Structured summary
Purpose of review
In the current review, we summarize recent progress on vasculature-specific function and regulation of integrins and integrin-associated proteins, including advances in our understanding of inside-out integrin activation. The studies on regulation of integrin activation received new impulse in 2009 with the identification of Kindlin protein family members as crucial mediators of integrin inside-out signaling. In the current review, we outline the recent findings on the role of Kindlins in the vascular system, as well as new studies that have begun shaping the mechanistic model of Kindlins’ function.
Recent findings
Several tissue-specific knockout models for integrins and genes associated with the integrin functions have been recently presented, including smooth muscle-specific ILK and endothelial-specific FAK and Talin-1 ablation. In the heterozygous animal knockout model, Kindlin-2 has been demonstrated as a crucial modulator of angiogenesis and vascular permeability. As a number of papers have advanced our understanding of Kindlin function, they are reviewed and discussed in further detail. New findings include an additional lipid binding site within the Kindlin molecule and preferential binding of non-phosphorylated form of β-integrins.
Summary
The role of integrins in angiogenesis has been demonstrated to include, in addition to cell adhesion and mechanotransduction, specific signaling functions. The importance of integrin inside-out pathway in vascular physiology has been unequivocally proven, and endothelial permeability is directly regulated by this process. Inhibition of Kindlin-dependent steps in the inside-out pathway as an approach to block platelet aggregation should be paralog-specific, as it may have adverse effects on vascular permeability.
Keywords: integrins, endothelial permeability, inside-out, adhesion, Kindlin
Introduction
Integrins are crucial in vasculogenesis, angiogenesis, and vascular integrity as major mediators of vascular cell adhesion and migration through the extracellular matrix, as well as signaling co-receptors of the receptor tyrosine kinases, including VEGFR – the “master switch” of angiogenesis [1]. While studies determining unique versus redundant roles of a particular integrin pair are currently under way, utilization of tissue specific knockouts of integrin subunits has allowed an accurate assessment of integrin functions in endothelial and smooth muscle cells, as reviewed here. An important feature of integrins is the regulation of their affinity towards the extracellular matrix (ECM) ligands. In endothelial cells, integrin activation is induced by growth factors, including VEGF [1], and mechanical stress [2, 3]. Activation is achieved by binding two FERMT domain containing proteins, Talin and Kindlin, to NxxY motifs within the β integrin cytoplasmic domain. The role of Talin in integrin activation has been reported [4], however, the significance of Kindlin is demonstrated more recently [5–8] and will be further discussed in this review. Kindlin paralogs Kindlin-1, -2, and -3 have a high degree of sequence identity among them, yet exhibit different patterns of expression [9]. The presence of at least one Kindlin paralog is required to achieve integrin activation. Once integrins are engaged by ECM, they function as docking modules for a plethora of intracellular signaling proteins, altogether known as “adhesome” [10]. Integrin Linked Kinase (ILK) and Focal Adhesion Kinase (FAK) are required for the integrin link with the cytoskeleton and signal transduction. As global knockouts of ILK and FAK are lethal [11, 12], recently reported inducible and tissue specific knockouts of ILK and FAK have demonstrated the unique roles of these genes in smooth muscle and endothelial cells, respectively [13, 14].
