Abstract
While moderate calorie restriction (CR) in the absence of malnutrition has been consistently shown to have a systemic, beneficial effect against aging in several animals models, its effect on the brain microstructure in a non-human primate model remains to be studied using post-mortem histopathologic techniques. In the present study, we investigated differences in expression levels of glial fibrillary acid protein (GFAP) and β-amyloid plaque load in the hippocampus and the adjacent cortical areas of 7 Control (ad libitum)-fed and 6 CR male rhesus macaques using immunostaining methods. CR monkeys expressed significantly lower levels (∼30% on average) of GFAP than Controls in the CA region of the hippocampus and entorhinal cortex, suggesting a protective effect of CR in limiting astrogliosis. These results recapitulate the neuroprotective effects of CR seen in shorter-lived animal models. There was a significant positive association between age and average amyloid plaque pathology in these animals, but there was no significant difference in amyloid plaque distribution between the two groups. Two of the seven Control animals (28.6%) and one of the six CR animal (16.7%) did not express any amyloid plaques, five of seven Controls (71.4%) and four of six CR animals (66.7%) expressed minimal to moderate amyloid pathology, and one of six CR animals (16.7%) expressed severe amyloid pathology. That CR affects levels of GFAP expression but not amyloid plaque load provides some insight into the means by which CR is beneficial at the microstructural level, potentially by offsetting the increased load of oxidatively damaged proteins, in this non-human primate model of aging. The present study is a preliminary post-mortem histological analysis of the effects of CR on brain health, and further studies using molecular and biochemical techniques are warranted to elucidate underlying mechanisms.
Keywords: calorie restriction, GFAP, amyloid, aging, rhesus macaque
1. Introduction
Calorie restriction (CR) without malnutrition slows the aging process and prolongs median and maximum lifespan in yeast, worms, flies, fish, and rodents (Kennedy et al., 2007; Anderson and Weindruch, 2010). Three independent studies of CR in non-human primates (Macaca mulatta) have demonstrated that CR confers a protective effect against aging-related diseases (Bodkin et al., 2003; Colman et al., 2009; Mattison et al., 2012), although consensus has not been reached regarding the beneficial effect of CR on survival. The beneficial effects of CR in nonhuman primates include prevention of age-related loss of muscle mass, fewer incidences of cardiovascular disease, increased insulin sensitivity and glucose tolerance, lower cancer incidence, preservation of critical gray and white matter regions, and protective changes in gene expression (Colman and Anderson, 2011; Kemnitz, 2011). The specific mechanisms by which CR delays aging and the onset of age-associated disease have yet to be elucidated. It has been well established that CR is associated with reduced levels of oxidative damage and reduced inflammatory tone (Heilbronn and Ravussin, 2003; Weindruch, 2003), outcomes that may be particularly important with respect to brain aging.
Recently, our group has demonstrated the wide-ranging effects of CR in the rhesus macaque brain using neuroimaging techniques. Brain atrophy, a characteristic aging change in both humans and non-human primates, is attenuated by CR in rhesus macaques, particularly in the midcingulate cortex, bilateral lateral temporal cortex, and right dorsolateral frontal cortex (Colman et al., 2009). CR also preserves white matter integrity in the fronto-occipital fasciculus, superior longitudinal fasciculus, external capsule, and brainstem (Bendlin et al., 2011). In addition, age-related iron accumulation in the basal nuclei, red nucleus, and parietal, temporal, and perirhinal cortices is attenuated with CR, and this in turn is associated with improved performance on motor function tests (Kastman et al., 2010). Consistent with improved inflammatory tone, CR moderates the effect of important plasma-based inflammatory (e.g. IL-6) and vascular (e.g. homocysteine) markers on gray and white matter changes in several brain regions that are sensitive to aging (Willette et al., 2010; Willette et al., 2012a). Furthermore, CR improves glucoregulatory profiles in these animals and positively influences gray matter volume in the hippocampus and motor task performance (Willette et al., 2012b). While these findings point to an overall salubrious effect of CR on the brain, it is important to confirm and extend the effect of CR by examining neuropathological indices using post-mortem histologic techniques.
