Abstract
Overexpression of LIN28A is associated with human germ cell tumors and promotes primordial germ cell (PGC) development from embryonic stem cells in vitro and in chimeric mice. Knockdown of Lin28a inhibits PGC development in vitro, but how constitutional Lin28a deficiency affects the mammalian reproductive system in vivo remains unknown. Here, we generated Lin28a knockout (KO) mice and found that Lin28a deficiency compromises the size of the germ cell pool in both males and females by affecting PGC proliferation during embryogenesis. Interestingly however, in Lin28a KO males the germ cell pool partially recovers during postnatal expansion, while fertility remains impaired in both males and females mated to wild type mice. Embryonic overexpression of let-7, a microRNA negatively regulated by Lin28a, reduces the germ cell pool, corroborating the role of the Lin28a/let-7 axis in regulating the germ lineage.
Keywords: Germ Cells, Lin28a, miR-let7, Fertility
INTRODUCTION
The RNA-binding protein lin-28 was originally identified in C. elegans as a heterochronic gene that regulates developmental timing [1, 2]. We and others have shown that mammalian Lin28a/b regulates the biogenesis of the let-7 family of tumor-suppressor microRNAs [3–7]. LIN28 additionally functions as a cellular reprogramming factor, as an oncogene, and in the regulation of organismal growth and glucose metabolism [8–10]. By loss-of-function analysis with shRNA, we previously defined a molecular pathway whereby Lin28a influences primordial germ cell (PGC) development in vitro via the let-7 target Blimp1, a key regulator of PGC specification [11], but to date there has been no assessment of the lifelong effects of Lin28a-deficiency on the germ lineage. Here we generated Lin28a knockout mice and found that Lin28a deficiency reduces the size of the germ cell pool during embryogenesis, leading to impaired fertility in adults. Interestingly, in males the germ cell pool size recovers with age due to postnatal expansion of germ cells, indicating compensatory mechanisms regulating spermatogonial stem cells in adults, though fertility remains reduced. Although Lin28 protein can exert effects on protein translation independently of let-7 through mRNA binding, we demonstrate that overexpression of let-7 phenocopies Lin28a deficiency, confirming a role for the Lin28/let-7 axis in the regulation of the germ lineage.
MATERIALS AND METHODS
Mice
All animal procedures were approved by the Institutional Animal Care and Use Committee. The conditional Lin28a knockout mouse was reported previously [9]. We used a Ddx4-Cre strain (The Jackson Laboratory) to delete the floxed allele and generated conventional Lin28a KO mice. In parallel, we developed another Lin28a knockout strain (Fig. S1). A targeting construct with loxP sites flanking exon 2 (of 4) of Lin28a was electroporated into 129/SvEv Embryonic Stem (ES) cells. Southern analysis confirmed that clone H7 had the predicted hybridization pattern for both wild type and targeted alleles using 5-prime, 3-prime, and NeoR probes. Transient cre expression and subsequent clone selection was used to derive a targeted allele where the floxed exon and NeoR cassette was deleted. This deletion ES line was verified by PCR and used for blastocyst injection. Heterozygous mice were obtained by breeding with C57BL/6NTac female mice. The allele Lin28atm1Egm lacks exon 2 which encodes the conserved CSD domain of Lin28a. Exons 3 and 4 are out of frame with exon 1. The phenotypes observed were consistent between these strains. iLet-7 mice were previously reported [9]. Doxycycline (1g/mL) was given to mice in water. For all experiments, littermate controls were used.
Sperm analysis
Epididymides were harvested. Cauda epididymis and vas deferens were cut with a razor blade in HTF media (Millipore) and the number of cells released was counted after 1 hour. Sperm motility and morphology was determined according to the criteria from The Jackson Laboratory web site (http://reproductivegenomics.jax.org/maleprotocol.html).
Fertility test and superovulation
One 2–3 month-old female of either Lin28a +/− or −/− was placed into a cage with a fertility-proven wild-type male. Cages were monitored daily and the number of pups and litters were recorded for 10 weeks. One 7–10 week-old or 7–8 month-old male of either Lin28a +/+, +/−, or −/− was placed into a cage with 2 fertility-proven wild-type females. Cages were monitored daily and the number of pups and litters were recorded for 13 weeks. For the female plugging test, one 5 month-old male of either Lin28a +/+, +/−, or −/− was placed into a cage with two wild-type females. Cages were monitored daily and the number of plugs was recorded for 4 weeks. Females were replaced every 7 days. Superovulation was performed by successive injections of pregnant mare serum gonadotropin (10 units/mouse, Calbiochem) and human chorionic gonadotropin (10 units/mouse, Sigma) with a 48 hour interval. Oocytes were harvested and counted on the following day.
In vitro fertilization
In vitro fertilization was performed as described previously [12]. Briefly, cumulus masses were collected from the ampullae of 3–4 week-old superovulated 57 female mice. Sperm from cauda epididymis of 4–6 month-old Lin28a +/+, +/−, and −/− males (N=2–3) were collected and counted after 10 minutes of capacitation. 5×105 sperm/ml concentration was used. Embryos were cultured with 5% CO2. Developing embryos were counted on the following day (day 1) for two-cell embryos, and on days 3 and 4 for expanded blastocysts.
