Abstract
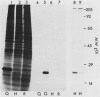
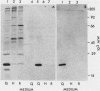
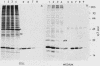
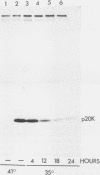
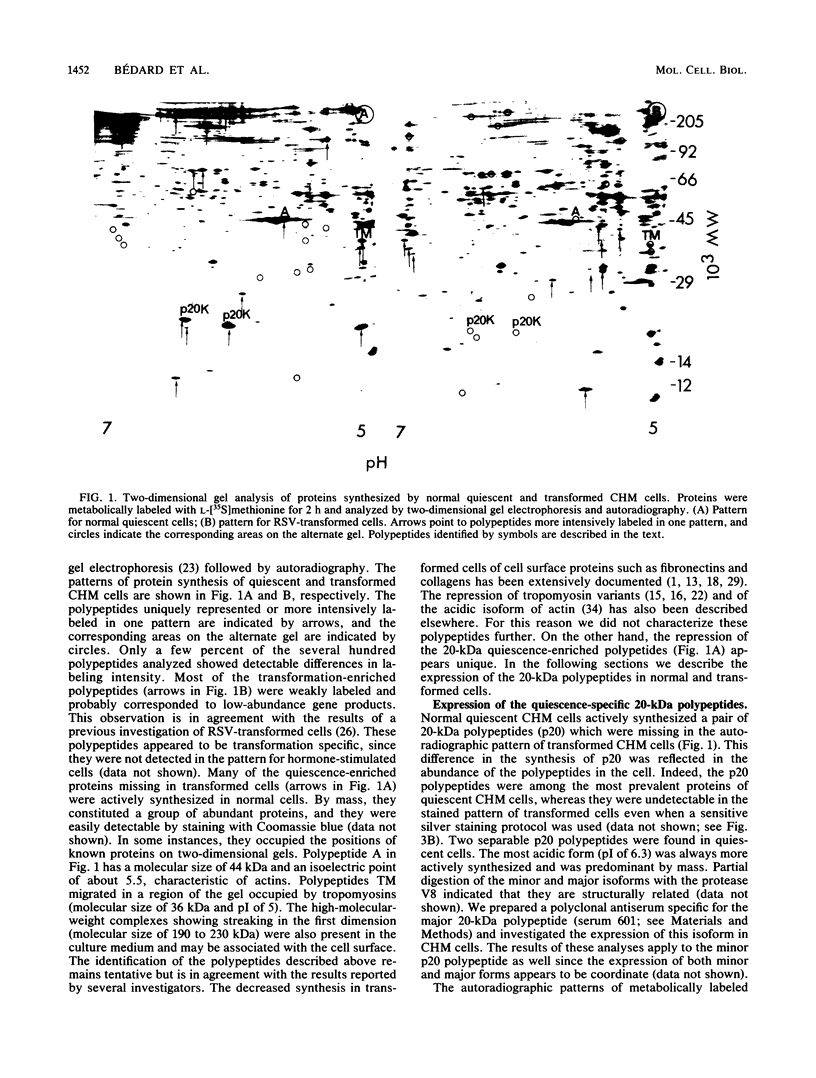
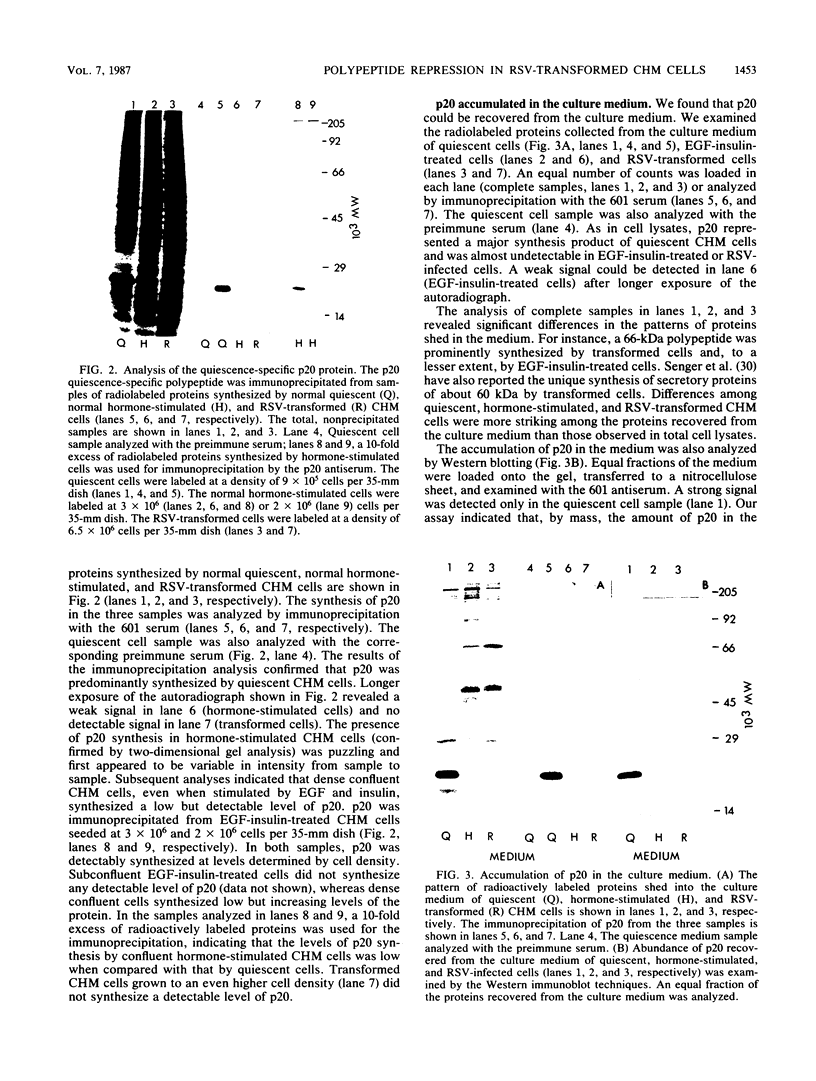
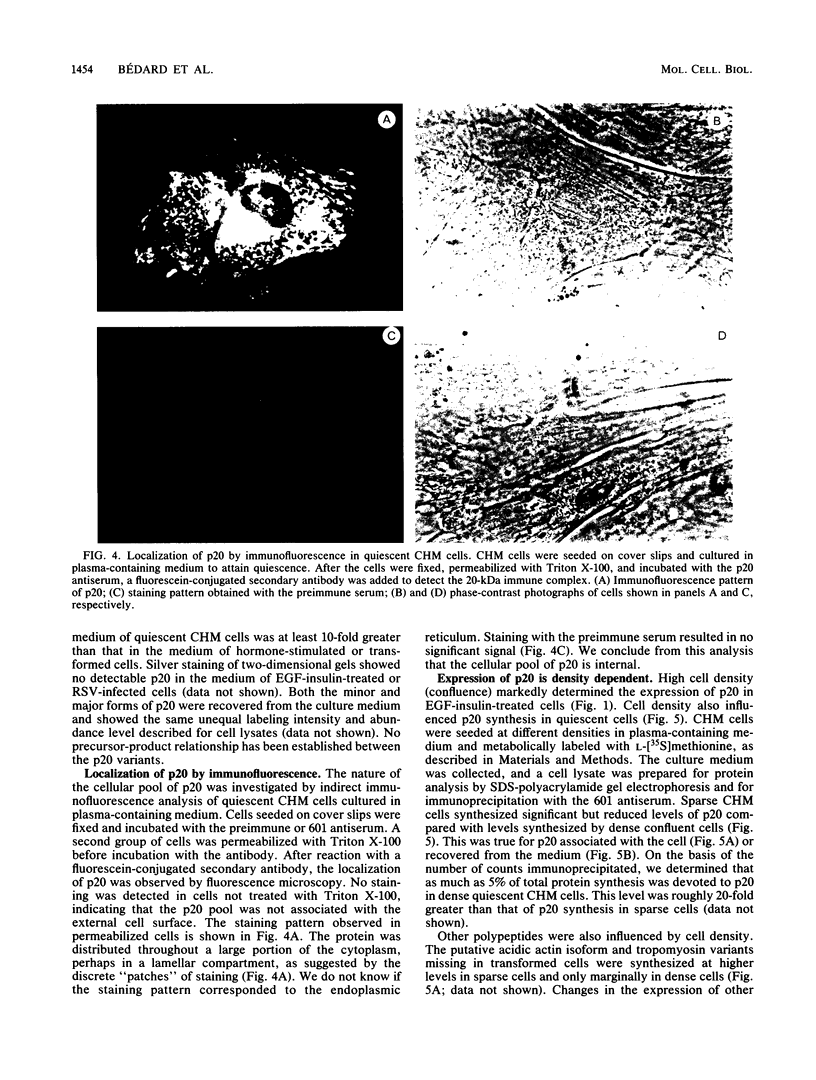
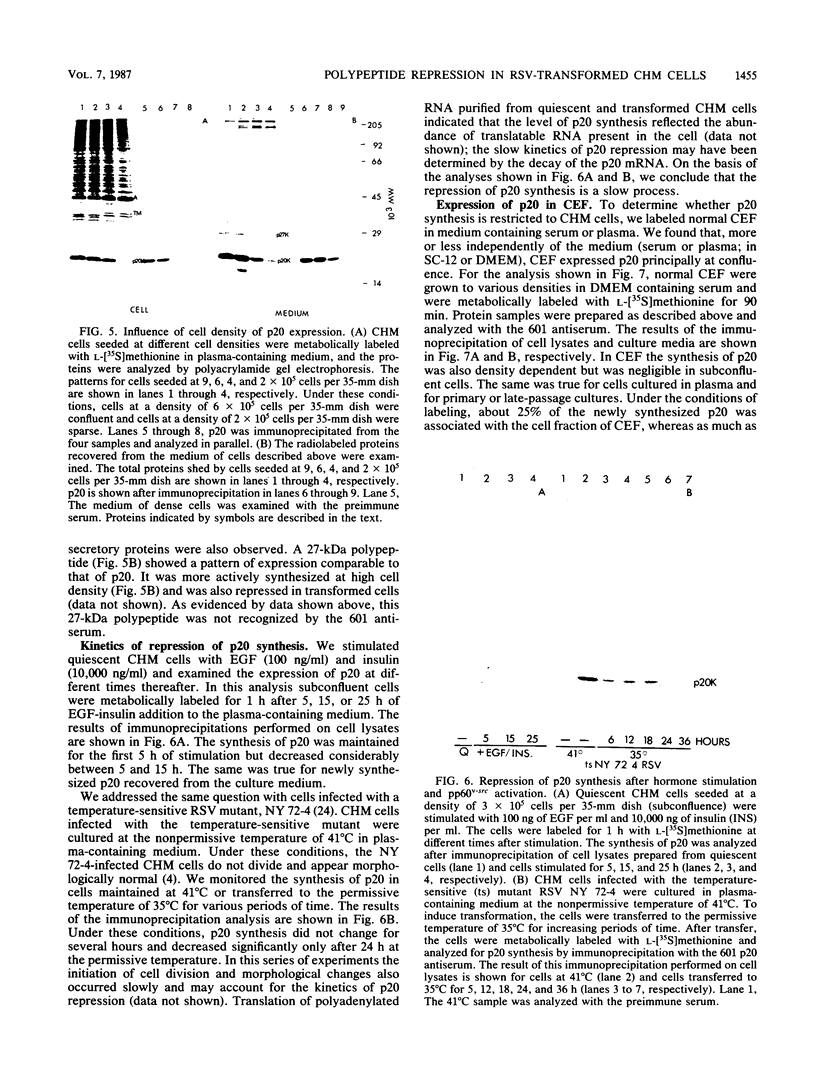
Chicken heart mesenchymal cells do not proliferate in medium of physiological composition containing plasma (S. Balk, Proc. Natl. Acad. Sci. USA 77:6606-6610, 1980). To understand the molecular events involved in cell quiescence and in the initiation of cell division under physiological conditions, we examined the differences in the patterns of protein synthesis of quiescent, hormone-stimulated, and Rous sarcoma virus-transformed chicken heart mesenchymal cells. We describe the expression of a 20,000-kilodalton (kDa) polypeptide actively synthesized by quiescent cells but not by their transformed counterparts. Normal chicken heart mesenchymal cells stimulated with epidermal growth factor and insulin also repressed the synthesis of the 20,000-kDa polypeptide while actively growing but synthesized increasing amounts of the protein at high cell density (confluence). The synthesis of the 20,000-kDa protein is not restricted to chicken heart mesenchymal cells, since confluent, density-arrested chicken embryo fibroblasts also expressed high levels of the protein. Transformed chicken heart mesenchymal cells and embryo fibroblasts did not synthesize the protein even at high cell density. The 20,000-kDa polypeptide accumulated in the culture medium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Balk S. D. Active proliferation of Rous sarcoma virus-infected, but not normal, chicken heart mesenchymal cells in culture medium of physiological composition. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6606–6610. doi: 10.1073/pnas.77.11.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Gunther H. S., Morisi A. Morphological transformation, autonomous proliferation and colony formation by chicken heart mesenchymal cells infected with avian sarcoma, erythroblastosis and myelocytomatosis viruses. Life Sci. 1984 Sep 10;35(11):1157–1171. doi: 10.1016/0024-3205(84)90186-3. [DOI] [PubMed] [Google Scholar]
- Balk S. D., Morisi A., Gunther H. S., Svoboda M. F., Van Wyk J. J., Nissley S. P., Scanes C. G. Somatomedins (insulin-like growth factors), but not growth hormone, are mitogenic for chicken heart mesenchymal cells and act synergistically with epidermal growth factor and brain fibroblast growth factor. Life Sci. 1984 Jul 23;35(4):335–346. doi: 10.1016/0024-3205(84)90643-x. [DOI] [PubMed] [Google Scholar]
- Balk S. D., Shiu R. P., LaFleur M. M., Young L. L. Epidermal growth factor and insulin cause normal chicken heart mesenchymal cells to proliferate like their Rous sarcoma virus-infected counterparts. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1154–1157. doi: 10.1073/pnas.79.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Bédard P. A., Brandhorst B. P. Patterns of protein synthesis and metabolism during sea urchin embryogenesis. Dev Biol. 1983 Mar;96(1):74–83. doi: 10.1016/0012-1606(83)90312-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Whitfield J. F. The selective induction of a small number of proteins during G1 transit results from the mitogenic action of pp60v-src in tsASV-infected rat cells. J Cell Physiol. 