Abstract
Purpose
Poly(methyl methacrylate) (PMMA) has been widely used as a denture-base acrylic resin due to its excellent physical and mechanical properties. However, the material is highly prone to microbial fouling that often leads to Candida-associated denture stomatitis. Incorporation of phosphate groups into PMMA could facilitate adsorption of salivary antimicrobials and inhibit microbial adherence on the polymer surface. An in vitro study evaluated PMMA polymers containing varying amounts of phosphate group for their efficacy to inhibit Candida albicans adhesion, adsorb salivary histatin 5, and exhibit candidacidal activity.
Methods
Six PMMA polymers containing 0%, 5%, 15%, 10%, 20%, and 25% of phosphate group were synthesized by bead (suspension) polymerization technique using mixtures of methyl methacrylate and methallyl phosphate as monomers. The efficacy of the polymers to inhibit the adherence of C. albicans was examined by using human saliva-coated polymer beads and radio-labeled C. albicans cells, as compared with that of PMMA. The potency of the phosphated PMMA polymers to adsorb histatin 5 was determined by measuring the radioactivity of the adsorbed labeled-peptide on the polymer surface. The candidacidal activity of the histatin 5-adsorbed polymers was assessed by using the fluorescence technique. The percent release of the fluorescent probe calcein from the C. albicans membrane caused by the disruption of the cell membrane was determined. The data were analyzed statistically by one-way ANOVA followed by Scheffé’s test (α = 0.05 and n = 6).
Results
The presence of ≥15% phosphate content in PMMA significantly reduced the saliva-mediated adhesion of C. albicans. Phosphated PMMA polymers showed significantly enhanced adsorption of histatin 5 in a phosphate density-dependent manner. The candidacidal activity of the histatin 5-bound polymers increased significantly with the increase in the phosphate content of the polymer.
Conclusion
Phosphated PMMA polymers have the potential to serve as novel denture-base resins, which may reduce C. albicans colonization and prevent denture stomatitis.
Keywords: phosphated poly(methyl methacrylate) polymers, denture infection, Candida albicans adhesion, salivary antimicrobials, histatins
Introduction
Acrylic polymers have generally been used to replace missing teeth and periodontal tissues in edentulous or partially dentate patients. Poly(methyl methacrylate) (PMMA) is the most commonly used material, since it satisfies most physical, mechanical, and biocompatibility criteria for an intraoral prosthesis. However, the absence of ionic charge on the resin surface substantially reduces selective adsorption of salivary antimicrobials.1–3 Salivary antimicrobials such as defensins and histatins are cationic peptides, and they are strongly adsorbed onto the tooth surface by electrostatic interaction. 4 Absence of an anionic surface on PMMA minimizes the adsorption of defense molecules on the denture surface. This may facilitate the adherence of C. albicans and other pathogens leading to Candida-associated denture stomatitis, a complex condition with a multifactorial etiology.4–9 This chronic inflammatory condition is prevalent among prosthesis users particularly the elderly and institutionalized. Treatment of this clinical condition is problematic because of incomplete disinfection of the acrylic surface and rapid microbial recolonization.10
The absence of ionic charge on PMMA denture surface has been reported to contribute to a decline in natural salivary defense functions mediated by the tooth enamel surface, when teeth are replaced by a denture.1 PMMA modified by surface polymerization of methyl methacrylic acid, has shown that the carboxylate anionic surface enhanced the adsorption of salivary histatin 5 and inhibition of C. albicans adhesion.1 However, mere surface modified PMMA cannot retain the surface anionic charge, since it might be removed during denture fabrication and polishing.11 Therefore, incorporation of phosphate groups in PMMA may provide a natural and biocompatible anionic surface to overcome microbial adhesion and chronic inflammation.
This article describes the efficacy of a few PMMA polymers containing varying amounts of phosphate to inhibit the adhesion of C. albicans onto the polymer surface. Further, this research suggests a relationship between the phosphate content of the polymers with their efficacy to adsorb the antimicrobial histatin 5 and the candidacidal activity of the histatin 5-bound polymers.