Several neuropathological alterations are associated with aging in the brain. The expression level of glial fibrillary acidic protein (GFAP), a marker of astrocytic activation, increases with age in rodents, rabbits, monkeys, and humans (Finch, 2003). This increased GFAP expression during aging has been suggested to be a consequence of the increased load of oxidatively damaged proteins, which occur in tissues throughout the body (Finch, 2003; Middeldorp and Hol, 2011). In rats, CR attenuates the age-associated increase in glial activation (Morgan et al., 1997; Kaur et al., 2008). Additionally, reactive astrogliosis is also predominant in several neurodegenerative diseases, including Down syndrome (Trisomy 21), Parkinson disease, Huntington disease, and Alzheimer disease (AD), and is suggested to be secondary to marked neurodegeneration and neuronal death characteristic of these diseases (Middeldorp and Hol, 2011). Higher GFAP expression levels are associated with AD in humans (Beach et al., 1989). Moreover, subjects with the ApoE ε4 allele (a risk factor for developing AD) express higher levels of GFAP than non-APOE ε4 carriers (Overmyer et al., 1999).
Another common histological change that occurs with aging in both monkeys and humans is a progressive increase in the formation of amyloid plaques (Heilbroner and Kemper, 1990; Sloane et al., 1997; Anderton, 2002). These are formed by the abnormal aggregation of amyloid-β peptide (Aβ), a small peptide that is involved in the pathogenesis of AD in humans (Zhang et al., 2012). Although Aβ deposition and plaque formation are signature pathologies of AD in humans, they are also commonly present in the brains of cognitively normal older adults (Rodrigue et al., 2009). Rhesus monkeys do not develop AD, but amyloid plaques are detected in the cortex in aged animals (Heilbroner and Kemper, 1990; Sloane et al., 1997; Uno, 1997). In this way, AD is associated with amyloid plaques but plaques are not always associated with cognitive impairment. Interestingly, both in rodent models of AD and in human AD, there is increased GFAP expression adjacent to Aβ plaques (Hanzel et al., 1999; Gallagher et al., 2012). Human AD amyloid plaques are surrounded by astrocytes expressing high levels of antioxidant enzymes, including superoxide dismutase, suggesting a role for oxidative stress in mediating age-associated astrocytic activation (Furuta et al., 1995).
The current report is an interim post-mortem histological analysis of the effects of age and CR on brain health. We examined GFAP expression and Aβ plaque distribution in the male rhesus macaque hippocampus and the adjacent entorhinal cortex (EC). The hippocampus exhibits extensive astrogliosis both during normal aging and in neurodegenerative states such as AD (Nichols et al., 1993). Since amyloid plaques in the aged rhesus brains preferentially accumulate in association cortical areas (Sani et al., 2003), we also examined the overlying neocortex (mainly temporal) for Aβ plaque distribution. We hypothesized that CR would lead to a decrease in the expression of GFAP and Aβ plaque frequency.
2. Results
2.1. Subject Characteristics
Table 1 shows the demographic characteristics of the animals. There was no significant difference in age distribution between Control and CR animals. Age-related causes of death included adenocarcinoma (N = 3 Controls, 2 CR), cardiomyopathy (N = 1 Control), immune suppression (N = 1 Control), renal and cardiac disease (N = 1 Control), and cerebral edema (N = 1 Control), whereas non age-related causes included viral cardiomyopathy (N = 1 CR), viral myocarditis (N = 1 CR), anastomosis dehiscence (N = 1 CR), and abdominal hemangioma (N = 1 CR).
Table 1. Subject characteristics.
