Abstract
Autoreactive B lymphocytes that are not culled by central tolerance in the bone marrow frequently enter the peripheral repertoire in a state of functional impairment, termed anergy. These cells are recognized as a liability for autoimmunity, but their contribution to disease is not well-understood. Insulin-specific 125Tg B cells support T cell-mediated Type 1 diabetes (T1D) in nonobese diabetic (NOD) mice, despite being anergic to B cell mitogens and T cell dependent immunization. Using this model, the potential of anergic, autoreactive B cells to present antigen and activate T cells was investigated. The data show that: a) insulin is captured and rapidly internalized by 125Tg BCRs, b) these antigen-exposed B cells are competent to activate both experienced and naïve CD4+ T cells, c) anergic 125Tg B cells are more efficient than naïve B cells at activating T cells when antigen is limiting, and d) 125Tg B cells are competent to generate low-affinity insulin B chain epitopes necessary for activation of diabetogenic anti-insulin BDC12-4.1 T cells, indicating the pathological relevance of anergic B cells in T1D. Thus, phenotypically tolerant B cells that are retained in the repertoire may promote autoimmunity by driving activation and expansion of autoaggressive T cells via antigen-presentation.
Introduction
Autoreactive B lymphocytes in the developing repertoire are subject to central tolerance in the bone marrow that includes receptor editing and clonal deletion. However, a number of B cells escape central tolerance and enter the mature repertoire in a functionally silent, or anergic, state (1–3). Anergy is considered the principal mechanism that keeps peripheral B cell autoreactivity in check, since anergic cells fail to proliferate or produce antibody in T cell dependent responses(4). However, their role in autoantigen-presentation is not clear.
Transgenic mice (125Tg), in which B cells express anti-insulin B cell receptors (BCRs), have enabled the study of tolerance in B cells that recognize a physiologically relevant hormone antigen that is a critical target in T1D (5, 6). 125Tg BCRs bind rodent insulin with a Kd < 10−7 L/M (7), and the majority of BCRs in 125Tg mice are occupied by insulin in-vivo (8). Proliferative responses to anti-IgM, LPS, or CD40 are significantly impaired in vitro (9), and 125Tg B cells fail to produce insulin-specific antibody responses following immunization (8) in vivo. Nevertheless, these B cells are able to support the development of diabetes in NOD mice (5, 9). T1D in both mice and humans results from T cell-mediated destruction of insulin-producing β cells in pancreatic islets. B cells are necessary for T1D pathogenesis, and several studies indirectly support their role in antigen-presentation, in a capacity that is not redundant with other APCs (10–16). However, the functional status of B cells in the polyclonal populations used in these studies is not clear.
To directly address the function of tolerant B cells in antigen presentation, we used 125Tg B cells and insulin, or insulin conjugated to peptide mimotopes, to probe for antigen-specific responses from functionally distinct CD4+ T cells populations. While the anergic state of anti-insulin B cells was confirmed in studies of calcium mobilization, these B cells nevertheless capture and rapidly internalize insulin for processing and presentation. Anergic 125Tg B cells are competent to activate disease-relevant T cells from NOD mice, including anti-insulin T cells, which require APCs to process and present a critical low-affinity B chain epitope(17). We find that tolerant 125Tg B cells are also competent to present specific epitopes to nonautoreactive, naïve T cells, which have not been previously primed. When compared to naïve B cells, anergic B cells prove efficient for activating T cells when transiently exposed to antigen, indicating that they may be particularly effective when antigen is present intermittently, or at low levels. Since B cells exhibiting a similar functional state are present in normal repertoires, these findings indicate that anergic B cells are an ever-present liability for activating autoaggressive T cells. In contrast to the common assertion that autoimmunity arises from a breach in immune tolerance, we find the pernicious actions of anergic B cells are a consequence of their tolerant state and not its loss.
Materials and Methods
Calcium Flux
Calcium transients were measured using MACS LS Column (Miltenyi)-purified B cells loaded with the ratiometric dye, Fura2AM (Molecular Probes) and a Flexstation II scanning fluorometer (Molecular Devices). The FlexStation II fluorometer was used to measure calcium fluxes following the addition of ligands at 5 seconds (insulin 5µg/mL, hen egg lysozyme (HEL; Sigma) 5µg/mL or ionomycin 1µg/mL) and of 2mM calcium to the calcium-free buffer at 20 seconds. Measurements (340/380 nm excitation ratios) were obtained at 5 s intervals.
Cells for antigen presentation
Lymphocytes were purified by negative selection using MACS LS Columns, following manufacturer’s instructions. CD4+ T cells were selected using a CD4+ T cell Sort Kit II (Miltenyi). For B lymphocyte purification, biotinylated anti-CD43 (1 µL/106 cells), anti-CD11c (0.25 µL/106 cells) and anti-CD11b (0.25 µL/106 cells) were used, followed by streptavidin beads (Miltenyi) prior to negative selection. Cell purity was confirmed by flow cytometry (>85% for T cells, and >90% for B cells).
