Abstract
Spinal muscular atrophy (SMA), a recessive neuromuscular disorder, is caused by diminished function of the Survival Motor Neuron (SMN) protein. To define the cellular processes pertinent to SMA, parallel genetic screens were undertaken in Drosophila and Caenorhabditis elegans SMA models to identify modifiers of the SMN loss of function phenotypes. One class of such genetic modifiers was the small conductance, Ca2+-activated K+ (SK) channels. SK channels allow efflux of potassium ions when intracellular calcium increases and can be activated by the neuroprotective drug riluzole. The latter is the only drug with proven, albeit modest, efficacy in the treatment of amyotrophic lateral sclerosis. It is unclear if riluzole can extend life span or ameliorate symptoms in SMA patients as previous studies were limited and of insufficient power to draw any conclusions. The critical biochemical target of riluzole in motor neuron disease is not known, but the pharmacological targets of riluzole include SK channels. We examine here the impact of riluzole in two different SMA models. In vertebrate neurons, riluzole treatment restored axon outgrowth caused by diminished SMN. Additionally, riluzole ameliorated the neuromuscular defects in a C. elegans SMA model and SK channel function was required for this beneficial effect. We propose that riluzole improves motor neuron function by acting on SK channels and suggest that SK channels may be important therapeutic targets for SMA patients.
Introduction
Spinal muscular atrophy (SMA), an autosomal recessive neuromuscular disorder, is the leading genetic cause of infant death in the United States (Pearn, 1978; Crawford and Pardo, 1996). SMA primarily affects the α-motor neurons in the anterior horn of the spinal cord and is characterized by progressive muscle degeneration, loss of neuromuscular function, paralysis, and/or death. SMA is caused by Survival Motor Neuron 1 (SMN1) homozygous loss of function mutations that lead to decreased Survival Motor Neuron (SMN) protein levels (Lefebvre et al., 1995). Amyotrophic lateral sclerosis (ALS) has commonalities with SMA as both target spinal cord motor neurons and share phenotypic, genetic, and molecular similarities. For example, SMN gene variants have been associated with sporadic ALS (Veldink et al., 2001; Corcia et al., 2009; Blauw et al., 2012), overexpression of TDP-43, an ALS protein, increases SMN mRNA levels (Bose et al., 2008), and mutation of the vesicle-trafficking protein VAPB can cause late-onset SMA and ALS (Nishimura et al., 2004). Therefore, these neuromuscular disorders may share a common neurodegenerative pathway and respond to similar treatments.
We have previously reported that small conductance Ca2+-activated K+ (SK) channels are cross-species invertebrate SMN modifiers (Chang et al., 2008; Dimitriadi et al., 2010). SK channels are activated by intracellular calcium; are potassium selective; have been implicated in epilepsy, ataxias, and other disorders (Pedarzani and Stocker, 2008); and play roles in after-hyperpolarization, repetitive firing, dendritic integration, synaptic transmission, and synaptic plasticity (Xia et al., 1998; Keen et al., 1999; Schumacher et al., 2001). In neurons, SK channel activity is regulated by calcium entry through voltage-gated calcium channels. The overall impact of SK channels on neuronal activity can be difficult to predict. Increased potassium efflux can reduce excitability, but when coupled with depolarization-induced calcium influx, SK channels can increase firing rates by accelerating repolarization. SK2 channels are often found in a complex with L-type Ca2+ channels and α-actinin, an actin-binding protein (Lu et al., 2007, 2009). α-actinin interacts directly with SMN in Drosophila adult muscle (Rajendra et al., 2007), and α-actinin orthologs are invertebrate SMN modifier genes (Chang et al., 2008; Dimitriadi et al., 2010).
SK channels are among the pharmacological targets of riluzole. Riluzole ameliorates the aberrant cytoskeletal organization of synaptic terminals in mice lacking SMN exon 7 (Haddad et al., 2003). Riluzole had no impact on severely affected SMA patients in a short duration study with a limited number of subjects (Russman et al., 2003). This study was of insufficient power to determine whether riluzole might benefit other SMA patient populations. Elucidating the biochemical target of riluzole may increase our understanding of ALS and SMA pathogenesis. Here, we test the hypothesis that riluzole can ameliorate SMN loss-of-function defects across species.
Materials and Methods
Caenorhabditis elegans strains.