Tissue-specific functions of integrins
We summarize here the recent findings in gene knockout (KO) studies addressing integrin function. Previously, knockout of β1-integrin has been reported as peri-implantation lethal [15], and endothelial-specific gene ablation resulted in severe vascular development defects [16, 17]. Two new reports addressed the mechanism underlying the impaired vascular development in β1 integrin KO using blastocyst-derived embryonic cells [18] and endothelial-specific knockout [19]**. The first study shows CD31+ endothelial precursors from wild type, but not β1 KO, embryos spontaneously undergo clustering and sprouting in vitro. In striking difference to wild type, the basal membrane of β1−/− embryonic bodies (EB) was absent, as a result of diminished expression of ECM proteins [18]. Re-introduction of beta1 expression under the CD31 promoter rescued all of the defects of tubulogenesis observed in EB, demonstrating a cell-autonomous effect of β1 ablation in the endothelial cells [18]. The other study addressed the β1 integrin’s role in vasculogenesis using VE-cadherin (VE-Cad) driven Cre expression [19]**. Unlike Tie-2-driven β1 integrin ablation [16], which is lethal at E10, the VE-Cad-driven knockout resulted in lethality around E17, allowing studies of embryos [19]**. Similar to Tie-2-driven KO, the VE-Cad KO of the β1 integrin resulted in vascular rupture and hemorrhage. The remarkable feature of these VE-Cad KO embryos was the defective lumen formation. The lumen was formed in the aorta, but not in mid-size vessels, which is likely due to the timing of the β1 excision. In agreement with the other report, the effect is endothelial cell-autonomous. An unexpected consequence of the β1 ablation was the loss of Par3 expression, but not other proteins of the polarity complex. Therefore, vacuoles over-accumulated in the endothelial cells’ cytoplasm due to the lack of coordinated vacuoles movement and fusion. Over-expression of Par3 partially rescued the phenotype, thereby solidifying the role of β1 integrin-Par3 axis in vascular lumen formation [19]**. Alpha subunits of integrins have important roles in vascular functions, including lower vertebrates [20]. Using Tie-2-driven ablation of gene expression, Richard Hynes’s group has shown α5 and αv integrin subunits have overlapping functions in developmental angiogenesis [21]**. In the double knockout, development appeared normal until E11.5, then exhibited impaired vascular remodeling, resulting in death at midgestation. Surprisingly, a low percentage of double KO mice survived, indicating functional compensation by other alpha subunits. As Tie-2-driven ablation of the β1 integrin results in death at earlier stages than in α5/αv double KO, some other alpha subunits function in a complex with β1 at the earlier stages [21]**. Another interesting study utilized endothelial-specific ablation of α3 and demonstrated a suppressive effect of this integrin on tumor angiogenesis [22].
Together, these elegant in vivo experiments with tissue specific deletions demonstrate not only the importance of individual integrin subunits, but also high plasticity and adaptability of cell adhesion machinery. The multiple levels of functional redundancy underscore the key role of integrins in vascular biology.
Tissue-specific role of integrin modulators in vivo
Talin-1 appears to be the only isoform expressed by endothelial cells [23, 24]. Since global deletion of Talin-1 results in early embryonic arrest [25], inducible and endothelial specific Talin-1 KO are of interest [23]. Talin-1 ablation at E8.5 and endothelium specific ablation resulted in vascular defects and embryo death. Vasculature was reported to be dilated and poorly branching as a result of the endothelial cells’ inability to spread [23]. A recent study [26] using embryoid bodies from Talin-1 null embryos suggests that Talin-1 is needed for the maintenance of the β1 integrin expression, but not its activation. Most surprisingly, either expression of the β1 integrin or proteosome inhibition largely rescued defective cell adhesion in Talin-1 null cells. Thus, Talin-1 binding to the integrin β1 appears to merely prevent β1 degradation by proteosome in K48 ubiquitination-dependent manner. In this connection, identification of the SHARPIN protein as a novel negative modulator of integrin activation is particularly intriguing [27], as SHARPIN has been previously identified within linear ubiquitin chain assembly complex LUBAC [28]. SHARPIN binds to a conservative peri-membrane GFFRK motif of at least three alpha subunits, presumably “locking” integrins in inactive conformation [27].
Similar to Talin, ILK and FAK are essential for integrin dependent adhesion and signaling. Both global and endothelial specific knockouts were reported for the FAK gene [12, 29]. Hodivala-Dilke and colleagues, using inducible endothelial-specific FAK ablation, have now reported a dramatic decrease in tumor neo-vascularization. As expected, the effect was not confined to tumor angiogenesis, but was also present in other in vivo settings [14]. ILK knockout models have been reported previously [11, 30, 31]. In the new report, ablation of ILK expression governed by SM22 smooth muscle specific promoter resulted in aneurismal dilation of the aorta, lethal at postnatal day P1 [13]. The effects are cell autonomous and correlated with decreased expression of genes associated with an SMC contractile phenotype [13].