Insulin Internalization Assays
For cell labeling, insulin (Eli Lilly & Company) or HEL (Sigma Aldrich) were modified using sulfo-MBS (Sigma), reacted with NHS-biotin (Sigma), or directly conjugated with Alexa488 succinimide ester (Molecular Probes). These conjugation reactions for insulin target the lysine at B30 for both Alexa488 and biotin, and these labeled insulins have been interchangeably used for flow cytometry. Biotin-insulin or Alexa-488-insulin (50ng/mL final) were incubated on ice with 106/100µL cells in 1.5 ml microfuge tubes, then washed. Pellets were resuspended in 0.5 mL culture medium and returned to ice or transferred to pre-warmed tubes and incubated at 37°C for the time indicated. Reactions were stopped by the addition of 1 mL cold FACS buffer containing EDTA and azide, and cells were stained for flow cytometry. For confocal microscopy, total splenocytes were collected from 125Tg/NOD mice and treated as above with Alexa-488-insulin. Following incubation with antigen, cells were fixed with 1 mL PBS containing 4% formaldehyde, then stained for B220 using B220-biotin and TexasRed-avidin. Cells were then washed with 1% FBS in PBS, and mounted to glass slides (Cytospin 4, Thermo Shandon, Waltham MA; 1 minute at 750 rpm) using fluorescent mounting medium (Dako, Carpinteria, CA). Visualization was performed at 488nm and 561nm by confocal microscopy using a LSM 710 (Zeiss) equipped with a 63X / 1.4 NA plan apochromat objective lens to image 200 um fields. At least two fields were collected from each sample. Z-stacks of 6–11 slices were prepared at .83–.86µm intervals. Slices with distinct B220 staining were selected for further analysis. Images were prepared for publication using Zen2011 software. Contrast, brightness, and image magnification were adjusted equally across all images to enable optimal visualization. Images were then exported to Photoshop (Adobe) for organization without further manipulation.
Antigen-conjugation
Pork insulin (Eli Lilly & Company) or HEL (Sigma Aldrich) were modified using the heterobifunctional reagent 3-maleimidobenzoic acid N-hydroxy-succinimide ester (MBS) (Sigma Aldrich) in dimethyl formamide (DMF;Sigma Aldrich)(18). The MBS-modified antigens were isolated and reacted with OVA323–329 peptide (KISQAVHC, Sigma-Genosys), or BDC2.5 1040-63 peptide (RTRPLWVRMEC, Schafer-N Inc.) (19) with N-terminal cysteines to form stable thioether bonds. The conjugates were purified by dialysis and chromatography under sterile conditions and protein concentration determined by spectrophotometry.
Antigen-presentation Assays
105 CD4+ T cells were plated in 96 well flat-bottom plates (Costar) with 105 B cells (irradiated, 6 Gys) in 200µL culture medium. Tritiated-thymidine (3H; PerkinElmer) added on day 3 (1µCi/well), was measured on day 4 as counts per minute (CPM) using a Beckman Coulter LS 6500. Anti-insulin 125Tg, control 281Tg B cells, and anti-HEL B cells on C57BL/6 background were used for assays in which responding transgenic T cells were also C57BL/6 (OTII). For T cells from the NOD background (BDC2.5 and BDC12-4.1), 125Tg/NOD and 281Tg/NOD B cells were used. For antigen-pulsing assays, B cells were incubated with antigen at 37°C for 30 minutes, then washed extensively, prior to co-culture with T cells. CFSE labeling was performed as previously described (20). CFSE-labeled T cells (5×105) were plated with B cells (non-irradiated, 5×105) in 24 well plates (Costar) with 1 mL culture medium for 4 days. Number of cells in each peak was determined using cell enumeration for each well, multiplied by relevant subset percentages, determined by flow cytometry. Mitotic events were calculated as previously described (20–22). Briefly, the formula [N×(2n − 1)]/2nwas used to determine mitotic events for each generational peak, “n”, in which “N” is the total number of cells under each peak. This formula allows determination of the original number of precursors for each peak (N×1/2n), and extrapolation of the number of mitotic events for the precursors from each peak by incorporating [2n-1] into the formula. For example, if 8 cells (N) were present in the 2nd divided peak (n), they would have arisen from 2 precursors (8×1/22=2). Each precursor would have undergone one mitotic event to give rise to the first generation of daughter cells and each of those daughter cells would have undergone a second mitotic event, expressed as 22-1=3 mitotic events. In this example, [8×(22 − 1)]/22 =6 mitotic events required to give rise to the 8 cells found in the second generation.
Flow Cytometry
Splenocytes were stained with fluorochrome-conjugated Abs to B220, IgMaCD4, CD69, and 7-AAD (BD Pharmingen). Biotinylated insulin followed by streptavidin-fluorochrome, or insulin-Alexa488 was used to identify insulin-specific B cells. Data were collected on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo (Tree Star) software.
Enzyme linked immunosorbent spot analysis (ELISPOT)
ELISPOT was performed as previously described, using IFNγ ELISPOT kit (BD Biosciences, San Diego, CA), following manufacturer’s instructions (23). Briefly, Purified BDC12-4.1/NOD T cells (105) and 125Tg/NOD or 281Tg/NOD B cells (105) were co-cultured for 48 hours at 37° C in anti-IFNγ-coated 96 well plates (Millipore). IFNγ-producing T cells were enumerated using CTL ImmunoSpot analyzer and CTL ImmunoSpot software, version 3.2 (Cellular Technology).
Cytokine analysis
Cell supernatants from CFSE-labeled cells in antigen-presentation experiments were analyzed using a Mouse Cytokine/Chemokine Millipore multiplex panel (cat.#MPXMCYTO-70K), following manufacturer's instructions. The plate was read on a Luminex 100 instrument, using Exponent 3.2 software. Raw fluorescent values were analyzed using Milliplex Analyst 3.4 software. A five parameter log curve fit was used.
Animals
125Tg and VH281Tg mice were developed as previously described, harboring non-targeted VH and Vk Tgs on C57BL/6 (B6) or NOD backgrounds (6, 9). BDC2.5/NOD mice, OTII/C57Bl/6 mice, and MB4/C57Bl/6 mice were purchased from Jackson labs as breeding pairs, then bred and housed in the Vanderbilt University specific-pathogen-free facility. BDC12-4.1/NOD mice were developed as previously described, bred and housed at the University of Colorado (23). All studies were approved by the Institutional Animal Care and Use Committee of Vanderbilt University, fully accredited by the AAALAC.