1985 Oct;125(1):51–60. doi: 10.1002/jcp.1041250108. [DOI] [PubMed] [Google Scholar]
- Green H., Todaro G. J., Goldberg B. Collagen synthesis in fibroblasts transformed by oncogenic viruses. Nature. 1966 Feb 26;209(5026):916–917. doi: 10.1038/209916a0. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Multiple tropomyosin polypeptides in chicken embryo fibroblasts: differential repression of transcription by Rous sarcoma virus transformation. Mol Cell Biol. 1984 Sep;4(9):1823–1833. doi: 10.1128/mcb.4.9.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. doi: 10.1073/pnas.78.9.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., Adams S. L., Sobel M. E., Pastan I., de Crombrugghe B. Decreased levels of collagen mRNA in rous sarcoma virus-transformed chick embryo fibroblasts. J Biol Chem. 1978 Aug 25;253(16):5869–5874. [PubMed] [Google Scholar]
- Hynes R. O., Bye J. M. Density and cell cycle dependence of cell surface proteins in hamster fibroblasts. Cell. 1974 Oct;3(2):113–120. doi: 10.1016/0092-8674(74)90114-7. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. Neoplastic transformation of human diploid fibroblast cells by chemical carcinogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1334–1338. doi: 10.1073/pnas.75.3.