Abstract
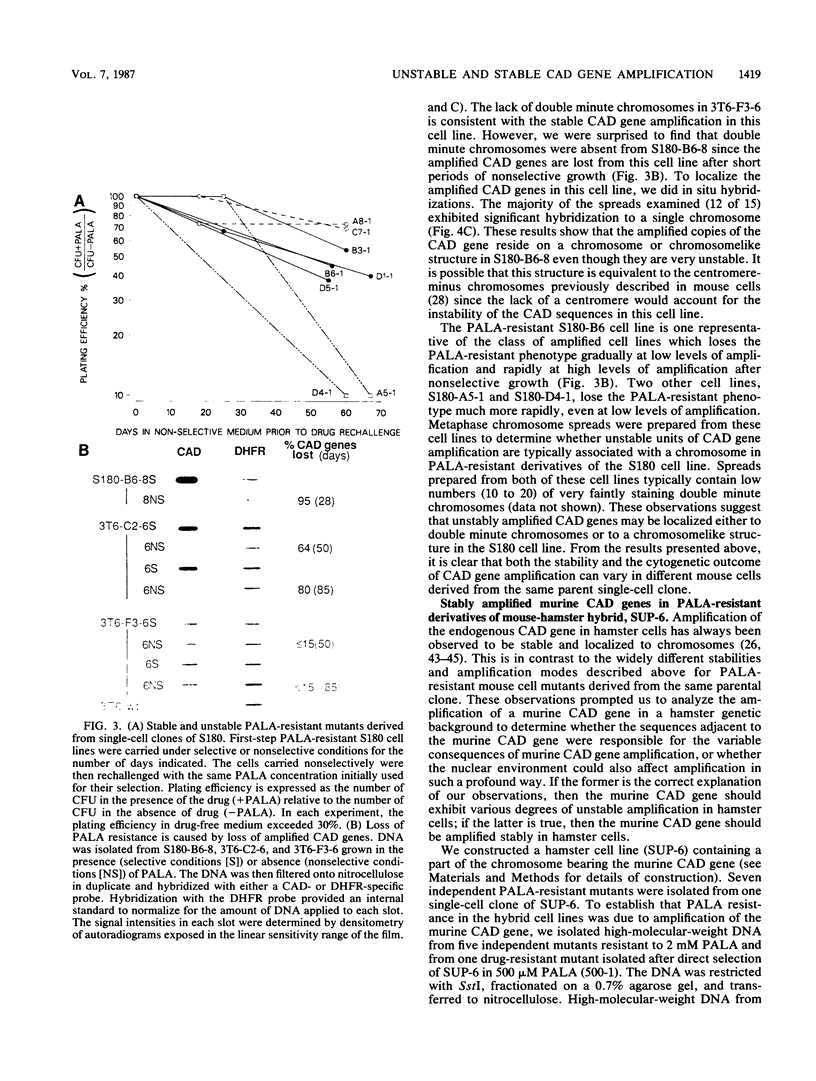
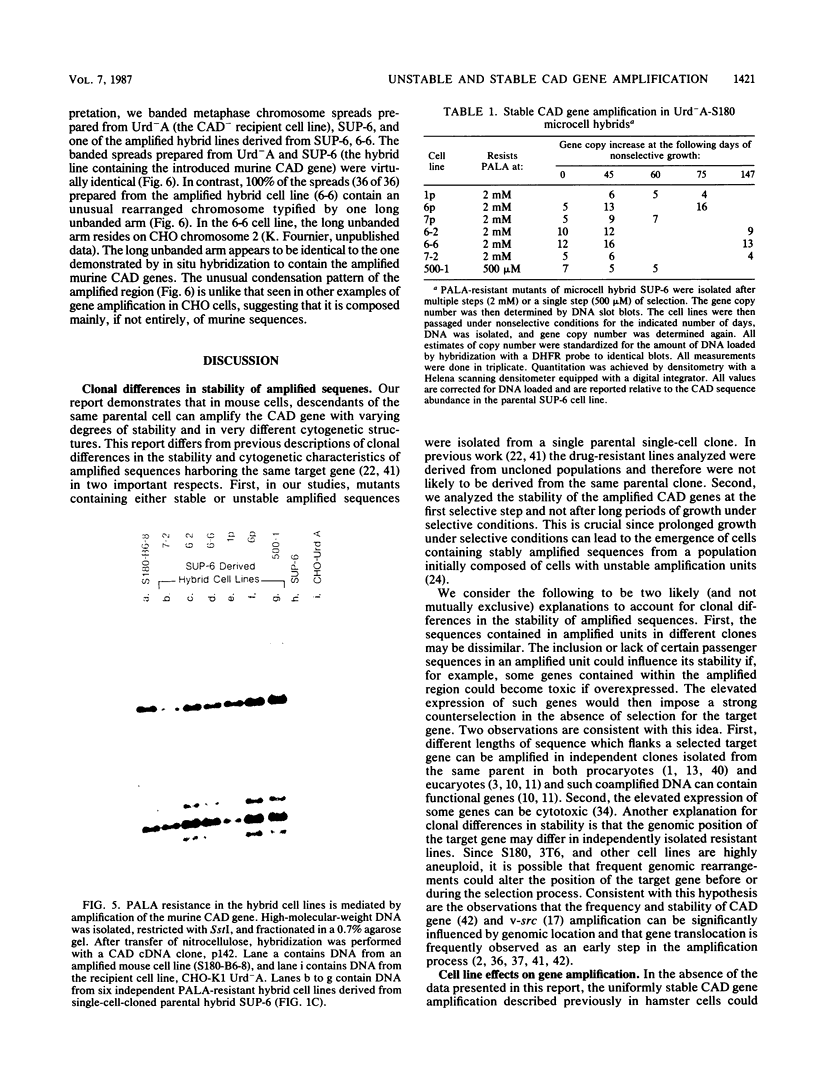
We analyzed the amplification of the CAD gene in independently isolated N-(phosphonacetyl)-L-aspartate-resistant clones derived from single parental clones in two mouse cell lines. We report for the first time that the CAD gene is amplified unstably in mouse cells, that the degree of instability varies greatly between clones, and that minute chromosomes and highly unstable chromosomelike structures contain the amplified sequences. These data are most consistent with the idea that the amplified unit in each clone consists of different flanking DNA and that such differences engender amplified sequences with unequal stability. We also introduced the mouse chromosome containing the CAD gene into hamster cells by microcell-mediated chromosome transfer to determine whether the propensity for unstable extrachromosomal amplification of the mouse CAD gene would prevail in the hamster cell nuclear environment. We report that the mouse CAD gene was amplified stably in expanded chromosomal regions in each of seven hybrids that were analyzed. This observation is consistent with the idea that the nuclear environment influences whether mutants containing intra- or extrachromosomally amplified sequences will be isolated.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Andrulis I. L., Duff C., Evans-Blackler S., Worton R., Siminovitch L. Chromosomal alterations associated with overproduction of asparagine synthetase in albizziin-resistant Chinese hamster ovary cells. Mol Cell Biol. 1983 Mar;3(3):391–398. doi: 10.1128/mcb.3.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshir F., Giulotto E., Zieg J., Brison O., Liao W. S., Stark G. R. Structure of amplified DNA in different Syrian hamster cell lines resistant to N-(phosphonacetyl)-L-aspartate. Mol Cell Biol. 1983 Nov;3(11):2076–2088. doi: 10.1128/mcb.3.11.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayusawa D., Iwata K., Seno T. Unusual sensitivity to bleomycin and joint resistance to 9-beta-D-arabinofuranosyladenine and 1-beta-D-arabinofuranosylcytosine of mouse FM3A cell mutants with altered ribonucleotide reductase and thymidylate synthase. Cancer Res. 1983 Feb;43(2):814–818. [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Cowell J. K. Double minutes and homogeneously staining regions: gene amplification in mammalian cells. Annu Rev Genet. 1982;16:21–59. doi: 10.1146/annurev.ge.16.120182.000321. [DOI] [PubMed] [Google Scholar]
