Abstract
In low dimensional cuprates several interesting phenomena, including high Tc superconductivity, are deeply connected to electron correlations on Cu and the presence of the Zhang-Rice (ZR) singlet state. Here, we report on direct spectroscopic observation of the ZR state responsible for the low-energy physical properties in two isostructural A-site ordered cuprate perovskites, CaCu3Co4O12 and CaCu3Cr4O12 as revealed by resonant soft x-ray absorption spectroscopy on the Cu L3,2- and O K-edges. These measurements reveal the signature of Cu in the high-energy 3+ (3d8), the typical 2+ (3d9), as well as features of the ZR singlet state (i.e., 3d9L, L denotes an oxygen hole). First principles GGA + U calculations affirm that the B-site cation controls the degree of Cu-O hybridization and, thus, the Cu valency. These findings introduce another avenue for the study and manipulation of cuprates, bypassing the complexities inherent to conventional chemical doping (i.e. disorder) that hinder the relevant physics.
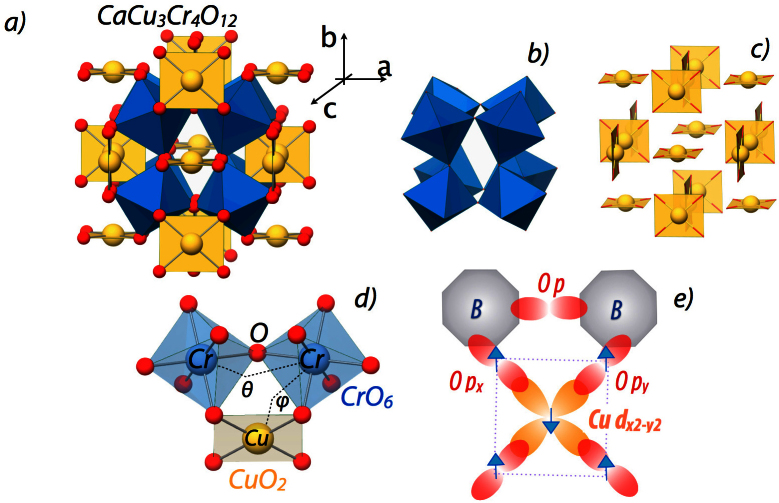
The many-body ZR singlet state1,2,3,4,5,6,7,8,9, a doped hole on the oxygen site coupled antiferromagnetically with a hole on the copper site, can be manipulated with conventional solid-state chemistry methods by partial removal of oxygen or by non-isovalent cation exchange (e.g. La3+ → Sr2+). Such chemical routes of manipulation make the the doped cuprates prone to chemical disorder causing strong structural distortions or even changes in crystal symmetry, which in turn may severely alter the properties associated with Cu d-electron derived electronic and magnetic structures. En route to the goal of realizing the ZR state without chemical disorder or lattice distortion, while the majority of the Cu oxide compounds have the Cu ions in the B-site of the perovskite ABO3 structure, the A-site ordered perovskites with chemical formula (ACu3)B4O12 are intriguing candidates for investigation due to the unique Cu A-site arrangement (see Fig. 1(a)). In this structure, a surprisingly rich set of interesting physics phenomena10,11,12,13,14,15,16 have been demonstrated by replacement of the B-site 3d transition metal ion via experiment and theory including colossal dielectric constant17,18, ferrimagnetism19, charge, orbital, and spin ordering20, non-fermi liquid behavior21, enhanced electronic correlations22 and possibly the Zhang-Rice state23. In addition, previous reports hinted at the presence of a mixed-valency on Cu for B = Cr and Co23,24. Despite the large variation in the B-site ions, all the members of this family belong to the same space group Im-3 built upon two sub-lattices of octahedral BO6 units and the planar CuO4 units (see Fig. 1(b)–(c). As seen in Fig. 1(a and d), the two structural units are connected via apical oxygens to form a BO6 - CuO4 - BO6 cluster. In this configuration, the effect of the B-site cation has been shown to be two-fold: first, its valence state controls the nominal valence state of the A′-site Cu ion and second, most importantly, its tendency for covalent mixing alters the charge distribution between Cu d- and O p-states11,15. The structural similarity of the local ionic environment of Cu in these ordered perovskites with that of high Tc cuprates, and, in close analogy to chemical doping, the above-mentioned B-site's potential to engineer the electronic structure prompts one to use the A-site ordered perovskites to gain unique insight into the development of the cuprate electronic structure. Construction of such manipulated valencies suggests the ability to tune the formation of ligand holes on oxygen and possibly to achieve the formation of the Zhang-Rice singlet state - an important ingredient for high temperature superconductivity.
Figure 1. Crystal structure of A-site ordered perovskites.
(a) The entire crystal structure of CaCu3Cr4O12. (b) CrO6 octahedral sub-lattice. (c) CuO4 distorted square planar sub-lattice. (d) Cr-Cr and Cr-Cu exchange pathways and (e) a sketch of B-site electronic states coupled to the ZR singlet state on Cu.