LM99 smn-1(ok355)I/hT2 (I;III) (Briese et al., 2009), HA2207 kcnl-2(tm1885) smn-1(ok355)/hT2, HA2415 kcnl-2(ok2818) smn-1(ok355)/hT2, HA2400 smn-1(ok355)/hT2; slo-1(js118)V, HA2402 smn-1(ok355)/hT2; slo-2(nf100)X, and HA2404 smn-1(ok355)/hT2; sup-9(n180)II strains were cultivated at 20°C under standard conditions (Brenner, 1974). kcnl-2 alleles tm1885 and ok2818 were backcrossed six and three times, respectively. tm1885 removes three transmembrane domains; ok2818 perturbs transmembrane domains and the calmodulin-binding domain. kcnl-2(tm1885) pumping rates are slightly lower than kcnl-2(ok2818) (p = 3 × 10−4). All assays used the progeny of hT2 parents to control genetic background.
C. elegans assays.
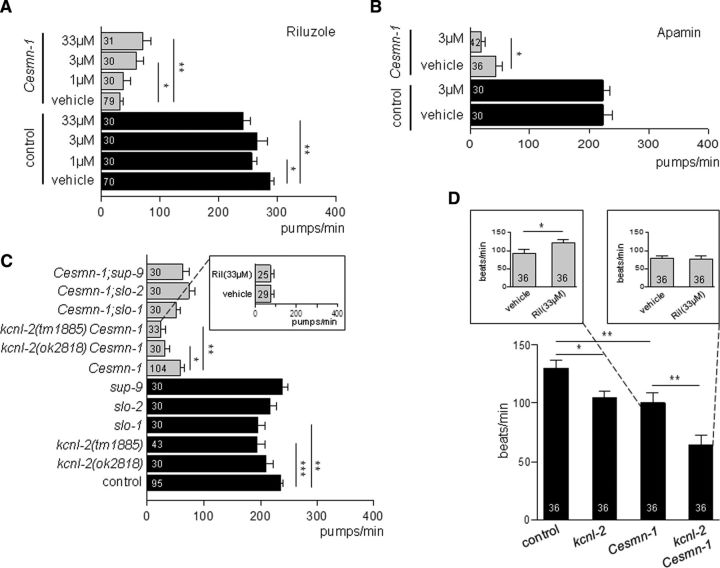
The pharyngeal pumping assay was performed as previously described (Dimitriadi et al., 2010). Eggs hatched on L4440 control vector (Kamath and Ahringer, 2003) were reared at 2 d at 25°C and 1 d at 20°C. Pumping rates were determined at the last larval stage. Average pumping rates (±SEM) were derived from at least three independent trials (n ≥ 25 animals in total). Experimenters were blinded to genotype/treatment for at least one trial. For Figure 2, A and C, more than three independent trials were performed and were pooled together for the final figure. Unpooled results Figure 2A: Trials 1–3 Control/DMSO 302 ± 7, Control/3 μm 266 ± 17, Control/33 μm 242 ± 12, Cesmn-1/DMSO 23 ± 5, Cesmn-1/3 μm 59 ± 14, Cesmn-1/33 μm 70 ± 13; Cesmn-1/DMSO vs Cesmn-1/3 μm, p = 0.04 and Cesmn-1/DMSO vs Cesmn-1/33 μm, p = 0.007; Trials 4–5 Control/DMSO 268 ± 9, Control/1 μm 257 ± 9, Cesmn-1/DMSO 48 ± 11, Cesmn-1/1 μm 38 ± 11. Unpooled results Figure 2C: Trials 1–4 Control 244 ± 6, kcnl-2(tm1885) 194 ± 13, Cesmn-1 57 ± 10, kcnl-2(tm1885) Cesmn-1 24 ± 8; p = 0.01 Cesmn-1 vs kcnl-2(tm1885) Cesmn-1; Trials 5–7 Control 238 ± 7, kcnl-2(ok2818) 210 ± 12, Cesmn-1 74 ± 14, kcnl-2(ok2818) Cesmn-1 36 ± 10; p = 0.01 Cesmn-1 vs kcnl-2(ok2818) Cesmn-1; Trials 8–10 Control 224 ± 9, slo-1 195 ± 13, slo-2 217 ± 11, sup-9 238 ± 11, Cesmn-1 47 ± 11, Cesmn-1;slo-1 51 ± 8, Cesmn-1;slo-2 74 ± 11, Cesmn-1;sup-9 62 ± 11; p = 0.04 Cesmn-1 vs Cesmn-1;slo-2.
Figure 2.