Materials and methods
General materials and methods
Chemicals and solvents were of the highest purity available and used without further purification. Amino acid derivatives, methyl methacrylate and chemicals for the synthesis of histatin 5 were purchased from Sigma Chemical Co. (St. Louis, MO) and Bachem (Torrance, CA). Calcein-AM was obtained from Molecular Probes (Eugene, OR). Histatin 5 synthesis was carried out using a peptide synthesizer (ACT Model-90; Advanced Chemtech, Louisville, KY). High-performance liquid chromatography (HPLC) was carried out on a Rainin-HPXL system interfaced to a Macintosh SE/30 computer, using a Dynamax C18 column (10 × 250 mm; Rainin, Woburn, MA) coupled to a guard column (10 × 50 mm). Amino acid analysis was performed on a 420A derivatizer and analyzer system (Perkin- Elmer, Waltham, MA). The amino acid sequence of histatin 5 was checked by sequence analysis, using a Perkin-Elmer Model 477A Protein Sequencer. The procedure used for the collection of human submandibular sublingual secretion (HSMSL) and human whole saliva has been described previously. 1 It was approved by the Institutional Review Board of Marquette University and informed consent was obtained from all volunteers.
Synthesis of histatin 5
Salivary histatin 5 (Histatin 5: D-S-H-A-K-R-H-H-G-Y-K-R-K-F-H-E-K-H-H-S-H-R-G-Y) was synthesized by standard solid-phase procedures using 9-fluorenylmethoxy carbonyl (Fmoc) chemistry as previously described.12 It was purified by HPLC and its homogeneity was determined by analytical HPLC, sequence and amino acid analysis as reported previously.12,13 The identity of the synthetic histatin 5 was established by comparing the physical and biological properties with those of salivary histatin 5 as reported previously.12
Synthesis of methallyl phosphate monomer
Methallyl phosphate (MAP) was synthesized from methyl methacrylate (MMA) by reducing the ester function in MMA to primary alcohol with sodium borohydride in ethanol using the standard organic synthetic procedure. Briefly, methyl methacrylate (150 g) was refluxed with a three-fold molar excess of sodium borohydride (NaBH4) in 90% ethanol (200 mL) for 6 h. Ethanol was removed by rotary evaporation and the aqueous layer extracted with ethyl acetate (3 × 50 mL). Methallyl alcohol obtained as the residual liquid (130 mL) after complete evaporation of ethyl acetate was then phosphorylated by treating with ditertiarybutyl N,N-diethylphosphoramidite in the presence of 1H-tetrazole and m-chloroperbenzoic acid. The product was then treated with 95% trifluoroacetic acid to cleave the t-butyl groups as described previously.13
Synthesis of PMMA and phosphated PMMA polymers
PMMA and phosphated PMMAs were synthesized by using mixtures of methyl methacrylate (MMA) and methallyl phosphate (MAP) in various proportions as monomers [MMA:MAP = 100:0 (PMMA); 95:5 (PMMA 1); 90:10 (PMMA 2); 85:15 (PMMA 3); 80:20 (PMMA 4); 75:25 (PMMA 5)]. The bead (suspension) polymerization technique was employed.14 Briefly, in a 50 mL flask, 35 g of the monomer or the monomer mixture was stirred with 1.2 g of benzoyl peroxide (well ground). Then, 0.75 mL of dimethyl paratoluidine was added and stirred briefly. The mixture was poured into a 250 mL flask containing 1% poly(vinyl alcohol) at pH 3, and stirred well to prevent separation of two layers and the temperature was recorded. The reaction was allowed to continue for 15 min after the rise in temperature ceased. The polymer beads were filtered, washed with distilled water, and then dried.
