Eval Clin Pract. 2011;17:486–492. [DOI] [PubMed] [Google Scholar]
- 17. Chou AF, Scholle SH, Weisman CS, et al. Gender disparities in the quality of cardiovascular disease care in private managed care plans. Womens Health Issues. 2007;17:120–130. [DOI] [PubMed] [Google Scholar]
- 18. Piccini JP, Hernandez A, Zhao X, et al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009;54:1280–1289. [DOI] [PubMed] [Google Scholar]
- 19. Barocas D, Penson D. Racial variation in the pattern and quality of care for prostate cancer in the USA: mind the gap. BJU Int. 2010;106:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krupski TL, Kwan L, Afifi AA, et al. Geographic and socioeconomic variation in the treatment of prostate cancer. J Clin Oncol. 2005;23:7881–7888. [DOI] [PubMed] [Google Scholar]
- 21. Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris MI, Eastman RC, Cowie CC, et al. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22:403–408. [DOI] [PubMed] [Google Scholar]
- 23. Chan L, Giardino N, Rubenfeld G, et al. Geographic differences in use of home oxygen for obstructive lung disease: a national Medicare study. J Rural Health. 2010;26:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goodney PP, Travis LL, Malenka D, et al. Regional variation in carotid artery stenting and endarterectomy in the Medicare population. Circ Cardiovasc Qual Outcomes. 2010;3:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guadagnoli E, Landrum MB, Normand SL, et al. Impact of underuse, overuse, and discretionary use on geographic variation in the use of coronary angiography after acute myocardial infarction. Med Care. 2001;39:446–458. [DOI] [PubMed] [Google Scholar]
- 26. Kumar A, Fonarow G, Eagle K, et al. Regional and practice variation in adherence to guideline recommendations for secondary and primary prevention among outpatients with atherothrombosis or risk factors in the United States: a report from the REACH Registry. Crit Pathw Cardiol. 2009;8:104–111. [DOI] [PubMed] [Google Scholar]
- 27. Buccelletti F, Carroccia A, Marsiliani D, et al. Utility of routine thyroid‐stimulating hormone determination in new‐onset atrial fibrillation in the ED. Am J Emerg Med. 2011;29:1158–1162. [DOI] [PubMed] [Google Scholar]
- 28. Krahn AD, Klein GJ, Kerr CR, et al. How useful is thyroid function testing in patients with recent‐onset atrial fibrillation? The Canadian Registry of Atrial Fibrillation Investigators. Arch Intern Med. 1996;156:2221–2224. [PubMed] [Google Scholar]
- 29. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. [DOI] [PubMed] [Google Scholar]
- 30. Heeringa J, Hoogendoorn EH, van der Deure WM, et al. High‐normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219–2224. [DOI] [PubMed] [Google Scholar]
- 31. Danese MD, Powe NR, Sawin CT, et al. Screening for mild thyroid failure at the periodic health examination: a decision and cost‐effectiveness analysis. JAMA. 1996;276:285–292. [PubMed] [Google Scholar]
- 32. Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 33. Ellrodt AG, Conner L, Riedinger M, et al. Measuring and improving physician compliance with clinical practice guidelines. A controlled interventional trial. Ann Intern Med. 1995;122:277–282. [DOI] [PubMed] [Google Scholar]
- 34. Francke A, Smit M, de Veer AJ, et al. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta‐review. BMC Med Inform Decis Mak. 2008;8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tricoci P, Allen JM, Kramer JM, et al. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 2009;301:831–841. [DOI] [PubMed] [Google Scholar]
- 36. Makar GA, Weiner MG, Kimmel SE, et al. Incidence and prevalence of abnormal liver associated enzymes in patients with atrial fibrillation in a routine clinical care population. Pharmacoepidemiol Drug Saf. 2008;17:43–51. [DOI] [PubMed] [Google Scholar]
- 37. Lip GY, Frison L, Halperin JL, et al. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. [DOI] [PubMed] [Google Scholar]
- 38. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 39. Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47:S51–S55. [DOI] [PubMed] [Google Scholar]
- 40. Hansen K. Medicare and the laboratory. Clin Lab Sci. 2009;22:94–98. [PubMed] [Google Scholar]