Riluzole improves the neuromuscular function in Cesmn-1(lf) animals via SK channels. A, Riluzole, an SK channel activator, increases Cesmn-1(lf) pumping rates. Cesmn-1(lf) and control animals were reared on riluzole (1, 3, and 33 μm) and pumping rates were scored at day 3, post hatching. Control: p = 0.007, F = 4.2; Cesmn-1(lf): p = 0.02, F = 3.5; one-way ANOVA. B, Apamin, an SK2 and SK3 channel blocker, exacerbates Cesmn-1(lf) pumping defects. Animals were reared on apamin (3 μm) and pumping rates were scored at day 3, post hatching. C, Loss of the C. elegans SK gene ortholog kcnl-2 enhanced Cesmn-1(lf) pumping defects and blocked the beneficial effect of riluzole (inset, kcnl-2(tm1885) Cesmn-1(lf) double mutant animals). Loss of other potassium channel genes (slo-1, slo-2, sup-9) did not exacerbate Cesmn-1(lf) defects. Control: p = 9 × 10−4, F = 4.3; Cesmn-1(lf): p = 0.005, F = 3.5; one-way ANOVA. For presentation purposes, A and C combine results from independent experiments; see Materials and Methods for details and results of independent experiments. D, Loss of kcnl-2(tm1885) exacerbated Cesmn-1(lf) locomotion defects; riluzole significantly improved Cesmn-1(lf) motility (left inset, Cesmn-1(lf) animals). kcnl-2 function is required for riluzole to improve Cesmn-1(lf) performance (right inset, kcnl-2(tm1885) Cesmn-1(lf) animals) p = 3.5 × 10−7, F = 12.3; one-way ANOVA. SEM is shown; paired t-test or Mann–Whitney U test: *p < 0.05, **p <0.01, ***p <0.001.
The motility assay was described previously (Briese et al., 2009). Here, C. elegans was reared on plates for 2 d at 25°C and 1 d at 20°C. Motility was assessed manually after 2 min in M9 buffer at day 3 post hatching regardless of developmental stage. A complete bend at mid-body was scored as a beat. At least three independent trials were performed.
Compounds.
Riluzole (R116) and apamin (A9459) were purchased from Sigma-Aldrich. Riluzole dramatically decreased egg laying (data not shown).
Hippocampal cell culture.
Experimental procedures were performed in compliance with animal protocols approved by Children's Hospital Institutional Animal Care and Use Committee, Boston. Hippocampi were dissected from E18 Sprague Dawley rat embryos (Charles River). Neurons were dissociated with papain, triturated, and plated onto poly-d-lysine/laminin (Sigma/Invitrogen)-coated plates at 250,000 cells/6-well culture plates for Western blotting and 20,000 cells/24-well culture plates for immunostaining. Neurons were cultured in Neurobasal medium with B27 supplement (Invitrogen), 500 μm l-glutamine (Invitrogen), and 1× penicillin/streptomycin (Invitrogen) at 37°C in a humidified incubator with 5% CO2.
Inhibition of SMN, riluzole treatment, and measurement of neuronal morphology.
SMN was knocked down using conventional siRNA technique (Applied Biosystems), Lipofectamine 2000 (Invitrogen), and Opti-MEM (Invitrogen). Briefly, neurons were transiently transfected with siRNA and treated with riluzole or DMSO after 24 h. Four days post-transfection, protein lysates were collected; SMN (BD Biosciences) and synaptophysin (Cell Signaling Technology) protein levels were measured by Western blot using GAPDH (Life Technologies) as a control. For immunohistology, neurons were fixed with 4% paraformaldehyde and stained with Tau antibody (Millipore) (Choi et al., 2008). Length of Tau-positive axons was measured using ImageJ.
Statistical analysis.
Significance was determined with Mann–Whitney U (two-tailed) or one-way ANOVA. After ANOVA, paired t-tests were used to identify significantly different pairs. Corresponding p and F values are reported.