Results
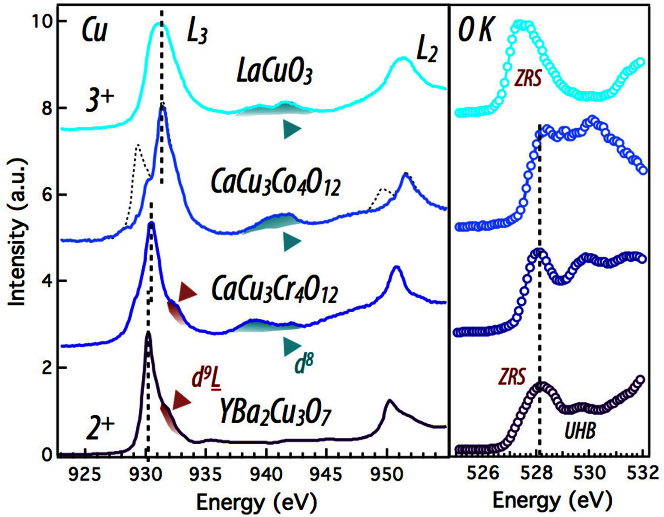
Resonant x-ray absorption spectroscopy (XAS) in the soft X-ray regime is an ideal tool, extensively used in the past, to investigate the Cu and O electronic structure and the effects of electron-electron correlations in high Tc cuprates2,6,7,8,25,26,27. Results of our soft XAS measurements on the Cu L3,2- and O K-edge are shown in Fig. 2 for the two A-site ordered perovskites CaCu3Co4O12 (CCCoO) and CaCu3Cr4O12 (CCCrO) along with that of the optimally doped superconducting YBa2Cu3O7−δ (YBCO) and metallic LaCuO3 (LCO) perovskite with a unique formal Cu3+ (3d8) oxidation state. From the absorption line shape in Fig. 2, it is readily apparent that in CCCoO and CCCrO a mixed valency of Cu is present; we will discuss the CCCrO compound first. The sharp peak ~ 930 eV corresponds to the transition from the d9 ground state, featuring a single hole in the Cu eg band, to the cd10 (here c denotes a core hole) excited state. The shoulder seen at 931.5 eV, a signature of the ZR singlet state, is a transition from the ground state d9L to the cd10L excited state2,5,7,26,27. The key difference between these two transitions being that while the Cu still maintains a nearly 2+ (or d9) valency, a ligand hole is distributed over the neighboring oxygens as illustrated in Fig. 1(e). The interaction between this ligand hole and the core hole created by the photon absorption raises the energy required to promote the core electron to the unoccupied state in the eg band resulting in the observed high-energy state marked by red arrows. Furthermore, to corroborate this, we show the absorption data on LCO with the formal Cu charge state of 3+. Indeed, in-line with our previous statement, the main feature of the LCO XAS around the L3-edge at ~ 931.5 eV is the transition from the d9L state, thus making the ZR state the dominant contribution to the ground state in good agreement with previous reports28,29. The aforementioned doped ligand holes are the charge carriers essential to the physics of high Tc cuprates; for instance, in hole doped cuprates the superconducting transition temperature is strongly dependent on the amount of doping present2,30,31. To clarify the connection between these compounds and the high-temperature superconducting cuprates, XAS data was taken for an optimally doped YBCO sample. As seen in Fig. 2, the Cu L-edge spectrum of CCCrO shows remarkable resemblance to YBCO, namely the dominant d9 initial states followed by the higher energy ZR state shoulder. This result highlights the capacity of the A-site ordered cuprate perovskites to mimic the effects of chemical doping without provoking unwanted lattice and stoichiometry deviations inherent to conventional chemical doping. In addition, the multiplet split peak at ~ 940 eV corresponds to a transition from the metastable 3d8 state to the cd9 excited state, indicating the presence of the ionically Cu3+ state entirely absent in YBCO.
Figure 2. XAS measurements on Cu L- and O K-edges.
Left panel displays the Cu L-edge for all samples with the Zhang-Rice and d8 ground states highlighted. LCO and YBCO are shown as references for Cu 3+ and Cu 2+ respectively, highlighting the change in valence for Cu with B-site change36,37. Right panel shows the corresponding O K-edge spectra displaying the Zhang-Rice ligand hole state. In LCO, the higher energy peak around ~ 940 eV is due to the presence of the unusual Cu d8 state. In CCCoO, a previously reported impurity peak around 929 eV32 is subtracted from both the L3 and L2 edges by fitting to a Voigt function. This peak corresponds to the d9 peak observed on the other samples, however it has a very small spectral weight (~ 1/50 ratio between d9 and d9L states).
Next, we turn our attention to the CCCoO electronic structure. As clearly seen in Fig. 2, CCCoO shows a dominant white absorption line analogous to CCCrO, but, surprisingly, it is shifted ~ 1 eV away from the d9 white line of CCCrO and YBCO. When compared with the formally Cu3+ compound LCO (shown immediately above it in Fig. 2) it becomes readily apparent that the Cu ground state is predominantly of d9L character, implying a dominant Cu3+ formal valence as in LCO. To quantify the prevalence of the d9L state, a three peak fit is used for the L3 data, which are centered at 929.3 eV (impurity)32, 930 eV (d9), and 931.4 eV (d9L) which gave a very small spectral weight ratio of .0203 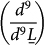 . The impurity peak was subtracted from the original data (dotted line) for clarity. Further demonstrating the 3+ formal valence, the multiplet split peak at ~940 eV provides strong evidence for the admixture of the d8 state. When considered alongside CCCrO, CCCoO also contains all three Cu states, but with obviously significantly different weights, further testifying to the ability of the B-site to effectively hole dope the Cu sites.