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Poirier F., Calothy G., Karess R. E., Erikson E., Hanafusa H. Role of p60src kinase activity in the induction of neuroretinal cell proliferation by rous sarcoma virus. J Virol. 1982 Jun;42(3):780–789. doi: 10.1128/jvi.42.3.780-789.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Development and characterization of antisera specific for amino- and carboxy-terminal regions of pp60src. J Virol. 1985 Jul;55(1):242–245. doi: 10.1128/jvi.55.1.242-245.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D., Yarden A., Zipori D., Kimchi A. Autocrine beta-related interferon controls c-myc suppression and growth arrest during hematopoietic cell differentiation. Cell. 1986 Jul 4;46(1):31–40. doi: 10.1016/0092-8674(86)90857-3. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Bornstein P. Declining procollagen mRNA sequences in chick embryo fibroblasts infected with rous sarcoma virus. Correlation with procollagen synthesis. J Biol Chem. 1979 Jun 25;254(12):4950–4953. [PubMed] [Google Scholar]
- Senger D. R., Wirth D. F., Hynes R. O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979 Apr;16(4):885–893. doi: 10.1016/0092-8674(79)90103-x. [DOI] [PubMed] [Google Scholar]
- Sigel M. B., Sinha Y. N., VanderLaan W. P. Production of antibodies by inoculation into lymph nodes. Methods Enzymol. 1983;93:3–12. doi: 10.1016/s0076-6879(83)93031-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt D. P., Brown D. J., Gordon J. A. Transformation-sensitive isoactin in passaged chick embryo fibroblasts transformed by Rous sarcoma virus. J Cell Biol. 1983 Jun;96(6):1766–1771. doi: 10.1083/jcb.96.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]