Characterization of polymer beads
The experimental conditions such as temperature and stirring speed were maintained during polymer synthesis to obtain polymer beads of uniform size verified by X-ray diffraction. The surface area of the beads were assessed by Brunauer–Emmett–Teller nitrogen adsorption analysis described previously.13 The incorporation of phosphate in the polymers was examined by using Fourier transform infrared spectroscopy (FTIR). FTIR spectral changes in the region 1000–1200 cm−1 (P=O and P–O IR bands) provided a measure of the phosphate content of the polymers. 31P-nuclear magnetic resonance (NMR) was also employed to assess phosphate levels. A mixture of chloroform and dimethyl sulfoxide was used as solvents for spectroscopic studies as described previously.1,13
Organisms and culture conditions
C. albicans strain DS1 isolated from the palate of a denture stomatitis patient was used for this present study. This C. albicans strain possesses the surface properties to adhere to denture surface.1 The identity of the clinical isolate as C. albicans was verified by the Yeast System (Flow Laboratories, McLean, VA, USA) as described previously.12 Organisms were streaked onto Sabouraud Dextrose Agar plates (Difco Laboratories, Detroit, MI, USA), grown at 30°C and later maintained at 4°C. One colony of C. albicans from this plate was inoculated into 10 mL of sucrose-salts-biotin yeast synthetic medium and incubated for 48 h at 30°C in a shaker rotating at 200 rpm. After this period, the population of yeast cells was in the late-exponential-phase growth. This has been verified from the cell growth versus incubation time curve. Cell morphology was determined by phase contrast microscopy and the cells were found to be uniformly blastospores. Only cultures that showed the presence of blastospores were used for the assay. Subcultures were carried out every three days by inoculating 100 μL of 3 days old culture into 10 mL of fresh media. Cells were harvested by centrifugation at 1500 × g for 10 min, washed twice with 10 mM tris-buffereed saline (TBS), 10 mM tris-hydrochloride, pH 7.5, and re-suspended in TBS at a concentration of 107 cells/mL using a Petroff–Hausser counting chamber (Hausser Scientific Partnership, Horsham, PA, USA).
Measurement of adhesion of C. albicans to PMMA resins
The assays were performed using [35S]methionine labeled C. albicans as described previously.15 Previous studies have established that C. albicans do not adhere to the naked surface of PMMA polymer beads.1,15 However, HSMSL has been shown to mediate and promote C. albicans adhesion to PMMA surface.1,15 Hence, for consistency, we used HSMSL coated polymers for the assay. Briefly, each group of PMMA beads (50 mg) was hydrated in 1.5 mL polypropylene tubes and coated with 500 μL of HSMSL separately for 2 h at 4°C as described previously.1 The HSMSL coated beads (4 mg) were placed into 1.5 mL polypropylene tubes. Radiolabeled C. albicans blastospore cells (105) in 50 μL were added and incubated with an orbital shaker for 2 h at 25°C. The free cells were separated by treating with 75 μL of TBS, placed on 0.5 mL of 100% percoll and centrifuged in a microfuge for 10 min at 500 × g. Cells adhering to the beads were carried to the bottom of the tube in a pellet, while non-adherent cells remained near the top of the percoll and removed by aspiration. The pellet was transferred to scintillation vials containing 5 mL of Liquiscint and radioactivity of bound cells was measured in a Beckman LS-7000 liquid scintillation counter. Control experiments verified the consistency of the bead counts. Parallel experiments were also carried out with human whole saliva coated beads. The assay was performed by treating the polymer beads with varying cell concentrations (0.5 × 107, 1.0 × 107, 1.5 × 107, and 2.0 × 107 cells/mL). All test samples were assayed by three separate independent experiments and each experiment was performed in duplicate. To determine background binding, labeled cells were added to tubes without beads and processed as described earlier.
Assessment of adsorption of histatin 5 to PMMAs
The extent of adsorption of histatin 5 onto PMMA and phosphated PMMA polymers was determined by incubating histatin 5 (50–300 ng) with 4 mg of beads in 50 μL of phosphate-buffered saline (PBS) for 2 h at 25°C as described previously.1,13 The chloramine T method was used to label histatin 5.16 Briefly, each batch of hydrated polymer beads (4 mg) were equilibrated in 50 μL of PBS and a range of 50–300 ng of histatin 5 was added per tube. Histatin 5 was allowed to adsorb to the beads for 2 h at 25°C. Adsorption kinetics experiments showed that equilibrium was achieved in less than 2 h.1 After adsorption, the beads were separated by centrifugation in a Beckman Microfuge at 250 × g for 10 min, washed three times with 1 mL PBS to remove buffer and un-adsorbed histatin 5 and then the separated beads were measured in a γ-counter to determine the amount of histatin 5 adsorbed. For adsorption experiments, [125I] histatin 5 was used for the range 50–150 ng of histatin 5 and the extent of adsorption measured in a γ-counter. For the adsorption studies involving >150–300 ng of histatin 5, unlabeled peptide was used and the adsorption levels determined by amino acid analysis.