Results
B lymphocytes that express an anti-insulin B cell receptor are refractory to insulin stimulation
Insulin-specific 125Tg B cells have impaired proliferative responses to B cell mitogens (9). To confirm that uncoupling of the BCR from antigen-specific signals, the hallmark of B cell tolerance, is present in 125Tg B cells, we assessed calcium mobilization by purified B cells in response to their cognate antigen. Since the essential physiologic requirement for insulin in vivo precludes the availability of naïve anti-insulin B cells, anti-HEL MD4 transgenic B cells were used as controls for an antigen-specific response in naïve B cells. Mobilization of Ca++ by anti-insulin and anti-HEL B cells was measured using dual-wavelength ratiometry on fura 2-AM-loaded B cells (Figure 1A). Calcium flux was initially measured in the absence of extracellular calcium, then following the addition of 2mM Ca++to assess mobilization from intracellular stores and channels in the cell membrane, respectively. The data show that encounter of HEL by naïve anti-HEL control B cells triggers rapid release of Ca++ from intracellular stores. This is followed by a rapid influx through store-operated channels in the membrane upon addition of extracellular Ca++. In contrast, anti-insulin B cells fail to demonstrate any Ca++ mobilization following encounter with ligand. This failure to respond to cognate antigen is highly consistent across a wide range of insulin concentrations (1–500 ug/mL, not shown). Slight elevation of basal Ca++ levels is sometimes observed in anti-insulin B cells but this finding is not consistent. As expected, anti-HEL control B cells do not respond to insulin. Although unable to signal in response to antigen, anti-insulin B cells are competent to flux calcium as demonstrated by the response to the calcium ionophore, ionomycin (Figure 1B). These data and prior functional studies indicate that exposure of anti-insulin B cells to their antigen in vivo is sufficient to uncouple antigen-specific BCR signaling, and render anti-insulin B cells tolerant (8, 9).
Figure 1. Tolerant anti-insulin B lymphocytes internalize antigen, despite failure to flux calcium in response to antigen-engagement.
Purified anti-insulin 125Tg B cells and anti-HEL B cells, were loaded with the ratiometric dye Fura2AM, then plated in triplicate wells containing calcium-free medium. Calcium flux was measured every 5 seconds. A) Calcium flux after the addition of cognate antigen (5 µg/mL) at 5 seconds, followed by addition of extracellular calcium (2mM) at 20 seconds. B) Calcium flux in parallel samples after the addition of the calcium ionophore, Ionomicin (1µg/mL), at 5 seconds and of extracellular calcium at 20 seconds. C&D) Flow cytometry for cell surface analysis: 125Tg B lymphocytes expressing the anti-insulin BCR (IgMa) transgene were incubated with biotinylated insulin on ice, then washed and incubated on ice (C) or at 37°C (D) for 5 minutes. Secondary staining with Avidin-FITC identifies IgM-associated insulin-biotin remaining on the cell surface. E&F) Flow cytometry for cell-associated insulin analysis: 125Tg B cells were incubated with insulin-Alexa-488, on ice, then washed and incubated on ice (E) or at 37°C(F) for 5 minutes, followed by washing and FACS analysis. The insulin-Alexa 488 allows visualization of antigen which remains cell associated. G&H) Confocal images of B cells treated as in E&F to measure insulin-Alexa-488 (green) internalization. Cells were counterstained with B220 (red) to discriminate B cell surface, and mounted on glass slides for direct visualization. Red line down the center of the merged cell images in the x,y plane indicate the section shown in the y,z plane to the right. Representative B cells incubated on ice (G) or internalized after incubation at 37°C for 5 minutes (H). Data are representative of 3–5 experiments.
B cell receptors on tolerant anti-insulin B cells efficiently capture and internalize antigen
To investigate the potential for antigen internalization by 125Tg B cells, labeled insulins were used to track the fate of BCR-associated antigen. Purified B cells were chilled on ice, then incubated with antigen (50ng/ml). After washing in cold buffer, cells were either incubated at 37°C to allow internalization of the antigen, or returned to ice (controls). To provide differential staining, insulin was conjugated to either biotin or Alexa-488. Insulin-Alexa-488 permits identification of internalized antigen by flow cytometry. Biotinylated insulin, however, only allows detection of antigen remaining on the cell surface and is revealed by the addition of fluorochorome-conjugated streptavidin following incubations. Cells incubated on ice do not internalize antigen, and served as controls. As shown in Figure 1, Panel C, 125Tg B cells loaded with biotinylated insulin and incubated on ice, followed by staining with Avidin-FITC, bind insulin in proportion to surface IgM, as do those loaded with insulin-Alexa-488 (Figure 1E). After incubation at 37°C, however, loss of fluorescence (FITC) indicates insulin is no longer present on the surface of cells incubated with biotinylated insulin (Figure 1D). IgM on the surface remains unchanged, consistent with BCR turnover known to continuously replace surface IgM. In contrast, insulin-Alexa-488 allows visualization of the fluorescent signal even after antigen-internalization (Figure 1F), indicating that the antigen has not simply dissociated from the surface, but remains diffusely cell associated. Confocal microscopy was used to confirm internalization of insulin-Alexa-488 by anti-insulin B cells. Splenocytes from 125Tg mice, incubated on ice with insulin-Alexa-488 then washed, were then returned to ice or placed at 37°C to allow for antigen internalization. Cells were counterstained with B220 to discriminate the B cell perimeter. Cell imaging shows that most of the antigen remains on the surface of cells maintained in the cold, whereas antigen is readily visualized internal to the perimeter after warming the cells for 5 minutes (Figure 1Gx and 1HA), respectively). Similar antigen internalization was also observed by confocal microscopy after 10 and 20 minutes at 37°C (Supplemental Figure 1). These findings indicate that tolerant anti-insulin B cells efficiently capture and internalize their ligand in vitro under conditions that reflect monovalent BCR encounter with antigen in vivo. Findings do not differ between 125Tg B cells on C57BL/6 vs. NOD backgrounds for either calcium flux or antigen-internalization.