. The impurity peak was subtracted from the original data (dotted line) for clarity. Further demonstrating the 3+ formal valence, the multiplet split peak at ~940 eV provides strong evidence for the admixture of the d8 state. When considered alongside CCCrO, CCCoO also contains all three Cu states, but with obviously significantly different weights, further testifying to the ability of the B-site to effectively hole dope the Cu sites.
Because of the large degree of charge transfer between oxygen and copper and its importance to the ZR state, studies of the O K-edge can lend supporting information about the electronic structure of Cu. As seen in Fig. 2(b), O K-edge data in all samples show the oxygen specific signature of the 3d9L state, a necessary ingredient of the ZR state, which is particularly evident from the comparison to the well studied case of YBCO, where the ligand hole on oxygen appears as a large, doping dependent pre-peak around 528 eV6,7,8,25. This observation of the pre-peak intensity indicates that the ZR state is the first ionization state at the Fermi energy. This further evidences our conclusion on the presence of the ZR state inferred from the Cu L-edge analysis.
Although our prime focus is on the electronic structure of Cu and O, Co and Cr L3,2-edge absorption can serve to independently validate the Cu oxidation state. With this goal in mind, we performed L-edge XAS measurements on Co and Cr and compared this to the previous results on reference Cr and Co compounds. As shown in Fig. 3(a), a direct comparison to the Cr3+ and Cr4+ absorption confirms that in CCCrO the Cr valency is 4+ or very close to it33. This verifies the conclusion that, in this compound, if Cr is 4+ then Cu must be ~2+ (assuming O2− and Ca2+). Any deviation of Cr valency towards 3+ must then be compensated by raising the Cu charge state, thus Cu must be 2 + or very close to it, consistent with the Cu L-edge results. The Co L-edge data shown in Fig. 3(b) bear strong similarities with previous studies of LaCoO3, which consists of ionically bound Co3+34. Specifically, strong, high energy multiplet shoulders on the L3 and L2 white lines are present in both CCCoO and LaCoO3. In variance to this, Co4+ shows a smaller shoulder shifted to even higher energy. While the high energy side of the CCCoO Co L-edge is consistent with Co3+, the low energy multiplet peak does not match either 3+ or 4+, implying CCCoO is neither ionically pure 3+ or 4+. Based upon the Cu L-edge conclusion of the Cu3+ charge state, we can independently assess the Co valency to be ~ 3.25+. In this context, the Co L-edge data is compatible with the Cu L-edge results and implies the presence of a mixed 3+/4+ Co valency, although it cannot confirm the exact 3.25+ valence state needed to conserve charge. Lending further support to this conclusion, Co L edge data in total electron yield (TEY) mode is shown in the Supplemental Figure S1.
Figure 3. XAS measurements on the Cr and Co L-edges.
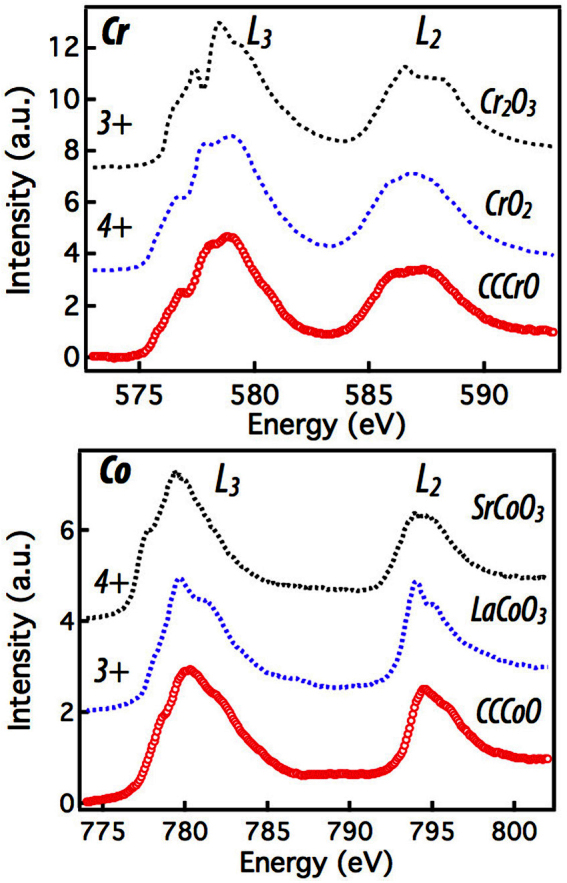
(a) The Cr L-edge along with 3+ and 4+ references from33, the CCCrO and the 4+ reference samples show strong similarities. (b) The Co L-edge with valence references from34 showing a similar, but not identical line shape. No standards were available meaning any energy shift between the samples could not be analyzed, all spectra were shifted to ~ align the white lines.