To determine the extent of desorption of the adsorbed histatin 5 over time, the polymer beads, after determining the adsorption, were re-suspended in 1 mL PBS and incubated at 0°C to minimize peptide degradation. The amount of adsorbed histatin 5 remaining on the beads was measured at 12 h intervals for 80 h. The sample was removed and washed in PBS and the remaining adsorbed histatin 5 that was not desorbed from the beads was measured. The percent retention of histatin 5 on the polymer beads was calculated as [(μg of adsorbed peptide/μg of added peptide)/mg of polymer] × 100.
Determination of candidacidal activity of histatin 5 adsorbed PMMAs
The cidal activity of salivary histatins and defensins has been correlated well with their efficacy to induce loss of cell membrane integrity.1,5,6,17,18 Hence, the efficacy of the peptide-bound polymer to induce disruption of C. albicans membrane and cause the release of the fluorescent probe calcein, was monitored as a measure of cidal activity as described previously.1 The fluorescence intensity (Fa) of cell suspension and polymer beads was measured at 520 nm using an excitation wavelength of 495 nm every 5 min for 1 h, then every 15 min for 10 h. The cell suspension was stirred gently for 10 s after each measurement to ensure cell-bead contact. The fluorescence intensity at 520 nm of cells and free polymer beads with no adsorbed histatin 5 measured after 15 min was taken as the background fluorescence (Fb). The total fluorescent content of the cell suspension was determined by incubating the cells at 100°C for 5 min (Ft). The minimal nonspecific leakage of fluorescence from cells alone was determined after 6 h and subtracted from that obtained from cells and histatin 5-bound polymer. The percent release of calcein was determined from the formula, % release of calcein = [(Fa−Fb)/Ft] × 100. The experiment was performed at pH 7.2, and at 37°C.
Statistical analysis
In order to assess the relevance of the results, the following sets of experimentally determined data for the five phosphated PMMAs and the control PMMA polymers were subjected to statistical analysis:
the number of C. albicans cells adhered to the HSMSL/saliva-coated polymer surface at four cell concentrations (0.5 × 107, 1.0 × 107, 1.5 × 107, and 2.0 × 107 cells/mL);
the amount of histatin 5 adsorbed onto the polymer surface at six equilibrium concentrations of histatin 5 (50, 100, 150, 200, 250, and 300 ng/50 μL); and
the percent release of calcein observed from the histatin 5-bound polymer surface over five intervals of time (200, 300, 400, 500, and 600 min).
The mean, median, and mode were calculated for each experimental polymer group. Distribution curves were analyzed for normality and one-way analysis of variance (ANOVA) and Scheffé’s Post test were used to evaluate and compare means between the polymer groups. To determine whether the differences in the magnitude of the three assayed properties of the polymers are statistically significant, a threshold significance level was set at α = 0.05.
Results
Synthesis of PMMA and phosphated PMMA
Bead-suspension polymerization technique14 using methyl methacrylate as the major monomer component and methallyl phosphate as the minor monomer component resulted in good yield of polymer beads of uniform size. The integrated intensity of 31P-NMR resonances increased linearly with increasing phosphate monomer substitution and found to be consistent with the amount of phosphate in the phosphated PMMAs. The surface area of the polymer beads was found to be consistent for all polymers (0.32 ± 0.02 m2 g−1).