Naïve T cells are activated by anergic B cells
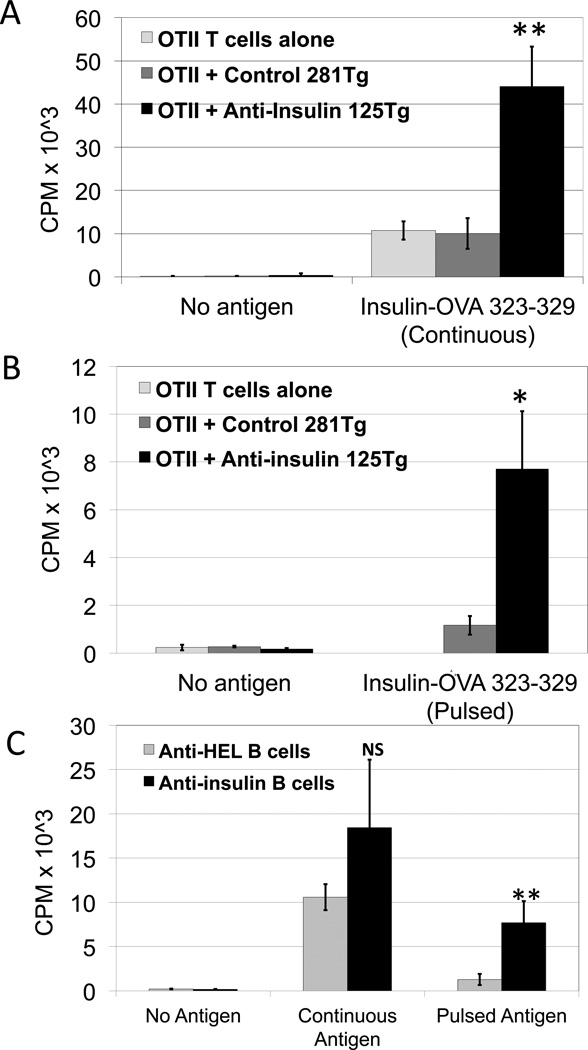
Internalized antigen must be processed for presentation to T cells. To test the ability of tolerant anti-insulin B cells to perform this function, we conjugated insulin to a peptide recognized by OTII CD4+ T cells, OVA323–329, for internalization by the insulin-specific BCR of 125Tg B cells. All cells used in these studies were from mice fully backcrossed onto C57BL/6 backgrounds. Purified OTII CD4+ T cells were co-cultured with irradiated, purified, B cells, with or without insulin-OVA323–329, and T cell proliferation was measured using tritiated thymidine. Figure 2A shows robust proliferative responses of OTII CD4+ T cells activated by 125Tg B cells in the presence of 1µg/mL conjugate antigen (black bars). This is not due to non-specific cellular activation, as indicated by poor proliferation in antigen-free co-cultures. 281Tg noninsulin-binding B cells (dark gray bars), do not stimulate T cells to proliferate above background of T cells and antigen alone (light gray bars), indicating that antigen-specific uptake by tolerant 125Tg B cells, not fluid phase pinocytosis, drives activation of naïve T cells (p<0.01, 125Tg vs. 281Tg or vs. T cells alone, with continuous antigen). These data indicate that the antigen-processing and presentation function of tolerant 125Tg B cells is intact.
Figure 2. Tolerant anti-insulin B lymphocytes induce proliferation of naïve OTII CD4+ T cells.
A) Proliferative responses of purified, naïve OTII CD4+ T cells cultured with an insulin-OVA323–329 peptide conjugate antigen (1µg/mL) and irradiated anti-insulin 125Tg B cells (black), non-insulin-binding 281Tg B cells (dark gray), or without B cells (light gray). CPM represents proliferating T cell incorporation of 3H-thymidine. B) Irradiated 125Tg B cells (black) and 281Tg control B cells (gray) were pulsed with antigen for 30 minutes, then washed prior to co-culture with OTII CD4+ T cells. C) Comparison of OTII CD4+ T cell proliferation in the presence of anti-insulin 125Tg B cells (black bars) and insulin-OVA323–329 peptide conjugate antigen, or naïve anti-HEL B cells (gray bars) and HEL-OVA323–329 peptide conjugate antigen. T cells were co-cultured with B cells in the continuous presence of antigen, or with B cells pulsed with antigen, then washed prior to co-culture. Bars represent the average of three samples, with standard deviations as shown, representative of 2–5 experiments, n=2–3 pooled mice per genotype, per sample. NS=nonsignificant, *p=0.01, **p<0.01.
Antigen-pulsed anergic B cells are sufficient for T cell activation
B cells occupy a unique antigen-presenting niche, as they are able to internalize and concentrate specific antigen. We therefore examined the capability of anergic B cells to process and present limited amounts of physiologic antigen. Purified B cells were pulsed with insulin-OVA323–329 conjugate at 37° C for 30 minutes, then washed to mimic antigen acquisition under temporal conditions. These antigen-loaded B cells were irradiated and co-cultured with OTII CD4+ T cells. No free antigen was added to the cultures. Data presented in Figure 2B show that OTII CD4+ T cells are induced to proliferate by antigen-pulsed 125Tg B cells (black bars). In the absence of antigen exposure, 125Tg B cells do not induce T cell proliferation, and antigen-pulsed control 281Tg B cells induce only low levels of proliferation, which may be due to limited antigen uptake via pinocytosis (dark gray bars; p=0.01, pulsed 125Tg vs. pulsed 281Tg). These data indicate that tolerant B cells specific for a physiologic antigen, insulin, can present antigen and activate naïve cognate T cells in a setting wherein antigen is present only in small amounts, or for limited periods of time.