To gain further understanding of the B-site influence on the unusual electronic states on Cu, we performed first-principles spin-polarized DFT calculations within the GGA+U approximation. Fig. 4 summarizes the first-principles results for CCCoO and CCCrO, respectively. Note, although no long-range magnetic ordering is reported in these compounds, spin-polarized calculations are essential for proper assignment of both charge and spin states of various constituents15. The three panel Fig. 4(a) and (b) show an inspection of the calculated density of states (DOS) projected onto B-d (B = Co/Cr), Cu-d and O-p states respectively. A direct inspection of the states in the vicinity to the Fermi energy, EF demonstrates the existence of strong mixing between B and Cu d-states and O p-states. This strong covalency effect makes both CCCoO and CCCrO systems metallic, in agreement with experimental observations. In addition, as shown in the inset of Fig. 4(a)–(b), the octahedral crystal field of the oxygen atoms around the B ion splits the B-t2g states from the B-eg
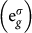 states; the trigonal distortion present in the BO6 octahedra breaks the t2g levels further into doubly degenerate,
states; the trigonal distortion present in the BO6 octahedra breaks the t2g levels further into doubly degenerate, 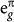 and singly degenerate a1g states. The distorted square planar environment of oxygen atoms surrounding the Cu ion, on the other hand, lifts the 5-fold degeneracy of Cu d-levels, with large energy separation of Cu
and singly degenerate a1g states. The distorted square planar environment of oxygen atoms surrounding the Cu ion, on the other hand, lifts the 5-fold degeneracy of Cu d-levels, with large energy separation of Cu 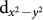 state from the rest. Here we note that the nominal d8 (i.e. Cu3+) would imply empty Cu:
state from the rest. Here we note that the nominal d8 (i.e. Cu3+) would imply empty Cu: 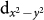 states in both spin up and spin down channels, and for d9 Cu:
states in both spin up and spin down channels, and for d9 Cu: 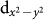 states would be occupied in one of the spin channels and be empty in the other spin channel. From the DOS plots shown in Fig. 4(a) and (b), we find that Cu:
states would be occupied in one of the spin channels and be empty in the other spin channel. From the DOS plots shown in Fig. 4(a) and (b), we find that Cu: 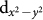 states are empty in the down spin channel, while in the up spin channel it is indeed close to being empty for CCCoO, but mostly occupied for CCCrO. We note that DFT calculation deals with single configuration nature of the wavefunction and therefore, delivers the state with most dominant weight. Interestingly enough, the dominance of Cu 3+ state in CCCoO and Cu 2+ state in CCCrO is evident even at the level of single configuration theory. This result lends strong theoretical support for the conclusion inferred from our XAS measurements that the Cu state is predominantly in the 3+ state for CCCoO and closer to 2+ for CCCrO.
states are empty in the down spin channel, while in the up spin channel it is indeed close to being empty for CCCoO, but mostly occupied for CCCrO. We note that DFT calculation deals with single configuration nature of the wavefunction and therefore, delivers the state with most dominant weight. Interestingly enough, the dominance of Cu 3+ state in CCCoO and Cu 2+ state in CCCrO is evident even at the level of single configuration theory. This result lends strong theoretical support for the conclusion inferred from our XAS measurements that the Cu state is predominantly in the 3+ state for CCCoO and closer to 2+ for CCCrO.
Figure 4. The GGA + U density of states, projected onto Co d (Cr d), Cu d and O p states for the A-site ordered perovskites.
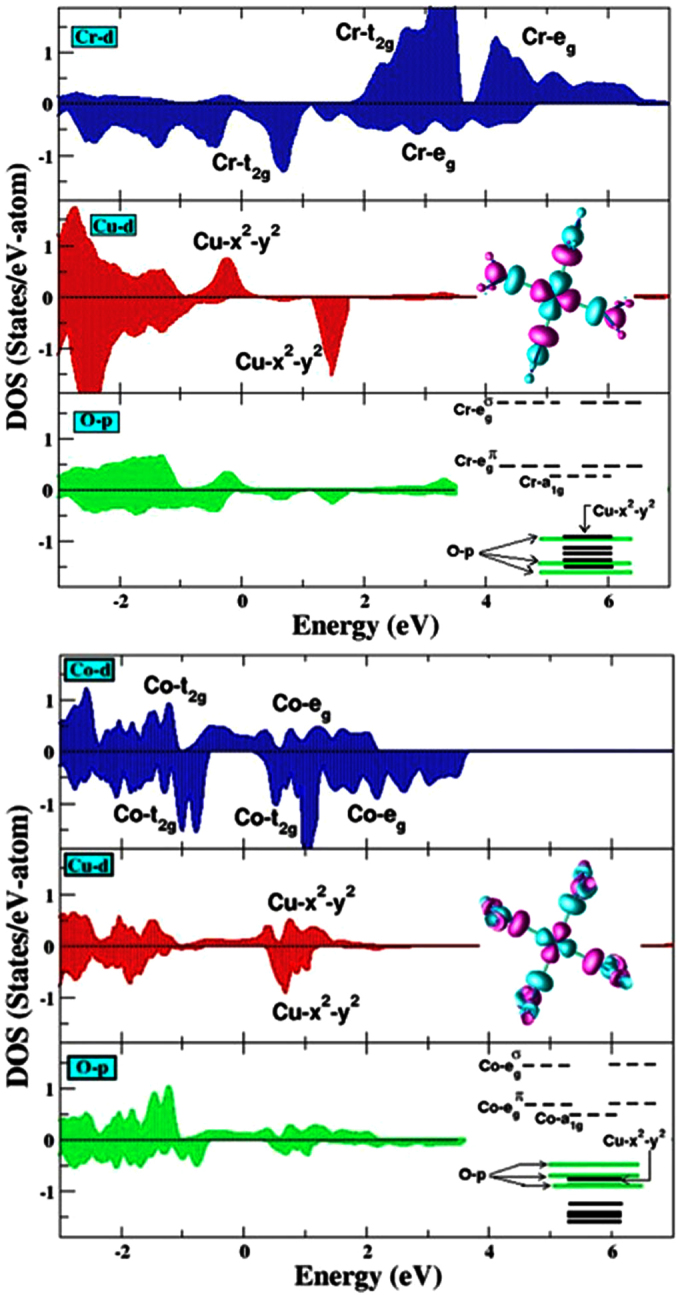
(a) CaCu3Co4O12 ((b) CaCu3Cr4O12) compound. The positive and negative values in y-axis correspond to density of states inup and down spin channels, respectively. The zero of the energy is set at EF. The features due to dominant contributions of various degrees of freedom have been marked. The inset in the middle panel shows the plot of the effective Cu x2 − y2 Wannier function, calculated by NMTO-downfolding. Shown are the isosurface with lobes of opposite signs are colored differently. The inset in the bottom panel shows the relative positions of Co d, Cu d and O p energy levels.