Results
Riluzole prevents axonal defects in vertebrate neurons
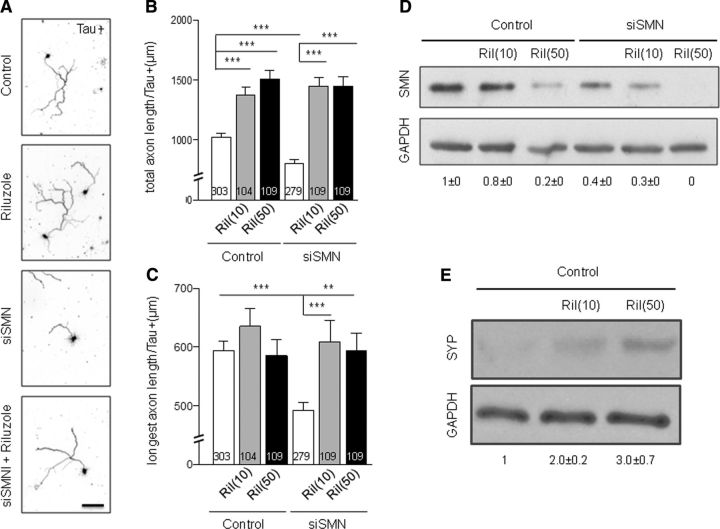
We examined the impact of riluzole on rat embryonic hippocampal neurons with reduced SMN levels. SMN knockdown reduced axon outgrowth in these neurons based on overall process length (p = 6.7 × 10−5). Treatment with 10 and 50 nm riluzole increased control and restored SMN-deficient axons to comparable levels (Fig. 1A,B). Longest axon length was also significantly reduced upon SMN knockdown (p = 1.4 × 10−5), but restored to wild-type levels after riluzole treatment (Fig. 1C). Riluzole does not ameliorate SMN knockdown defects by increasing SMN protein levels; riluzole treatment actually decreased SMN protein levels in both control and SMN-deficient neurons (Fig. 1D). SMN protein levels are tightly regulated during development; expression is high in embryonic tissues, but the concentration of SMN decreases as cells differentiate (La Bella et al., 1998; Grice and Liu, 2011). To address the possibility that riluzole might accelerate hippocampal neuron maturation, thereby lowering SMN, we examined synaptophysin levels (which normally increase as hippocampal neurons mature in culture) (Daly and Ziff, 1997). Riluzole raised synaptophysin levels consistent with accelerated maturation (Fig. 1E). Therefore, the riluzole neuroprotection is not due to increased SMN levels, and riluzole may accelerate neuronal maturation.
Figure 1.
Riluzole treatment rescues axonal outgrowth defects in SMN-deficient vertebrate neurons. A, Representative images of rat hippocampal neurons (days in vitro 5, Tau staining, 50 nm riluzole). Scale bar, 100 μm. B, Analysis of total axon length for SMN-deficient and control rat hippocampal neurons after riluzole treatment. SMN siRNA was followed by treatment with 10 nm or 50 nm riluzole. Number of neurons scored is reported in each bar; p = 2.2 × 10−16, F = 36.6; one-way ANOVA. C, Analysis of longest axon length for SMN-deficient and control rat hippocampal neurons after riluzole treatment; p = 6.75 × 10−6, F = 6.4; one-way ANOVA. Four independent experiments; >50 neurons each condition. Error bars indicate SEM, **p <0.01, ***p <0.001 (paired t-test). D, Treatment with riluzole decreases SMN protein levels. Representative Western blot comparing SMN and GAPDH protein levels in SMN-deficient and control rat hippocampal neurons in the presence of riluzole. E, Riluzole treatment (10 nm or 50 nm) increases levels of the maturation marker synaptophysin (SYP) in rat hippocampal neurons. Representative Western blot. For two independent biological samples, fold change of SMN and SYP levels normalized to GAPDH by densitometry are shown ± SD.
Riluzole ameliorates SMN loss of function defects in C. elegans
To address the mechanism of riluzole protection, we turned to a C. elegans SMA model. The C. elegans genome harbors a single ortholog of SMN, smn-1, referred to here as Cesmn-1 for clarity. Complete loss of Cesmn-1 causes slow growth, larval lethality, and impairs neuromuscular function in locomotion and in pharyngeal pumping during feeding (Briese et al., 2009; Dimitriadi et al., 2010). C. elegans feed on microorganisms using a discrete subset of muscles and neurons in the pharynx (Avery, 1993). Animals pump continuously at >250 beats per minute on food. The pumping rates of SMN loss-of-function animals (Cesmn-1(lf)) are significantly reduced; these defects are progressive and not a developmental defect. Cesmn-1(lf) is recessive and heterozygous animals are overtly normal (Briese et al., 2009; Dimitriadi et al., 2010). To assess the impact of riluzole, control and Cesmn-1(lf) animals were reared on plates containing the drug. Riluzole partially rescued SMN loss-of-function defects as treatment increased Cesmn-1(lf) pharyngeal pumping rates (p = 0.044 for 3 μm, p = 0.004 for 33 μm; Fig. 2A). In contrast, riluzole lowered the pumping rates in controls (p = 0.027 for 1 μm, p = 0.001 for 33 μm) suggesting that riluzole is only beneficial when neuromuscular function is perturbed. The efficacy of riluzole was also tested using motility, a neuromuscular assay that measures the frequency of body bends during swimming (Briese et al., 2009). Riluzole significantly increased the Cesmn-1(lf) motility (p = 0.04 for 33 μm; Fig. 2D) consistent with riluzole ameliorating Cesmn-1 loss of function neuromuscular defects.