| Controls | CR | |
|---|---|---|
| N | 7 | 6 |
| Age (years) | ||
| Mean ± SEM | 23.25 ± 1.74 | 25.77 ± 1.20 |
| Range | 15-28 | 21-29 |
| Cause of death | ||
| Age-related | 7 | 2 |
| Non age-related | 0 | 4 |
2.2. GFAP Immunoreactivity
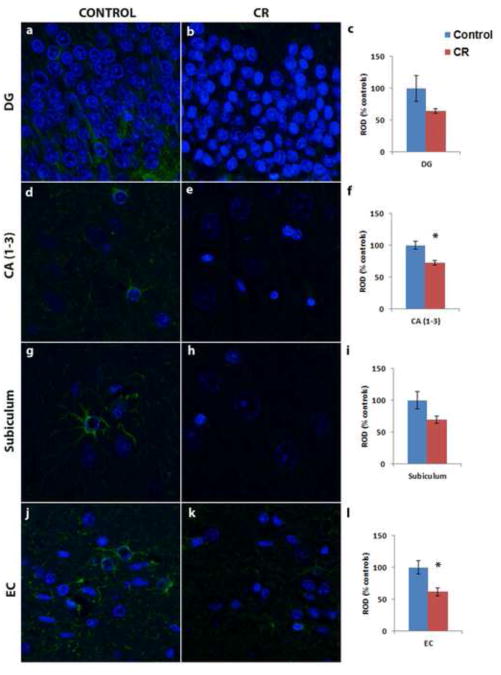
There was no significant relationship between GFAP expression and age in a combined analysis that included all Control and CR animals (p > 0.05). GFAP was detected at significantly higher levels in Control animals in the hippocampus compared to CR animals (Fig 1). Specifically, GFAP immunoreactivity in the CA region of the hippocampus (Fig 1d-f, control = 100 ± 6.07%, CR = 72.77 ± 3.76%, p = 0.004) and entorhinal cortex (Fig 1j-l, control = 100 ± 10.34%, CR = 62.05 ± 6.18%, p = 0.032) was significantly greater in Controls than CR. A trend in a similar direction (Controls > CR) was detected in the granule cell layer of the dentate gyrus (Fig 1a-c, control = 100 ± 20.13%, CR = 64.37 ± 3.85%, p = 0.24) and subiculum (Fig 1g-i, control = 100 ± 13.61%, CR = 69.72 ± 5.52%, p = 0.13), but did not reach significance. There was a significant correlation in GFAP expression among the different regions examined within individuals (p < 0.01).
Figure 1.
GFAP immunoreactivity (green) in the dentate gyrus (DG) (a-c), CA (1-3) (d-f), and subiculum (g-i) of the hippocampus and EC (j-l) of representative Control (a, d, g, j) and calorie restricted (CR) (b, e, h, k) animals. GFAP expression is significantly reduced in the CR animals (N = 6) compared to Controls (N = 6). * denotes a significant difference between the two dietary groups (p < 0.05). Nuclei were counterstained with DAPI (blue). Relative optical density (ROD) is expressed as mean percent (±SEM) relative to average control expression.
2.3. Aβ plaque distribution
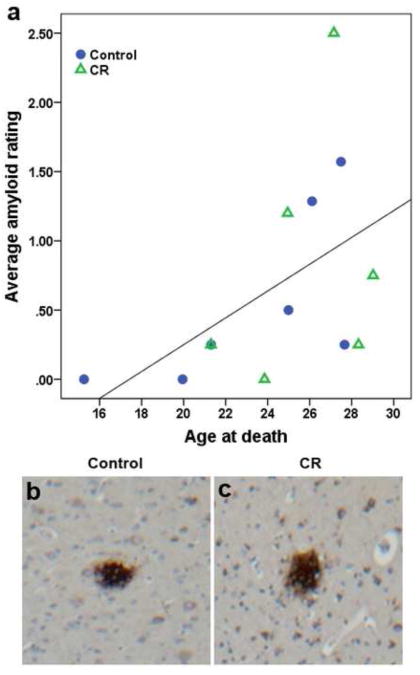
There was a significant correlation between average amyloid rating across all regions examined and age in a combined analysis of all Control and CR animals (Spearman's coefficient = 0.572, p = 0.041, Fig 2a). The distribution of amyloid plaques was variable across animals (Figure 2b, c). Plaques of both neuritic and diffuse types were included in the analyses. Two Control (aged 15 and 20) and one CR (aged 24 years) animals did not have any plaques in any of the regions examined. None of the animals exhibited plaque pathology in the CA region of the hippocampus. Five animals (2 Controls, 3 CR) displayed plaques in the subiculum region, four (1 Control, 3 CR) in EC, and ten (5 Controls, 5 CR) in one or more regions of the neocortex. Table 2 lists Aβ plaque ratings for animals with positive amyloid pathology in the specified location. Nonparametric tests revealed no difference in ratings of amyloid distribution between the two dietary conditions.