Next, we discuss the calculated magnetic states in connection to Cu. First, we note that occupancy of the square planar Cu d-states according to the formal valence of d8 would give rise to a paired electronic configuration with zero magnetic moment at Cu sites, while that of formal valence of d9 would give rise to one unpaired electron with magnetic moment of 1 μB in the fully spin polarized limit. In comparison, the calculation yields magnetic moments on Cu site 0.07 μB for CCCoO and 0.50 μB for CCCrO, thus lending additional validation of our experimental conclusion on the exotic 3+ state for CCCoO and closer to 2+ valency in CCCrO. Moreover, from the simple ionic charge count, the nominal 3+ and 2+ valences of Cu (assuming Ca2+ and O2−), would set the formal valences of the B-site ion to 3.25+ and 4+ respectively, and result in d-electron occupancies of 5.75 for Co and 2 for Cr. The computed magnetic moment at the Cr site is 2.2 μB, in good agreement with the nearly 2+ valence of Cu and 4+ valence of Cr in CCCrO. The d-occupancy of 5.75 on Co can lead to either low or high spin magnetic state; our computed Co magnetic moment of ~1.7 μB, however, is in conformity with the intermediate rather than low- or high-spin state.
By examining the energy level positions, as calculated in DFT, shown in the insets in the bottom panels of Figs. 4(a–b), we find that Cu 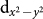 energy level is nearly degenerate with O-p states, leading to a situation very similar to that in high Tc cuprates. This electronic configuration naturally supports strong mixing of Cu
energy level is nearly degenerate with O-p states, leading to a situation very similar to that in high Tc cuprates. This electronic configuration naturally supports strong mixing of Cu 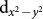 and O px and py states by forming a strong pdσ anti-bonding combination, which may further bind to d-states at the B site as shown in Fig. 1(e). The formation of such a mixed state is also evident in the Wannier function plot of an effective Cu x2 − y2 Wannier function obtained by keeping only Cu
and O px and py states by forming a strong pdσ anti-bonding combination, which may further bind to d-states at the B site as shown in Fig. 1(e). The formation of such a mixed state is also evident in the Wannier function plot of an effective Cu x2 − y2 Wannier function obtained by keeping only Cu 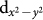 state active and downfolding or integrating out all the other degrees of freedom (see inset of the middle panel of Fig. 4). We notice that for the Co compound mixing of Cu-O hybridized states with B-site d-states is much stronger compared to that of Cr compound, which is driven by the strong hybridization of empty
state active and downfolding or integrating out all the other degrees of freedom (see inset of the middle panel of Fig. 4). We notice that for the Co compound mixing of Cu-O hybridized states with B-site d-states is much stronger compared to that of Cr compound, which is driven by the strong hybridization of empty 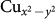 orbital of Cu 3+ state with empty Co, eg orbitals. In CCCrO, this leads to vanishing contribution of the B-site states for the selected iso-surface value of the Wannier plot, showing the typical Cu-O antibonding wavefunction, making the situation more like high Tc cuprates with dominant Cu 2+ state. The strong mixing between
orbital of Cu 3+ state with empty Co, eg orbitals. In CCCrO, this leads to vanishing contribution of the B-site states for the selected iso-surface value of the Wannier plot, showing the typical Cu-O antibonding wavefunction, making the situation more like high Tc cuprates with dominant Cu 2+ state. The strong mixing between 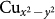 and O p states, which is a prerequisite for formation of the ZR state - observed as the feature around 931 eV in Cu L-edge spectra, is further corroborated by the presence of a 0.2 μB fraction of the magnetic moment on the O site and 0.5 μB on Cu. We thus conclude that the formation of a ZR like many-body state similar to what is observed on the high Tc cuprates is indeed highly favorable in these systems with enhanced probability for the Cr compound.
and O p states, which is a prerequisite for formation of the ZR state - observed as the feature around 931 eV in Cu L-edge spectra, is further corroborated by the presence of a 0.2 μB fraction of the magnetic moment on the O site and 0.5 μB on Cu. We thus conclude that the formation of a ZR like many-body state similar to what is observed on the high Tc cuprates is indeed highly favorable in these systems with enhanced probability for the Cr compound.