Two new in vivo studies are focused on “the master switch” of integrin-dependent functions, Rap1 small G protein. Rap1b KO and VEGFR2 inhibition have an additive inhibitory effect on angiogenesis in vivo, indicating non-overlapping functions and emphasizing that integrin activation is required for the VEGFR2 function, thus providing a positive feedforward loop [32]. In the other study, Zhang et al. demonstrated that αIIbβ3-dependent outside-in signaling activates Rap-1b, which is crucial for platelet spreading and clot retraction [33]. Thus, activation of Rap-1 plays a dual role in platelet function: agonist-induced Rap-1 leads to integrin activation and platelet granule secretion, while integrin-mediated Rap-1 activation is required for clot retraction and platelet spreading [33].
Kindlin-2 mechanistic and structural studies
The Kindlin proteins have been implicated in integrin activation [4, 9]. The Kindlin family consists of three members: Kindlin-1 (FERMT1), Kindlin-2 (FERMT2), and Kindlin-3 (FERMT3), all of which are structurally very similar. However, it appears that Kindlins cannot compensate one another, as deficiencies of each Kindlin show distinct phenotypes [9]. Since Kindlins were demonstrated to be crucial for integrin activation [5–8], the focus of follow-up studies branched into two major directions – the role of Kindlin paralogs in different physiological settings, and the mechanistic studies of Kindlin function and regulation. Kindlins, similar to the Talin head region, are largely composed of FERM domains. In Kindlins, however, these FERM domains are further intersected with the pleckstrin homology (PH) domain [4, 9]. Structurally, Kindlins’ integrin binding site resembles a phosphotyrosine binding domain (PTB) [34]. As the integin is phosphorylated in the Kindlin binding motif NxxY [35], Bledzka et al. have now demonstrated that phosphorylation of this motif in the β3 cytoplasmic tail disrupts its interaction with Kindlin-2 [36]**. These studies suggest that NxxY motifs might act as a dual switch in a control of integrin activation.
To propose the mechanistic model of the Kindlin function, the crystal structure of the Kindlin protein in a complex with the β-integrin cytoplasm tail is essential, however, it is not yet reported. Crystallization of the PH domain of Kindlin-2 has been announced, although the structural model is not finalized [37]. Kindlin-2 PH domain is reported to bind phosphatidylinositol phosphate with preference for PIP3, and overexpression of PH-deficient Kindlin-2 reduced integrin activation in podocytes [37, 38]. Apparently, the PH domain is not the only lipid binding region within the Kindlin-2 protein, as the F0 sub-domain of the Kindlin-2 FERM domain has been demonstrated to interact with PIP2 [39]*. Kindlin-2 F0 is unlikely to contribute to the protein microdomain targeting, as the depletion of PIP2 from focal adhesions impeded recruitment of Talin-1 and Vinculin, but not Kindlin-2 [40].