Tolerant 125Tg B cells present antigen more efficiently than naïve B cells under antigen limiting conditions
We next compared the efficiency of antigen presentation by anergic 125Tg B cells with that of naïve B cells. Because their antigen is present physiologically, mature, naïve, anti-insulin B cells are not available for study. We therefore used transgenic B cells that recognize HEL (MD4) as naïve comparators (C57BL/6 background). Conjugated HEL-OVA323–329 antigen was used to test antigen-presenting capacity of naïve anti-HEL B cells to OTII CD4+ T cells, in continuous and pulsed conditions, in parallel assays with anergic 125Tg B cells and insulin-OVA323–329. As shown in Figure 2C, OTII CD4+ T cell proliferation in response to continuous antigen is not significantly different when activated by anergic 125Tg B cells (black bars) or naïve anti-HEL B cells (gray bars). However, when B cells are pulsed with antigen, then washed prior to co-culture with T cells, naïve anti-HEL B cells are unable to stimulate T cell proliferation as well as anergic anti-insulin 125Tg B cells can (p=0.01). This finding for anti-insulin B cells is consistent with previously published studies indicating that naive anti-HEL B cells internalize antigen less efficiently than anergic ones (24). Thus, under conditions of limited or intermittent antigen availability, tolerant B cells may have an advantage in activating their cognate T cells compared to their naïve counterparts.
Tolerant 125Tg B cells drive anti-insulin BDC12-4.1 T cells to produce IFNγ
To determine the pathogenic relevance of antigen-processing and presentation by tolerant anti-insulin B cells, we next used the NOD mouse model of T1D. In studies published to date, 125Tg B cells are the only Ig transgenic B cells that fully support diabetes development in NOD mice (5). To directly test their ability to present autoantigen in this setting, we used BDC12-4.1/NOD CD4+ T cells, which recognize a key insulin epitope that mediates diabetes development in NOD mice (23). Importantly, BDC12-4.1 CD4+ T cells were recently shown to recognize a truncated peptide from insulin B:9–23, that binds IAg7 in register 3 with low affinity(17). Therefore, antigen-handling by APCs is critical to the activation of BDC12-4.1 T cells, as processing must generate this low-affinity epitope to be effective. We used ELISPOT assays to determine if anti-insulin 125Tg/NOD B cells could drive BDC12-4.1/Rag−/−/NOD T cells to produce IFNγ, as this response is known to report splenocyte APC and peptide stimulation (23). As shown in Figure 3, co-culture of 125Tg/NOD B cells (black bars) with BDC12-4.1/Rag−/−/NOD CD4+ T cells, in the presence of intact insulin, induced increased numbers of T cells to produce IFNγ, compared with those co-cultured with antigen and 281Tg/NOD B cells (gray bars, p<0.01). These data directly demonstrate that tolerant anti-insulin B cells are capable of driving effector function of pathogenic T cells. Further, the findings indicate that processing of insulin by tolerant 125Tg B cells permits presentation of low-affinity pathogenic epitopes from insulin.
Figure 3. Anti-insulin B lymphocytes stimulate anti-insulin BDC12-4.1 CD4+ T cells to produce IFNγ.
ELISPOT analysis of IFNγ production by purified BDC12-4.1/NOD CD4+ T cells co-cultured with insulin and 125Tg/NOD B cells (black bars), or non-insulin-binding 281Tg/NOD control B cells (gray bars). Bars represent the average of three samples, with three pooled mice per genotype, with standard deviations, indicated by error bars, **p<0.01.
Cognate interaction with tolerant 125Tg/NOD B cells induces proliferation and TH1-predominant cytokine production by islet-specific BDC2.5/NOD T cells
To investigate the ability of tolerant B cells to fully activate diabetogenic T cells, we used another well-characterized TCR transgenic model of T1D, BDC2.5/NOD CD4+ T cells that recognize chromogranin A (CGA) (25), with 125Tg/NOD or 281Tg/NOD B cells. For these experiments, insulin was conjugated to a BDC2.5 peptide mimotope, 1040-63, known to activate these T cells (19). The data show that BDC2.5/NOD T cells proliferate better when co-cultured with antigen and 125Tg/NOD B cells (Figure 4A, black bars), than with non-insulin binding controls (dark gray bars). In pulsed conditions, T cells are induced to proliferate by antigen-pulsed 125Tg/NOD B cells (Figure 4B, black bars), but not antigen-pulsed 281Tg/NOD control B cells (dark gray bars; p=0.01). To understand the extent of this cellular activation, additional experiments were performed using CFSE-labeled BDC2.5/NOD CD4+ T cells. This approach allows analysis of all cells that proliferate during the full four-day course of the culture, as well as confirmation of cellular activation, and cytokine analysis of supernatants. Figure 5A shows flow cytometric analysis of CD4+ gated, CFSE-labeled BDC2.5/NOD T cells after co-culture with 125Tg/NOD B cells. T cells that have undergone division are represented by CFSE+ peaks of decreasing fluorescence (x-axis), and are also activated, indicated by increased expression of CD69 (y-axis). The histogram overlay depicts CFSE+ peaks from CD4+ gated T cells after co-culture with 125Tg/NOD B cells (blue), 281Tg/NOD control B cells (red), or alone (black), in the presence of antigen. Enumeration of CD4+ T cells in each CFSE+ generational peak confirms the robust proliferative responses of BDC2.5/NOD T cells co-cultured with 125Tg/NOD B cells compared to control 281Tg/NOD (Figure 5B, p<0.05 for the first generation, and <0.01 for generations 2–5). Total mitotic events were calculated for each peak, and summed, as shown in Figure 5C (7.89 +/− 1.48×105 total mitotic events for cells co-cultured with 125Tg, vs 2.19 +/−1.48×105 for those with 281Tg, p<0.01). To determine the functional outcome of interactions between tolerant B cells and BDC2.5/NOD CD4+ T cells, cytokine analysis was performed by Luminex assay on cellular supernatants from the above studies. As shown in Figure 5D, IFNγ predominates the cytokine profile from T cells co-cultured with 125Tg/NOD (4514 +/− 594 pg/mL) vs. 281Tg/NOD (762 +/− 54 pg/mL, p<0.01). Other cytokines shown to be differentially produced are: IL-10 (821 +/−58 pg/mL vs. 340 +/− 25 pg/mL p<0.01), IL-17 (278 +/− 43 pg/mL vs. 193 +/− 23 pg/mL p<0.05), TNFα (100 +/− 10 pg/mL vs. 53 +/− 7 pg/mL p<0.01) and IL-4, which was found only in extremely small amounts (7.5 +/−2.2 pg/mL vs. 3.2 +/− 0.0 pg/mL, p<0.05). Other cytokines tested that were not statistically different between groups included IL-2, IL-6 and IL-12 (not shown).