Figure 2.
(a) There is a significant direct association between average amyloid plaque rating and age in a combined analysis that included both control-fed and calorie restricted monkeys (Spearman's coefficient = 0.572, p = 0.041). Amyloid plaque staining in the temporal cortex showing sparse amyloid pathology (score = 1) in Control (b) and CR (c) animals.
Table 2. Amyloid pathology rating.
| ID | Age | Group | CA | Subiculum | EC | Neocortex |
|---|---|---|---|---|---|---|
| C1 | 15 | C | 0 | 0 | 0 | 0 |
| C2 | 20 | C | 0 | - | - | 0 |
| C3 | 21 | C | 0 | 0 | 0 | 1 |
| C4 | 25 | C | 0 | 0 | 0 | 2 |
| C5 | 26 | C | 0 | 1 | 0 | 3 |
| C6 | 27 | C | 0 | 2 | 1 | 3 |
| C7 | 28 | C | 0 | 0 | 0 | 1 |
| CR1 | 21 | CR | 0 | 0 | 0 | 1 |
| CR2 | 24 | CR | 0 | 0 | 0 | - |
| CR3 | 25 | CR | 0 | 1 | 2 | 2 |
| CR4 | 27 | CR | - | 1 | 3 | 3 |
| CR5 | 28 | CR | 0 | 0 | 0 | 1 |
| CR6 | 29 | CR | 0 | 1 | 1 | 1 |
Notes: 0 = none, 1 = sparse, 2 = moderate, 3 = severe amyloid pathology; C = Control; CR = calorie restricted. Blank values denote areas that were not rated due to tissue damage during processing. Neocortex rating consists of highest rating from one or more of the following regions: dorsal and ventral inferotemporal cortex, superior temporal gyrus, and parietal cortex.
2.4. Neurofibrillary tangles
One section each from the two CR animals with significant amyloid pathology was processed for silver staining to assess the presence of neurofibrillary tangles. There was no evidence of neurofibrillary tangles in either animal.
3. Discussion
In the present study, we demonstrate that CR animals have significantly lower GFAP immunoreactivity in the CA region of the hippocampus and entorhinal cortex of rhesus macaques. However, we did not find a regulatory effect of CR on the development of amyloid plaques in the aged monkey hippocampus and adjacent cortical areas. While there have been numerous reports examining the effects of CR on the brain using neuroimaging methods in this non-human primate model, to our knowledge, this is the first study to report the effect of CR on the expression of brain markers of aging using post-mortem immunohistochemical techniques.
Astrogliosis occurs during the normal aging process in rodents, rabbits, monkeys, and humans (Sloane et al., 2000; Finch, 2003; Haley et al., 2010). Previous studies have shown that there is an increase in GFAP immunoreactivity in subcortical white matter of the frontal, temporal, and parietal cortices (Sloane et al., 2000), and in the hippocampus of the aged macaques compared to young animals (Haley et al., 2010). This increase in expression occurs in conjunction with increases in GFAP synthesis and degraded products, suggesting alterations in both production and degradation of GFAP with age (Sloane et al., 2000). There was no significant age correlation in GFAP expression given the limited age range of the current sample, with only two animals below the age of 20. However, we show that CR monkeys display decreased GFAP immunoreactivity in the CA region of the hippocampal formation and entorhinal cortex compared to Controls, which is similar to CR studies in rodents (Morgan et al., 1997). While a similar trend (Controls > CR) was detected in the granule cell layer of the dentate gyrus and subiculum, this difference did not reach significance, possibly due to the small sample size and increased variance in GFAP expression in these regions.
