Abstract
Background
The moderately homologous (~60%) proteins, Ara h 2 and Ara h 6, are the most potent peanut allergens. This study was designed to define the relative individual contributions of Ara h 2 and Ara h 6 to the overall allergenic activity of a crude peanut extract (CPE).
Methods
Ara h 2 and Ara h 6 were removed from CPE by gel filtration chromatography. Ara h 2.01, Ara h 2.02, and Ara h 6 were further purified (>99%). The potency of each allergen and the ability of these allergens to reconstitute the allergenic activity of CPE depleted of Ara h 2 and Ara h 6 was measured with RBL SX-38 cells sensitized with IgE from sensitized peanut allergic patients.
Results
The potency of the native proteins were significantly different (p<0.0001) although not dramatically so, with a rank order of Ara h 2.01 > Ara h 2.02 > Ara h 6. The addition of either purified Ara h 2 or Ara h 6 independently at their original concentration to CPE depleted of both Ara h 2 and Ara h 6 restored 80–100% of the original CPE allergenic activity. Addition of both Ara h 2 and Ara h 6 consistently completely restored the allergenic activity of CPE.
Conclusions
These studies indicate that either Ara h 2 or Ara h 6 independently can account for most of the allergenic activity in a CPE and demonstrate important redundancy in the allergenic activity of these related molecules.
Keywords: Food allergy, peanut, Ara h 2, Ara h 6, allergenic activity, RBL SX-38
Introduction
The prevalence of peanut allergy in the Western world has been increased over the last decade, representing a main health concern affecting more than 1 % of children {Liu, 2010 #1952;Ben-Shoshan, 2009 #1588;Sicherer, 2010 #1987}. Eleven peanut allergens have been identified {Pons, 2002 #1039;Pele, 2010 #1996}. Of these allergens, the 2S albumins, Ara h 2 and Ara h 6, are the most important peanut allergens in peanut-allergic patients, as determined by a combination of immunoblots, skin prick tests, ex vivo basophil histamine release assays, and in vitro experiments with RBL cells expressing human FcεRI {Koppelman, 2005 #1029;Flinterman, 2007 #1489;Blanc, 2009 #1859;Porterfield, 2009 #1582;Chen, 2011 #1994}. Furthermore, Ara h 2 and Ara h 6 are the major elicitors of anaphylaxis in a murine model of peanut allergy and can effectively desensitize peanut-allergic mice {Kulis, 2012 #1997}.
Ara h 2 has two isoforms, Ara h 2.01 and Ara h 2.02, coded by homologous genes with molecular sizes of 17 and 19 kD respectively. The latter has a duplication of 12 amino acids in the middle of the sequence that contains a linear epitope that strongly binds IgE {Shreffler, 2005 #1867;Burks, 1992 #331;de Jong, 1998 #332;Sen, 2002 #623;Chatel, 2003 #1048}. Similarly, multiple genes for Ara h 6 with minor differences in primary structure are expressed in a seed-specific fashion and during seed development {Kang, 2007 #1846;Ramos, 2006 #1661;Bernard, 2007 #1488;Paik-Ro, 2002 #1845}. Both Ara h 2 and Ara h 6 contain multiple disulphide–bridged cysteine residues, resulting in a tightly coiled, heat-stable, protease resistant core structure that may be important for allergenicity. Overall, Ara h 2 and Ara h 6 have 59% amino acid homology and this is higher (75%) in the alpha–helical regions accounting for their immunologic cross-reactivity {Ramos, 2006 #1661;Koppelman, 2005 #1029}.
In order to elucidate the most clinically important peanut allergens, we have worked to define the relative contributions of specific peanut allergens towards the overall allergenic activity of crude peanut extract using RBL SX-38 cells, an in vitro model of mast cell activation. We have defined the “allergenic activity” of an allergen as its ability to cross-link complexes of IgE and the high affinity receptor for IgE, FcεRI (IgE/FcεRI), which is likely a more precise measure of clinical relevance than the ability of the allergen to simply bind IgE (immunoreactivity). In our previous studies, we have demonstrated that specifically removing Ara h 2 and Ara h 6 together from a CPE either by size exclusion chromatography or by immunodepletion with specific rabbit antibodies results in significant decrease in the allergenic activity of a CPE {Porterfield, 2009 #1582;Chen, 2011 #1994}. Importantly, removing either Ara h 2 or Ara h 6 alone has little effect {Chen, 2011 #1994}. This suggested that these two moderately homologous allergens could act either synergistically or in a redundant fashion.
In this study, we further investigated the individual contributions of Ara h 2 and Ara h 6 to the potency of a crude peanut extract. To do this we first purified native Ara h 2.01, Ara h 2.02, and Ara h 6 from CPE, determined the concentrations of these allergens in our CPE, and compared their potencies in the RBL SX-38 cell assay. Then, we removed >99% of Ara h 2 and Ara h 6 from CPE by gel filtration chromatography and assessed quantitatively the effect of adding back purified Ara h 2 and Ara h 6 separately and together. The data demonstrate that Ara h 2.01, Ara h 2.02, and Ara h 6 have similar potency and that there is significant functional redundancy between Ara h 2 and Ara h 6.
Materials and Methods
Human sera
Individuals were selected on the basis of a strong history of systemic allergic reactions to peanuts and having high concentrations of peanut-specific IgE (>14 kU/L) to peanuts, a level associated with a 95% confidence of a positive double-blind placebo controlled challenge {Sampson, 2001 #1183}. A pool of 10 sera was prepared in which each serum was combined proportionally based on the concentration of anti-peanut IgE. This serum pool is different from that described in reference [10]. In addition, we studied 6 individual sera, 2 of which had slightly higher levels of anti-Ara h 2 IgE than anti-Ara h 6 IgE (anti-Ara h 2 IgE: anti-Ara h 6 IgE ratio was 1.8:1.0), 2 of which had slightly higher levels of anti-Ara h 6 IgE than that of anti-Ara h 2 (anti-Ara h 6 IgE: anti-Ara h 2 IgE was 1.7:1.0) and 2 of which had equal levels of anti-Ara h 2 and anti-Ara h 6. Clinical characteristics, the total IgE, the peanut-specific IgE of the individual sera and the sera in the serum pool are shown in Table 1. This study was approved by the Institutional Review Board of the University of Colorado Denver. All subjects signed informed consent and, for minors, assent.
Table 1.
The total IgE and the peanut-specific IgE of the individual sera and serum pool
| Serum# | Gender | Age | Race** | Other food allergies* | Total IgE (IU/ml) |
Anti-peanut IgE (kU/L) |
Anti - Ara h 2/6 ratio*** |
|---|---|---|---|---|---|---|---|
| D67 | M | 9 | C | E | 1418 | 254 | 1.8 : 1.0 |
| D120 | M | 3 | C | T | 171 | 54 | 1.7 : 1.0 |
| D121 | M | 4 | C | T | 6490 | 2020 | 1.2 : 1.0 |
| D122 | F | 17 | C | Soy | 341 | 97 | 1.2 : 1.0 |
| D123 | M | 3 | AA | T, S | 568 | 137 | 1.0: 1.6 |
| D124 | M | 7 | C | T, Sesame | 245 | 53 | 1.0 : 1.7 |
| Serum pool | 501 | 71 | 1.0 : 1.2 | ||||
| D60 | M | 16 | C | T, D, S, E, P, B, fish | 454 | 159 | ND |
| D64 | F | 9 | C | T, S | 2803 | 591 | ND |
| D65 | F | 11 | C | D | 2421 | 180 | ND |
| D68 | F | 16 | C | T | 212 | 25.2 | ND |
| D70 | M | 13 | C | T, D, Mushroom | 1423 | 500 | ND |
| D71 | M | 7 | PI | T, S, D, E | 4939 | 88.4 | ND |
| D80 | M | 13 | C | T | 545 | 164 | ND |
| D81 | M | 16 | C | T, Sesame | 268 | 67.5 | ND |
| D103 | F | 9 | C | T | 1797 | 787 | ND |
| D107 | F | 32 | C/H | None | 199 | 65.3 | ND |
T, Tree nut allergy; D, Dairy allergy; Shell fish allergy; E, Egg allergy; Peas allergy; B, Beef allergy.
C, Caucasian; AA, African American; PI, Pacific Islander; C/H, Caucasian/Hispanic
ND, not done.
Antibodies
Rabbit peptide-specific affinity purified anti-Ara 1, anti-Ara h 2, and anti-Ara h 6 antibodies used for immunoblots have been described previously {McDermott, 2007 #1259;Chen, 2011 #1994}.
ELISA and competitive inhibition ELISA
Relative binding of serum IgE to Ara h 2 and Ara h 6 was measured by ELISA. Ninety six well plates were coated with 10 ng of purified native Ara h 2 (a mixture (~1:1) of Ara h 2.01 and 2.02) or Ara h 6 in 0.1M bicarbonate buffer, pH 9.6, at 4 °C overnight. The plates were washed and blocked. Subject sera, diluted from 1:10 to 1: 1280 with 1% BSA in PBS was added to the wells and incubated at room temperature for 2h. Plates were washed and incubated with mouse anti-human IgE-HRP (SouthernBiotech, Birmingham, Alabama) at room temperature for 1h and developed. The optical density of IgE binding to Ara h 2 and Ara h 6 were compared at linear portions of binding curves to assess relative binding.
Quantification of Ara h 2 and Ara h 6 in our crude peanut extract was performed by modification of an inhibitory ELISA previously described independently by De Ceuninck and Schmitt {De Ceuninck, 2001 #1866;Schmitt, 2004 #1047}. Briefly, plates were coated with 10 ng of purified Ara h 2 (an ~1:1 mixture of Ara h 2.01 and 2.02) or Ara h 6 at a concentration of 0.2 µg/ml in 0.1M bicarbonate buffer, pH 9.6, at 4 °C overnight. The plates were washed and blocked. Varying concentrations of purified Ara h 2 and Ara h 6 (0–20 µg/ml) and CPE (0 – 500 µg/ml) were incubated with anti-Ara h 2 and Ara h 6 antibodies respectively for 1 h at room temperature (RT) before the samples were added to the wells. The plates were incubated for 3 h at RT and, after extensive washing, the plates were incubated with HPR-Goat anti-rabbit IgG antibody and developed. The content of allergens in CPE was calculated by reference to the purified allergen curve and expressed as percentage of the total protein in the CPE.
Crude peanut extract
Crude peanut extracts (CPEs) were prepared from Georgia Green peanuts as previously described {Porterfield, 2007 #1298}. Protein concentrations were determined with the Pierce BCA kit (Pierce, Rockford, IL, USA).
Removal of Ara h 2 and Ara h 6 together by gel filtration chromatography
All steps were performed at 4°C. Chromatography was carried out using an AKTA FPLC system (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Gel filtration (GF) was performed on a HiLoad 26/60 Superdex 75 Prep-Grade Column as previously described {Porterfield, 2009 #1582}. The fraction (13–25 kD) consisting (~97%) of Ara h 2 and Ara h 6 (referred as the 20 kD fraction or 20 kD) was saved for further purification and the remaining fractions were recombined to yield a CPE without either Ara h 2 or Ara h 6 (CPE w/o 20 kD). In previous studies we demonstrated that recombining all fractions from the gel filtration column including 20 kD (CPE recombined) gave activity that was indistinguishable from the original CPE itself {Porterfield, 2009 #1582}. For this reason, the original CPE is used as the control for these experiments.
Purification of Ara h 2 and Ara h 6
All steps were performed at 4°C. The fractions from gel filtration (above) containing Ara h 2 and Ara h 6 were collected and dialyzed against 0.1M phosphate, 1.5 M ammonium sulphate, pH 7.0 buffer and further purified by hydrophobic interaction chromatography (HIC) using HiLoad 16/13 Phenylsepharose column (Amersham Biosciences, Piscataway, NJ, USA). Elution was performed using linear and step-wise gradients of ammonium sulfate in 0.1 phosphate, pH 7.0 at 2 ml/min. The resultant fractions were dialyzed against 1X PBS (Gibco) and stored in aliquots at ~1mg/ml at −70C.
Electrophoresis and immunoblot
Allergens were separated by SDS-PAGE and transferred to PVDF membranes. Immunoblotting was performed with anti-Ara h 2 and Ara h 6 antibodies, and followed by a goat anti-rabbit IgG conjugated to HRP (Bio-Rad, Hercules, CA) as previously described {Porterfield, 2009 #1582}. Coomassie-blue staining was performed using Bio-Safe Coomassie (Bio-Rad, Hercules, CA) per manufacturer’s instructions. For assessment of IgE binding, 2 µg of CPE, CPE recombined, or CPE w/o 20kD, and 0.5 µg of 20kD fraction were run on the gel, probed with the human serum pool (1:10), and developed with biotinylated murine anti-Human IgE (Invitrogen, Carlsbad, CA) and HRP-avidin (Sigma, St. Louis, MO) as previously described {Porterfield, 2009 #1582}.
RBL SX-38 cell release assay
RBL SX-38 cells were grown, sensitized, triggered, and the data analyzed as previously described except they were first cultured in MEM medium containing 3% of human AB serum (Gemini Bio-products, West Sacramento, CA) and 7 % of FBS for at least one month before being sensitized with the pooled serum {Dibbern, 2003 #376;Palmer, 2005 #969;Chen, 2011 #1994}. Briefly, two × 105 cells/ml were labeled with 1µCi/ml of tritium- 5-hydroxytryptamine (5-HT, serotonin; PerkinElmer Life Sciences, Covina, California) and passively sensitized overnight with MEM containing either 7.5% pooled serum or 5–10% of individual sera. The sensitized cells were then washed and challenged with CPE (200 ng/ml) or varying doses of Ara h 2 or Ara h 6. The data from replicate experiments were normalized to the amount of release with 200 ng/ml of CPE within each experiment and are presented as the percent of maximal (100%) release. Potency of these proteins were compared by determining the concentration of each that gave 50% of the maximal degranulation seen with CPE (EC50) {Porterfield, 2009 #1582}. For reconstitution experiments we studied the effect of adding Ara h 2 and/or Ara h 6 proportionately to the CPE w/o 20kD at a sub-optimal CPE dose of 10 ng/ml. The amounts of purified Ara h 2 and Ara h 6 to be added to the CPE w/o 20kD were determined based on data from a competitive ELISA assay. For these experiments, the sub-optimal signal at 10 ng/ml was set to 100% to allow comparison of replicate experiments.
Statistics
One-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was used to compare the EC50 values for Ara h 2.01, Ara h 2.02 and Ara h 6. All statistical comparisons were two tailed. Data were considered to be significantly different when the P-value was < 0.05. All statistical comparisons, the best-fit lines, and EC50 values were generated with GraphPad Prism 5.0c for the MacIntosh (GraphPad Software, USA).
Results
Removal of Ara h 2 and Ara h 6 by gel filtration chromatography
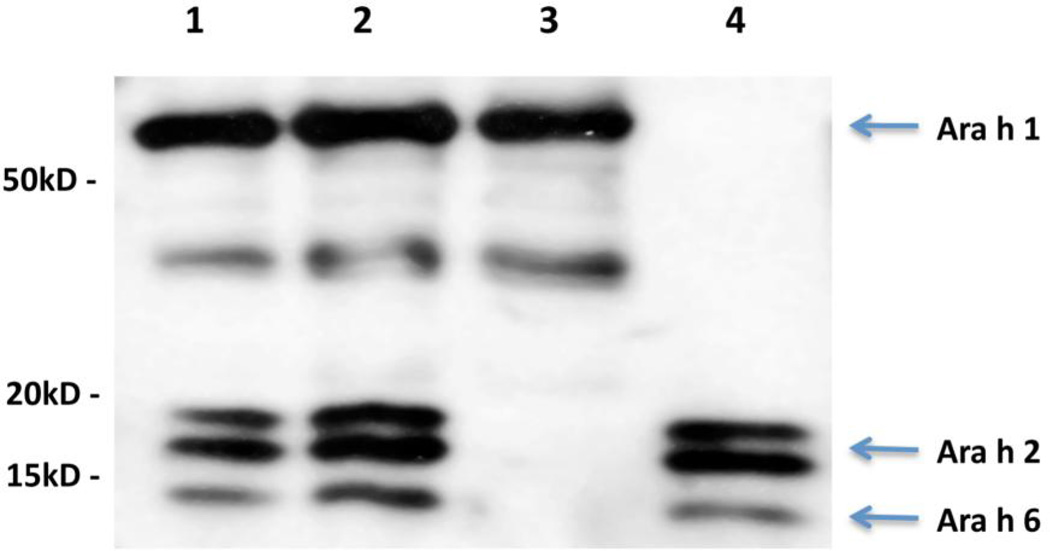
An IgE immunoblot of CPE, CPE recombined, CPEw/o 20kD, and purified 20 kD fraction containing Ara h 2 and Ara h 6 is shown in Figure 1. This was probed with a pool of 10 peanut-allergic sera and developed with anti-IgE as described in the Materials and Method section. The CPE (lane 1), the CPE recombined (lane 2) and the 20 kD fraction (lane 4), all contain bands at 15, 17 and 19kD compatible with Ara h 6, Ara h 2.01, and Ara h 2.02 respectively. The presence of Ara h 2 and Ara h 6 in the 20 kD fraction was confirmed by immunoblot with specific rabbit antibodies (data not shown). Similarly, the CPE w/o 20 kD (lane 3) shows absence of Ara h 2 and Ara h 6 and the presence of Ara h 1.
Figure 1. IgE Immunoblots.
The blot (representative of 3 replicates) was probed with a 1:10 dilution of a serum pool as described in the materials and methods section. Lane 1, CPE; Lane 2, CPE recombined; Lane 3, CPE w/o 20kD; and Lane 4, 20kD fraction.
Purification of native Ara h 2 and Ara h 6 from crude peanut extracts
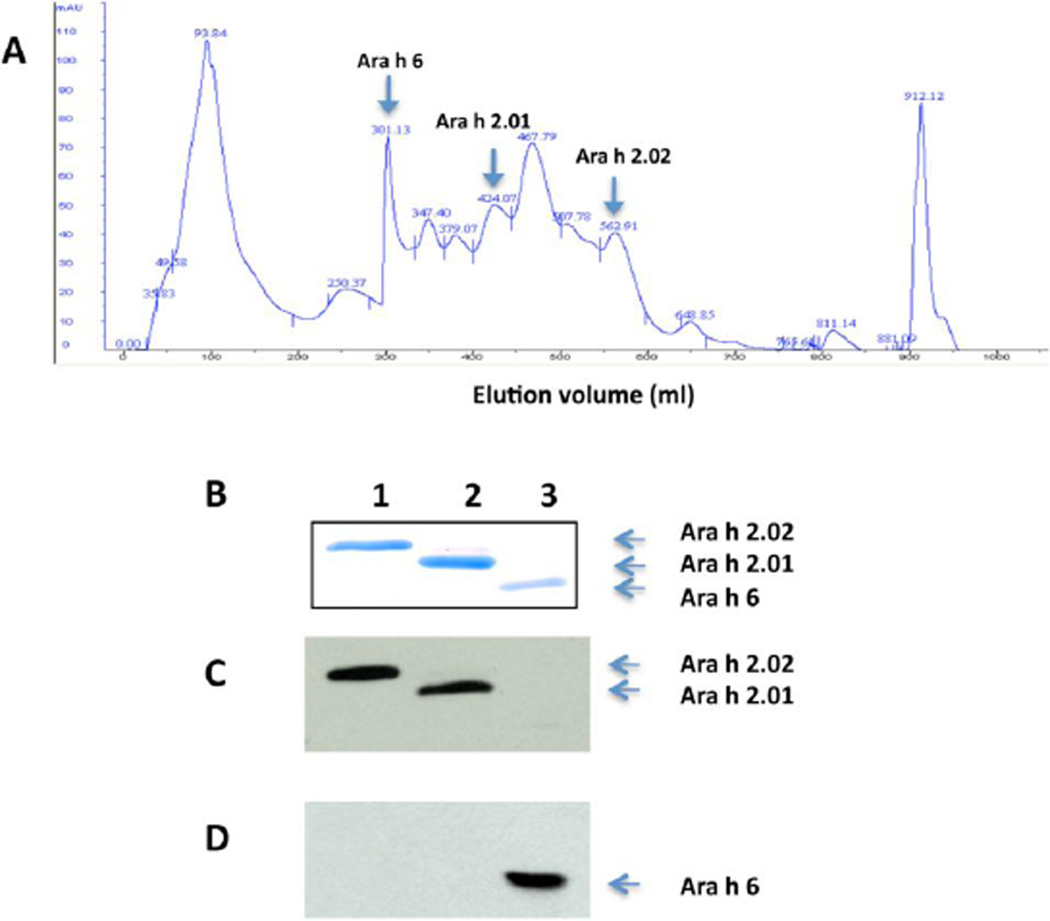
Ara h 2.01, 2.02 and 6 were purified in 2 simple, non-denaturing steps (GF and HIC chromatography) as described in the Materials and Methods section. Figure 2A shows the elution pattern from the HIC column. This method yielded highly purified (>99%) Ara h 2.01, Ara h 2.02 and Ara h 6 as detected by Coomassie blue staining (Fig. 2B) and immunoblot analysis with rabbit anti-Ara h 2 antibody (Fig. 2C) and anti-Ara h 6 antibody (Fig. 2D).
Figure 2. Purification of native Ara h 2.01, Ara h 2.02 and Ara h 6 from the 20kD fraction by hydrophobic interaction chromatography (HIC).
A) Absorption at 280 nm is shown on the vertical axis (mAU). Highly purified Ara h 2.01, 2.02 and Ara h 6 fractions were found in fractions at 305–340 ml, 428–435 ml and 570–580 ml respectively as noted. B) Coomassie blue staining of an SDS-PAGE gel:lane 1, Ara h 2.02(~19 kD); Lane 2, Ara h 2.01 (~ 17 kD; and Lane 3, Ara h 6 (~ 15 kD). C) Immunoblot of purified Ara h 2 and 6 with rabbit anti-Ara h 2 antibody, and D) Immunoblot of purified Ara h 2 and 6 with rabbit anti- Ara h 6 antibody. This purification scheme has been repeated more than 5 times with similar results.
Ara h 2.01, Ara h 2.02 and Ara h 6 have similar allergenic activity
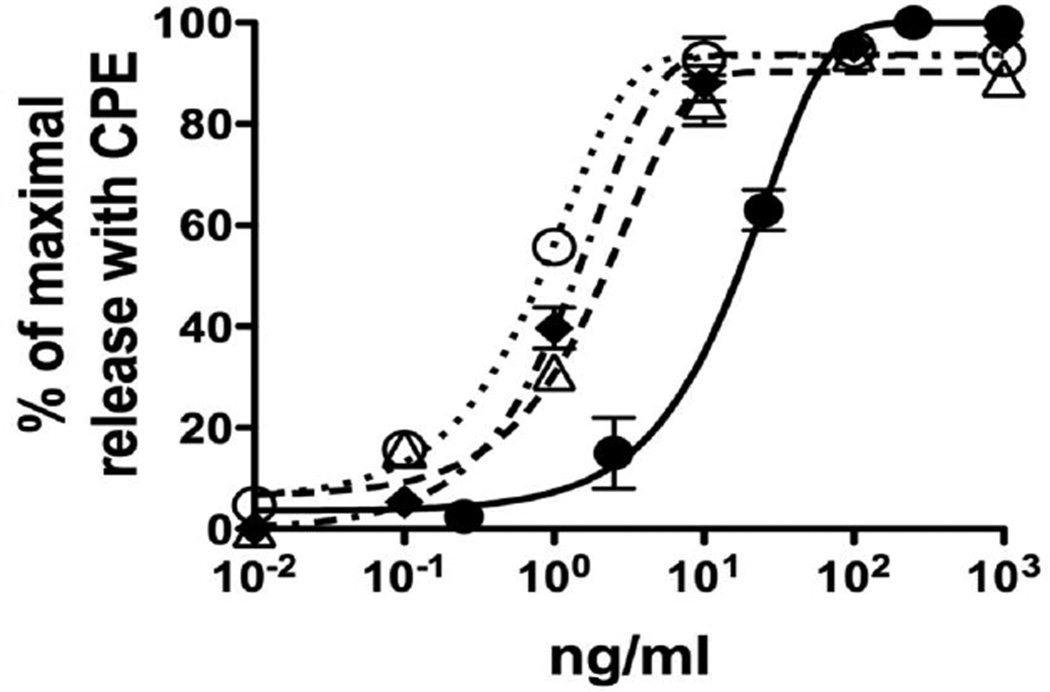
To examine the allergenic activity of each purified allergen, RBL SX-38 cells were sensitized with IgE from a pool of 10 peanut-allergic sera and triggered with either purified Ara h 2.01, Ara h 2.02 or Ara h 6 at different concentrations (0.01 to 10 ng/ml) as described in the Materials and Methods section. The EC50 values of CPE, Ara h 2.01, Ara h 2.02 and Ara h 6 were 16.95±2.55 (n=3), 0.83±0.1 (n= 3), 1.53±0.26 (n=4) and 2.57±0.48 (n= 6) ng/ml respectively (p<0.0001 for all comparisons) (Fig. 3). These purified proteins are clearly more potent than is the CPE and their rank order of potency is Ara h 2.01>Ara h 2.02>Ara h 6 with Ara h 2.01, Ara h 2.02 and Ara h 6 having approximately 20-fold, 11-fold, and 7–fold respectively more potency than the original CPE. Although the differences in molecular size among the 2S albumins are small, we converted the data to moles of protein yielding EC50 values of 4.7 ± 0.12×10−11 M, 8 ± 0.17×10−11 M and 1.7 ± 0.19×10−10 M for Ara h 2.01, Ara h 2.02 and Ara h 6 respectively (p<0.0001 for all comparisons). However, it is important to point out that, given the several logs encompassed by the dose response curves, these differences, albeit statistically different, are not large.
Figure 3. Allergenic activity of purified Ara h 2.01, Ara h 2.02 and Ara h 6.
Cells were sensitized with IgE from the serum pool. Sensitized cells were then triggered with serial dilutions of allergens. Values are expressed as per cent of maximal net degranulation seen with 200 ng/ml of CPE (set to 100%). In these experiments the total degranulation with CPE was 35–45% with < 10% background. CPE (●), Ara h 2.01 (○); Ara h 2.02 (■) and Ara h 6 (∆).
Quantification of Ara h 2 and Ara h 6 in CPE by competitive ELISA
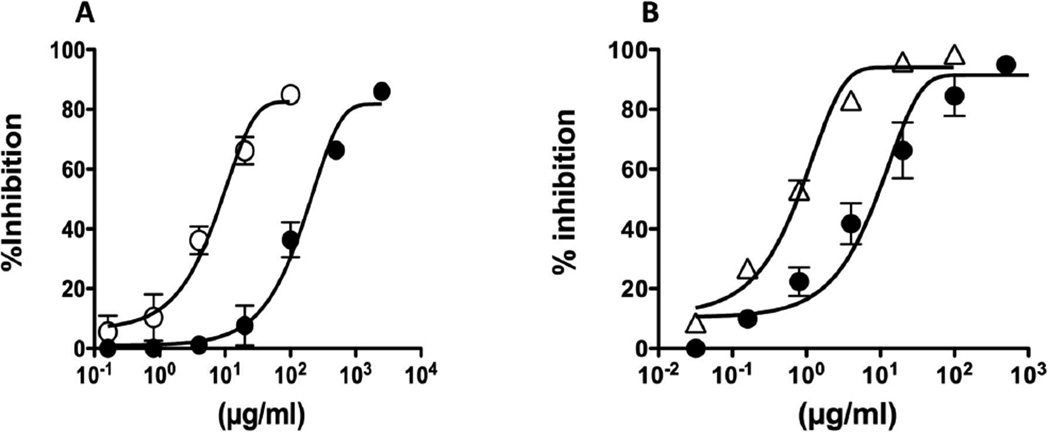
The binding of anti-Ara h 2 and anti-Ara h 6 antibodies to immobilized Ara h 2 and Ara h 6 was specifically inhibited with CPE or purified allergens (Fig. 4). To determine the relative content of each allergen in crude peanut extracts, the concentration of inhibitory allergens at 50% of inhibition (I50) were determined and compared. Based on these calculations, we estimated that the content of Ara h 2 in our CPE is 4 ± 0.3%, which is consistent with previous reports {Koppelman, 2001 #377}. Based on a similar assay, we estimate that Ara h 6 accounts for 6 ± 1.5 % of the total protein in CPE.
Figure 4. Quantification of Ara h 2 and Ara h 6 in CPE by competitive ELISA.
The binding of Ara h 2 to rabbit anti-Ara h 2 antibody (A) or Ara h 6 to rabbit anti-Ara h 6 antibody (B) was inhibited by crude peanut extract and by Ara h 2 and Ara h 6 respectively. CPE (●); Ara h 2. (○);Ara h 6 (∆). The X-axis represents allergen concentration and Y-axis represents % inhibition induced by allergens. This analysis was repeated 3 times.
Restoration of CPE allergenic activity by reconstitution with Ara h 2 and Ara h 6
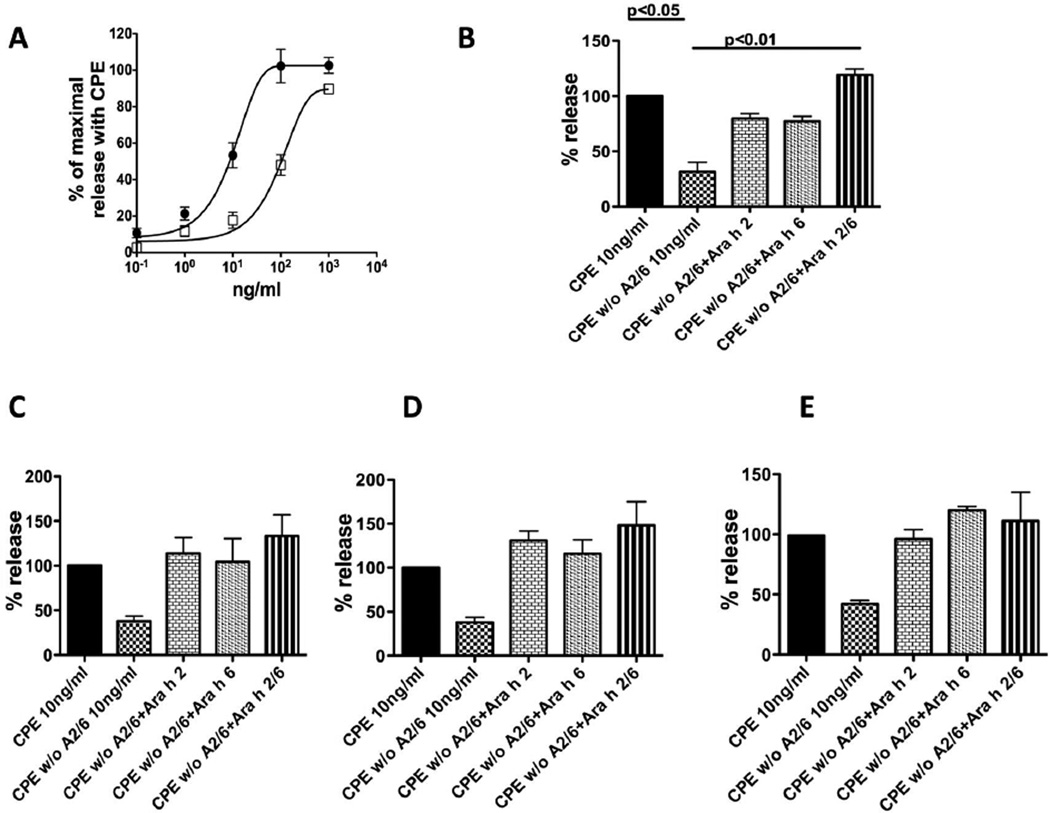
Based on the preceding experiments, we considered the possibilities that Ara h 2 and Ara h 6 could act in a synergistic, additive, or redundant fashion. We first repeated earlier work showing that, compared to CPE, the allergenic activity of CPE w/o 20 kD is approximately 10% of the total activity of the original CPE (a shift in potency of ~1 log(10)) (Fig. 5A) {Porterfield, 2009 #1582}. In the reconstitution experiments, we decided to study the effect of adding Ara h 2 and/or Ara h 6 proportionately to the CPE w/o 20kD, at a CPE dose of 10 ng/ml, a sub-optimal concentration slightly lower than 50% of the maximum allergenic activity (EC50) of CPE. Based on the data from Figure 4, the concentrations of purified Ara h 2 and Ara h 6 in 10 ng/ml of CPE were approximately 0.4 ng/ml (4%) and 0.6 ng/ml (6%) respectively. With pooled sera (Fig 5B), adding either Ara h 2 or Ara h 6 alone at these concentrations restored the activity to ~80 % of the activity in the original CPE (Fig. 5B, bars 3 and 4) and adding both Ara h 2 and Ara h 6 together at these doses to CPE w/o 20kD fully restored the total allergenic activity of CPE (p<0.01 compared with CPE w/o 20 kD) (Fig. 5B, bar 5). When cells were sensitized with individual sera (Fig. 5C –E), similar patterns were seen regardless of whether the IgE in these sera bound better to Ara h 2 (Fig. 5C), equally to Ara h 2 and Ara h 6 (Fig. 5D), or better to Ara h 6 (Fig. 5E). Of note, in all graphs shown in Figure 5 the maximal signal seen with repletion of both Ara h 2 and Ara h 6 was always numerically (but not statistically) greater than the original CPE. This likely reflects minor overestimation of the content of Ara h 2 and Ara h 6 from the inhibitory ELISA in Figure 4.
Figure 5. Restoration of CPE allergenic activity by reconstitution with Ara h 2 and Ara h 6.
Cells were sensitized with IgE from the serum pool (A, B) or with individual sera (C, D121; D, D123; E; D67). Sensitized cells were triggered with serial dilutions of CPE (●) or CPE w/o 20 kD (□) (A) or CPE (10 ng/ml) or CPE w/o 20 kD (10 ng/ml) plus either Ara h 2 (0.4 ng/ml) or Ara h 6 (0.6 ng/ml) or both as labeled (B–E). Values are expressed as per cent of maximal net degranulation seen with 200 ng/ml of CPE (set to 100%) for A and as percent of maximal net degranulation seen with 10 ng/ml of CPE (set to 100%) for B–E. In (A) the total degranulation with 200 ng/ml of CPE was 35–45% with < 10% background. The data are the average ± SEM of 4 separate experiments (A), 5 separate experiments (B), and 2 separate experiments (C–E).
Discussion
We have previously shown that removal of both Ara h 2 and Ara h 6, but neither alone, is necessary to significantly reduce the allergenic activity of a CPE {Porterfield, 2009 #1582;Chen, 2011 #1994}. To study the relative contributions of Ara h 2 and Ara h 6 to the allergenic activity of CPE, we chose to study the activity of adding either Ara h 2 and Ara h 6 or both to a CPE preparation that was depleted of both Ara h 2 and Ara h 6 by gel filtration chromatography (CPE w/o 20 kD). This approach was chosen to remove Ara h 2 and Ara h 6 rather than removal by immunodepletion since the removal of Ara h 2 and Ara h 6 by gel filtration is technically easier. In this report, we first describe a simple, non-denaturing, two step method for purification of Ara h 2.01, Ara h 2.02, and Ara h 6 (Fig. 2). It is possible that our purified Ara h 2 and Ara h 6 contain minor impurities. However, the gels and immunoblots shown in Figure 2 demonstrate that impurities are <1% of the recovered protein.
We then demonstrated that these allergens have strong allergenic activity (Fig. 3). Because Ara h 2.02 isoform contains an extra copy of a reported immunodominant IgE-binding epitope, DPYSPS, it has been speculated that Ara h 2.02 could be more potent than Ara h 2.01 {Chatel, 2003 #1048;Stanley, 1997 #1123}. In fact, Hales and colleagues reported an increased frequency of binding of IgE to Ara h 2.02 compared with Ara h 2.01 and that Ara h 2.02 was more efficient in an IgE competition assay {Hales, 2004 #1036}. However, our finding that Ara h 2.01 has slightly greater potency than Ara h 2.02 is contrary to these earlier reports and underscores the concept that the capacity of an allergen to bind IgE in an immunochemical assay may not be indicative of its ability to cross-link IgE/FcεRI complexes on mast cells/basophils leading to mast cell activation. Furthermore, this finding suggests that the repeating linear sequence, DPYSPS, does not contribute substantially to the allergenic activity of Ara h 2. It is important to point out that the data in Fig 3 were obtained with RBL SX-38 cells sensitized with IgE from a pool of 10 peanut allergic sera. It is possible that some individual sera have a different rank order of sensitivity to Ara h 2.01, 2.02, and Ara h 6. However, given the small differences in the EC50 values, to determine this with accuracy would entail multiple assays with each serum and not accomplish very much. Finally, our repletion assays (Figure 5) did not reveal any significant serum to serum variability in the activity of Ara h 2 and Ara h 6 (see below).
We next found that, based on their concentrations in the original CPE, either Ara h 2 or Ara h 6 alone could greatly restore the allergenic activity (about 80 –100 %) of a crude peanut extract (CPE) that had been depleted of both Ara h 2 and Ara h 6, and that the proportionate addition of Ara h 2 and 6 together to CPE depleted of Ara h 2 and Ara h 6 fully restored the allergenic activity (Figure 5) (p<0.01). It is interesting that the repletion of Ara h 2 and Ara h 6 consistently resulted in final activities that were slightly in excess of 100% (Fig 5). This could be due to underestimation of the concentrations in our purified samples or overestimation of the amount of allergen in the CPE or possibly due to the unveiling of new epitopes in the purified materials that may have been inhibited by other proteins in the extract. These findings were true if the cells were sensitized with IgE from a pool of 10 sera or from individual sera containing IgE that bound more Ara h 2 than Ara h 6 and from sera containing IgE that bound more Ara h 6 than Ara h 2. Thus, these allergens have substantial redundant functional activity.
To our knowledge, this is the first study to examine the allergenic activity of purified native Ara h 2.01, Ara h 2.02 and Ara h 6 in parallel and to determine the relative contributions of Ara h 2 and Ara h 6 to the activity of a CPE. We did this quantitatively by removal of both allergens followed by reconstitution with purified allergens. These studies are also distinctive in that we used native allergens purified under gentle conditions. Of note, we did not determine the relative content of Ara h 2.01 and 2.02 and did not reconstitute with purified preparations of these isoforms. However, the Ara h 2 used in these studies did contain the same relative amounts of Ara h 2.01 and Ara h 2.02 as found in the original CPE.
To better understand the possible molecular basis of this redundancy we aligned the primary amino acid sequences of Ara h 2.01 and Ara h 6 and found that three prominent IgE-binding linear epitopes of Ara h 2.01 previously reported in the literature are conserved in Ara h 6 (CEALQQIMENQ, KRELRNLP, QRCDLDVE) and may explain in great part the redundancy between Ara h 2 and Ara h 6 described in this study {Shreffler, 2005 #1455}.
In conclusion, our studies demonstrate in a quantitative fashion that Ara h 2 and Ara h 6 account for the great majority of the allergenic activity in a CPE and have redundant biologic activity. Given their overall 60% sequence homology, we predict that the similarities between these proteins are more important than their differences. An important implication of the findings in this study and our previous work {McDermott, 2007 #1259;Porterfield, 2009 #1582;Chen, 2011 #1994} is that depletion or genetic alteration of either Ara h 2 or Ara h 6 alone from peanuts should not have a significant impact on reducing the clinical severity to peanut or on decreasing threshold of reactivity. For the benefit of general peanut allergic population, it will be necessary to eliminate or dramatically alter both of these major peanut allergens.
Acknowledgements
The authors also thank our study coordinator, Darcy G. Schlichting, and Drs. Allen Adinoff, Karen Andrews, Sandy Avner, Alan Bock, Leon Greos, John James, Grant Olson, Jerry Koepke, Jeffery Rumbyrt, and Catherine Van Kerkhove for referring patients, and our study subjects for their participation.
Support
RO1-AI052164 and a supplemental ARRA grant from National Institute of Allergy and Infectious Diseases to Dr. Dreskin and divisional funds
Footnotes
In this manuscript “allergenic activity” refers to the ability of an allergen to cross-link IgE/FcεRI complexes leading to activation of mast cells.
References
- 1.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC. National prevalence and risk factors for food allergy and relationship to asthma: Results from the national health and nutrition examination survey 2005-2006. J Allergy Clin Immunol. 2010;126:798–806 e713. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Shoshan M, Kagan RS, Alizadehfar R, Joseph L, Turnbull E, St Pierre Y, Clarke AE. Is the prevalence of peanut allergy increasing? A 5-year follow-up study in children in montreal. J Allergy Clin Immunol. 2009;123:783–788. doi: 10.1016/j.jaci.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. Us prevalence of self-reported peanut, tree nut, sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Pons L, Chery C, Romano A, Namour F, Artesani MC, Gueant JL. The 18 kda peanut oleosin is a candidate allergen for ige-mediated reactions to peanuts. Allergy. 2002;57 Suppl 72:88–93. doi: 10.1034/j.1398-9995.57.s72.16.x. [DOI] [PubMed] [Google Scholar]
- 5.Pele M. Peanut allergens. Romanian Biotechnological Letters. 2010;15:5204–5212. [Google Scholar]
- 6.Koppelman SJ, de Jong GA, Laaper-Ertmann M, Peeters KA, Knulst AC, Hefle SL, Knol EF. Purification and immunoglobulin e-binding properties of peanut allergen ara h 6: Evidence for cross-reactivity with ara h 2. Clin Exp Allergy. 2005;35:490–497. doi: 10.1111/j.1365-2222.2005.02204.x. [DOI] [PubMed] [Google Scholar]
- 7.Flinterman AE, van Hoffen E, den Hartog Jager CF, Koppelman S, Pasmans SG, Hoekstra MO, Bruijnzeel-Koomen CA, Knulst AC, Knol EF. Children with peanut allergy recognize predominantly ara h2 and ara h6, which remains stable over time. Clin Exp Allergy. 2007;37:1221–1228. doi: 10.1111/j.1365-2222.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 8.Blanc F, Adel-Patient K, Drumare MF, Paty E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and ara h 6 are the most efficient elicitors. Clin Exp Allergy. 2009;39:1277–1285. doi: 10.1111/j.1365-2222.2009.03294.x. [DOI] [PubMed] [Google Scholar]
- 9.Porterfield HS, Murray KS, Schlichting DG, Chen X, Hansen KC, Duncan MW, Dreskin SC. Effector activity of peanut allergens: A critical role for ara h 2, ara h 6, and their variants. Clin Exp Allergy. 2009;39:1099–1108. doi: 10.1111/j.1365-2222.2009.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Zhuang Y, Wang Q, Moutsoglou D, Ruiz G, Yen SE, Dreskin SC. Analysis of the effector activity of ara h 2 and ara h 6 by selective depletion from a crude peanut extract. J Immunol Methods. 2011;372:65–70. doi: 10.1016/j.jim.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulis M. The 2s albumin allergens of arachis hypogaea, ara h 2 and ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice. Clin Exp Allergy. 2012 doi: 10.1111/j.1365-2222.2011.03934.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shreffler WG, Lencer DA, Bardina L, Sampson HA. Ige and igg4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, ara h 2. J Allergy Clin Immunol. 2005;116:893–899. doi: 10.1016/j.jaci.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Burks AW, Williams LW, Connaughton C, Cockrell G, O'Brien TJ, Helm RM. Identification and characterization of a second major peanut allergen, ara, h ii, with use of the sera of patients with atopic dermatitis and positive peanut challenge. J Allergy Clin Immunol. 1992;90:962–969. doi: 10.1016/0091-6749(92)90469-i. [DOI] [PubMed] [Google Scholar]
- 14.de Jong EC, Van Zijverden M, Spanhaak S, Koppelman SJ, Pellegrom H, Penninks AH. Identification and partial characterization of multiple major allergens in peanut proteins. Clin Exp Allergy. 1998;28:743–751. doi: 10.1046/j.1365-2222.1998.00301.x. [DOI] [PubMed] [Google Scholar]
- 15.Sen M, Kopper R, Pons L, Abraham EC, Burks AW, Bannon GA. Protein structure plays a critical role in peanut allergen stability and may determine immunodominant ige-binding epitopes. J Immunol. 2002;169:882–887. doi: 10.4049/jimmunol.169.2.882. [DOI] [PubMed] [Google Scholar]
- 16.Chatel JM, Bernard H, Orson FM. Isolation and characterization of two complete ara h 2 isoforms cdna. Int Arch Allergy Immunol. 2003;131:14–18. doi: 10.1159/000070429. [DOI] [PubMed] [Google Scholar]
- 17.Kang IH, Srivastava P, Ozias-Akins P, Gallo M. Temporal and spatial expression of the major allergens in developing and germinating peanut seed. Plant Physiol. 2007;144:836–845. doi: 10.1104/pp.107.096933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos ML, Fleming G, Chu Y, Akiyama Y, Gallo M, Ozias-Akins P. Chromosomal and phylogenetic context for conglutin genes in arachis based on genomic sequence. Mol Genet Genomics. 2006;275:578–592. doi: 10.1007/s00438-006-0114-z. [DOI] [PubMed] [Google Scholar]
- 19.Bernard H, Mondoulet L, Drumare MF, Paty E, Scheinmann P, Thai R, Wal JM. Identification of a new natural ara h 6 isoform and of its proteolytic product as major allergens in peanut. J Agric Food Chem. 2007;55:9663–9669. doi: 10.1021/jf071424g. [DOI] [PubMed] [Google Scholar]
- 20.Paik-Ro OG, Seib JC, Smith RL. Seed-specific, developmentally regulated genes of peanut. Theor Appl Genet. 2002;104:236–240. doi: 10.1007/s001220100763. [DOI] [PubMed] [Google Scholar]
- 21.Sampson HA. Utility of food-specific ige concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 22.McDermott RA, Porterfield HS, El-Mezayan R, Schlichting D, Hansen KC, Duncan MW, Solomon B, Redzic J, Simpson M, Dreskin SC. Contribution of ara h 2 to peanut-specific immunoglobulin emediated, cell activation. Clinical & Experimental Allergy. 2007;37:752–763. doi: 10.1111/j.1365-2222.2007.02701.x. [DOI] [PubMed] [Google Scholar]
- 23.De Ceuninck F, Pastoureau P, Agnellet S, Bonnet J, Vanhoutte PM. Development of an enzymelinked immunoassay for the quantification of ykl-40 (cartilage gp-39) in guinea pig serum using hen egg yolk antibodies. J Immunol Methods. 2001;252:153–161. doi: 10.1016/s0022-1759(01)00352-0. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt DA, Cheng H, Maleki SJ, Burks AW. Competitive inhibition elisa for quantification of ara h 1 and ara h 2, the major allergens of peanuts. J AOAC Int. 2004;87:1492–1497. [PubMed] [Google Scholar]
- 25.Porterfield HS, Duncan MW, Hansen KC, Dreskin SC. The majority of the effector activity of a crude peanut extract co-purifies with ara h 2 but not ara h 1 or ara h 3. J Allergy and Clinical Immunology. 2007;119:S193. [Google Scholar]
- 26.Dibbern DJ, Palmer G, Williams P, Bock S, Dreskin S. Rbl cells expressiing human fcεri are a sensitive tool for exploring functional ige-allergen interactions. J Immunol Methods. 2003;274:37–45. doi: 10.1016/s0022-1759(02)00369-1. [DOI] [PubMed] [Google Scholar]
- 27.Palmer GW, Dibbern DA, Burks AW, Bannon GA, Bock SA, Porterfield HS, McDermott RA, Dreskin SC. Comparative potency of ara h 1 and ara h 2 in immunochemical and functional assays of allergenicity. Clinical Immunology. 2005;115:301–312. doi: 10.1016/j.clim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Koppelman SJ, Vlooswijk RA, Knippels LM, Hessing M, Knol EF, van Reijsen FC, Bruijnzeel-Koomen CA. Quantification of major peanut allergens ara h 1 and ara h 2 in the peanut varieties runner, spanish, virginia, and valencia, bred in different parts of the world. Allergy. 2001;56:132–137. doi: 10.1034/j.1398-9995.2001.056002132.x. [DOI] [PubMed] [Google Scholar]
- 29.Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, Helm RM, West CM, Bannon GA. Identification and mutational analysis of the immunodominant ige binding epitopes of the major peanut allergen ara h 2. Arch Biochem Biophys. 1997;342:244–253. doi: 10.1006/abbi.1997.9998. [DOI] [PubMed] [Google Scholar]
- 30.Hales BJ, Bosco A, Mills KL, Hazell LA, Loh R, Holt PG, Thomas WR. Isoforms of the major peanut allergen ara h 2: Ige binding in children with peanut allergy. Int Arch Allergy Immunol. 2004;135:101–107. doi: 10.1159/000080652. [DOI] [PubMed] [Google Scholar]