Adhesion of C. albicans to PMMA and phosphated PMMAs
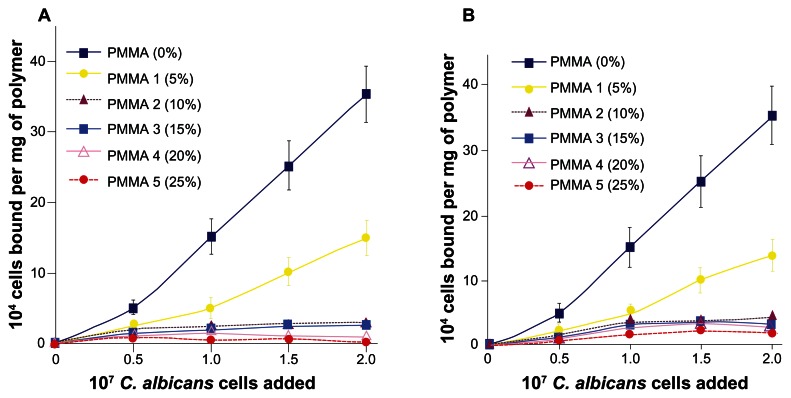
The extent of adhesion of labeled C. albicans to each polymer surface at varying concentration of cell suspension was determined using HSMSL-coated and human whole saliva-coated polymers. The results are summarized in Figure 1. The amount of cells adhered to HSMSL-coated PMMA that has no phosphate group on the surface, was significantly higher than that observed for phosphated PMMAs (Figure 1A). Uncoated PMMA and phosphated PMMA polymer beads showed only minimal adhesion of C. albicans. The adhesion of C. albicans to HSMSL-coated PMMA 1, which has only 5% phosphate content, was relatively reduced as compared to that of PMMA. The extent of inhibition of C. albicans adhesion observed for PMMA 1 was found to be statistically significant (P = 0.039) as compared to the control PMMA. When compared with the control PMMA, PMMA 2, PMMA 3, PMMA 4, and PMMA 5 with increasing phosphate content (10%, 15%, 20%, and 25%, respectively) exhibited statistically significant reduction in the adhesion of C. albicans (P < 0.001). Among PMMA 1, PMMA 2, the extent of inhibition of cell adhesion was found to be statistically significant (P < 0.05) between each other. The extent of inhibition among PMMA 3, PMMA 4, and PMMA 5 is statistically not significant (P > 0.05) between each other (15%–20% and 20%–25%). The presence of ≥15% phosphate content in PMMA substantially inhibited C. albicans adhesion. The whole saliva coated polymer beads also showed the same significant trend of decreased cell adhesion with the increased phosphate content of the polymers (Figure 1B). Collectively, the data suggest that the amount of phosphate present on the surface of the polymer diminished the HSMSL and saliva- mediated adherence of C. albicans.
Figure 1.
Adhesion of C. albicans to (A) HSMSL-coated PMMA polymer beads and (B) human whole saliva-coated PMMA polymer beads. The adhesion assay was performed as described in the Materials and methods section. The phosphate content of each polymer is indicated within parentheses. Each data point is the mean (±SD) of duplicate determinations from three separate independent experiments.
Adsorption of histatin 5 to PMMA and phosphated PMMAs
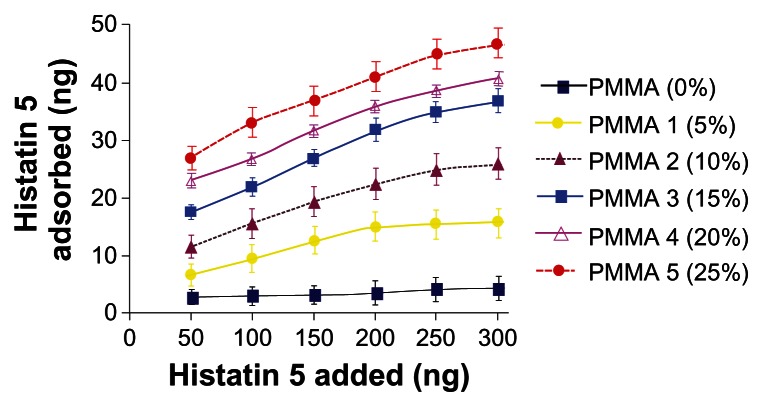
The extent of histatin 5 adsorbed onto various polymers was determined and the results are shown in Figure 2. An increase in the phosphate content of the polymer increased the adsorption of histatin 5. PMMA, which has no phosphate group, exhibited minimal adsorption of histatin 5, whereas PMMA 1–5 showed a dose dependent increase in histatin 5 adsorption (Figure 2). The extent of histatin 5 adsorption was higher and statistically significant for PMMA 1 (P < 0.001) than that observed for the control PMMA that has no phosphate group. Other phosphated polymers (10%, 15%, 20%, and 25% phosphated PMMAs) exhibited much higher adsorption of histatin 5 than that observed for the control PMMA and found to be statistically significant (P < 0.0001). The increase in the extent of adsorption observed among 5%, 10%, 15%, 20%, and 25% phosphated PMMAs was found to be statistically significant (P < 0.05) between each other (5%–10%, 10%–15%, 15%–20%, 20%–25%). Collectively, the data suggest that the adsorption of histatin 5 on the polymer surface is proportional to the phosphate content of the polymer.