Kindlin-2 in heart and vasculature
Kindlin-2 is the most widely expressed Kindlin family member, and multiple lines of evidence point to its crucial role in integrin activation in vascular cells. In vitro, the Kindlin-2 knockdown in endothelial cells led to a defect in adhesion, spreading, and migration on the β3 integrin ligands [41]. Studies by Dowling and colleagues show the essential role of Kindlin-2 in heart development [42]. The depletion of Kindlin-2 led to severe myocardial defects resulting from disrupted intercalated disc formation [42]. Early embryonic lethality of the Kindlin-2 KO mice did not permit the detailed analysis of the role of this molecule in endothelium or pathological vascularization. However, analysis of embryonic bodies generated from the Kindlin-2 deficient embryonic stem cells revealed impaired adhesion, spreading, and integrin activation [43]. Unlike homozygous mice, Kindlin-2 heterozygous mice are viable and, when challenged, showed significantly reduced angiogenesis in growing tumors and Matrigel implants [44]**. The RM1 prostate tumors grown in the Kindlin-2+/− mice were smaller and had reduced vessel density and vascular area. New blood vessels formed in these mice were immature, had thinner basement membrane, and severely reduced pericyte content – all leading to vascular leakage [44]**. Likewise, Kindlin-2 knockdown in zebrafish resulted in multiple abnormalities in developmental angiogenesis, including thin, shortened intersegmental vessels and failure to form a continuous dorsal longitudinal anastomotic vessel. Aortic endothelial cells (ECs) isolated from the Kindlin-2+/− mice showed diminished β3 integrin activation and, consequently, all of the β3 integrin-dependent cellular responses were impaired, while the β1 integrin function appeared to be preserved [44]**. Since Kindlin-2 is known to interact with and activate both β1 and β3 integrins, this differential integrin specificity in ECs is intriguing. Interestingly, the baseline permeability of preexisting blood vessels was enhanced by 70% in Kindlin-2+/− mice, while VEGF, a potent inducer of vascular permeability, robustly increased permeability in the WT vasculature; the Kindlin-2+/− blood vessels remained unresponsive [44]**. Remarkably, VEGF and PLGF did not induce intracellular signaling in Kindlin-2+/− ECs and VEGF did not rescue aberrant angiogenesis observed in zebrafish with Kindlin-2 knockdown. Another interesting question is the mechanism by which Kindlin-2 regulates vascular permeability. It has been demonstrated that Kindlin-2 co-localizes with E-cadherin in keratinocyte cell-cell junctions and is present in adherence junctions in the colon and heart [42, 45]. However, the functions of Kindlin-2 at these sites or the mechanism underlying its recruitment are not clear and remain an exciting field for investigation. Kindlin-3, which is robustly expressed in hematopoietic cells, is a key molecule in the control of hemostasis and inflammation. Bialkowska et al. demonstrated that in addition to Kindlin-2, Kindlin-3 is also present in ECs [46]*. While Kindlin-2 is needed for αVβ3-mediated adhesion to vitronectin, Kindlin-3 is required for α5β1-dependent adhesion to fibronectin, suggesting that the two Kindlins do not have overlapping functions in ECs. Additionally, although both Kindlins co-localize with the β1 and β3 integrins, they do not co-localize with each other, as Kindlin-2 was detected in the focal adhesions, where Kindlin-3 was absent [46]*. What segregates Kindlin-2 and Kindlin-3 and the molecular mechanism responsible for functional differences between these two proteins in the same cell remains to be elucidated.
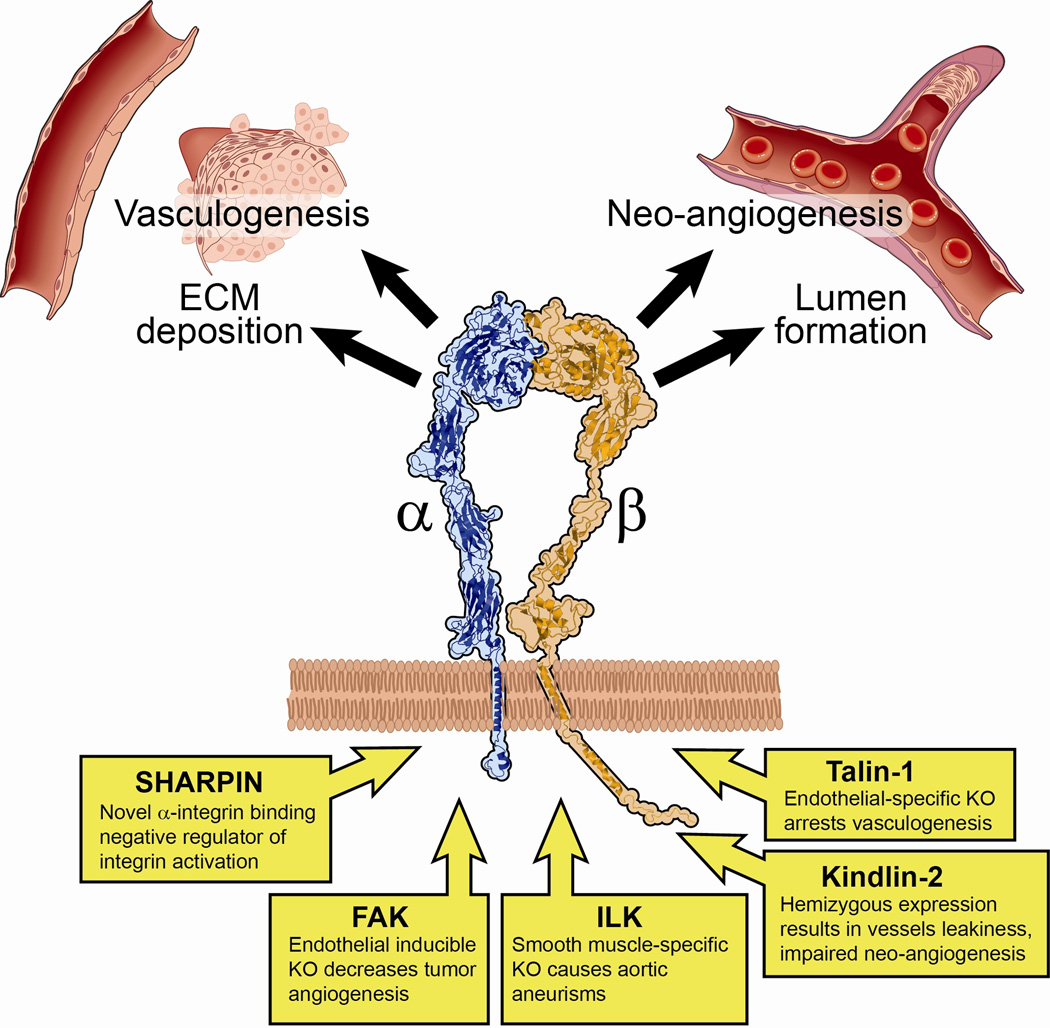
On the mechanistic side, new cases of Leukocyte Adhesion Disorder type III(LADIII) help to shape the structure-functional studies on Kindlin-3, as the G308R point mutation has been reported as specifically affecting cell migration, but not cell adhesion, signifying the finely tuned regulation of several inter-related processes by Kindlin proteins [47]*. Recent studies in leukocytes shed light on the signaling events preceding the final steps of integrin activation. The authors identified two types of protein complexes: one containing RAPL/Mst1, and the second one containing RIAM/Mst1/Kindlin-3 [48]. These results laid a foundation for future work on protein-protein interaction studies for Kindlin paralogs. (figure 1)
Figure 1. Integin and integrin-binding proteins in vasculature biology.
New reports identify the unique role of integrins and integrin-binding proteins in the vasculature. Ablation or decreased expression of integrin modulators Talin-1 and Kindlin-2, respectively, disable vasculature development and regulation of permeability. Focal Adhesion Kinase or Integrin Linked Kinase gene ablation result in the unique phenotypes as indicated. Integrin β1 gene ablation has a profound effect on vasculogenesis through disabled cell signaling for secretion of the extracellular matrix proteins and lumen formation, as well as through impaired cell adhesion and spreading. Novel α-integrin binding modulator of integrin activity, SHARPIN, is shown as well.
Source: original
Conclusions and future directions
Most recent studies further demonstrated the importance of integrins and their signaling in vascular biology. Deletions of integrin subunits and key regulators of integrin function result in distinctive vascular phenotypes. However, a substantial functional redundancy remains between several integrin subunits. Talin-1’s essential role for vasculogenesis is clearly demonstrated using in vivo knockout approach. However, we may witness a revision of Talin function from the integrin activation to a link between the integrin and cytoskeleton required for normal levels of integrin expression. Kindlin paralogs were shown to serve as essential regulators of integrin-dependent functions in various cell types. It became clear that the expression levels of Kindlin-2 are critical for the endothelial cell functions as shown in the heterozygous animal model. The authors expect a series of reports on conditional knockouts of Kindlin paralogs expanding our knowledge on the proteins’ function. From the mechanistic perspectives, the next breakthrough in integrin signaling is expected to result from structural studies. As Kindlin studies progress further, the big picture of the molecular events leading to integrin activation remains under constant revision. As Kindlins are found in the nucleus and at cell-cell junctions, identification integrin-independent functions of these proteins remain an intriguing possibility.
Key points.
-
◆
Plethora of integrins heterodimers is crucial for both development and homeostasis of the circulatory system. There is a considerable degree of functional redundancy between integrin subunits on endothelial cells.
-
◆
Integrins as signaling entities are crucial for ECM deposition and vessel lumen formation.
-
◆
Endothelial expression of Talin-1 is essential for vasculogenesis.
-
◆
Kindlins are implicated in vasculogenesis and vascular permeability and postnatal angiogenesis.
-
◆
Conditional/inducible knockouts of Kindlin genes are required to fully address their roles in vascular biology.
Acknowledgments
We thank Dr. Elizabeth Klipfell and Emelye Crehore for the help with the manuscript preparation.
Disclosure of funding received: N.L.M. is supported by American Heart Association 10SDG4300062 grant, T.V.B. is a recipient of NIH grant HL073311 and HL071625-07.
Abbreviations
- VEGFR
vascular endothelial growth factor receptor
- ILK
Integrin Linked Kinase
- FAK
Focal Adhesion Kinase
- PIP2
phosphatidylinositol-(4,5)-biphosphate
Footnotes
Conflicts of interest
Authors declare no conflict of interest.
References
- 1.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12(2):177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiosses WB, Shattil SJ, Pampori N, et al. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat Cell Biol. 2001 Mar;3(3):316–320. doi: 10.1038/35060120. [DOI] [PubMed] [Google Scholar]
- 3.Thodeti CK, Matthews B, Ravi A, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009 May 8;104(9):1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moser M, Legate KR, Zent R, et al. The tail of integrins, talin, and kindlins. Science. 2009 May 15;324(5929):895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 5.Malinin NL, Zhang L, Choi J, et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009 Mar;15(3):313–318. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moser M, Bauer M, Schmid S, et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009 Mar;15(3):300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 7.Moser M, Nieswandt B, Ussar S, et al. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008 Mar;14(3):325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 8.Svensson L, Howarth K, McDowall A, et al. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009 Mar;15(3):306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood. 2010 May 20;115(20):4011–4017. doi: 10.1182/blood-2009-10-239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, et al. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007 Aug;9(8):858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai T, Li S, Docheva D, et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003 Apr 1;17(7):926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilic D, Furuta Y, Kanazawa S, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995 Oct 12;377(6549):539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 13.Shen D, Li J, Lepore JJ, et al. Aortic aneurysm generation in mice with targeted deletion of integrin-linked kinase in vascular smooth muscle cells. Circ Res. 2011 Sep 2;109(6):616–628. doi: 10.1161/CIRCRESAHA.110.239343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavora B, Batista S, Reynolds LE, et al. Endothelial FAK is required for tumour angiogenesis. EMBO Mol Med. 2010 Dec;2(12):516–528. doi: 10.1002/emmm.201000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens LE, Sutherland AE, Klimanskaya IV, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995 Aug 1;9(15):1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 16.Carlson TR, Hu H, Braren R, et al. Cell-autonomous requirement for beta1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice. Development. 2008 Jun;135(12):2193–2202. doi: 10.1242/dev.016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei L, Liu D, Huang Y, et al. Endothelial expression of beta1 integrin is required for embryonic vascular patterning and postnatal vascular remodeling. Mol Cell Biol. 2008 Jan;28(2):794–802. doi: 10.1128/MCB.00443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malan D, Wenzel D, Schmidt A, et al. Endothelial beta1 integrins regulate sprouting and network formation during vascular development. Development. 2010 Mar;137(6):993–1002. doi: 10.1242/dev.045377. [DOI] [PubMed] [Google Scholar]
- 19. Zovein AC, Luque A, Turlo KA, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010 Jan 19;18(1):39–51. doi: 10.1016/j.devcel.2009.12.006. Loss of arteriola lumen formation upon beta1 integrin ablation was shown as a consequence of loss of cell polarity due to decrease of Par3 expression.
- 20.Ablooglu AJ, Tkachenko E, Kang J, et al. Integrin alphaV is necessary for gastrulation movements that regulate vertebrate body asymmetry. Development. 2010 Oct;137(20):3449–3458. doi: 10.1242/dev.045310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Flier A, Badu-Nkansah K, Whittaker CA, et al. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010 Jul;137(14):2439–2449. doi: 10.1242/dev.049551. Using Tie2 driven Cre, the study shows roles of α5 and αv subunits in developmental angiogenesis.
- 22.da Silva RG, Tavora B, Robinson SD, et al. Endothelial alpha3beta1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am J Pathol. 2010 Sep;177(3):1534–1548. doi: 10.2353/ajpath.2010.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monkley SJ, Kostourou V, Spence L, et al. Endothelial cell talin1 is essential for embryonic angiogenesis. Dev Biol. 2011 Jan 15;349(2):494–502. doi: 10.1016/j.ydbio.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp PM, Bate N, Hansen TM, et al. Studies on the morphology and spreading of human endothelial cells define key inter- and intramolecular interactions for talin1. Eur J Cell Biol. 2010 Sep;89(9):661–673. doi: 10.1016/j.ejcb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monkley SJ, Zhou XH, Kinston SJ, et al. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn. 2000 Dec;219(4):560–574. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, He X, Qi Y, et al. Talin1 regulates integrin turnover to promote embryonic epithelial morphogenesis. Mol Cell Biol. 2011 Aug;31(16):3366–3377. doi: 10.1128/MCB.01403-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rantala JK, Pouwels J, Pellinen T, et al. SHARPIN is an endogenous inhibitor of beta1-integrin activation. Nat Cell Biol. 2011 Nov;13(11):1315–1324. doi: 10.1038/ncb2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerlach B, Cordier SM, Schmukle AC, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011 Mar 31;471(7340):591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 29.Shen TL, Park AY, Alcaraz A, et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005 Jun 20;169(6):941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kogata N, Tribe RM, Fassler R, et al. Integrin-linked kinase controls vascular wall formation by negatively regulating Rho/ROCK-mediated vascular smooth muscle cell contraction. Genes Dev. 2009 Oct 1;23(19):2278–2283. doi: 10.1101/gad.535409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedrich EB, Liu E, Sinha S, et al. Integrin-linked kinase regulates endothelial cell survival and vascular development. Mol Cell Biol. 2004 Sep;24(18):8134–8144. doi: 10.1128/MCB.24.18.8134-8144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakshmikanthan S, Sobczak M, Chun C, et al. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alphavbeta. Blood. 2011 Aug 18;118(7):2015–2026. doi: 10.1182/blood-2011-04-349282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G, Xiang B, Ye S, et al. Distinct roles for Rap1b protein in platelet secretion and integrin alphaIIbbeta3 outside-in signaling. J Biol Chem. 2011 Nov 11;286(45):39466–39477. doi: 10.1074/jbc.M111.239608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderwood DA, Yan B, de Pereda JM, et al. The phosphotyrosine binding-like domain of talin activates integrins. J Biol Chem. 2002 Jun 14;277(24):21749–21758. doi: 10.1074/jbc.M111996200. [DOI] [PubMed] [Google Scholar]
- 35.Phillips DR, Prasad KS, Manganello J, et al. Integrin tyrosine phosphorylation in platelet signaling. Curr Opin Cell Biol. 2001 Oct;13(5):546–554. doi: 10.1016/s0955-0674(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 36. Bledzka K, Bialkowska K, Nie H, et al. Tyrosine phosphorylation of integrin beta3 regulates kindlin-2 binding and integrin activation. J Biol Chem. 2010 Oct 1;285(40):30370–30374. doi: 10.1074/jbc.C110.134247. This is the first evidence that tyrosine phosphorylation in the membrane distal NxxY motif in the β3 integrin cytoplasmic tail negatively regulates kindlin-2 binding and integrin activation.
- 37.Lee JH, An JY, Park H, et al. Crystallization and preliminary X-ray crystallographic analysis of the human kindlin-2 PH domain. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Jun 1;67(Pt 6):696–699. doi: 10.1107/S1744309111013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu H, Tu Y, Shi X, et al. Kindlin-2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. J Cell Sci. 2011 Mar 15;124(Pt 6):879–891. doi: 10.1242/jcs.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perera HD, Ma YQ, Yang J, et al. Membrane Binding of the N-Terminal Ubiquitin-Like Domain of kindlin-2 Is Crucial for Its Regulation of Integrin Activation. Structure. 2011 Nov 9;19(11):1664–1671. doi: 10.1016/j.str.2011.08.012. Terminal Ubiquitin-Like Domain of kindlin-2 Is Crucial for Its Regulation of Integrin Activation.
- 40.Legate KR, Takahashi S, Bonakdar N, et al. Integrin adhesion and force coupling are independently regulated by localized PtdIns(4,5)(2) synthesis. EMBO J. 2011 Sep 16;30(22):4539–4553. doi: 10.1038/emboj.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma YQ, Qin J, Wu C, et al. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol. 2008 May 5;181(3):439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dowling JJ, Gibbs E, Russell M, et al. Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ Res. 2008 Feb 29;102(4):423–431. doi: 10.1161/CIRCRESAHA.107.161489. [DOI] [PubMed] [Google Scholar]
- 43.Montanez E, Ussar S, Schifferer M, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008 May 15;22(10):1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pluskota E, Dowling JJ, Gordon N, et al. The integrin coactivator kindlin-2 plays a critical role in angiogenesis in mice and zebrafish. Blood. 2011 May 5;117(18):4978–4987. doi: 10.1182/blood-2010-11-321182. This is the first in vivo evidence showing that Kindlin-2 is a critical regulator of angiogenesis and vascular permeability as shown on heterozygous mouse model.
- 45.Lai-Cheong JE, Ussar S, Arita K, et al. Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J Invest Dermatol. 2008 Sep;128(9):2156–2165. doi: 10.1038/jid.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bialkowska K, Ma YQ, Bledzka K, et al. The integrin co-activator Kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell. J Biol Chem. 2010 Jun 11;285(24):18640–18649. doi: 10.1074/jbc.M109.085746. This is the first paper demonstrating that expression of kindlin-3 is not restricted to hematopoietic cells. Kindlin-3 is found in human endothelial cells, where it regulates α5β1-dependent cellular responses, while kindlin-2 is required for αVβ3 –mediated functions.
- 47. McDowall A, Svensson L, Stanley P, et al. Two mutations in the KINDLIN3 gene of a new leukocyte adhesion deficiency III patient reveal distinct effects on leukocyte function in vitro. Blood. 2010 Jun 10;115(23):4834–4842. doi: 10.1182/blood-2009-08-238709. Identification of a point mutation in Kindlin-3 that differentially affect cell adhesion and migration
- 48.Kliche S, Worbs T, Wang X, et al. CCR7-mediated LFA-1 functions in T cells are regulated by two independent ADAP/SKAP55-modules. Blood. 2012 Nov 23;119(3):777–785. doi: 10.1182/blood-2011-06-362269. [DOI] [PubMed] [Google Scholar]