Figure 4. Anti-insulin B lymphocytes induce proliferation of BDC2.5/NOD T cells.
A) BDC2.5/NOD CD4+ T cell proliferative response to titrated concentrations of conjugate antigen and 125Tg/NOD B cells (black bars), 281Tg/NOD non-insulin binding control B cells (dark gray bars) or with antigen alone (light gray bars), by 3H-thymidine incorporation. B) Proliferative BDC2.5/NOD T cell responses in response to co-culture with antigen-pulsed B cells (as in figure 2). Bars indicate the average of triplicate samples, with error bars demarking standard deviations. *p<0.05, **p<0.01. Data are representative of more than 3 independent experiments, n=2–3 pooled mice per genotype per sample.
Figure 5. CFSE labeling further defines BDC2.5/NOD T cell responses to antigen-presentation by anti-insulin B lymphocytes.
A) Flow cytometric analysis of CFSE-labeled BDC2.5 T cells. Left panel shows CD4+ gated cells after co-culture with 125Tg/NOD B cells and conjugate antigen. CFSE peaks of descending MFI indicate cells that have undergone sequential division (x-axis). Y-axis indicates activation marker CD69. Right panel shows a histogram overlay of CD4+ T cells co-cultured with 125Tg/NOD B cells (blue), 281Tg/NOD B cells (red), or with antigen alone (black). Images are representative of four experiments. B) Total number of BDC2.5/NOD T cells present in each CFSE+ peak after co-culture with 125Tg/NOD B cells (black), 281Tg /NOD B cells (dark gray), or with antigen alone (light gray). C) Mitotic events per well. D) Cytokine production measured by Luminex assay from the supernatant fluid of CFSE-labeled cells. Bars represent the average of triplicate samples, with error bars indicating standard deviations. N=15 mice (3 mouse per genotype, 1 mouse per sample for B cells, and 9 pooled BDC2.5/NOD mice). *p<0.05, **p<0.01. Data are representative of 3 experiments.
Discussion
Tolerance, or anergy, in self-reactive B cells is expected to protect against autoimmunity, and a breach in tolerance is generally considered necessary for initiation of autoimmune disorders. However, the data presented here indicate that the anergic state does not prevent anti-insulin B cells from presenting antigen for both conventional and autoimmune T cell epitopes. Rather, BCR internalization results in effective delivery of antigen to endosomal compartments and loading of MHCII as evidenced by the ability of anti-insulin B cells to activate antigen-specific T cells. Using insulin conjugated to known T cell epitopes, anti-insulin B cells are shown to be effective APCs for both naïve CD4+ T cells that recognize ovalbumin peptide (OTII) and for potentially experienced autoreactive (BDC2.5) T cells that recognize a peptide mimotope (1040-63) for chromogranin (CGA). Anti-insulin B cells that present this mimotope to BDC2.5 T cells support a TH1 effector response in this model. In addition to conjugated epitopes, anti-insulin B cells are also competent to generate and present epitopes from intact insulin and drive IFNγ production from diabetogenic anti-insulin (BDC12.4-1) T cells. These in vitro studies are consistent with the finding that anti-insulin transgenic BCRs provide NOD B cells with an essential component that promotes T-cell-mediated T1D in vivo, despite their functionally anergic state (5, 9).
The interaction of 125Tg B cells with anti-insulin T cells allows new insight into the question of how the critical epitopes recognized by these T cells may be presented in vivo. BDC12-4.1 T cells recognize distinct residues from the B:9–23 peptide of insulin, register 12–20, that binds IAg7 with a low affinity. This low-affinity interaction is proposed to permit T cells to escape deletion in the thymus (26, 27). Some studies suggest that this type of low-affinity epitope may only be generated within the islets, where insulin concentrations are sufficiently high to load weakly binding peptides onto diabetogenic MHCII, IAg7. Our findings identify an additional mechanism for generating low-affinity epitopes that is mediated by the efficient capture and processing of autoantigen by specific B cells in which the continuous delivery of insulin to endosomal compartments aid MHCII loading of low-affinity epitopes. As B cells heavily populate inflamed islets, and many are insulin-specific (28, 29), this may, in fact, be a major means by which pathogenic T cells are activated in islets.
Fully functional, antigen-specific B cells are recognized to be highly efficient APCs because of their ability to capture and concentrate antigens for processing and presentation to T cells (30, 31). The present study on anti-insulin B cells, and recent data on tolerant B cells in anti-HEL/sHEL or anti-ssDNA models, clearly demonstrate that facilitated BCR internalization is a component of the tolerant state (24, 32, 33). Tolerance in the HEL model is characterized by developmental arrest and rapid turnover of B cells at the transitional stage (34). In contrast, anti-insulin B cells are not blocked in development and enter mature B cell subsets (5, 8, 9). Thus, receptor internalization remains intact in tolerant B cells from these two different models, independent of the maturational subset in which they reside. Similarly, internalization of BCRs is also intact in anergic anti-ssDNA and in ArsA1 B cells (32). However, the anergic state of B cells in these models includes a reversible block in late endosomal entry of BCR and TLR necessary for effective antigen presentation (32). This level of control is not evident in either anti-HEL or anti-insulin models (this report and (35, 36)). Thus, the diverse mechanisms by which B cell anergy is maintained for different self-molecules also includes differences in how tolerogenic signaling regulates antigen processing and presentation.