- Davidson J. N., Carnright D. V., Patterson D. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: III. Association of carbamyl phosphate synthetase, aspartate transcarbamylase, and dihydroorotase in mutants of cultured Chinese hamster cells. Somatic Cell Genet. 1979 Mar;5(2):175–191. doi: 10.1007/BF01539159. [DOI] [PubMed] [Google Scholar]
- Debatisse M., Hyrien O., Petit-Koskas E., de Saint-Vincent B. R., Buttin G. Segregation and rearrangement of coamplified genes in different lineages of mutant cells that overproduce adenylate deaminase. Mol Cell Biol. 1986 May;6(5):1776–1781. doi: 10.1128/mcb.6.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debatisse M., de Saint Vincent B. R., Buttin G. Expression of several amplified genes in an adenylate-deaminase overproducing variant of Chinese hamster fibroblasts. EMBO J. 1984 Dec 20;3(13):3123–3127. doi: 10.1002/j.1460-2075.1984.tb02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolnick B. J., Berenson R. J., Bertino J. R., Kaufman R. J., Nunberg J. H., Schimke R. T. Correlation of dihydrofolate reductase elevation with gene amplification in a homogeneously staining chromosomal region in L5178Y cells. J Cell Biol. 1979 Nov;83(2 Pt 1):394–402. doi: 10.1083/jcb.83.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier R. E., Moran R. G. Complementation mapping in microcell hybrids: localization of Fpgs and Ak-1 on Mus musculus chromosome 2. Somatic Cell Genet. 1983 Jan;9(1):69–84. doi: 10.1007/BF01544049. [DOI] [PubMed] [Google Scholar]
- Fournier R. E., Ruddle F. H. Microcell-mediated transfer of murine chromosomes into mouse, Chinese hamster, and human somatic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):319–323. doi: 10.1073/pnas.74.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N. Unstable expression and amplification of a transfected oncogene in confluent and subconfluent cells. Mol Cell Biol. 1985 Jun;5(6):1456–1464. doi: 10.1128/mcb.5.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M., Bleile B., Tseng B. Y. Methotrexate-induced misincorporation of uracil into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1956–1960. doi: 10.1073/pnas.77.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Belli T. A., Zaczkiewicz L. T., Belli J. A. High-level, unstable adriamycin resistance in a Chinese hamster mutant cell line with double minute chromosomes. Cancer Res. 1984 Sep;44(9):4023–4029. [PubMed] [Google Scholar]
- Jakobsson A. H., Dahllöf B., Martinsson T., Levan G. Transfer of methotrexate resistance by somatic cell hybridization. Hereditas. 1983;99(2):293–302. doi: 10.1111/j.1601-5223.1983.tb00901.x. [DOI] [PubMed] [Google Scholar]
- Kanalas J. J., Hutton J. J., Suttle D. P. Characterization of pyrazofurin-resistant HeLa cells with amplification of UMP synthase gene. Somat Cell Mol Genet. 1985 Jul;11(4):359–369. doi: 10.1007/BF01534413. [DOI] [PubMed] [Google Scholar]
- Kano-Tanaka K., Higashida H., Fukami H., Tanaka T. Double minutes in mouse neuroblastoma cells and their hybrids. Cancer Genet Cytogenet. 1982 Feb;5(1):51–62. doi: 10.1016/0165-4608(82)90040-1. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5669–5673. doi: 10.1073/pnas.76.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Schimke R. T. Amplification and loss of dihydrofolate reductase genes in a Chinese hamster ovary cell line. Mol Cell Biol. 1981 Dec;1(12):1069–1076. doi: 10.1128/mcb.1.12.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe T. D., Swyryd E. A., Bruist M., Stark G. R. Stable mutants of mammalian cells that overproduce the first three enzymes of pyrimidine nucleotide biosynthesis. Cell. 1976 Dec;9(4 Pt 1):541–550. doi: 10.1016/0092-8674(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Levan A., Levan G., Mandahl N. A new chromosome type replacing the double minutes in a mouse tumor. Cytogenet Cell Genet. 1978;20(1-6):12–23. doi: 10.1159/000130836. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Moyer J. D., Smith P. A., Levy E. J., Handschumacher R. E. Kinetics of N-(phosphonacetyl)-L-aspartate and pyrazofurin depletion of pyrimidine ribonucleotide and deoxyribonucleotide pools and their relationship to nucleic acid synthesis in intact and permeabilized cells. Cancer Res. 1982 Nov;42(11):4525–4531. [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Stark G. R. Structure of the gene for CAD, the multifunctional protein that initiates UMP synthesis in Syrian hamster cells. Mol Cell Biol. 1982 Mar;2(3):293–301. doi: 10.1128/mcb.2.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D., Carnright D. V. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: I. Isolation of a mutant defective in the early steps of de novo pyrimidine synthesis. Somatic Cell Genet. 1977 Sep;3(5):483–495. doi: 10.1007/BF01539120. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G., Dieckmann B., Lee F. Co-expression and amplification of dihydrofolate reductase cDNA and the Escherichia coli XGPRT gene in Chinese hamster ovary cells. J Mol Appl Genet. 1981;1(3):165–175. [PubMed] [Google Scholar]
- Shigesada K., Stark G. R., Maley J. A., Niswander L. A., Davidson J. N. Construction of a cDNA to the hamster CAD gene and its application toward defining the domain for aspartate transcarbamylase. Mol Cell Biol. 1985 Jul;5(7):1735–1742. doi: 10.1128/mcb.5.7.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y., Shipley J., Brodeur G. M., Bruns G., Korf B., Donlon T., Schreck R. R., Seeger R., Sakai K., Latt S. A. Differential amplification, assembly, and relocation of multiple DNA sequences in human neuroblastomas and neuroblastoma cell lines. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3761–3765. doi: 10.1073/pnas.82.11.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E., Onen M., Perkins F. T., Hayflick L. Karyological and morphological characteristics of human diploid cell strain WI-38 infected with mycoplasmas. Exp Cell Res. 1969 Oct;57(2):397–410. doi: 10.1016/0014-4827(69)90166-9. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Tlsty T. D., Albertini A. M., Miller J. H. Gene amplification in the lac region of E. coli. Cell. 1984 May;37(1):217–224. doi: 10.1016/0092-8674(84)90317-9. [DOI] [PubMed] [Google Scholar]
- Tyler-Smith C., Bostock C. J. Gene amplification in methotrexate-resistant mouse cells. III. Interrelationships between chromosome changes and DNA sequence amplification or loss. J Mol Biol. 1981 Dec 5;153(2):237–256. doi: 10.1016/0022-2836(81)90276-x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wahl G. M., Robert de Saint Vincent B., DeRose M. L. Effect of chromosomal position on amplification of transfected genes in animal cells. Nature. 1984 Feb 9;307(5951):516–520. doi: 10.1038/307516a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Padgett R. A., Stark G. R. Single-copy and amplified CAD genes in Syrian hamster chromosomes localized by a highly sensitive method for in situ hybridization. Mol Cell Biol. 1982 Mar;2(3):308–319. doi: 10.1128/mcb.2.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieg J., Clayton C. E., Ardeshir F., Giulotto E., Swyryd E. A., Stark G. R. Properties of single-step mutants of Syrian hamster cell lines resistant to N-(phosphonacetyl)-L-aspartate. Mol Cell Biol. 1983 Nov;3(11):2089–2098. doi: 10.1128/mcb.3.11.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]