Riluzole likely acts via SK channels
We tested the hypothesis that riluzole acts via SK channels to ameliorate defects using C. elegans. First, SK channels were blocked pharmacologically. Application of the SK2/SK3 channel blocker apamin exacerbated Cesmn-1(lf) pumping defects, suggesting that blocking SK channels impairs the Cesmn-1(lf) neuromuscular function (Fig. 2B). Second, SK channels were tested using genetic tools. Previously, RNAi knockdown of the C. elegans SK channel ortholog (kcnl-2) enhanced Cesmn-1(lf) growth defects and ameliorated their pumping defects (Dimitriadi et al., 2010). As RNAi can have off-target effects and RNAi by feeding is inefficient in C. elegans neurons, we used two C. elegans kcnl-2 alleles (kcnl-2(tm1885) and kcnl-2(ok2818)) that likely cause complete loss of function, to accurately assess the impact of kcnl-2 loss on Cesmn-1. Pumping rates of kcnl-2(tm1885) Cesmn-1(lf) and kcnl-2(ok2818) Cesmn-1(lf) double mutant animals were both significantly decreased (p = 0.003 and p = 0.023, respectively; Fig. 2C). If riluzole activates SK channels to ameliorate SMN loss-of-function defects, then loss of kcnl-2 should abrogate the beneficial effects of riluzole. Consistent with this hypothesis, riluzole treatment did not increase the pumping rates of kcnl-2 Cesmn-1(lf) mutant animals (Fig. 2C, tm1885; data not shown ok2818). Therefore, riluzole requires kcnl-2 SK channel function to ameliorate the SMN loss of function neuromuscular defects. We confirmed this in another assay. kcnl-2(tm1885) and Cesmn-1(lf) animals show decreased motility when swimming compared with controls (p = 0.029 and p = 0.007, respectively). Loss of kcnl-2 exacerbated the motility defects of Cesmn-1(lf) (p = 0.001), and riluzole treatment did not benefit kcnl-2 Cesmn-1(lf) mutant animals (Fig. 2D, tm1885; data not shown ok2818). To address the specificity of kcnl-2, other potassium channels were examined. Loss of slo-1(js118), slo-2(nf100), or sup-9(n180) did not exacerbate Cesmn-1(lf) pumping defects, suggesting that riluzole requires kcnl-2 SK channels to ameliorate SMN loss-of-function neuromuscular defects.
Discussion
The early observations of abnormal glutamate metabolism and decreased glutamate transport in the brain and spinal cord of ALS patients led to the hypothesis that the excitatory amino acid neurotransmitter glutamate may be involved in the ALS pathogenesis (Plaitakis, 1991; Rothstein et al., 1992). Hence, drugs affecting the glutamatergic system were suggested as putative therapeutic agents. Riluzole was initially identified as a paralytic agent (Domino et al., 1952) and was later shown to indirectly modulate glutamatergic transmission (Benavides et al., 1985; Doble et al., 1992; Debono et al., 1993; Albo et al., 2004). It was subsequently found to significantly improve muscle strength and disease progression in ALS patients (Bensimon et al., 1994; Lacomblez et al., 1996). The mechanism of riluzole protection remains unclear (Kuo et al., 2005; Bellingham, 2011; Schuster et al., 2012), as riluzole has diverse direct targets. These include potassium channels: SK channels (Grunnet et al., 2001; Cao et al., 2002), large conductance Ca2+-activated BK channels (Wu and Li, 1999; Wang et al., 2008), or TREK-1 and TRAAK two-pore-domain channels (Fink et al., 1998; Duprat et al., 2000). Also, riluzole blocks voltage-dependent sodium channels (Benoit and Escande, 1991; Song et al., 1997; Zona et al., 1998) and voltage-gated N- and P/Q-type calcium channels (Huang et al., 1997). Additionally, riluzole may inhibit cholinergic receptors (Deflorio et al., 2012) and decrease protein kinase C (PKC) activity (Noh et al., 2000).