Discussion
In summary, resonant soft X-ray absorption data on Cu L3,2- and O K-edges revealed the presence of the ZR singlet state in the symmetrically invariant A-site-ordered cuprate compounds, CaCu3Co4O12 and CaCu3Cr4O12. Additionally, the data demonstrated the existence of a mixed valency for Cu modulated by the electronic state of the B-site cation and reflected in the d9, d9L, and the metastable d8 states of Cu d-electrons. First principles GGA + U calculations further validate the notion that within the A-site ordered perovskite family, the choice of B-site cation is an effective tool to manipulate the degree of B-Cu-O hybridization, adjusting the Cu valency towards the unusual 3+ valence state in CCCoO and away from 3+ closer to the stable 2+ valency in CCCrO. Additionally, Wannier function calculations support this direct experimental observation that in the CCCrO compound the ZR many-body state is particularly favorable and controls the low-energy physics. These findings demonstrate that the charge and spin state of Cu, fundamental to the intriguing physical properties the cuprates display, can be effectively altered by a careful choice of the B-site cation on isomorphic lattices that circumvent the chemical disorder and lattice modulation intrinsic to other doping methods.
Methods
Experimental details
Polycrystalline CaCu3B4O12 (B = Cr and Co) samples were prepared under high-pressure and high-temperature (HPHT) conditions with a Walker-type multianvil module (Rockland Research Co.). For B = Cr, stoichiometric amounts of CaO, CuO, and CrO2 powders were thoroughly mixed and then subjected to a HPHT treatment at 7 GPa and 900°C for 30 minutes. For B = Co, a precursor containing Ca, Cu, and Co ions in the 1:3:4 molar ratio obtained via a sol-gel route was mixed with 30 wt.% KClO4 acting as an oxidizing agent and then subjected to a HPHT treatment at 9 GPa and 1000°C for 30 minutes. The resultant KCl was washed away with deionized water. Details about the sample assembly and procedures for the HPHT experiment can be found in a previous paper35. Phase purity of the above samples was examined with powder X-ray diffraction (XRD) at room temperature with a Philips Xpert diffractometer (Cu Kα radiation). The B = Co sample contains a small amount of cobalt and copper oxides. The cubic lattice parameter was calculated to be a = 7.2385(5) and 7.1259(3) Å for M = Cr and Co, respectively. Reitveld refinement for the two a-site ordered compounds is available in the Supplemental Figure S2.
LCO was synthesized under high oxygen pressure to the rhombohedral symmetry, details of which can be found elsewhere36. YBCO was grown with pulsed laser deposition, the details of which can also be found elsewhere37.
In order to elucidate the transformation of the Cu valency across these compounds, soft XAS measurements were carried out at the soft x-ray branch at the 4-ID-C beamline in the bulk-sensitive total fluorescence yield (TFY) mode and TEY mode in the Advanced Photon Source at Argonne National Laboratory. Measurements were taken on the Cu L-edge and O-K edge for all samples. All measurements were accompanied by a CuO standard, which allows precise alignment of the energy for oxidation state comparison. The Co and Cr L-edges were also obtained for the corresponding samples. All measurements shown were obtained in TFY mode; except, the Cr L edge data and all the Cr reference absorption data were taken in TEY mode.
Computational details
In the first-principles DFT calculations we have used the plane wave basis set and pseudo-potentials as implemented in the Vienna Ab-initio Simulation Package (VASP)38. The exchange-correlation function was chosen to be that of generalized gradient approximation (GGA) implemented following the parametrization of Perdew-Burke-Ernzerhof39. The electron-electron correlation beyond GGA was taken into account through improved treatment of GGA + U calculation within the +U implementation of Dudarev et al.40. For the plane wave based calculations, we used projector augmented wave (PAW)41 potentials. The wave functions were expanded in the plane wave basis with a kinetic energy cutoff of 600 eV and Brillouin zone summations were carried out with a 6 × 6 × 6 k-mesh. The U value of 5 eV on Cu site and 4 eV on B site were used for GGA + U calculations while the Hund's rule coupling J was fixed to 0.8 eV. The obtained results were verified in terms of variation of U parameter.
In order to estimate the positions of the Cu-d, B-d and O-p energy levels as well as the plots of the effective Wannier functions for B-d states, we used muffin-tin orbital (MTO) based N-th order MTO (NMTO)42-downfolding calculations. Starting from a full DFT calculations, NMTO-downfolding arrives at a few-orbital Hamiltonian by integrating out degrees which are not of interest. It does so by defining energy-selected, effective orbitals which serve as Wannier-like orbitals defining the few-orbital Hamiltonian in the downfolded representation. NMTO technique which is not yet available in its self-consistent form relies on the self-consistent potential parameters obtained out of linear muffin-tine orbital (LMTO)43 calculations. The results were cross-checked among the plane wave and LMTO calculations in terms of total energy differences, density of states and band structures.