Previous studies have demonstrated widespread distribution of Aβ plaques in the aged macaque cortex, starting around the age of 25 and escalating significantly after 30 years in the frontal areas. Until the age of 30, plaques are only found in a few animals and are generally relatively sparse (Sloane et al., 1997; Uno, 1997; Sani et al., 2003; Shah et al., 2010). Our current findings are in agreement with these prior studies in that the density of Aβ plaques was variable across animals. Two Control and one CR animal showed no amyloid pathology. A majority of the animals (including two 21 year old animals) expressed relatively few amyloid plaques. Prior studies also reported that amyloid plaques in the aged rhesus brains generally form in association cortical areas and spare the primary motor and sensory cortices (Heilbroner and Kemper, 1990; Sani et al., 2003). Consistent with these findings, we found amyloid plaques in the inferotemporal and parietal cortices. Additionally, while the presence of neurofibrillary tangles comprises a hallmark feature of AD, it has not been reported in nonhuman primate brains (Shah et al., 2010). Accordingly, we did not observe any neurofibrillary tangles in the two aged macaque brains examined. Although CR has been shown to attenuate Aβ deposition in AD transgenic mouse models (Patel et al., 2005; Mouton et al., 2009), we did not find a significant effect of diet on the distribution of Aβ plaques in the rhesus macaque brain. A possible explanation is that many of these monkeys are too young to express significant age-associated amyloid pathology in the brain, thus precluding unbiased comparisons in Aβ plaque distribution between the two groups.
Although the exact mechanisms underlying CR remain to be investigated, differences in GFAP expression in the hippocampus between control-fed and CR animals might reflect a reduction in oxidative damage in the brain. In fact, CR attenuates the age-associated oxidative damage in brain membranes in rodent models (Tacconi et al., 1991; Dubey et al., 1996). Studies in primary rodent glial cultures have demonstrated that exposure of astrocytes to hydrogen peroxide and cysteamine induces oxidative stress and directly increases GFAP mRNA (Morgan et al., 1997). Decreased levels of macromolecular oxidation products in the liver and other tissues of aging rodents suggest that CR acts by reducing oxidative stress systemically (Hagopian et al., 2005; Seo et al., 2006; Browning et al., 2008).
There are some limitations to the current study. Our sample included only males, and future studies should explore these effects in both males and females to probe for potential contributions of gender on brain aging. A larger sample size would also enable controlling for age and cause of death influences. Several animals in this cohort are still living; as more animals come to post mortem, the project will undertake comprehensive histologic and biochemical assessment. Here we show that CR affects GFAP expression but not amyloid plaque load. These data pave the way for future studies aimed at identifying causative events in brain aging and in the mechanisms of delayed aging by CR.
4. Experimental Procedure
4.1. Animals
Post-mortem brain tissue from thirteen rhesus macaque males (Controls = 7, CR = 6), aged 15 to 29 years at the time of death, processed in this study were part of the longitudinal “Dietary Restriction and Aging Study” at the Wisconsin National Primate Research Center (WNPRC). Details of the dietary manipulation and experimental setup have been described extensively elsewhere (Kemnitz et al., 1993; Ramsey et al., 2000). Animals were either fed ad libitum (Controls) or were maintained on a moderately restricted diet (30% reduced intake from individualized baseline), with both groups receiving comparable diet supplements. Animals were initiated on the CR diet in young adulthood between 8.5 to 14 years of age and the length of CR diet ranged from 7 to 20 years prior to death. The study was approved by the Animal Care and Use Committee of the Graduate School of the University of Wisconsin-Madison. As the animals died, the cause of death was determined through necropsy by a qualified primate pathologist. Deaths were differentiated into age-related causes (e.g., adenocarcinoma) and non age-related causes (e.g., anesthesia). Brains were removed following a standard necropsy protocol. The left hemisphere was fixed in 10% buffered formalin solution, subsequently blocked, and embedded in paraffin using standard techniques, while the right hemisphere was cut in the coronal plane and frozen at -80°C Paraffin-embedded coronal brain blocks containing the left hippocampus at the level of the lateral geniculate nucleus were cut (5 μm) using a microtome.
4.2. GFAP Immunostaining
Two to three comparable coronal sections from each animal were used for assessment of GFAP immunoreactivity. Briefly, following standard deparaffinization and rehydration protocol, sections were heated in an autoclave in 100 mM citrate buffer (pH 6) for antigen retrieval, washed in phosphate buffer saline (PBS), permeabilized using 0.1% Triton X-100 in PBS (PBS/TX), blocked for 1 hour with 10% goat serum, 2% bovine serum albumin, and 0.1% PBS/TX, and incubated overnight in rabbit anti-GFAP (polyclonal; 1:500; Dako, Carpinteria, CA, USA) diluted in blocking solution at 4°C. After three washes in PBS on the following day, sections were incubated in blocking solution containing Alexa 488-conjugated anti-rabbit secondary antibody for 1 hr at room temperature (5 μg/mL; Molecular Probes-Invitrogen, Carlsbad, CA, USA). Nuclei were identified with 4′,6-diamidino-2-phenylindole (DAPI, Molecular Probes-Invitrogen) counterstain. Slides were mounted using Gel/Mount aqueous mounting medium (Electron Microscopy Sciences, Hatfield, PA, USA) and stored in the dark. One Control animal was excluded from the analyses due to the section drying out overnight during primary antibody incubation. GFAP labeling was imaged on a Leica microscope using ImagePro 6.3 software at 40× magnification. Quantification of relative optical density (ROD) of GFAP staining was performed in two to three non-overlapping fields of view from the granule cell layer of the dentate gyrus, CA (regions 1-3), and subiculum of the hippocampal formation and the neighboring EC.
4.3. Aβ Immunostaining
One comparable coronal section from each animal was used to assess the presence and distribution of Aβ plaques in the hippocampus, entorhinal, and adjacent neocortex. These slides were processed in BenchMark ULTRA automated slide preparation system (Ventana Medical Systems, Tucson, Arizona). Following deparaffinization, slides were heated to 95°C in a Tris-based cell conditioning solution (pH 8-8.5, Ventana) for antigen retrieval, washed in reaction buffer (pH 7.6, Ventana), and incubated in mouse monoclonal β-amyloid antibody (1:150, Biocare Medical, Concord, CA) for 1 hour. The target antigen was visualized using ultraView Universal DAB Detection Kit (Ventana), which contains horseradish peroxidase (HRP) labeled anti-mouse secondary antibody. The sections were then counterstained with hematoxylin. A board certified neuropathologist (MSS), blind to the animals' condition and age, assessed the processed slides at 5x magnification and rated the Aβ plaque burden on a 0-3 CERAD (The Consortium to Establish a Registry for Alzheimer's Disease) plaque score (0 = none, 1 = sparse, 2 = moderate, 3 = severe amyloid pathology) in the hippocampus and adjacent neocortical areas (Mirra et al., 1991; Mirra et al., 1993).
4.4. Statistics
All statistical analyses were performed using IBM SPSS 20 software (Chicago, IL). For correlational analyses, the Spearman Rank correlation coefficient was used. Group differences in GFAP immunoreactivity and amyloid plaque levels were assessed using nonparametric tests. Alpha was set at 0.05 (2-tailed) to be considered significant.
Highlights.
The rhesus macaque is useful for studying aging and aging interventions
We tested effect of calorie restriction on gliosis and amyloid plaque load
Calorie restriction attenuates astrogliosis in hippocampus
Calorie restriction does not affect amyloid plaque load
Acknowledgments
The authors acknowledge the assistance provided by the Animal Care, Veterinary and Pathology Staff of the Wisconsin National Primate Research Center. This study was supported by the National Institutes of Health grants RR000167, AG011915, AG000213 and AG028569. The study was also supported with resources and facilities at the W.S. Middleton Memorial Veterans Hospital. AS researched the data, analyzed the data, and wrote the manuscript. MP, MSS, TDP assisted with data collection and analysis. BBB, AAW, RA, and JWK offered expertise and reviewed/edited the manuscript. RJC, RHW, LP, and SCJ contributed resources, offered expertise, and reviewed/edited the manuscript.