SK channels were identified previously as cross-species genetic modifiers in invertebrate SMA models (Chang et al., 2008; Dimitriadi et al., 2010). Here, we provide evidence that riluzole has beneficial effects in two SMA models and may act via SK channels. Loss of the C. elegans SK channel ortholog kcnl-2 exacerbated Cesmn-1(lf) neuromuscular defects. Apamin, which blocks SK2 and SK3 channels, also exacerbated Cesmn-1 loss-of-function defects. Treatment with riluzole, whose actions include SK channel activation, improved the neuromuscular function of Cesmn-1(lf) animals and the axon outgrowth of SMN deprived rat hippocampal neurons.
Although Franks et al. (2002) identified a sodium current in pharyngeal muscles that is sensitive to sodium channel drugs, neither genes nor mRNAs encoding classical voltage-gated sodium channels have been found in C. elegans, suggesting that riluzole likely does not act via these channels to ameliorate Cesmn-1(lf) defects. Riluzole also restored axon outgrowth caused by diminished SMN in vertebrate neurons. Apamin, which blocks some classes of SK channels, had no impact on hippocampal neurons (data not shown), suggesting that either apamin-sensitive SK channels are not expressed at this stage or the beneficial effects of riluzole are not solely through SK channels in these mammalian neurons.
The present study reports that riluzole, while beneficial, does not increase SMN protein levels. However, riluzole treatment increased levels of a hippocampal neuron maturation marker, synaptophysin, suggesting that riluzole may accelerate maturation with consequent decreases in SMN levels. Therefore, it might be worthwhile to investigate the synergistic effects of riluzole in combination with drugs that directly increase SMN levels (Wadman et al., 2011a, b). In summary, our studies demonstrate the beneficial impact of riluzole in SMA models and suggest that riluzole acts via SK channels to ameliorate SMN loss-of-function defects, delineating an important therapeutic pathway for neuromuscular disease patients.
Footnotes
This work was supported by funding from SMA Foundation and National Institutes of Health (NIH)–National Institute of Neurological Disorders and Stroke (NS066888) to A.C.H, Slaney Family Fund and Children's Hospital Boston Translational Research Program to M.S., and the Boston Children's Hospital Intellectual and Development Disabilities Research Center (P30 HD18655). M.J.K. is supported by a fellowship from William Hearst Foundation. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which was funded by the NIH National Center for Research Resources. Helpful discussions with Drs. Spyros Artavanis-Tsakonas, David Van Vactor, Lee Rubin, Diane Lipscombe, and members of their laboratories were invaluable. We are grateful to C. elegans knock-out consortia and the Japanese National BioResource Project for reagents, to Luis Miguel Briseno for help with the measurement of neuronal morphology, and to Dr. George Poulogiannis for statistical analysis.
References
- Albo F, Pieri M, Zona C. Modulation of AMPA receptors in spinal motorneurons by the neuroprotective agent riluzole. J Neurosci Res. 2004;78:200–207. doi: 10.1002/jnr.20244. [DOI] [PubMed] [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther. 2011;17:4–31. doi: 10.1111/j.1755-5949.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides J, Camelin JC, Mitrani N, Flamand F, Uzan A, Legrand JJ, Gueremy C, Le Fur G. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission–II. Biochemical properties. Neuropharmacology. 1985;24:1085–1092. doi: 10.1016/0028-3908(85)90196-0. [DOI] [PubMed] [Google Scholar]
- Benoit E, Escande D. Riluzole specifically blocks inactivated Na channels in myelinated nerve fibre. Pflugers Arch. 1991;419:603–609. doi: 10.1007/BF00370302. [DOI] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Blauw HM, Barnes CP, van Vught PW, van Rheenen W, Verheul M, Cuppen E, Veldink JH, van den Berg LH. SMN1 gene duplications are associated with sporadic ALS. Neurology. 2012;78:776–780. doi: 10.1212/WNL.0b013e318249f697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose JK, Wang IF, Hung L, Tarn WY, Shen CK. TDP-43 overexpression enhances exon 7 inclusion during the survival of motor neuron pre-mRNA splicing. J Biol Chem. 2008;283:28852–28859. doi: 10.1074/jbc.M805376200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese M, Esmaeili B, Fraboulet S, Burt EC, Christodoulou S, Towers PR, Davies KE, Sattelle DB. Deletion of smn-1, the Caenorhabditis elegans ortholog of the spinal muscular atrophy gene, results in locomotor dysfunction and reduced lifespan. Hum Mol Genet. 2009;18:97–104. doi: 10.1093/hmg/ddn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YJ, Dreixler JC, Couey JJ, Houamed KM. Modulation of recombinant and native neuronal SK channels by the neuroprotective drug riluzole. Eur J Pharmacol. 2002;449:47–54. doi: 10.1016/S0014-2999(02)01987-8. [DOI] [PubMed] [Google Scholar]