Flow cytometry
Genital ridges were isolated from E12.5 or E13.5 embryos and dissociated in the “EB dissociation enzyme mix” described previously [13]. Cells were then washed once and resuspended in staining buffer (2% serum/PBS, SB) with primary antibodies (FITC mouse anti-SSEA1 [BD Pharmingen] and APC rat anti-c-Kit [BD Pharmingen]) and incubated for 20 minutes on ice. Cells were washed twice and resuspended in 500 ul SB, followed by addition of PI (1:1000) or DAPI (1:1000). Samples were sorted and analyzed on a FACS Calibur system or a FACS Aria system and via BD CellQuest or BD FACS Diva software (BD Biosciences).
Quantitative RT-PCR
RNA was collected using Trizol reagent (Invitrogen). Total RNA was reverse-transcribed and subjected to Taqman miRNA assay (Applied Biosystems) followed by pre-amplification of cDNA using PreAmp master mix (Applied Biosystems) following the manufacturer’s instructions. miRNA expression was measured by quantitative PCR using the delta-delta CT method.
Histology
Tissue samples were fixed in 10% buffered formalin or Bouin’s solution and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E).
Immunohistochemistry and immunofluorescence
Immunohistochemistry was performed as described [10]. Immunofluorescence was performed in the same manner except using Alexa488 or Alexa568-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies. The primary antibodies used in this study include: rabbit anti-Lin28 (1:300, Cell Signaling Technology), rabbit anti-Mvh (1:1000, abcam), and mouse anti-Ki67 (1:50, Dako).
Follicle counting
Adult ovaries were collected during early estrus and estrus stages of the estrous cycle determined by analysis of vaginal smears. Follicle count was performed using ovary sections with H&E staining or immunostained by an anti-Mvh antibody for easier detection of oocytes. Follicles were classified as primordial (flattened or at least one cuboidal granulosa cells (GC)), primary (one complete layer of cuboidal GC), secondary (two or more complete layers of GC), and antral (antrum formation).
Plasma hormone measurement
Testosterone was measured by an enzyme immunoassay kit (Assay Designs). TSH and LH were measured by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Statistical analysis
Data is presented as mean ± SEM, and Student’s t test (two-tailed distribution, two-sample unequal variance) was used to calculate p values. Statistical significance is displayed as * p < 0.05 or ** p < 0.01.
RESULTS
Lin28a expression in juvenile and adult mice
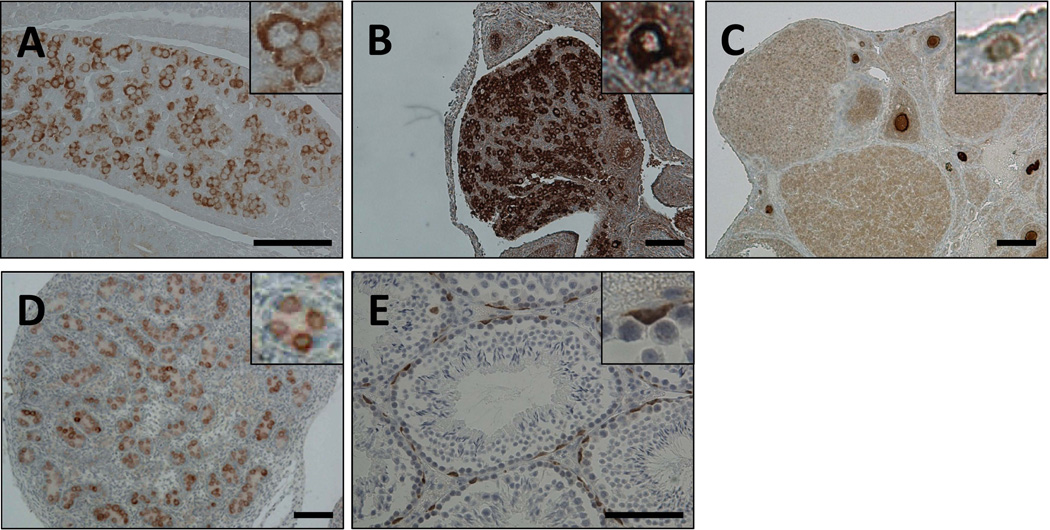
The localization of Lin28a in adult mouse testis was reported previously [14]. However its expression and localization during development remained to be characterized. We evaluated Lin28a expression patterns in the germ lineage from midgestation to adult stages in both males and females. Lin28a was prominently expressed in SSEA1+ PGCs of the developing embryo at day 13.5 (Fig. 1A and data not shown). In the ovary, oocytes expressed Lin28a in newborns and adults alike (Fig. 1B, C). In the testis, nearly all gonocytes defined as Mvh+ cells expressed Lin28a in newborns (Fig. 1D and data not shown), expression of which was limited to undifferentiated spermatogonia in adults (Fig. 1E). Lin28a localization remained cytoplasmic at all developmental stages analyzed.
Fig. 1.
Lin28a expression in normal gonadal tissues at different ages. (A) E13.5 genital ridge. (B) Newborn ovary. (C) 5-week-old ovary. (D) Newborn testis. (E) 8-month-old testis. Insets show higher magnification of Lin28a positive cells. Note that Lin28a expression in cytoplasm. Scale bars 100 µm.
Lin28a deficiency compromises germ cell pool size
To evaluate Lin28a in the germ cell lineage and reproductive system function in vivo, we generated two independent Lin28a knockout (KO) mouse strains (Supplementary Fig. 1 and [9]). We confirmed that the second exon of Lin28a was deleted in both strains and observed that the phenotypes such as higher let-7 levels in embryos, lower number of germ cells in neonates, and reduced fertility described below were consistent between these strains. The data presented in this article are mainly taken from the strain in Supplemental Fig. 1.