Footnotes
The authors report no conflicts of interest or relevant financial interests related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogrammin, calorie restriction and aging. Trends Endocrinol Metab. 2010;21:134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton BH. Ageing of the brain. Mech Ageing Dev. 2002;123:811–817. doi: 10.1016/s0047-6374(01)00426-2. [DOI] [PubMed] [Google Scholar]
- Beach TG, Walker R, McGeer EG. Patterns of gliosis in Alzheimer's disease and aging cerebrum. Glia. 1989;2:420–436. doi: 10.1002/glia.440020605. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Canu E, Willette A, Kastman EK, McLaren DG, Kosmatka KJ, Xu G, Field AS, Colman RJ, Coe CL, Weindruch RH, Alexander AL, Johnson SC. Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiol of Aging. 2011;32:2319 e2311–2311. doi: 10.1016/j.neurobiolaging.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 2003;58:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- Browning JD, Weis B, Davis J, Satapati S, Merritt M, Malloy CR, Burgess SC. Alterations in hepatic glucose and energy metabolism as a result of calorie and carbohydrate restriction. Hepatology. 2008;48:1487–1496. doi: 10.1002/hep.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM. Nonhuman primate calorie restriction. Antioxid Redox Signal. 2011;14:229–239. doi: 10.1089/ars.2010.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol of Aging. 2003;24(1):S123–127. doi: 10.1016/s0197-4580(03)00051-4. discussion S131. [DOI] [PubMed] [Google Scholar]
- Furuta A, Price DL, Pardo CA, Troncoso JC, Xu ZS, Taniguchi N, Martin LJ. Localization of superoxide dismutases in Alzheimer's disease and Down's syndrome neocortex and hippocampus. The American Journal of Pathology. 1995;146:357–367. [PMC free article] [PubMed] [Google Scholar]
- Gallagher JJ, Finnegan ME, Grehan B, Dobson J, Collingwood JF, Lynch MA. Modest amyloid deposition is associated with iron dysregulation, microglial activation, and oxidative stress. Journal of Alzheimer's disease. 2012;28:147–161. doi: 10.3233/JAD-2011-110614. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Harper ME, Ram JJ, Humble SJ, Weindruch R, Ramsey JJ. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E674–684. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- Haley GE, Kohama SG, Urbanski HF, Raber J. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus macaque prefrontal cortex and hippocampus. Age (Dordrecht, Netherlands) 2010;32:283–296. doi: 10.1007/s11357-010-9137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzel DK, Trojanowski JQ, Johnston RF, Loring JF. High-throughput quantitative histological analysis of Alzheimer's disease pathology using a confocal digital microscanner. Nat Biotechnol. 1999;17:53–57. doi: 10.1038/5225. [DOI] [PubMed] [Google Scholar]
- Heilbroner PL, Kemper TL. The cytoarchitectonic distribution of senile plaques in three aged monkeys. Acta Neuropathol. 1990;81:60–65. doi: 10.1007/BF00662638. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. The American Journal of Clinical Nutrition. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Kastman EK, Willette AA, Coe CL, Bendlin BB, Kosmatka KJ, McLaren DG, Xu G, Canu E, Field AS, Alexander AL, Voytko ML, Beasley TM, Colman RJ, Weindruch RH, Johnson SC. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci. 2010;30:7940–7947. doi: 10.1523/JNEUROSCI.0835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Sharma S, Kaur G. Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology. 2008;9:441–454. doi: 10.1007/s10522-008-9168-0. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW. Calorie restriction and aging in nonhuman primates. ILAR J. 2011;52:66–77. doi: 10.1093/ilar.52.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. Journal of Gerontology. 1993;48:B17–26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64:1323–1328. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzhiemer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzhiemer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mirra AA, Hart MN, Terry RD. Making the diagnosis of Alzhiemer's diease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993;117:132–144. [PubMed] [Google Scholar]
- Morgan TE, Rozovsky I, Goldsmith SK, Stone DJ, Yoshida T, Finch CE. Increased transcription of the astrocyte gene GFAP during middle-age is attenuated by food restriction: implications for the role of oxidative stress. Free Radical Biology & Medicine. 1997;23:524–528. doi: 10.1016/s0891-5849(97)00120-2. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci Lett. 2009;464:184–187. doi: 10.1016/j.neulet.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE. GFAP mRNA increases with age in rat and human brain. Neurobiol of Aging. 1993;14:421–429. doi: 10.1016/0197-4580(93)90100-p. [DOI] [PubMed] [Google Scholar]
- Overmyer M, Helisalmi S, Soininen H, Laakso M, Riekkinen P, Sr, Alafuzoff I. Astrogliosis and the ApoE genotype. an immunohistochemical study of postmortem human brain tissue. Dementia and Geriatric Cognitive Disorders. 1999;10:252–257. doi: 10.1159/000017128. [DOI] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol of Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Experimental Gerontology. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Park DC. Beta-amyloid deposition and the aging brain. Neuropsychol Rev. 2009;19:436–450. doi: 10.1007/s11065-009-9118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani S, Traul D, Klink A, Niaraki N, Gonzalo-Ruiz A, Wu CK, Geula C. Distribution, progression and chemical composition of cortical amyloid-beta deposits in aged rhesus monkeys: similarities to the human. Acta Neuropathol. 2003;105:145–156. doi: 10.1007/s00401-002-0626-5. [DOI] [PubMed] [Google Scholar]
- Seo AY, Hofer T, Sung B, Judge S, Chung HY, Leeuwenburgh C. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8:529–538. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- Shah P, Lal N, Leung E, Traul DE, Gonzalo-Ruiz A, Geula C. Neuronal and axonal loss are selectively linked to fibrillar amyloid-{beta} within plaques of the aged primate cerebral cortex. The American Journal of Pathology. 2010;177:325–333. doi: 10.2353/ajpath.2010.090937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane JA, Hollander W, Rosene DL, Moss MB, Kemper T, Abraham CR. Astrocytic hypertrophy and altered GFAP degradation with age in subcortical white matter of the rhesus monkey. Brain Res. 2000;862:1–10. doi: 10.1016/s0006-8993(00)02059-x. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Pietropaolo MF, Rosene DL, Moss MB, Peters A, Kemper T, Abraham CR. Lack of correlation between plaque burden and cognition in the aged monkey. Acta Neuropathol. 1997;94:471–478. doi: 10.1007/s004010050735. [DOI] [PubMed] [Google Scholar]
- Tacconi MT, Lligona L, Salmona M, Pitsikas N, Algeri S. Aging and food restriction: effect on lipids of cerebral cortex. Neurobiol of Aging. 1991;12:55–59. doi: 10.1016/0197-4580(91)90039-m. [DOI] [PubMed] [Google Scholar]
- Uno H. Age-related pathology and biosenescent markers in captive rhesus macaques. Age. 1997;20:1–13. doi: 10.1007/s11357-997-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R. Caloric restriction, gene expression, and aging. Alzheimer Disease and Associated Disorders. 2003;17(2):S58–59. doi: 10.1097/00002093-200304002-00008. [DOI] [PubMed] [Google Scholar]
- Willette AA, Bendlin BB, McLaren DG, Canu E, Kastman EK, Kosmatka KJ, Xu G, Field AS, Alexander AL, Colman RJ, Weindruch RH, Coe CL, Johnson SC. Age-related changes in neural volume and microstructure associated with interleukin-6 are ameliorated by a calorie-restricted diet in old rhesus monkeys. Neuroimage. 2010;51:987–994. doi: 10.1016/j.neuroimage.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Gallagher C, Bendlin BB, McLaren DG, Kastman EK, Canu E, Kosmatka KJ, Field AS, Alexander AL, Colman RJ, Voytko ML, Weindruch RH, Coe CL, Johnson SC. Homocysteine, neural atrophy, and the effect of caloric restriction in rhesus monkeys. Neurobiol Aging. 2012a;33:670–680. doi: 10.1016/j.neurobiolaging.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Bendlin BB, Colman RJ, Kastman EK, Field AS, Alexander AL, Sridharan A, Allison DB, Anderson R, Voytko ML, Kemnitz JW, Weindruch RH, Johnson SC. Calorie Restriction Reduces the Influence of Glucoregulatory Dysfunction on Regional Brain Volume in Aged Rhesus Monkeys. Diabetes. 2012b;61:1036–1042. doi: 10.2337/db11-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ma Q, Zhang YW, Xu H. Proteolytic processing of Alzheimer's beta-amyloid precursor protein. J Neurochem. 2012;120(1):9–21. doi: 10.1111/j.1471-4159.2011.07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]