- Chang HC, Dimlich DN, Yokokura T, Mukherjee A, Kankel MW, Sen A, Sridhar V, Fulga TA, Hart AC, Van Vactor D, Artavanis-Tsakonas S. Modeling spinal muscular atrophy in Drosophila. PLoS One. 2008;3:e3209. doi: 10.1371/journal.pone.0003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, He X. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22:2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcia P, Camu W, Praline J, Gordon PH, Vourch P, Andres C. The importance of the SMN genes in the genetics of sporadic ALS. Amyotroph Lateral Scler. 2009;10:436–440. doi: 10.3109/17482960902759162. [DOI] [PubMed] [Google Scholar]
- Crawford TO, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis. 1996;3:97–110. doi: 10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- Daly C, Ziff EB. Post-transcriptional regulation of synaptic vesicle protein expression and the developmental control of synaptic vesicle formation. J Neurosci. 1997;17:2365–2375. doi: 10.1523/JNEUROSCI.17-07-02365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debono MW, Le Guern J, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993;235:283–289. doi: 10.1016/0014-2999(93)90147-A. [DOI] [PubMed] [Google Scholar]
- Deflorio C, Palma E, Conti L, Roseti C, Manteca A, Giacomelli E, Catalano M, Limatola C, Inghilleri M, Grassi F. Riluzole blocks human muscle acetylcholine receptors. J Physiol. 2012;590:2519–2528. doi: 10.1113/jphysiol.2012.230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadi M, Sleigh JN, Walker A, Chang HC, Sen A, Kalloo G, Harris J, Barsby T, Walsh MB, Satterlee JS, Li C, Van Vactor D, Artavanis-Tsakonas S, Hart AC. Conserved genes act as modifiers of invertebrate SMN loss of function defects. PLoS Genet. 2010;6:e1001172. doi: 10.1371/journal.pgen.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble A, Hubert JP, Blanchard JC. Pertussis toxin pretreatment abolishes the inhibitory effect of riluzole and carbachol on D-[3H]aspartate release from cultured cerebellar granule cells. Neurosci Lett. 1992;140:251–254. doi: 10.1016/0304-3940(92)90114-M. [DOI] [PubMed] [Google Scholar]
- Domino EF, Unna KR, Kerwin J. Pharmacological properties of benzazoles. I. Relationship between structure and paralyzing action. J Pharmacol Exp Ther. 1952;105:486–497. [PubMed] [Google Scholar]
- Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks CJ, Pemberton D, Vinogradova I, Cook A, Walker RJ, Holden-Dye L. Ionic basis of the resting membrane potential and action potential in the pharyngeal muscle of Caenorhabditis elegans. J Neurophysiol. 2002;87:954–961. doi: 10.1152/jn.00233.2001. [DOI] [PubMed] [Google Scholar]
- Grice SJ, Liu JL. Survival motor neuron protein regulates stem cell division, proliferation, and differentiation in Drosophila. PLoS Genet. 7:e1002030. doi: 10.1371/journal.pgen.1002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Angelo K, Frøkjaer-Jensen C, Klaerke DA, Olesen SP, Jensen BS. Pharmacological modulation of SK3 channels. Neuropharmacology. 2001;40:879–887. doi: 10.1016/S0028-3908(01)00028-4. [DOI] [PubMed] [Google Scholar]
- Haddad H, Cifuentes-Diaz C, Miroglio A, Roblot N, Joshi V, Melki J. Riluzole attenuates spinal muscular atrophy disease progression in a mouse model. Muscle Nerve. 2003;28:432–437. doi: 10.1002/mus.10455. [DOI] [PubMed] [Google Scholar]
- Huang CS, Song JH, Nagata K, Yeh JZ, Narahashi T. Effects of the neuroprotective agent riluzole on the high voltage-activated calcium channels of rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1997;282:1280–1290. [PubMed] [Google Scholar]
- Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/S1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Keen JE, Khawaled R, Farrens DL, Neelands T, Rivard A, Bond CT, Janowsky A, Fakler B, Adelman JP, Maylie J. Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels. J Neurosci. 1999;19:8830–8838. doi: 10.1523/JNEUROSCI.19-20-08830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na(+) current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol. 2005;563:843–854. doi: 10.1113/jphysiol.2004.074138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Bella V, Cisterni C, Salaün D, Pettmann B. Survival motor neuron (SMN) protein in rat is expressed as different molecular forms and is developmentally regulated. Eur J Neurosci. 1998;10:2913–2923. doi: 10.1111/j.1460-9568.1998.00298.x. [DOI] [PubMed] [Google Scholar]
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res. 2007;100:112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- Lu L, Timofeyev V, Li N, Rafizadeh S, Singapuri A, Harris TR, Chiamvimonvat N. Alpha-actinin2 cytoskeletal protein is required for the functional membrane localization of a Ca2+-activated K+ channel (SK2 channel) Proc Natl Acad Sci U S A. 2009;106:18402–18407. doi: 10.1073/pnas.0908207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, Skehel P, Zatz M. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh KM, Hwang JY, Shin HC, Koh JY. A novel neuroprotective mechanism of riluzole: direct inhibition of protein kinase C. Neurobiol Dis. 2000;7:375–383. doi: 10.1006/nbdi.2000.0297. [DOI] [PubMed] [Google Scholar]
- Pearn J. Incidence, prevalence, and gene frequency studies of chronic childhood spinal muscular atrophy. J Med Genet. 1978;15:409–413. doi: 10.1136/jmg.15.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, Stocker M. Molecular and cellular basis of small–and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaitakis A. Altered glutamatergic mechanisms and selective motor neuron degeneration in amyotrophic lateral sclerosis: possible role of glycine. Adv Neurol. 1991;56:319–326. [PubMed] [Google Scholar]
- Rajendra TK, Gonsalvez GB, Walker MP, Shpargel KB, Salz HK, Matera AG. A Drosophila melanogaster model of spinal muscular atrophy reveals a function for SMN in striated muscle. J Cell Biol. 2007;176:831–841. doi: 10.1083/jcb.200610053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Russman BS, Iannaccone ST, Samaha FJ. A phase 1 trial of riluzole in spinal muscular atrophy. Arch Neurol. 2003;60:1601–1603. doi: 10.1001/archneur.60.11.1601. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bächinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Schuster JE, Fu R, Siddique T, Heckman CJ. Effect of prolonged riluzole exposure on cultured motoneurons in a mouse model of ALS. J Neurophysiol. 2012;107:484–492. doi: 10.1152/jn.00714.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Huang CS, Nagata K, Yeh JZ, Narahashi T. Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther. 1997;282:707–714. [PubMed] [Google Scholar]
- Veldink JH, van den Berg LH, Cobben JM, Stulp RP, De Jong JM, Vogels OJ, Baas F, Wokke JH, Scheffer H. Homozygous deletion of the survival motor neuron 2 gene is a prognostic factor in sporadic ALS. Neurology. 2001;56:749–752. doi: 10.1212/WNL.56.6.749. [DOI] [PubMed] [Google Scholar]
- Wadman RI, Bosboom WM, van den Berg LH, Wokke JH, Iannaccone ST, Vrancken AF. Drug treatment for spinal muscular atrophy type I. Cochrane Database Syst Rev. 2011a;12:CD006281. doi: 10.1002/14651858.CD006281.pub3. [DOI] [PubMed] [Google Scholar]
- Wadman RI, Bosboom WM, van den Berg LH, Wokke JH, Iannaccone ST, Vrancken AF. Drug treatment for spinal muscular atrophy types II and III. Cochrane Database Syst Rev. 2011b;12:CD006282. doi: 10.1002/14651858.CD006282.pub3. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Lin MW, Lin AA, Wu SN. Riluzole-induced block of voltage-gated Na+ current and activation of BKCa channels in cultured differentiated human skeletal muscle cells. Life Sci. 2008;82:11–20. doi: 10.1016/j.lfs.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Wu SN, Li HF. Characterization of riluzole-induced stimulation of large-conductance calcium-activated potassium channels in rat pituitary GH3 cells. J Investig Med. 1999;47:484–495. [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- Zona C, Siniscalchi A, Mercuri NB, Bernardi G. Riluzole interacts with voltage-activated sodium and potassium currents in cultured rat cortical neurons. Neuroscience. 1998;85:931–938. doi: 10.1016/S0306-4522(97)00604-0. [DOI] [PubMed] [Google Scholar]