Author Contributions
D.M., J.W.F. and J.C. acquired the experimental data. S.M. and T.S.-D. did the theoretical calculations. J.G.C., J.S.Z. and J.B.G. grew the samples. S.M. and B.A.G. analyzed data and provided notes on the initial versions of the manuscript. D.M., T.S.-D. and J.C. wrote the final version of the manuscript.
Supplementary Material
Supplemental
Acknowledgments
JC is supported by DOD-ARO Grant No. 0402-17291. JSZ and JBG is supported by NSF Grant. No. DMR-1122603. TS-D would also like to thank, CSIR and DST, India for funding. Work at the Advanced Photon Source, Argonne is supported by the U.S. Department of Energy, Office of Science under Grant No. DEAC02-06CH11357. Thanks to Dr. Bogdan Dabrowski for the SrCoO2.9 (~4+) sample. JGC acknowledges the support from the Japan Society for the Promotion of Science (Grant No. 12F02023) and the Chinese Academy of Sciences.
References
- Zaanen J., Sawatzky G. A. & Allen J. W. Band gaps and electronic structure of transition-metal compounds. Phys. Rev. Lett. 55, 418 (1985). [DOI] [PubMed] [Google Scholar]
- Nikolay High-Temperature Cuprate Superconductors. Berlin: Springer-Verlag, 2010. [Google Scholar]
- Chakhalian J. et al. Orbital Reconstruction and covalent bonding at an oxide interface. Science 318, 1114–1117 (2007). [DOI] [PubMed] [Google Scholar]
- Chakhalian J. et al. Magnetism at the interface between ferromagnetic and superconducting oxides. Nat. Phys. 2, 244–8 (2006). [Google Scholar]
- Karpinen M. & Yamauchi H. The doping routes and distribution of holes in layered cuprates: a novel bond-valence approach. Phil. Mag. B 79, 2, 343–66 (1999). [Google Scholar]
- Nücker N., Fink J., Fuggle J. C., Durham P. J. & Temmerman W. M. Evidence for holes on oxygen sites in the high Tc superconductors La2−xSrxCuO4 and YBa2Cu3O7−γ. Phys. Rev. B 37, 10 (1988). [DOI] [PubMed] [Google Scholar]
- Nücker N. et al. Site-specific and doping-dependent electronic structure of YBa2Cu3Ox probed by O1s and Cu 2p x-ray-absorption spectroscopy. Phys. Rev. B 51, 13 (1995). [DOI] [PubMed] [Google Scholar]
- Kuiper P. et al. X-ray absorption study of the O 2p hole concentration dependence on O stoichiometry in YBa2Cu3Ox. Phys. Rev. B 38, 10 (1988). [DOI] [PubMed] [Google Scholar]
- Zhang F. C. & Rice T. M. Effective hamiltonian for the superconducting Cu oxides. Phys. Rev. B 37, 3759–3761 (1988). [DOI] [PubMed] [Google Scholar]
- Xiang H. P., Liu X. J., Meng J. & Wu Z. J. Structural stability and magnetic coupling in CaCu3Co4O12 from first principles. J. Phys.: Condens Matter 21, 045501 (2009). [DOI] [PubMed] [Google Scholar]
- Shimakawa Y. A-site-ordered perovskites with intriguing physical properties. Inorg. Chem 47, 8562–70 (2008). [DOI] [PubMed] [Google Scholar]
- Alippi P. & Fiorentini V. Magnetism and unusual Cu valency in quadruple perovskites. Eur, Phys. J. B. 85, 82 (2012). [Google Scholar]
- Tran T. T., Takubo K., Mizokawa T., Kobayashi W. & Terasaki I. Electronic structure of CaCu3Ru4O12 studied by x-ray photoemission spectroscopy. Phys. Rev. B 73, 193105 (2006). [Google Scholar]
- Shiraki H., Saito T., Azuma M. & Shimakawa Y. Metallic behavior n A-site-ordered perovskites ACu3V4O12 with A = Na+, Ca2+, and Y3+. J. Phys. Soc. Jpn. 77, 6 (2008). [Google Scholar]
- Mukherjee S., Sarkar S. & Saha-Dasgupta T. First Principles study of CaCu3B4O12 (B = Co, Rh, Ir). J. Mater. Sci. 47, 7660–4 (2012). [Google Scholar]
- Long Y. W. et al. Temperature-induced A-B intersite charge transfer in a A-site-ordered LaCu3Fe4O12 perovskite. Nature 458, 60 (2009). [DOI] [PubMed] [Google Scholar]
- McGuinness C. et al. X-ray spectroscopic study of the electronic structure of the high-dielectric-constant material CaCu3Ti4O12. Phys. Rev. B 71, 19511 (2005). [Google Scholar]
- Lin Y., Chen Y. B., Garret T., Liu S. W. & Chen C. L. Epitaxial growth of dielectric CaCu3Ti4O12 thin films on (001) LaAlO3 by pulsed laser deposition. Appl. Phys. Lett. 81, 631–3 (2002). [Google Scholar]
- Yamada I. et al. A perovskite containing quadrivalent iron as a charge-disproportionated ferrimagnet. Angew. Chem. Int. Ed 47, 7032–5 (2008). [DOI] [PubMed] [Google Scholar]
- Prodi A. et al. Charge, orbital and spin ordering phenomena in the mixed valence manganite (NaMn3+3)(Mn3+2Mn4+2)O12. Nat. Mater. 3, 48–52 (2004). [DOI] [PubMed] [Google Scholar]
- Tanaka S., Shimazui N., Takatsu H., Yonezawa S. & Maeno Y. Heavy-Mass Behavior of Ordered Perovskites ACu3Ru4O12 (A = Na, Ca, La). J. Phys. Soc. Jpn. 78, 024706 (2009). [Google Scholar]
- Hollmann N. et al. Correlation effects in CaCu3Ru4O12. arXiv: 1211.2984v1.