The question of how these various models relate to anergy in the physiologic setting is still open. Anergic, autoreactive-prone B cells have been shown to be part of the normal repertoire, in both mice and humans, and may be increased in some disease states(1–3). B cell anergy can be reversed in certain circumstances(9, 37, 38), raising the question of whether interaction with cognate T cells could deliver signals that would reverse B cell anergy and lead, in turn, to more efficient T cell activation by those B cells. In fact, the underlying assumption regarding anergic B cell contributions to disease is that this reversal is a necessary component. Elegant studies in the anti-HEL model have shown that T cell help can effectively rescue those cells from antigen-induced anergy, and that this rescue is more effective in NOD than B6 B cells(38). Anti-insulin B cells used in the studies presented here, however, retain their anergic properties in most circumstances, with the exception of in vitro stimulation with anti-CD40 together with IL4(9). Co-culture with cognate T cells in the assays presented in Figure 5, which did not require B cell irradiation, failed to expand B cell numbers, indicating lack of proliferation even in the setting of direct interaction with cognate helper T cells (Supplemental Figure 2A). We have also performed adoptive transfer studies using CFSE-labeled anti-insulin B cells and find that they can successfully populate inflamed islets with little evidence of proliferation even as late as five days after transfer (Supplemental Figure 2B). Furthermore, as we have previously shown, even T cell dependent immunization using insulin-CFA fails to induce them to produce insulin antibodies in vivo in either C57Bl/6 or NOD mice(8). Nevertheless, they are able to support the development of T1D in the NOD model(9). Therefore, it does not seem likely that the antigen-presenting function of 125Tg B cells depends upon their loss of anergy.
A related question concerns the anergic state of B cells that promote T1D in the endogenous BCR repertoire of WT NOD mice, and in humans. Since anti-insulin antibodies are the hallmark, and predictive, of T1D, then by definition there are non-anergic anti-insulin B cells present. It is possible that these antibodies may arise in the wake of somatic hypermutation (SH) from nonanergic cells that originally recognized insulin with lower affinity. This idea is supported by the fact that the 125Tg was originally created from an anti-insulin antibody that emerged with 2 CDR changes post-immunization from a germline heavy chain (281Tg) that does not detect insulin at high enough affinity to be detected by flow cytometry. However, antibody production may be a separate issue from antigen-presentation in terms of disease promotion. While the findings presented here do not prove that anergic cells in the WT NOD repertoire promote disease development in T1D, they support the idea that anergy need not necessarily be reversed to contribute to autoimmune disease via antigen-presentation.
These findings contribute to a body of emerging data on the importance of B cells in T1D in both NOD mice and in humans (10, 15, 16, 20, 39–42). Furthermore, our data emphasize the important autoantigen-presenting function of B lymphocytes that have not emerged from their tolerant state. We propose the presence of such B cells expands the pool of autoreactive T cells prior to the appearance of autoantibodies. Therefore, the limited success of T1D interventions in subjects with autoantibodies to insulin, GAD65, or other beta cell antigens, may reflect autoreactive T cell expansion occurring in response to anergic B cells, before overt loss of tolerance in the B cell compartment gives rise to autoantibodies. Understanding the regulation of these events in tolerant anti-insulin B cells may provide new targets for preventing the expansion of autoaggressive T cells in T1D.
Supplementary Material
Acknowledgements
We would like to acknowledge Dr. George Eisenbarth for support and encouragement throughout the development of this project. We would like to thank Chrys Hulbert and Hunter Houston at Vanderbilt University Medical Center for technical assistance. The Vanderbilt Hormone Assay and Analytical Services Core performed the Luminex cytokine analysis. Flow Cytometry experiments were performed in the VMC Flow Cytometry Shared Resource. Confocal experiments were performed in part through the use of the VUMC Cell Imaging Shared Resource. We thank Chrys Hulbert and Hunter Houston at Vanderbilt University Medical Center for technical assistance, and Maki Nakayama, University of Colorado, Denver, for her role in developing the BDC12-4.1/NOD mouse model.
Footnotes
This work was supported by National Institutes of Health Grants R01 DK 084246, R01 AI 051448, K08 DK 070924, and R21 DK 084568, as well as JDRF grant numbers APC 4-2007-1056 and 1-2008- 108. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center(DK058404). The VUMC Cell Imaging Shared Resource is supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126.
References
- 1.Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, Mathias M, Garman L, Helms C, Nakken B, Smith K, Farris AD, Wilson PC. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quach TD, Manjarrez-Orduno N, Adlowitz DG, Silver L, Yang H, Wei C, Milner EC, Sanz I. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. Journal of immunology. 2011;186:4640–4648. doi: 10.4049/jimmunol.1001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, cote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nature reviews. Immunology. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulbert C, Riseili B, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J Immunol. 2001;167:5535–5538. doi: 10.4049/jimmunol.167.10.5535. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroer JA, Bender T, Feldmann RJ, Kim KJ. Mapping epitopes on the insulin molecule using monoclonal antibodies. Eur J Immunol. 1983;13:693–700. doi: 10.1002/eji.1830130902. [DOI] [PubMed] [Google Scholar]
- 8.Rojas M, Hulbert C, Thomas JW. Anergy and not clonal ignorance determines the fate of B cells that recognize a physiological autoantigen. J Immunol. 2001;166:3194–3200. doi: 10.4049/jimmunol.166.5.3194. [DOI] [PubMed] [Google Scholar]
- 9.Acevedo-Suarez CA, Hulbert C, Woodward EJ, Thomas JW. Uncoupling of anergy from developmental arrest in anti-insulin B cells supports the development of autoimmune diabetes. J. Immunol. 2005;174:827–833. doi: 10.4049/jimmunol.174.2.827. [DOI] [PubMed] [Google Scholar]
- 10.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new"speed congenic" stock of NOD.Ig mu null mice. J Exp. Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noorchashm H, Lieu YK, Noorchashm N, Rostami SY, Greeley SA, Schlachterman A, Song HK, Noto LE, Jevnikar AM, Barker CF, Naji A. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163:743–750. [PubMed] [Google Scholar]
- 12.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46:941–946. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 13.Serreze DV, Silveira PA. The role of B lymphocytes as key antigen-presenting cells in the development of T cell-mediated autoimmune type 1 diabetes. Curr.Dir.Autoimmun. 2003;6:212–227. doi: 10.1159/000066863. [DOI] [PubMed] [Google Scholar]
- 14.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–1168. [PubMed] [Google Scholar]
- 15.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 16.Silveira PA, Johnson E, Chapman HD, Bui T, Tisch RM, Serreze DV. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur.J Immunol. 2002;32:3657–3666. doi: 10.1002/1521-4141(200212)32:12<3657::AID-IMMU3657>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 17.Crawford F, Stadinski B, Jin N, Michels A, Nakayama M, Pratt P, Marrack P, Eisenbarth G, Kappler JW. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci U S A. 2011;108:16729–16734. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peeters JM, Hazendonk TG, Beuvery EC, Tesser GI. Comparison of four bifunctional reagents for coupling peptides to proteins and the effect of the three moieties on the immunogenicity of the conjugates. J Immunol Methods. 1989;120:133–143. doi: 10.1016/0022-1759(89)90298-6. [DOI] [PubMed] [Google Scholar]
- 19.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 20.Kendall PL, Moore DJ, Hulbert C, Hoek KL, Khan WN, Thomas JW. Reduced diabetes in btk-deficient nonobese diabetic mice and restoration of diabetes with provision of an anti-insulin IgH chain transgene. Journal of immunology. 2009;183:6403–6412. doi: 10.4049/jimmunol.0900367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noorchashm H, Moore DJ, Noto LE, Noorchashm N, Reed AJ, Reed AL, Song HK, Mozaffari R, Jevnikar AM, Barker CF, Naji A. Impaired CD4 T cell activation due to reliance upon B cell-mediated costimulation in nonobese diabetic (NOD) mice. J.Immunol. 2000;165:4685–4696. doi: 10.4049/jimmunol.165.8.4685. [DOI] [PubMed] [Google Scholar]
- 22.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J.Clin.Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jasinski JM, Yu L, Nakayama M, Li MM, Lipes MA, Eisenbarth GS, Liu E. Transgenic insulin (B:9-23) T-cell receptor mice develop autoimmune diabetes dependent upon RAG genotype, H-2g7 homozygosity, and insulin 2 gene knockout. Diabetes. 2006;55:1978–1984. doi: 10.2337/db06-0058. [DOI] [PubMed] [Google Scholar]
- 24.Blery M, Tze L, Miosge LA, Jun JE, Goodnow CC. Essential role of membrane cholesterol in accelerated BCR internalization and uncoupling from NF-kappa B in B cell clonal anergy. The Journal of experimental medicine. 2006;203:1773–1783. doi: 10.1084/jem.20060552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K. Chromogranin A is an autoantigen in type 1 diabetes. Nature immunology. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an insulin peptide-MHC complex allows diabetogenic T cells to escape thymic deletion. J Exp Med. 2011;208:2375–2383. doi: 10.1084/jem.20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nature immunology. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendall PL, Yu G, Woodward EJ, Thomas JW. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. J Immunol. 2007;178:5643–5651. doi: 10.4049/jimmunol.178.9.5643. [DOI] [PubMed] [Google Scholar]
- 29.Henry RA, Kendall PL. CXCL13 blockade disrupts B lymphocyte organization in tertiary lymphoid structures without altering B cell receptor bias or preventing diabetes in nonobese diabetic mice. Journal of immunology. 2010;185:1460–1465. doi: 10.4049/jimmunol.0903710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 31.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984;160:1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Neill SK, Veselits ML, Zhang M, Labno C, Cao Y, Finnegan A, Uccellini M, Alegre ML, Cambier JC, Clark MR. Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic cells. Proc Natl Acad Sci U S A. 2009;106:6262–6267. doi: 10.1073/pnas.0812922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou P, Araujo E, Zhao T, Zhang M, Massenburg D, Veselits M, Doyle C, Dinner AR, Clark MR. B cell antigen receptor signaling and internalization are mutually exclusive events. PLoS Biol. 2006;4:e200. doi: 10.1371/journal.pbio.0040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornall RJ, Goodnow CC. B cell antigen receptor signalling in the balance of tolerance and immunity. Novartis Found Symp. 1998;215:21–30. doi: 10.1002/9780470515525.ch3. discussion 30–40. [DOI] [PubMed] [Google Scholar]
- 35.Eris JM, Basten A, Brink R, Doherty K, Kehry MR, Hodgkin PD. Anergic self-reactive B cells present self antigen and respond normally to CD40-dependent T-cell signals but are defective in antigen-receptor-mediated functions. Proc Natl Acad Sci U S A. 1994;91:4392–4396. doi: 10.1073/pnas.91.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark MR, Tanaka A, Powers SE, Veselits M. Receptors, subcellular compartments and the regulation of peripheral B cell responses: the illuminating state of anergy. Mol Immunol. 2011;48:1281–1286. doi: 10.1016/j.molimm.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat. Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 38.Cox SL, Stolp J, Hallahan NL, Counotte J, Zhang W, Serreze DV, Basten A, Silveira PA. Enhanced responsiveness to T-cell help causes loss of B-lymphocyte tolerance to a beta-cell neo-self-antigen in type 1 diabetes prone NOD mice. European journal of immunology. 2010;40:3413–3425. doi: 10.1002/eji.201040817. [DOI] [PubMed] [Google Scholar]
- 39.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagafuchi S, Katsuta H, Anzai K. Rituximab, B-lymphocyte depletion, and beta-cell function. N Engl J Med. 2010;362:761. doi: 10.1056/NEJMc0912877. author reply 761. [DOI] [PubMed] [Google Scholar]
- 41.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kendall PL, Woodward EJ, Hulbert C, Thomas JW. Peritoneal B cells govern the outcome of diabetes in non-obese diabetic mice. Eur. J Immunol. 2004;34:2387–2395. doi: 10.1002/eji.200324744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.