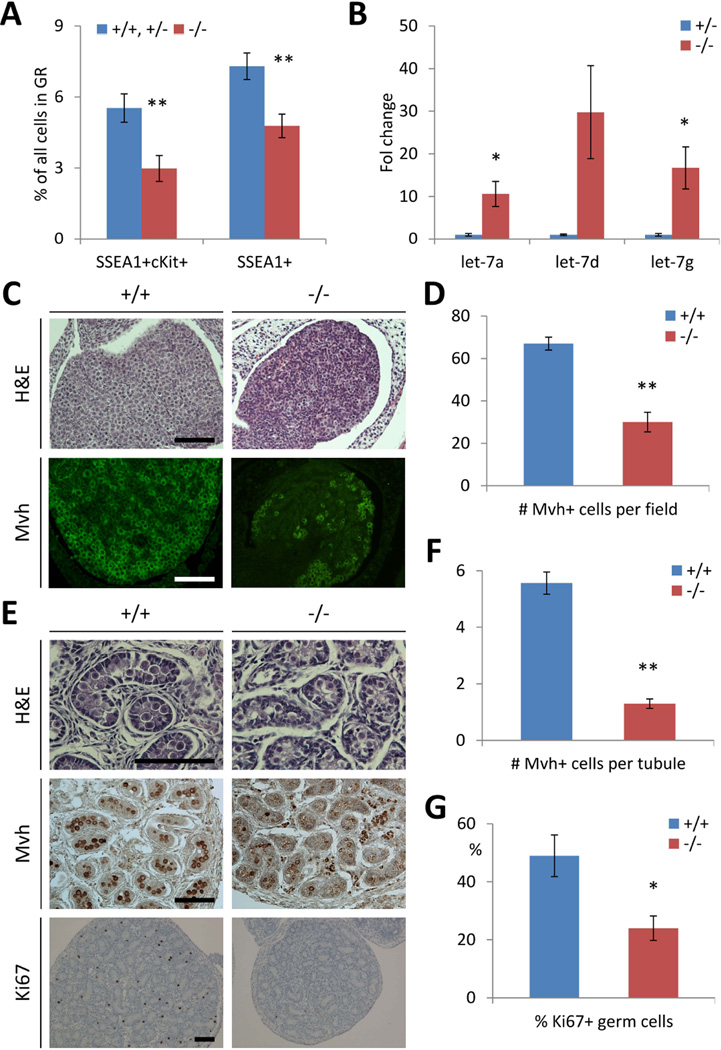
We examined PGC development in midgestation embryos, as our previous experiments using an in vitro embryonic stem cell differentiation system indicated that knockdown of Lin28a significantly diminished the numbers of PGCs in vitro [11]. We found markedly fewer SSEA-1+ and SSEA-1+c-Kit+ PGCs in the genital ridges of embryonic day 13.5 Lin28a KO embryos compared to controls (Fig. 2A). qPCR revealed that Lin28a KO PGCs at this stage express higher let-7 miRNAs, consistent with the loss of Lin28a suppression (Fig. 2B). Correspondingly, neonatal mouse testes and ovaries also contained fewer gametes positive for the germ cell-specific marker Mvh (Fig. 2C–F). Ki-67 staining revealed fewer proliferating germ cells in the newborn KO testes compared to wild type controls (Fig. 2E, G). Together, these results indicate a role for Lin28a in regulating germ cell pool size in embryonic and neonatal stages of both sexes.
Fig. 2.
Lin28a deficiency reduces germ cell pool size. (A) Percentage of primordial germ cells in Lin28a −/− and control genital ridges at E13.5. N=4–15. (B) let-7 levels of primordial germ cells in E13.5 embryos. N=3. (C) Lin28a +/+ and −/− newborn ovaries. (D) Number of Mvh+ germ cells in Lin28a +/+ and −/− newborn ovaries. N=4–5. (E) Lin28a +/+ and −/− newborn testes. (F–G) Number of Mvh+ germ cells per tubule (F) and % Ki67+ germ cells (G) in Lin28a +/+ and −/− newborn testes. N=64–74 from 3–5 mice in (F) and N=3–4 in (G). * p<0.05, ** p<0.01. Error bars represent SEM. Scale bars 100 µm.
Lin28a KO females have reduced fertility
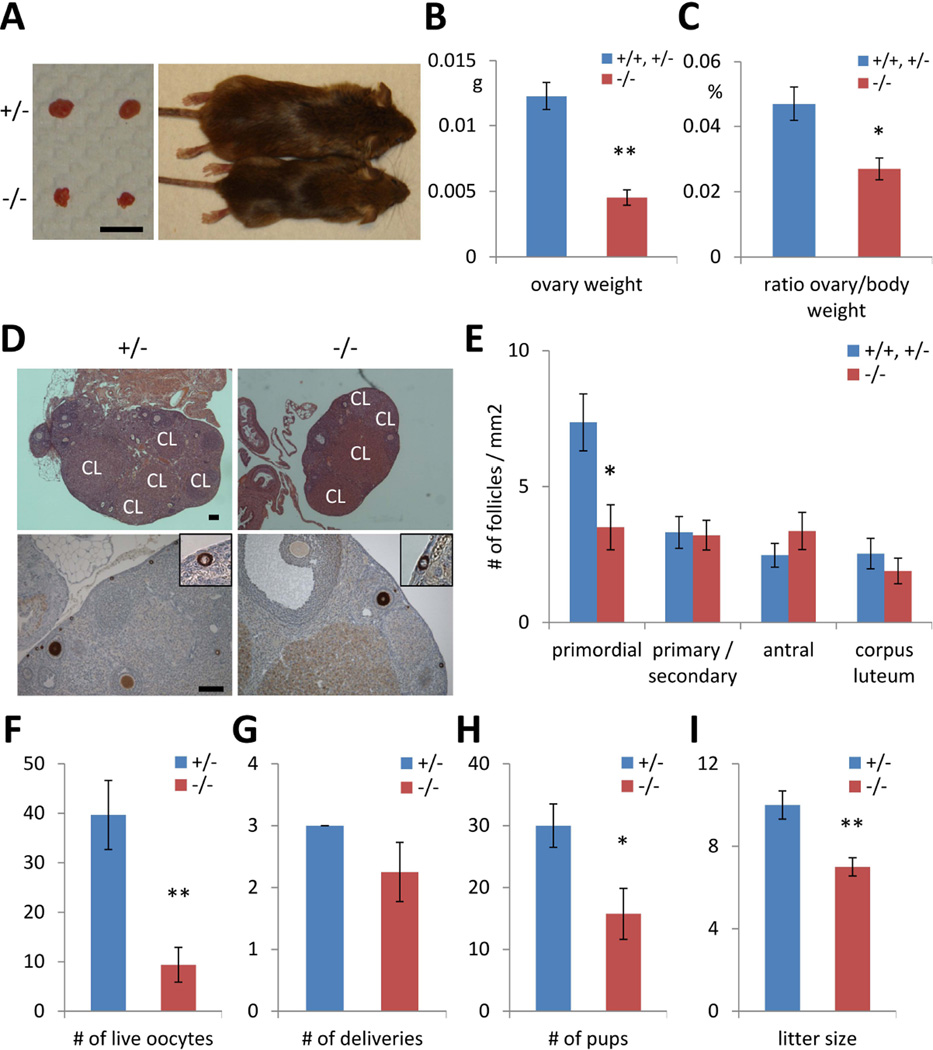
Next, we examined female Lin28a KO mice to determine whether reduced germ cell pool size impacts fertility. Lin28a KO mice were runted compared to controls (30–50% smaller body size) whereas average ovary weight of Lin28a KO mice was approximately one-third that of controls; thus, relative to body weight, the Lin28a KO ovary is disproportionally small (Fig. 3A–C). Lin28a KO ovaries harbored reduced numbers of primordial ovarian follicles per given area (Fig. 3D), whereas the number of maturing follicles and corpora lutea was indistinguishable from controls (Fig. 3E), indicating that despite a reduced pool of primordial follicles, morphological oocyte maturation remained intact in Lin28a KO animals. However, we noted a reduction in the numbers of live oocytes collected from KO animals following hormone-induced superovulation (Fig. 3F). Ultimately, fertility tests yielded lower total numbers of pups and smaller litter sizes in spite of comparable frequencies of delivery, suggesting fewer functionally mature oocytes in the KO females (Fig. 3G–I). These data indicate that in Lin28a KO females, the germ cell pool size is reduced at embryonic and adult stages, resulting in reduced numbers of mature oocytes and decreased fertility.
Fig. 3.
Lin28a knockout females have reduced fertility. (A) A representative photographs of 5-month-old Lin28a +/− and −/− mice and their ovaries. Bar 5mm. (B–C) Ovary weight (B), ovary weight relative to body weight (C) in 5-month-old Lin28a +/− and −/− females. N=4–6. (D) H&E staining (top) and immunostaining with Mvh (bottom) of 3-month-old adult ovaries. Inset: higher magnification of primordial follicles. Note that corpus luteum (CL) are found in both Lin28a +/− and −/− ovaries. Scale bars 100 µm. (E) Number of different stages of ovarian follicles per 1 mm2 in 3-month-old Lin28a +/+, +/− and −/− ovaries. N=8–10. (F) Number of live oocyte collected from 2-month-old Lin28a +/− and −/− females after superovulation. N=5–6. (G–I) Female fertility tests at 2–3 months old. Number of deliveries (G) and number of pups born (H) per Lin28a +/− and −/− female during 10-week experimental period. (I) Litter size of Lin28a +/− and −/− females. N=3–4. * p<0.05, ** p<0.01. Error bars represent SEM.