- Mizokawa T. et al. Metallic versus insulating behavior in the A-site ordered perovskite oxides ACu3Co4O12 (A = Ca and Y) controlled by Mott and Zhang-Rice physics. Phys. Rev. B 80, 125105 (2009). [Google Scholar]
- Subramanian M. A., Marshall W. J., Calvarese T. G. & Sleight A. W. Valence degeneracy in CaCu3Cr4O12. J. Phys. and Chem. Solids 64, 1569–71 (2003). [Google Scholar]
- Chen C. T. et al. Electronic States in La2−xSrxCuO4+δ probed by soft-x-ray absorption. Phys. Rev. Lett. 66, 1 (1991). [DOI] [PubMed] [Google Scholar]
- Grioni M. et al. Studies of copper valence states with Cu L3 x-ray absorption spectroscopy. Phys. Rev. B 39, 3 (1989). [DOI] [PubMed] [Google Scholar]
- Bianconi A. et al. Linearly polarized Cu L3-edge x-ray-absorption near-edge structure of Bi2CaSr2Cu2O8. Phys. Rev. B 44, 18 (1991). [DOI] [PubMed] [Google Scholar]
- Mizokawa T., Fujimori A., Namatame H., Takeda Y. & Takano M. Electronic structure of tetragonal LaCuO3 studied by photoemission and x-ray-absorption spectroscopy. Phys. Rev. B 57, 16 (1998). [DOI] [PubMed] [Google Scholar]
- Sarangi R. et al. X-ray absorption edge spectroscopy and computational studies on LCuO2 species. J. Am. Chem. Soc. 128, 8286–96 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoeuf D. et al. Electron pockets in the Fermi surface of hole-doped high-Tc superconductors. Nature 450, 533–6 (2007). [DOI] [PubMed] [Google Scholar]
- Ando Y., Komiya S., Segawa K., Ono S. & Kurita Y. Electronic phase diagram of high-Tc cuprate superconductors from a mapping of the in-plane resistivity curvature. Phys. Rev. Lett. 93, 267001 (2004). [DOI] [PubMed] [Google Scholar]
- Sarma D. D. et al. Electronic structure of high-Tc superconductors from soft-x-ray absorption. Phys. Rev. B 37, 16 (1988). [DOI] [PubMed] [Google Scholar]
- Debkov Y. S. et al. Correlations in the electronic structure of half-metallic ferromagnetic CrO2 films: An x-ray absorption and resonant photoemission spectroscopy study. Phys. Rev. B 72, 060401(R) (2005). [Google Scholar]
- Medling S. et al. Evolution of magnetic oxygen states in Sr-doped LaCoO3. Phys. Rev. Lett. 109, 157204 (2012). [DOI] [PubMed] [Google Scholar]
- Cheng J.-G., Zhou J.-S. & Goodenough J. B. Evolution of ferromagnetism in orthorhombic perovskites Sr1−xPbxRuO3. Phys. Rev. B 81, 134412 (2010). [Google Scholar]
- Zhou J.-S., Archibald W. & Goodenough J. B. Approach to Curie-Weiss paramagnetism in the metallic perovskites La1−xNdxCuO3. Phys. Rev. B 61, 3196–3199 (2000). [Google Scholar]
- Gray B. A. et al. arXiv:1301.3736.
- Kresse G. & Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996). [DOI] [PubMed] [Google Scholar]
- P. Perdew J., Burke K. & Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865 (1996). [DOI] [PubMed] [Google Scholar]
- Dudarev S. L., Botton G. A., Savrasov S. Y., Humphreys C. J., & Sutton A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA + U study. Phys. Rev. B 57, 1505 (1998). [Google Scholar]
- Blöchl P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994). [DOI] [PubMed] [Google Scholar]
- Andersen O. K. & Saha-Dasgupta T. Muffin-tin orbitals of arbitrary order. Phys. Rev. B 62, R16219 (2000). [Google Scholar]
- Andersen O. K. & Jepsen O. Explicit, First-Principles Tight-Binding Theory. Phys. Rev. Lett. 53, 2571 (1984). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental