Abstract
Background
Periodontitis is prevalent in older humans. Limiting the inflammation associated with periodontitis may provide a therapy for this condition, because Gram-negative bacteria expressing lipopolysaccharide (LPS) have a key role in initiation of inflammation by activating macrophage functions. Because oxidized galectin-1 regulates macrophage functions in other systems, we sought to establish whether this galectin-1 mRNA is expressed in the oral cavity, and whether it could dampen LPS-induced macrophage activation in vitro.
Methods
Using the reverse transcriptase polymerase chain reaction (RT-PCR), we measured galectin-1 mRNA expression to clarify its localization to rat gingival tissues and studied the effect of Porphyromonas gingivalis challenge on galectin-1 expression. Next, we tested the effects of adding oxidized galectin-1 to cultured LPS-activated peritoneal macrophages on mRNA expression of proinflammatory factors by RT-PCR and real-time RT-PCR.
Results
We established that galectin-1 mRNA is expressed in gingival tissues and also showed that galectin-1 mRNA was significantly increased by challenge with P. gingivalis, indicating that galectin-1 may regulate oral inflammation. On the other hand, LPS 100 ng/mL in serum-containing medium induced macrophages to upregulate mRNA associated with a proinflammatory response, ie, interleukins 1β and 6, and inducible nitric oxide synthase. We showed that application of 0.1–10 ng/mL of oxidized galectin-1 to LPS-treated macrophages reduced the intense LPS- induced increase by serum in proinflammatory mRNA expression in a concentration-dependent manner. Furthermore, application of oxidized galectin-1 10 ng/mL to LPS-treated macrophages in serum-free medium also showed a similar effect on LPS activity.
Conclusion
Oxidized galectin-1 restricts the proinflammatory actions of LPS, and this protein could limit the negative effects of inflammation.
Keywords: periodontitis, inflammation, macrophage, lipopolysaccharide, galectin-1, proinflammatory factors
Introduction
Periodontitis, an inflammatory disease in the tissues that support the teeth, is prevalent in older humans. Periodontal diseases are infections caused by pathogenic bacteria that colonize the subgingival area and instigate an inflammatory response, which may lead to destruction of periodontal tissue. These bacteria, eg, Porphyromonas gingivalis, secrete a variety of virulence factors.1 One of these factors is lipopolysaccharide (LPS), which activates macrophages to augment inflammation by promoting mRNA synthesis of proinflammatory cytokines and inducible nitric oxide synthase (iNOS).2 Factors that reduce LPS-induced macrophage activation are candidates for prevention of periodontitis. Oxidized galectin-1 regulates macrophage function in injured nerves3,4 and in other systems,5 indicating that oxidized galectin-1 could be one such candidate molecule.
Galectins are a family of proteins defined by their affinity for β-galactosides and by conserved sequence elements in their carbohydrate recognition domains. Galectin-1, the first of this family to be discovered, is expressed ubiquitously.6,7 Galectin-1 shows β-galactoside binding activity only in the reduced form, which has a variety of important functions.6,7 The oxidized form of galectin-1, which occurs under certain oxidation states in the environment, exhibits no lectin activity,8,9 but has peripheral nerve regeneration-promoting activity.10,11 Oxidized galectin-1 regulates macrophage function in rat models of peripheral nerve injury and promotes nerve regeneration.3 Galectin-1 is thought to be upregulated and released into the oxidizing extracellular space following injury, and oxidized galectin-1 then binds to macrophages and activates signaling cascades that enhance axonal regeneration. Moreover, oxidized galectin-1 promotes macrophage accumulation in injured nerves.4 These observations suggest that oxidized galectin-1 may regulate macrophage functions to limit the negative effect of inflammation.
Here we examine galectin-1 expression in gingival tissues, and establish whether oxidized galectin-1 affects LPS-induced macrophage activation. We show that galectin-1 mRNA is expressed in both control and periodontal tissue. Periodontitis-induced gingival inflammation is partly due to bacterial release of LPS, which activates macrophages to produce proinflammatory cytokines that exacerbate the disease.12,13 We studied the effect of oxidized galectin-1 treatment on LPS-activated peritoneal macrophages in vitro, and found oxidized galectin-1 tempered LPS-induced increases in proinflammatory mRNAs, including interleukin (IL)-1β, IL-6, and iNOS, suggesting that oxidized galectin-1 treatment may reduce periodontal inflammation.
Material and methods
Animals and experimental periodontitis
The experiments were performed as previously described.14 We used 20 male, pathogen-free, three-week-old Sprague Dawley rats (40–60 g) obtained from Nihon SLC (Shizuoka, Japan). The rats were given sulfamethoxazole 1 mg/mL and trimethoprim 200 μg/mL in drinking water ad libitum for four days to reduce the original oral flora, followed by a four-day antibiotic-free period before being challenged with P. gingivalis (ATCC 33277). Rats were orally challenged with P. gingivalis suspended in 5% carboxymethylcellulose. Each rat received 0.5 mL (1.5 × 1010 cells/mL) by oral gavage (four times) at 48-hour intervals. The experimental procedures of this study were reviewed and approved by the committee of ethics on animal experiments for Kanagawa Dental College, and were carried out under the guidelines for animal experimentation.
Tissue preparation
At the end of the experimental period, all animals were killed by decapitation under anesthesia (veterinary Ketalar® 50, intramuscular). Tissue blocks containing all three maxillary molars, alveolar bone, and surrounding soft tissues were removed from the right side of the maxilla (split-mouth experimental design).
Preparation of recombinant human oxidized galectin-1
Recombinant human oxidized galectin-1 was obtained according to methods described elsewhere.11 Briefly, recombinant human oxidized galectin-1 was expressed in Escherichia coli and purified from the supernatant of the sonicated E. coli by DEAE diethylaminoethyl high-performance liquid chromatography. This bacterially-expressed recombinant human oxidized galectin-1 was oxidized by the air oxidization method, catalyzed by CuSO4. Specifically, diethylaminoethyl-purified recombinant human oxidized galectin-1 was diluted 20-fold with 20 mM Tris-HCl, at pH 8.0, then CuSO4 was added to a final concentration of 0.0001% (w/v), and the mixture was maintained overnight at 4°C to allow disulfide bond formation. Recombinant human oxidized galectin-1 was purified by reverse-phase high-performance liquid chromatography on a YMC-Pack Protein RP column (YMC Co Ltd, Kyoto, Japan) with a linear gradient of acetonitrile in 0.1% trifluoroacetic acid. Analysis by sodium dodecyl sulfate polyacrylamide gel electrophoresis and high-performance liquid chromatography showed that recombinant human oxidized galectin-1 did not degenerate, even after 10 days’ incubation at 37°C in phosphate-buffered saline 5 μg/mL.
Culture of peritoneal macrophages
Male Wistar rats (10 to 14 weeks old) were killed with ether, and the peritoneal cavity of the rats was washed with a RPMI 1640 medium (Invitrogen, Carlsbad, CA). Peritoneal macrophages were obtained from the medium after double centrifugation. Approximately 1 × 106 to 1.5 × 106 cells were then seeded onto individual, uncoated 35 mm culture dishes. The weakly attached cells, which were different from macrophages, were washed out 10 minutes after seeding. We recovered highly purified macrophages (>80%) using this method.3 These purified macrophages were cultured in 10% serum-containing medium or serum-free macrophage medium (Invitrogen). After two hours of preculture in serum-containing medium, we added LPS (E. coli LPS, Sigma-Aldrich, St Louis, MO) and one of three different concentrations (0.1 ng/mL, 1 ng/mL, or 10 ng/mL) of oxidized galectin-1 to the cells. Similarly, after two hours of preculture in serum-free medium, we performed one of the following nine treatments on cells in serum-free medium: control (without LPS or oxidized galectin-1); 10 ng/mL LPS for two hours; 10 ng/mL LPS for three hours; 10 ng/mL LPS + 10 ng/mL oxidized galectin-1 for two hours; 10 ng/mL LPS + 10 ng/mL oxidized galectin-1 for three hours; 100 ng/mL LPS for two hours; 100 ng/mL LPS for three hours; 100 ng/mL LPS + 10 ng/mL oxidized galectin-1 for two hours; and 100 ng/mL LPS + 10 ng/mL oxidized galectin-1 for three hours. We analyzed mRNA expression of three proinflammatory factors, ie, IL-1β, IL-6, and iNOS, in cells subjected to each treatment using reverse transcriptase polymerase chain reaction (RT-PCR) and real-time RT-PCR.
RT-PCR and real-time RT-PCR
Total RNA was isolated from cultured peritoneal macrophages in each dish using an RNeasy Mini Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s instructions. cDNA was reverse transcribed and amplified using the SuperScript III One-Step RT-PCR system with platinum Taq DNA polymerase (Invitrogen). The primer pairs were synthesized by Invitrogen, and their sequences, PCR product sizes, and GenBank acc are shown below:
Galectin-1: 5′-ATGGCCTGTGGTCTGGTCGCC-3′ and 5′-TCACTCAAAGGCCACACACTT-3′ (408 bp, NM_019904), GAPDH: 5′-TTCAACGGCACAGT CAAGG-3′ and 5′-CATGGACTGTGGTCATGAG-3′ (373 bp, NM_009084), IL-1β: 5′-GAAGCTGTGG CAGCTACCTATGTCT-3′ and 5′-CTCTGCTTGA GAGGTGCTGATGTAC-3′ (520 bp, NM_031512), IL-6: 5′-GACTGATGTTGTTGACAGCCACTGC-3′ and 5′-TAGCCACTCCTTCTGTGACTCTAACT-3′ (509 bp, NM_012589), and iNOS: 5′-GGAGAGATTTTTCACGA CACCC-3′ and 5′-CCATGCATAATTTGGACTTGCA-3′ (493 bp, NM_012611).
Each real-time RT-PCR was run along with a dilution series of the standard, and this dilution series served as the standard for calibration. A no-template control was also included with each run. All the real-time RT-PCR runs were conducted in a LightCycler® 480 Multiwell Plate 96 (Roche Diagnostics GmbH, Mannheim, Baden-Wurttemberg, Germany) in a final volume of 20 mL of LightCycler 480 SYBR Green 1-Master containing fast start Taq DNA polymerase for “hot start” and DNA intercalating SYBR Green 1 dye for detection in a LightCycler 480 thermocycler (Roche Diagnostics GmbH). In addition, individual reaction mixtures included 0.5 μM of a gene-specific primer, 6 mL of 1:5 dilution of cDNA or 100 ng of DNA as template, and PCR grade water.
The optimal thermal cycling parameters varied according to the gene, and included a preincubation at 95°C for 10 minutes; 40 cycles of amplification at 95°C for 10 seconds were followed by annealing and extension. Melting curve analysis was done at 95°C for five seconds (segment 1), 65°C for one minute (segment 2), and 97°C for 0 seconds, followed by cooling at 40°C for 10 seconds. Fluorescence acquisition was done at 72°C for eight seconds for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), IL-1β, IL-6, and iNOS. The primers used in this experiment are shown below. PCR product sizes and GenBank acc of the primer pairs are also shown.
GAPDH: 5′-CTCCCATTCTTCCACCTTTG-3′ and 5′-GGTCCAGGGTTTCTTACTCC-3′ (155 bp, NM_017008), IL-1β: 5′-TCCTGTGTGATGAAAGACGG-3′ and 5′-CTGCTTGAGAGGTGCTGATG-3′ (175 bp, NM_031512), IL-6: 5′-GCCAGAGTCATTCAGAGCAA-3′ and 5′-TCCTTAGCCACTCCTTCTGT-3′ (145 bp, NM_012589), and iNOS: 5′-TGAGGAGCAGGTTGAGGATT-3′ and 5′-TCAGAGTCTTGTACCTTTGG-3′ (136 bp, NM_012611).
Statistical analysis
Data were statistically analyzed by analysis of variance or t-test. All values represent a mean ± standard error of the mean.
Results
Effect of P. gingivalis on galectin-1 mRNA expression in gingival tissue
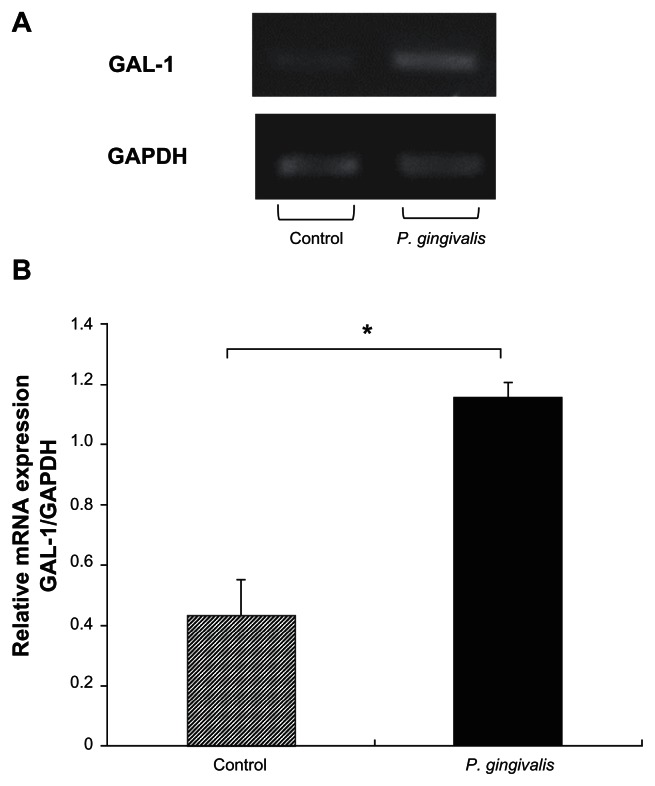
Analysis of gingival tissue by RT-PCR using seven 19- day- old rats shows that galectin-1 mRNA was weakly expressed in periodontal tissues (Figure 1A). This expression was confirmed in tissues from six other rats. Because galectin-1 mRNA expression was increased after injury or in case of inflammation, we studied the effect of P. gingivalis challenge on galectin-1 expression. We found that there was a significant increase in galectin-1 mRNA expression following P. gingivalis challenge (control 0.43 ± 0.11 and P. gingivalis 1.15 ± 0.04, P < 0.005, Figures 1A and 1B).
Figure 1.
GAL-1 mRNA is found within gingival tissues, and its expression is increased after Porphyromonas gingivalis challenge. A) GAL-1 and GAPDH mRNA expression in gingival tissue from control and P. gingivalis-challenged rats. B) Average values of GAL-1/GAPDH were 0.43 ± 0.11 (n = 3, mean ± standard error of the mean) in control gingival samples, and 1.15 ± 0.04 (n = 4) in samples from rats treated with P. gingivalis. There was a significant difference in gingival GAL-1 mRNA expression between the control and P. gingivalis-challenged rats (*analysis of variance, P < 0.005).
Abbreviations: GAL-1, galectin-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Concentration-dependent reduction of LPS-induced elevation of proinflammatory mRNAs by oxidized galectin-1
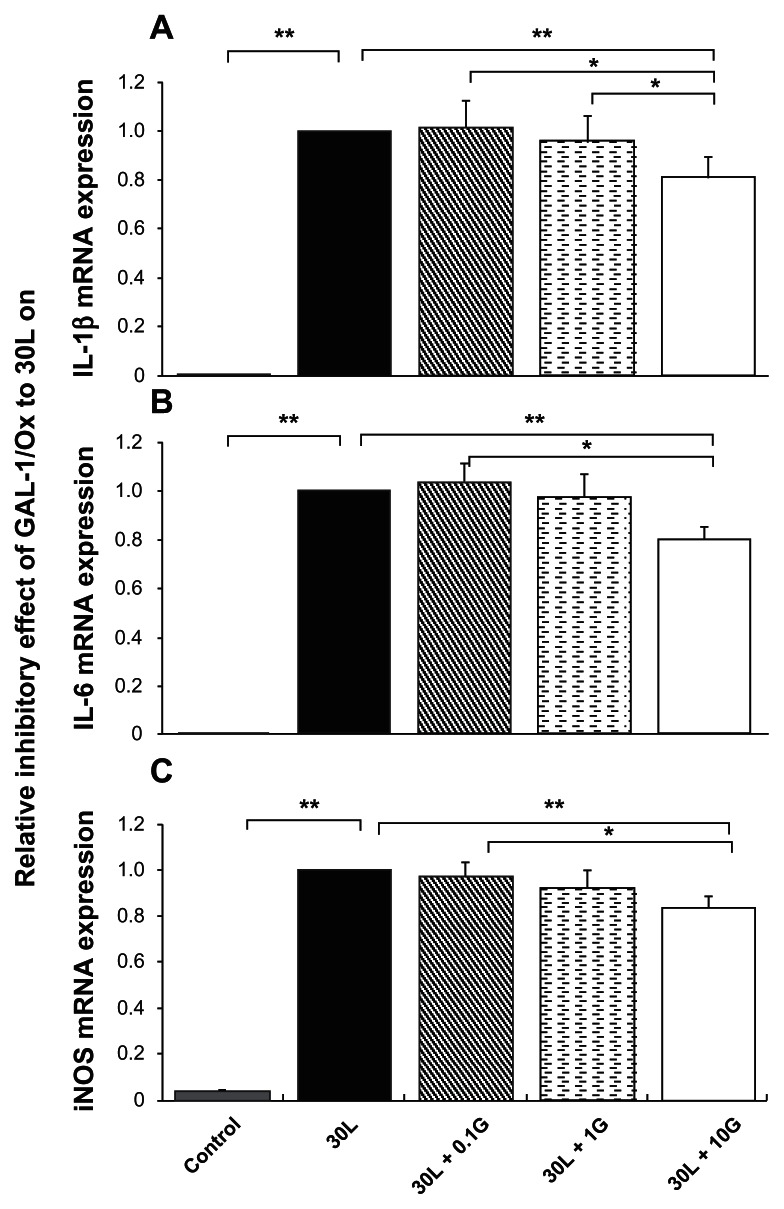
We cultured macrophages in the presence of LPS to model the inflammatory process in vitro. Macrophages from the rat peritoneal cavity were cultured in 10% serum-containing medium. Most macrophages survived for more than two days in culture. The macrophages were cultured for two hours before treatment with medium, LPS, and/or recombinant human oxidized galectin-1. We studied the effect of different concentrations of recombinant human oxidized galectin-1 (0.1 ng/mL, 1 ng/mL, and 10 ng/mL) on LPS-induced expression of three proinflammatory mRNAs, ie, IL-1β, IL-6, and iNOS, in macrophages cultured in serum-containing medium to determine whether the effects of oxidized galectin-1 were dose-dependent. The real-time RT-PCR analysis showed that 30 ng/mL LPS drastically increased mRNA expression of the three proinflammatory factors studied (IL-1β, IL-6, and iNOS, t-test, P < 0.01). We found that the relative expression of mRNA from macrophages treated with both LPS and 0.1 ng/mL recombinant human oxidized galectin-1 and the group that received both LPS and 1 ng/mL recombinant human oxidized galectin-1 were not statistically different from those exposed to 30 ng/mL LPS alone. In contrast, the expression of all three mRNAs encoding proinflammatory factors in macrophages exposed to both LPS and 10 ng/mL recombinant human oxidized galectin-1 were significantly reduced relative to their expression in macrophages treated with 30 ng/mL LPS alone (t-test, P < 0.05, Figure 2).
Figure 2.
Reduction of lipopolysaccharide-induced elevation of proinflammatory mRNAs by Gal-1/Ox was concentration-dependent in macrophages cultured in serum-containing medium. Real-time reverse transcriptase polymerase chain reaction shows that lipopolysaccharide 30 ng/mL intensely increased mRNA expression of three proinflammatory factors, ie, A) IL-1β, B) IL-6, and C) iNOS (**t-test, P < 0.01, n = 7). On the other hand, Gal-1/Ox 10 ng/mL significantly reduced the three upregulated proinflammatory mRNA expressions compared with the lipopolysaccharide-treated group (**t-test, P < 0.01, n = 7, in A, B, and C), from 0.1G (*P < 0.05, n = 7, in A, B, and C), and from 1G (*t-test, P < 0.05, in A). 30L: 30 ng/mL lipopolysaccharide, 30L + 0.1G: 30 ng/mL lipopolysaccharide + 0.1 ng/mL Gal-1/Ox, 1G: 1 ng/mL Gal-1/Ox, 10G: 10 ng/mL Gal-1/Ox.
Abbreviations: Gal-1/Ox, oxidized galectin-1; iNOS, inducible nitric oxide synthase; Il-6, interleukin 6; IL-1β, interleukin 1β.
Oxidized galectin-1 reduces LPS-induced elevation of proinflammatory mRNAs in serum-free conditions
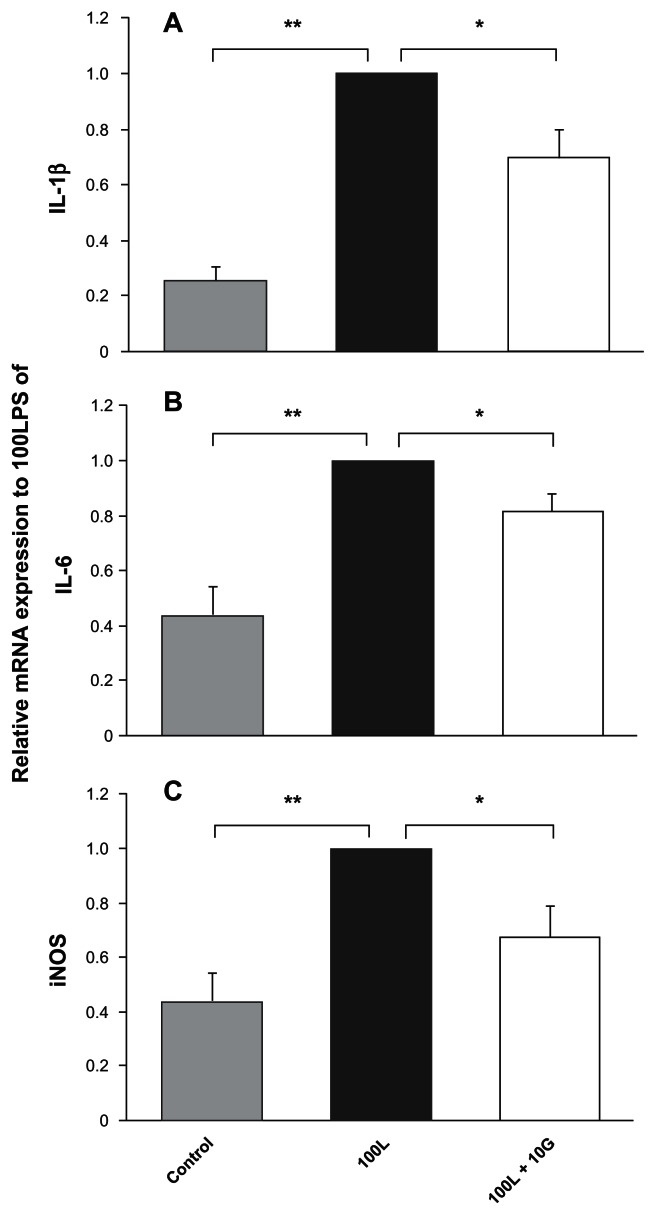
We performed parallel experiments in serum-free medium to limit the effects of serum components. Macrophages from the rat peritoneal cavity were cultured in serum-free defined medium. Because most of these macrophages survived for more than two days in culture, we could perform the eight-hour experiments in culture using the cells. The macrophages were cultured for two hours before treatment with medium, LPS, and/or recombinant human oxidized galectin-1. Using RT-PCR, we analyzed expression of three mRNA encoding proinflammatory factors in cultured macrophages under nine different treatment conditions. We found that LPS increased the expression of these mRNAs. We evaluated LPS activity by comparing treatment groups with control values (LPS/control). All of these relative values were larger than 1 (all six experiments, t-test, P < 0.05), indicating that LPS stimulated the macrophages to increase the expression of IL-1β mRNA. We found similar, statistically significant increases in IL-6 and iNOS mRNA expression in the macrophages after LPS treatment (both 10 ng/mL and 100 ng/mL) at both two and three hours (t-test, P < 0.05). The LPS effects were dose-dependent, ie, LPS 100 ng/mL induced significantly more proinflammatory factor mRNA expression in macrophages than did LPS 10 ng/mL. This LPS effect was confirmed by real-time RT-PCR analysis; all three proinflammatory factors were upregulated by treatment with LPS 100 ng/mL (Figure 4). On the other hand, oxidized galectin-1 had no effect on proinflammatory cytokine mRNA expression in macrophages not treated with LPS (data not shown).
Figure 4.
Lipopolysaccharide-induced increases in macrophage expression of proinflammatory factor mRNAs are restricted by cotreatment with oxidized galectin-1 in serum-free medium by real time polymerase chain reaction analysis. Real-time reverse transcriptase polymerase chain reaction analysis showed that treatment with 100L significantly increased proinflammatory mRNA expressions of A) IL-1β, B) IL-6, and C) iNOS (**t-test, P < 0.01, n = 6). The analysis also showed that cotreatment with 10G statistically reduced the normal lipopolysaccharide-induced increase in mRNA expressions of IL-1β, IL-6, and iNOS (*t-test, P < 0.05, n = 6). 100L: 100 ng/mL lipopolysaccharide, 10G: 10 ng/mL oxidized galectin-1.
Abbreviations: iNOS, inducible nitric oxide synthase; Il-6, interleukin-6; IL-1β, interleukin 1β.
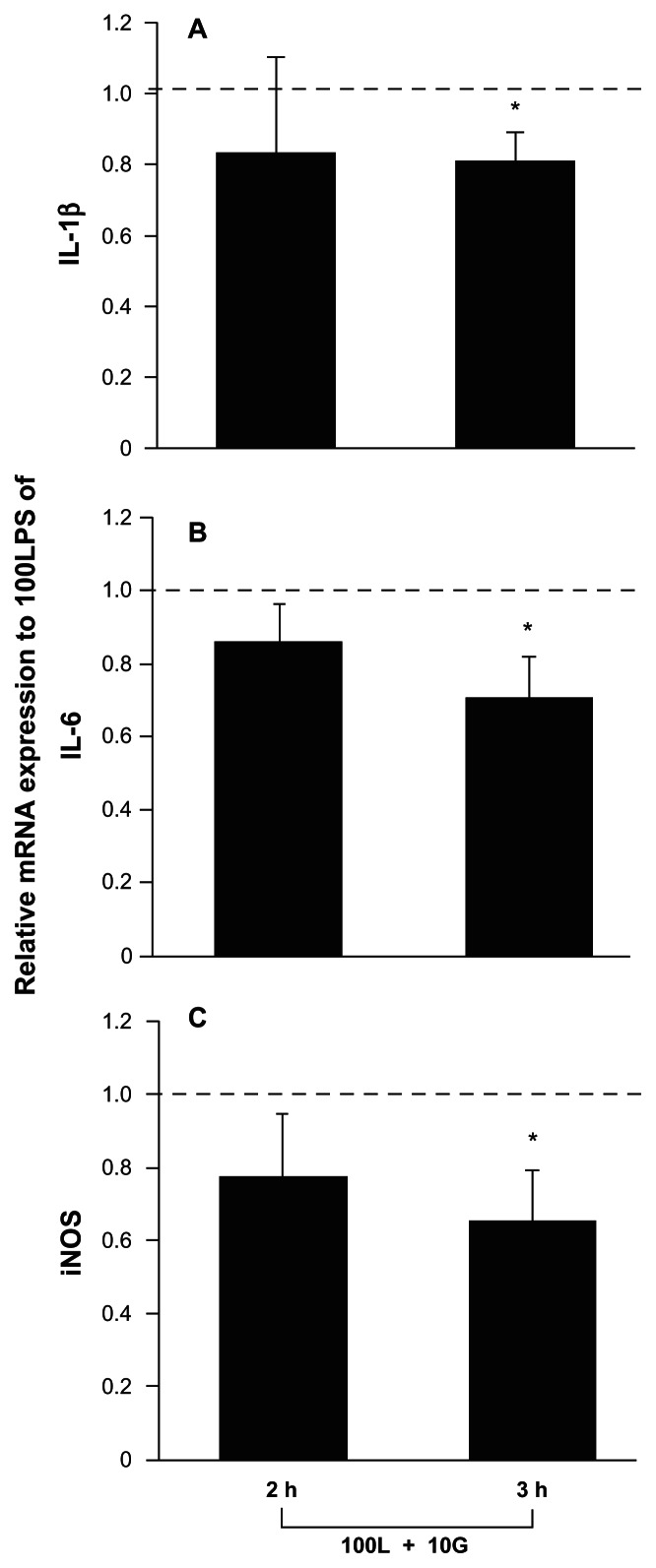
Next, we studied the effect of oxidized galectin-1 on LPS-induced expression of these three proinflammatory mRNAs. Relative values <1 indicated that oxidized galectin-1 inhibits LPS activity; therefore, inclusion of recombinant human oxidized galectin-1 10 ng/mL with LPS 100 ng/mL significantly inhibited the increases in mRNA expression normally induced by LPS alone. Notably, oxidized galectin-1 did not significantly change the small increase in proinflammatory mRNA production induced by LPS 10 ng/mL. The effects of oxidized galectin-1 on LPS-induced increases in expression of mRNAs encoding proinflammatory factors in macrophages were examined in this study. We found that macrophages treated with both 100 ng/mL of LPS and recombinant human oxidized galectin-1 had a lower expression of IL-1β, IL-6, and iNOS mRNA than did cells treated with LPS 100 ng/mL alone at three hours (t-test, P < 0.05), but not at two hours postculture (RT-PCR, Figure 3). These results suggest that oxidized galectin-1 dampens the inflammatory response of macrophages to LPS challenge. Corresponding samples analyzed with RT-PCR in Figures 3A, 3B and 3C were analyzed with real-time RT-PCR, and the results were shown in Figures 4A, 4B, and 4C. Each figure in Figure 4 indicates that treatment for three hours with LPS 100 ng/mL increased the expression of mRNAs encoding proinflammatory factors (t-test, P < 0.01) and that the LPS-induced increases were significantly reduced by treatment with recombinant human oxidized galectin-1 10 ng/mL (t-test, P < 0.05).
Figure 3.
Lipopolysaccharide-induced increases in macrophage expression of proinflammatory factor mRNAs are restricted by cotreatment with oxidized galectin-1 in serum-free medium by reverse transcriptase polymerase chain reaction analysis. Reverse transcriptase polymerase chain reaction analysis: Relative levels of proinflammatory mRNA expressions A) IL-1β, B) IL-6, and C) iNOS after coapplication of lipopolysaccharide and oxidized galectin-1 in the serum-free medium. A) Average values of IL-1β mRN A intensity after combinatorial treatment (100L + 10G)/100L were 0.83 ± 0.26 (mean ± standard error of the mean) at two hours and 0.81 ± 0.08 at three hours post-treatment. Oxidized galectin-1 reduced the lipopolysaccharide-induced increase in IL-1β mRN A expression at three hours (*t-test, P < 0.05, n = 6), but not two hours post-treatment. B) Average values of IL-6 mRNA intensity after combinatorial treatment (100L + 10G)/100LPS, were 0.85 ± 0.10 (mean ± standard error of the mean) at two hours and 0.70 ± 0.11 at three hours post-treatment. Oxidized galectin-1 reduced the lipopolysaccharideinduced increase in IL-6 mRNA expression at three hours (*t-test, P < 0.05, n = 6), but not two hours post-treatment. C) Average values of iNOS mRN A intensity after combinatorial treatment (100L + 10G)/100LPS were 0.77 ± 0.17 (mean ± standard error of the mean) at two hours and 0.65 ± 0.13 at three hours post-treatment. Oxidized galectin-1 reduced the lipopolysaccharide-induced increase in iNOS mRNA expression at three hours (*t-test, P < 0.05, n = 6), but not two hours post-treatment. 100L: 100 ng/mL lipopolysaccharide, 10G: 10 ng/mL oxidized galectin-1.
Abbreviations: iNOS, inducible nitric oxide synthase; Il-6, interleukin-6; IL-1β, interleukin 1β.
Discussion
In the current study, we have shown that galectin-1 mRNA is localized to gingival tissue, and that its expression is increased by P. gingivalis challenge. In addition, we found that oxidized galectin-1 reduced LPS-induced increases in IL-1β, IL-6, and iNOS mRNA in cultured macrophages.
Galectin-1 expression in gingival tissue
Although previous research has shown that galectin-1 is expressed in a variety of tissues (eg, lung, kidney, gut, nervous tissue),7,15 its expression in gingival tissue has not been examined. We found that galectin-1 mRNA is expressed in rat gingival tissues. We also showed that galectin-1 mRNA was significantly increased by challenge with P. gingivalis compared with controls. Because the subgingival accumulation of bacteria augments oral inflammation in periodontitis, the galectin-1 level may be increased to regulate inflammation, as seen in a number of models.11,16–19
Anti-inflammatory role for oxidized galectin-1
Although subgingival accumulation of bacteria is responsible for inflammation associated with periodontal disease, these bacteria do not invade affected tissues in large numbers. Bacterial release of soluble products interacts with host tissue and immune cells to elicit cytokine expression, inflammation, and onset of periodontitis. LPS is the key bacterial component that promotes upregulation of proinflammatory cytokine and iNOS mRNA.20–24 Because LPS from E. coli (E-LPS) shows similar effects to LPS from P. gingivalis (P-LPS) on gene expression of proinflammatory cytokines25 and the bioactive center, lipid A, of P-LPS induces cell activation via Toll-like receptor 4 (TLR4) common to E-LPS,26 we introduced an in vitro inflammatory model of E-LPS and cultured peritoneal macrophages. Proinflammatory factors, including IL-1β, IL-6, and iNOS, are believed to be the major pathological mediators of inflammatory periodontal disease.12,27 Therefore, we added oxidized galectin-1 in combination with LPS to cultured macrophages and studied the regulation of these proinflammatory factors, in order to establish whether oxidized galectin-1 had an effect on LPS- induced macrophage activation.
As has been shown previously,20–23 LPS 30 ng/mL elicited an increase in mRNA expression of the three inflammatory mediators of IL-1β, IL-6, and iNOS in macrophages cultured in serum-containing medium. Figure 2 shows that oxidized galectin-1 application to LPS-treated macrophages reduced the intense LPS-induced increase by serum of proinflammatory mRNA expression in a concentration-dependent manner. This LPS-induced increase in inflammatory factor mRNAs was limited when oxidized galectin-1 was included in culture, so oxidized galectin-1 has an anti-inflammatory role in this model. Our results suggest that oxidized galectin-1 may be one factor that could prevent periodontal inflammation caused by bacterial release of LPS. We then applied LPS to macrophages cultured in serum-free medium to isolate the effects of serum proteins, including LPS-binding protein. Because macrophages, which synthesize and express LPS receptor CD14, can respond to minute quantities of LPS,28 the application of LPS increased expressions of the three proinflammatory factors. Oxidized galectin-1 also dampened the LPS-induced increase in mRNA expression in the serum-free medium.
The mechanism underlying the anti-inflammatory effect of oxidized galectin-1 on LPS-induced activation has not been identified. It is plausible that oxidized galectin-1 binds to LPS to restrict LPS activity. However, oxidized galectin-1 loses lectin activity when it is oxidized,11 and therefore it is unlikely that it binds to LPS to block LPS activity. LPS acts by binding serum LPS-binding protein,28,29 which rapidly catalyzes the transfer of LPS to its receptors (eg, membrane- bound or soluble CD14). However, because oxidized galectin-1 reduces LPS activity in serum-free medium and LPS could bind to CD14 to signal transduction,16,18 oxidized galectin-1 may bind to CD14 to block LPS activity. This may be one of the possible mechanisms. The LPS receptor complexes initiate signaling cascades that lead to immune cell activation via LPS-activation receptor clusters comprised of CD14 and TLR4.28–31 The LPS-activation receptor clusters subsequently activate NF-kB, which leads to upregulation of proinflammatory factors, including IL-1β, IL-6, and iNOS.32,33 Multiple LPS activity inhibitors reduce LPS activity by inhibiting NF-kB signaling.34–37 Therefore, because oxidized galectin-1 binds to a macrophage membrane to induce changes in tyrosine phosphorylation of proteins,3 another possible mechanism is that oxidized galectin-1 may restrict the effects of LPS by inhibiting the NF-kB signaling pathway, thereby preventing upregulation of proinflammatory cytokines. These possible mechanisms remain to be clarified in future investigations.
Oxidized galectin-1 has several characteristics that seem to be important in periodontitis. Macrophages, major cellular contributors to periodontitis, are the targets of oxidized galectin-1,3 and oxidized galectin-1 inhibits LPS-induced activation of proinflammatory cytokine mRNA expression. Oxidized galectin-1 is also an endogenous protein, and works even at low concentrations of 10 ng/mL. Further examination of the anti-inflammatory effects of oxidized galectin-1 in periodontitis may enable development of useful therapy for this disease.
Conclusion
We established that galectin-1 mRNA is expressed in gingival tissues, and showed that galectin-1 mRNA is significantly increased after challenge with P. gingivalis. This indicates that galectin-1 may regulate oral inflammation. We cultured peritoneal macrophages in the presence of LPS in order to establish whether oxidized galectin-1 regulates the inflammatory response of these cells. LPS elicited IL-1ß, IL-6, and iNOS expression by macrophages, and we showed that cotreatment of macrophages with oxidized galectin-1 prevented LPS-induced increase in inflammatory factor mRNA expression in a concentration-dependent manner. Therefore, oxidized galectin-1 may work as an anti-inflammatory agent that suppresses macrophage activation in periodontal disease.
Acknowledgments
We thank Drs Takahashi and Watanabe for their help and advice with this research. This work was supported by the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohl CA, Pollack M. Lipopolysaccharide (LPS) antibodies regulate cellular uptake of LPS and LPS induced proinflammatory responses. Prog Clin Biol Res. 1998;397:227–234. [PubMed] [Google Scholar]
- 3.Horie H, Kadoya T, Hikawa N, et al. Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci. 2004;24:1673–1680. doi: 10.1523/JNEUROSCI.4483-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaudet AD, Leung M, Poirier F, Kadoya T, Horie H, Ramer MS. A role for galectin-1 in the immune response to peripheral nerve injury. Exp Neurol. 2009;220:320–327. doi: 10.1016/j.expneurol.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Okada T, Ichikawa M, Tokita Y, Horie H, Saito K, Yoshida J, et al. Intravitreal macrophage activation enables cat retinal ganglion cells to regenerate injured axons into the mature optic nerve. Exp Neurol. 2005;196:153–163. doi: 10.1016/j.expneurol.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Camby I, Mercier ML, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 7.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 8.Outenreath RL, Jones AL. Influence of an endogenous lectin substrate on cultured dorsal root ganglion cells. J Neurocytol. 1992;21:788–795. doi: 10.1007/BF01237904. [DOI] [PubMed] [Google Scholar]
- 9.Tracey BM, Feizi T, Abbott WM, et al. Subunit molecular mass assignment of 14,654 Da to the soluble beta-galactoside-binding lectin from bovine heart muscle and demonstration of intramolecular disulfide bonding associated with oxidative inactivation. J Biol Chem. 1992;267:10342–10347. [PubMed] [Google Scholar]
- 10.Horie H, Inagaki Y, Sohma Y, et al. Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J Neurosci. 1999;19:9964–9974. doi: 10.1523/JNEUROSCI.19-22-09964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki Y, Sohma Y, Horie H, Nozawa R, Kadoya T. Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. Eur J Biochem. 2000;267:2955–2964. doi: 10.1046/j.1432-1033.2000.01311.x. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MK, Reddi K, Henderson B. Cytokine-inducing components of periodonto-pathogenic bacteria. J Periodontal Res. 1996;31:393–407. doi: 10.1111/j.1600-0765.1996.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 13.Roberts FA, McCaffery KA, Michalek SM. Profile of cytokine mRNA expression in chronic adult periodontitis. J Dent Res. 1997;76:1833–1839. doi: 10.1177/00220345970760120501. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima K, Hamada N, Takahashi Y, et al. Restraint stress enhances alveolar bone loss in an experimental rat model. J Periodontal Res. 2006;41:527–534. doi: 10.1111/j.1600-0765.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 15.Akazawa C, Nakamura Y, Sango K, Horie H, Kohsaka S. Distribution of the galectin-1 mRNA in the rat nervous system; its transient up-regulation in rat facial motor neurons after facial nerve axotomy. Neurosci. 2004;125:171–178. doi: 10.1016/j.neuroscience.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Almkvist J, Karlsson A. Galectins as inflammatory mediators. Glycoconjugate J. 2004;19:575–581. doi: 10.1023/B:GLYC.0000014088.21242.e0. [DOI] [PubMed] [Google Scholar]
- 17.Gil CD, Cooper D, Rosignoli G, Perretti M, Oliani SM. Inflammation-induced modulation of cellular galectin-1 and -3 expression in a model of rat peritonitis. Inflamm Res. 2006;55:99–107. doi: 10.1007/s00011-005-0059-4. [DOI] [PubMed] [Google Scholar]
- 18.Hokama A, Mizoguchi E, Mizoguchi A. Roles of galectins in inflammatory bowel disease. World J Gastroenterol. 2008;14:5133–5137. doi: 10.3748/wjg.14.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinovich GA, Baum LG, Tinari N, et al. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23:313–320. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- 20.Benahmed M, Heymann D, Pilet P, Bienvenu J, Daculsi G. LPS increases biomaterial degradation by human monocytes in vitro. J Biomed Mater Res. 1997;34:115–119. doi: 10.1002/(sici)1097-4636(199701)34:1<115::aid-jbm15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 22.Sacco RE, Nibbelink SK, Baarsch MJ, Murtaugh MP, Wannemuehler MJ. Induction of interleukin (IL)-1beta and IL-8 mRNA expression in porcine macrophages by LPS from Serpulina hyodysenteriae. Infect Immun. 1996;64:4369–4372. doi: 10.1128/iai.64.10.4369-4372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson M. Biological activities of LPSs from oral bacteria and their relevance to the pathogenesis of chronic periodontitis. Sci Prog. 1995;78:19–34. [PubMed] [Google Scholar]
- 24.Sysmey-Durrant HJ, Hopps RM. Effect of lipopolysaccaride from Porphyromonas gingivalis on prostaglandin E2 and interleukin-1-beta release from rat periosteal and human gingival fibroblasts in vitro. Oral Microbiol Immunol. 1991;6:378–380. doi: 10.1111/j.1399-302x.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamaji Y, Kubota T, Sasaguri K, Sato S, Suzuki Y, Kumada H, et al. Inflammatory cytokine gene expression in human periodontal ligament fibroblasts stimulated with bacterial LPSs. Infect Immun. 1995;63:3576–3581. doi: 10.1128/iai.63.9.3576-3581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa T, Asai Y, Hashimoto M, et al. Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int Immunol. 2002;14:1325–1332. doi: 10.1093/intimm/dxf097. [DOI] [PubMed] [Google Scholar]
- 27.Lin SK, Kok SH, Lin LD, et al. Nitric oxide promotes the progression of periapical lesion via inducing macrophage and osteoblast apoptosis. Oral Microbiol Immunol. 2007;22:24–29. doi: 10.1111/j.1399-302X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 28.Gegner JA, Ulevitch RJ, Tobias PS. Lipopolysaccharide (LPS) signal transduction and clearance. J Biol Chem. 1995;270:5320–5325. doi: 10.1074/jbc.270.10.5320. [DOI] [PubMed] [Google Scholar]
- 29.Schumann RR, Leong SR, Flaggs GW, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 30.da Silva Correia, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. Transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 31.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 32.Kim JW, Kim C. Inhibition of LPS-induced NO production by taurine chloramine in macrophages is mediated through Ras-ERK-NF-kappaB. Biochem Pharmacol. 2005;70:1352–1360. doi: 10.1016/j.bcp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Lv N, Kim EK, Song MY, et al. JANEX-1, a JAK3 inhibitor, protects pancreatic islets from cytokine toxicity through downregulation of NF-kappaB activation and the JAK/STAT pathway. Exp Cell Res. 2009;315:2064–2071. doi: 10.1016/j.yexcr.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Liu YN, Pan SL, Peng CY, et al. Moscatilin repressed lipopolysaccaride-induced HIF-1alpha accumulation and NF-kappaB activation in murine RAW264.7 cells. Shock. 2010;33:70–75. doi: 10.1097/SHK.0b013e3181a7ff4a. [DOI] [PubMed] [Google Scholar]
- 35.Kim BH, Lee YG, Park TY, Kim HB, Rhee MH, Cho JY. Ginsenoside Rp1, a ginsenoside derivative, blocks LPS-induced interleukin-1beta production via suppression of the NF-kappaB pathway. Planta Med. 2009;75:321–326. doi: 10.1055/s-0028-1112218. [DOI] [PubMed] [Google Scholar]
- 36.Sugiyama K, Muroi M, Tanamoto K. A novel TLR4-binding peptide that inhibits LPS-induced activation of NF-kappaB and in vivo toxicity. Eur J Pharmacol. 2008;594:152–156. doi: 10.1016/j.ejphar.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 37.Wu GJ, Chen TL, Chang CC, Chen RM. Propofol suppresses tumor necrosis factor-alpha biosynthesis in LPS-stimulated macrophages possibly through downregulation of nuclear factor-kappa B-mediated toll-like receptor 4 gene expression. Chem Biol Interact. 2009;180:465–471. doi: 10.1016/j.cbi.2009.05.003. [DOI] [PubMed] [Google Scholar]