Spermatogenesis partially recovers in adult Lin28a KO testes
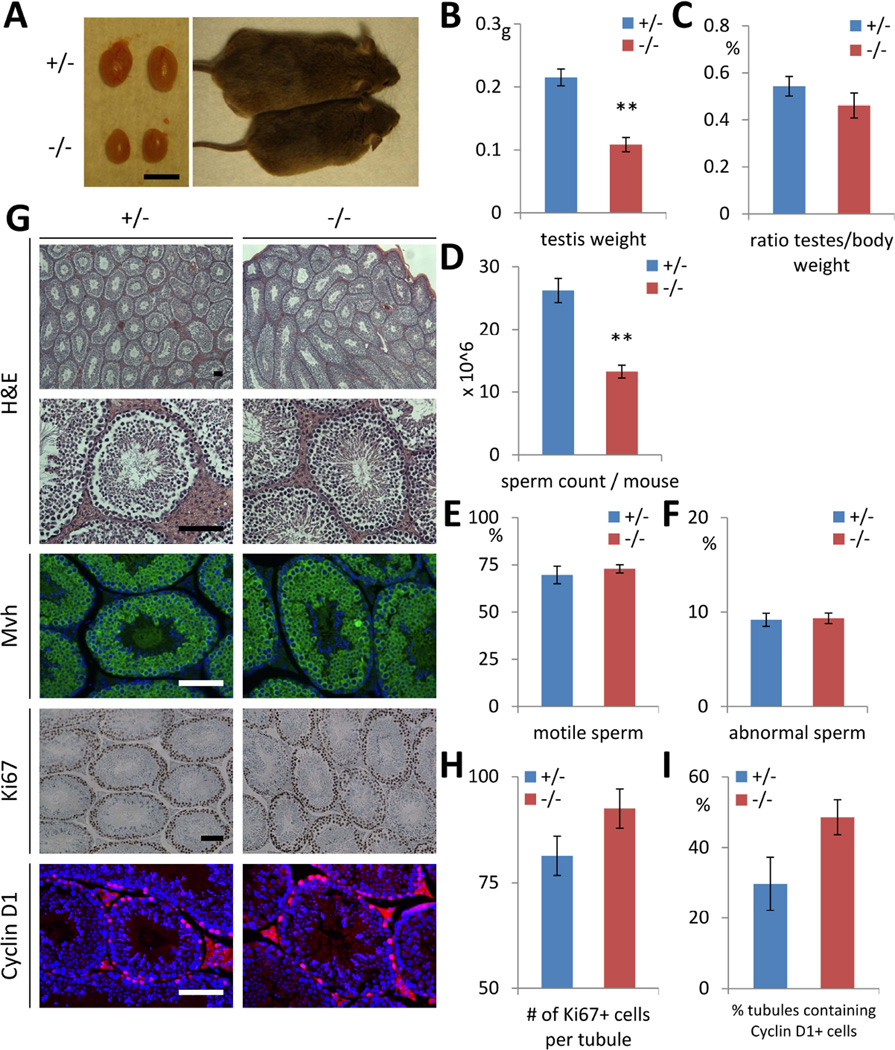
Like Lin28a KO females, males are runted. The testes of Lin28a KO adult males were approximately half the size of controls but proportional to their lower total body weight (Fig. 4A–C). Sperm counts were lower in KO males, whereas motility and morphology of the KO sperm were comparable to those of controls (Fig. 4D–F). The histology of KO testes was comparable to controls, showing normal maturation during spermatogenesis and comparable numbers of Ki-67+ proliferating germ cells and Cyclin D1+ proliferating spermatogonia (Fig. 4G–I). These data indicate a homeostatic recovery to near normal sperm production in adult Lin28a KO males.
Fig. 4.
Partial recovery of germ cell pool size in adult Lin28a KO males. (A) A representative photographs of 5-month-old Lin28a +/− and −/− mice and their testes. Bar 5mm. (B–F) Testis weight (B), testis weight relative to body weight (C) in 5- to 6-month-old Lin28a +/− and −/− mice. N=6. (D–F) Total epididymal sperm count per mouse (D), percentage of motile sperms (E), and percentage of abnormal sperms (F) in 7- to 8-month-old Lin28a +/− and −/− mice. N=3–4. (G) H&E and immunostaining of 6-month-old Lin28a +/− and −/− testes. (H–I) Number of Mvh+ germ cells per tubule (H) and number of tubules containing Ki67+ cells (I) in 6- to 8-month-old Lin28a +/− and −/− adult testes. N=5. ** p<0.01. Error bars represent SEM. Scale bars 100 µm.
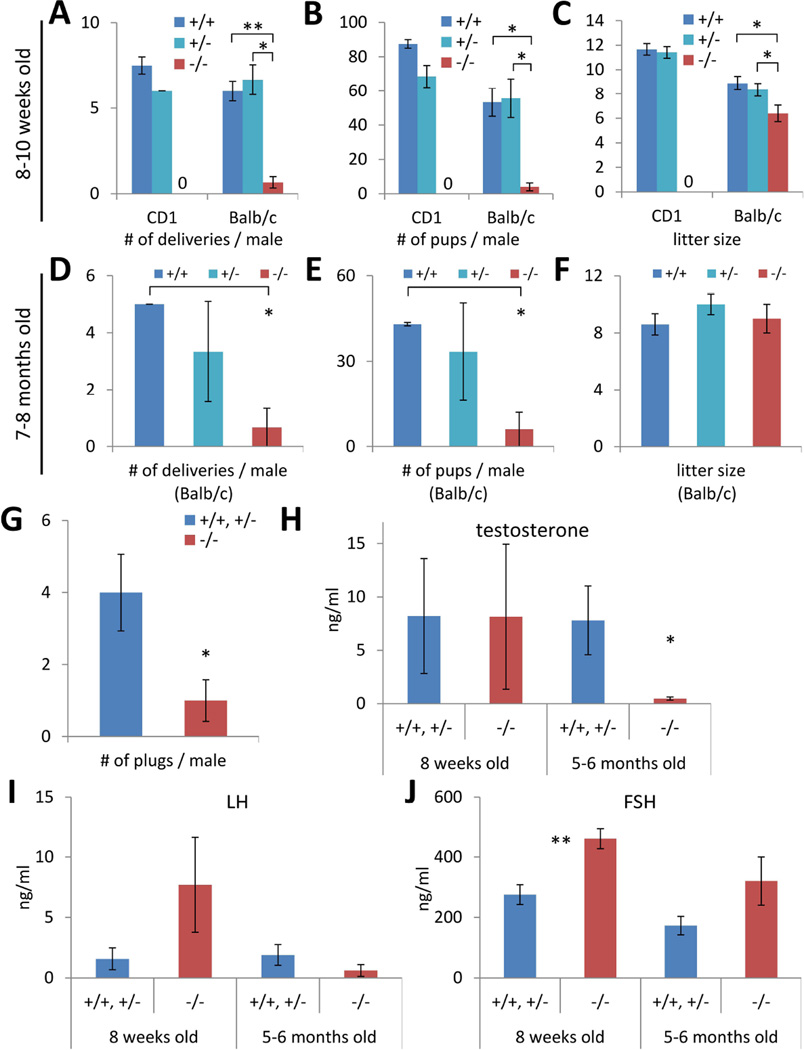
Lin28a KO males have reduced fertility
We then evaluated the fertility of Lin28a KO males. Given that the adult Lin28a KO testes showed a recovery of testis histology and sperm differentiation relative to neonates, we compared fertility in young post-weanling (7–10 weeks old) and older (7–8 months old) males. Both age groups sired fewer litters and produced lower overall numbers of pups during the experimental period relative to controls (Fig. 5A–E). We performed an in vitro fertilization experiment and confirmed that the lower numbers of pups did not result from less fertilizing capacity of Lin28a KO sperm (Table S1). Interestingly, while younger Lin28a KO males produced smaller litter sizes in two distinct strain backgrounds (Fig. 5C), older males were comparable to controls (Fig. 5F). The older Lin28a KO males had less frequent mating behavior as evidenced by reduced plugging in females, which could explain less frequent female deliveries and lower total numbers of pups in spite of similar litter sizes (Fig. 5G). In considering what may account for less frequent mating behavior in relatively older males, we found the plasma testosterone (T) levels were lower in Lin28a KO mice than in controls (Fig. 5H). Luteinizing hormone (LH) secreted from the pituitary gland positively controls T production from Leydig cells in testes [15]. Lin28a KO males had similar plasma LH levels to controls, indicating that the physiological origin of lower T levels was distal to pituitary function and likely originated from the Leydig cells themselves (Fig. 5I). The other gonadotropin, follicle-stimulating hormone (FSH), which stimulates sperm production through Sertoli cells in the testis, was higher in Lin28a KO plasma (Fig. 5J), consistent with the lower sperm count seen in the Lin28a KO mice (Fig. 4D), and the known feedback role of FSH in sperm production [15]. Thus, although Lin28a KO males recovered near normal spermatogenesis, they nonetheless manifest reduced fertility in the setting of lower sex hormone levels.
Fig. 5.
Lin28a −/− males are subfertile. (A–F) Male fertility tests at 7–10 weeks old (A–C) and 7–8 months old (D–F). Number of deliveries (A and D) and number of pups born (B and E) per Lin28a +/+, +/− and −/− male during 13-week experimental period. (C and F) Litter size of Lin28a +/+, +/− and −/− males. N=2–3. (G) Number of plugs per 5-month-old Lin28a +/+, +/− and −/− male during 4-week experimental period. N=3–6. (I–K) Plasma testosterone (I), LH (J), and FSH (K) levels in Lin28a +/+, +/− and −/− males. N=4–10. * p<0.05, ** p<0.01. Error bars represent SEM.
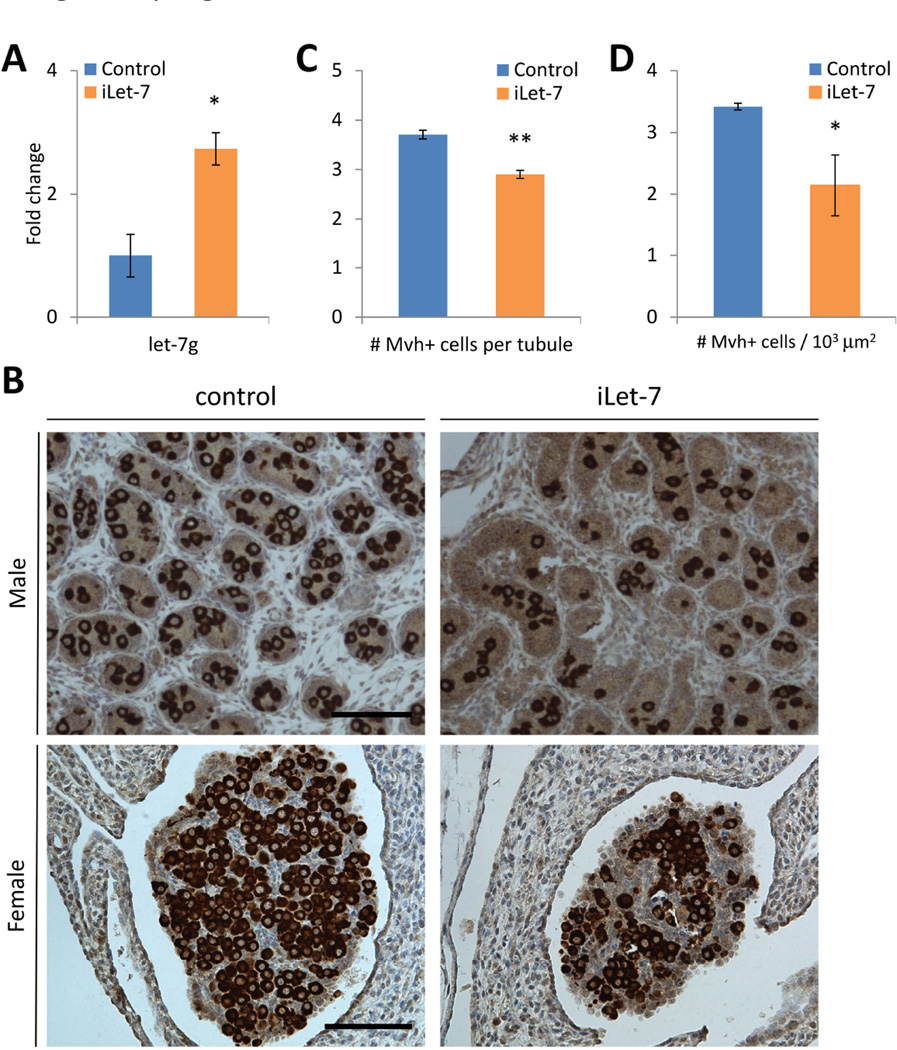
Embryonic overexpression of let-7 miRNA reduces germ cell pool size
Let-7 miRNAs are a major downstream target of Lin28a and known to regulate cell cycle and cell proliferation. We hypothesized that overexpression of let-7 during embryogenesis might compromise germ cell pool development similar to Lin28a KO mice. iLet-7 mice [9], which overexpress let-7g upon induction by doxycycline (dox), were fed dox in their drinking water throughout pregnancy, and we confirmed that SSEA-1+ PGCs from E13.5 embryos overexpressed let-7g 3-fold (Fig. 6A). Neonatal testes and ovaries of iLet-7 mice exposed to doxycycline during gestation showed a reduced frequency of Mvh+ germ cells compared to littermate controls (Fig. 6B–D), suggesting that Lin28a regulates the germ cell pool size in both males and females at least in part via modulation of the let-7 miRNA pathway.
Fig. 6.
Embryonic overexpression of let-7 miRNA reduces germ cell pool size. (A) let-7 levels of primordial germ cells in E13.5 embryos. N=3. (B) iLet-7 and control newborn testes and ovaries. (C) Number of Mvh+ germ cells per tubule in iLet-7 and control newborn testes. N=286–303 from 3 mice. (D) Number of Mvh+ germ cells per 1000 µm2 in iLet-7 and control ovaries. N=3–5. * p<0.05, ** p<0.01. Error bars represent SEM. Scale bars 100 µm.
DISCUSSION
In previous work, we performed an inhibitory shRNA screen during the in vitro differentiation of mouse embryonic stem cells into the germ lineage to implicate Lin28a as a regulator of PGC numbers via effects on the let-7 target gene Blimp1, a known master regulator of germ cell development [11]. Here we analyze Lin28a KO mice and reciprocal transgenic strains that overexpress let-7 to unequivocally establish a role for the Lin28/let-7 axis in establishing germ cell pools during embryogenesis and influencing reproductive function throughout adult life. While a marked reduction in the size of the germ cell pool is evident at birth in both males and females, our analysis of germ cell numbers and fertility in adults revealed interesting sex-specific differences in the mechanisms of lifelong reduced fertility. Coupled to our prior studies, which established that transgenic Lin28a induced marked delays in puberty [10], our work enriches our understanding of this pathway as one of the critical regulators of reproductive function through effects on germ cell development, timing of sexual maturation, and reproductive behavior throughout adult life.
PGCs, which emerge in the epiblast-derived extraembryonic mesoderm, migrate to the genital ridge and proliferate by E13.5 [16]. Our Lin28a KO and iLet-7 mice data indicate that repression of let-7 at this stage is necessary for establishing proper numbers of germ cells to populate the developing gonad in both males and females. Our prior in vitro study indicated that Lin28a and let-7 mediate opposing effects on PGC numbers, and that Lin28a functions in large part via regulation of the let-7 microRNAs and Blimp-1 [11]. We cannot exclude the possibility that other let-7 targets are involved or that Lin28a might bind to mRNAs that affect PGC development independently of let-7. These alternatives notwithstanding, our results illustrate the importance of the Lin28a/let-7 axis regulating the germ cell pool size as early as E13.5, and the effects are apparent throughout post-natal life.
Lin28a influences both male and female fertility in some common and some distinct ways. In fertility tests, young Lin28a KO males and females both yielded smaller litter sizes, reflecting fewer mature gametes, consistent with germ cell pool size reductions seen in the embryonic and neonatal gonads in both sexes. In male gametogenesis, spermatogonial stem cells proliferate and differentiate to form sperm throughout life. Our results showed that older (7–8 month-old) Lin28a KO mice had similar testis histology and litter sizes relative to control mice, although their sperm counts remained lower. This indicates that the size of the male germ cell pool at least partially recovers via postnatal expansion and that Lin28a is dispensable for the postnatal germ cell expansion. In Lin28a KO females, the germ cell pool does not change postnatally, consistent with the fact that female germ cells enter meiosis and then arrest midway through meiosis I during embryogenesis and do not produce new gametes postnatally. Although this traditional concept has been challenged recently [17], if females continue to produce oocytes in adults from germ line stem cells, we might have anticipated a similar recovery of the germ cell pool in females, as we did in males.
Another factor affecting fertility is regulation by sex hormones. Our results show that plasma testosterone levels in Lin28a KO males are lower despite comparable or even higher gonadotropin (LH, FSH) levels. The mechanism of the testosterone deficiency remains unknown. As Leydig cells do not express Lin28a (Fig. 1), other factor(s) outside of the pituitary/gonadal axis may regulate plasma testosterone levels. Genetic variation in LIN28B, a paralog of human LIN28A, has been linked to the timing of menarche in human genome-wide association studies (GWAS) [18–21], it will be interesting to explore endocrinological roles of Lin28a in the reproductive system.
Our current data reveal that the Lin28a/let-7 pathway is critical for proper germ cell pool development in vivo, primarily through effects at the PGC stage. Future studies may include analysis of human LIN28A levels that correlates with infertility, especially in patients with primary ovarian failure and short stature.
CONCLUSIONS
In this study we generated and analyzed Lin28a knock-out mice and found that Lin28a deficiency compromises the size of the germ cell pool during embryogenesis in both males and females, leading to reduced fertility in adults. Interestingly, the germ cell pool size partially recovers in adult males due to postnatal expansion of germ cells. Embryonic let-7 overexpression reduces the germ cell pool, reinforcing the role of the Lin28a/let-7 axis in regulating the germ lineage.
Supplementary Material
Fig. S1. Targeted generation of a Lin28a null allele. (A) Genomic map of the Lin28a locus and Lin28a targeting construct. PCR primer positions and sequences for genotyping are shown. (B) Western blot showing Lin28a expression in Lin28a +/− and −/− ESCs. (C) PCR genotyping showing wild type (wt) and deleted (null) alleles. This strain (Strain 1) differs from the other strain we reported previously (Zhu et al., 2011; Strain 2) in that we used different vector backbones and the Neo cassette remains within the Lin28a allele of Strain 2. Related to Experimental procedures.
Table S1 Results of in vitro fertilization using Lin28 +/+, +/−, and −/− sperm and wild type eggs. N=2–3. Numbers represent mean ± SEM. Related to Fig. 5.
ACKNOWLEDGMENTS
We thank Achia Urbach and Mathew William Lensch for invaluable discussions and advice, Roderick Bronson and the Harvard Medical School Rodent Histopathology Core for mouse tissue pathology, and The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for hormone measurement. This work was supported by grants from the Alex’s Lemonade Stand Foundation, the Ellison Medical Foundation, the NIDDK (Hematology center grant P30 DK049216-17) for Mouse Embryonic stem (ES) cells and Gene targeting Core, and the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934 for The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Footnotes
Author Contribution
G.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing and final approval of manuscript. T.Y.S. and M.T.S.: collection and/or assembly of data. A.Y., J.P.H., and R.I.G.: provision of study materials. Y.F. and P.Y.H.: collection and/or assembly of data, and data analysis and interpretation. E.G.M.: provision of study material or patients. G.Q.D.: conception and design, data analysis and interpretation, manuscript writing and final approval of manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 2.Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 3.Heo I, Joo C, Cho J, et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14:1539–1549. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rybak A, Fuchs H, Smirnova L, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 6.Piskounova E, Viswanathan SR, Janas M, et al. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem. 2008;283:21310–21314. doi: 10.1074/jbc.C800108200. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 2010;140:445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Zhu H, Shyh-Chang N, Segre AV, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu H, Shah S, Shyh-Chang N, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet. 2010;42:626–630. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West JA, Viswanathan SR, Yabuuchi A, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009;460:909–913. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagy A. Manipulating the mouse embryo : a laboratory manual. 3rd ed. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2003. p. 764. x. [Google Scholar]
- 13.West JA, Park IH, Daley GQ, et al. In vitro generation of germ cells from murine embryonic stem cells. Nat Protoc. 2006;1:2026–2036. doi: 10.1038/nprot.2006.303. [DOI] [PubMed] [Google Scholar]
- 14.Zheng K, Wu X, Kaestner KH, et al. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev Biol. 2009;9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. J Endocrinol. 2010;205:117–131. doi: 10.1677/JOE-10-0025. [DOI] [PubMed] [Google Scholar]
- 16.Ewen KA, Koopman P. Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol. 2010;323:76–93. doi: 10.1016/j.mce.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 17.White YA, Woods DC, Takai Y, et al. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong KK, Elks CE, Li S, et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet. 2009 doi: 10.1038/ng.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulem P, Gudbjartsson DF, Rafnar T, et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat Genet. 2009 doi: 10.1038/ng.383. [DOI] [PubMed] [Google Scholar]
- 20.He C, Kraft P, Chen C, et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009;41:724–728. doi: 10.1038/ng.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry JR, Stolk L, Franceschini N, et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat Genet. 2009;41:648–650. doi: 10.1038/ng.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Targeted generation of a Lin28a null allele. (A) Genomic map of the Lin28a locus and Lin28a targeting construct. PCR primer positions and sequences for genotyping are shown. (B) Western blot showing Lin28a expression in Lin28a +/− and −/− ESCs. (C) PCR genotyping showing wild type (wt) and deleted (null) alleles. This strain (Strain 1) differs from the other strain we reported previously (Zhu et al., 2011; Strain 2) in that we used different vector backbones and the Neo cassette remains within the Lin28a allele of Strain 2. Related to Experimental procedures.
Table S1 Results of in vitro fertilization using Lin28 +/+, +/−, and −/− sperm and wild type eggs. N=2–3. Numbers represent mean ± SEM. Related to Fig. 5.