Figure 2.
Adsorption of histatin 5 to PMMA and phosphated PMMA resins. Each group of polymer beads (4 mg) were incubated separately with [125I] histatin 5 (50–300 ng) in 50 μL of PBS for 2 hours at 25°C. The beads were washed three times with PBS (1 mL) and centrifuged (Beckmann Microfuge) for 10 min at 500 g to remove unadsorbed peptide. The [125I] peptide adsorbed to the beads was determined in a γ-counter. The phosphate content of each polymer is indicated within parentheses. Each data point is the mean (±SD) of duplicate determinations from three separate independent experiments.
Desorption profiles of adsorbed histatin 5 measured over a period of 80 h indicated that majority of release of histatin 5 occurred over a period of 48 h, and much smaller amounts desorbing after this time (data not shown). The desorption profile of histatin 5-adsorbed phosphated PMMA showed that 50% of the adsorbed peptide was released over the period of 4 days.
Candidacidal activity of histatin 5 adsorbed PMMA and phosphated PMMAs
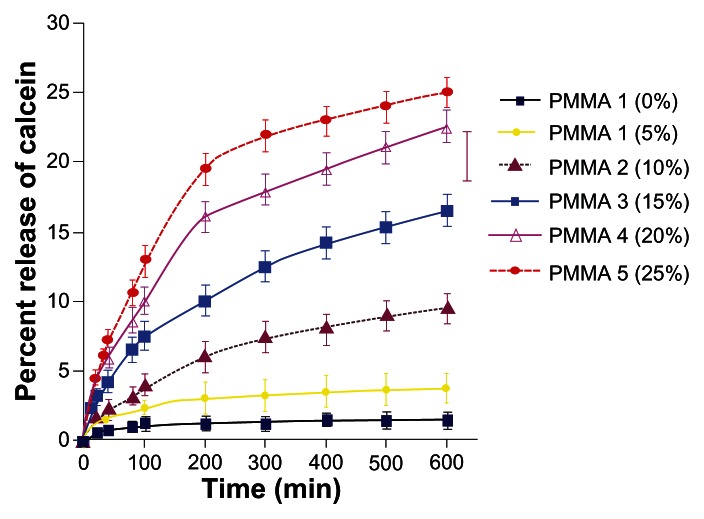
The percent release of calcein from C. albicans upon exposure to histatin 5-bound PMMA and phosphated PMMAs over time was assessed and the data are shown in Figure 3. PMMA that has no phosphate showed minimal increase in the % release of calcein over time. PMMA 1 that has 5% phosphate content exhibited a slight increase in the % release of calcein. For PMMA 2, PMMA 3, PMMA 4, PMMA 5 that have 10%, 15%, 20%, and 25% phosphate content, respectively, a significant increase in the percent release of calcein was observed (Figure 3). The measured increase in the fluorescence intensity is statistically significant and it is higher for the PMMA 1 (P = 0.00082) than that of the control PMMA. The increase in the fluorescence intensity over time observed for the polymer groups that contain 10%, 15%, 20%, and 25% was much higher than that observed for PMMA. The extent of enhancement of fluorescence observed for PMMA 2, PMMA 3, PMMA 4, and PMMA 5 was statistically significant (P < 0.0005) as compared to the control PMMA. The difference observed among the phosphated PMMA polymers is statistically significant (P < 0.05) when compared between each other (5%–10%, 10%–15%, 15%–20%, 20%–25%). The results suggest that an increase in the phosphate content of the histatin 5-bound polymer has increased the release of calcein as a consequence of the enhanced disruption of the C. albicans membrane.
Figure 3.
Candidacidal activity of histatin 5 adsorbed PMMA resins. Fluorescence intensity was measured at 520 nm using an excitation wavelength of 495 nm every 5 minutes for 60 minutes, then every 15 minutes for 600 minutes. The assays were performed at pH 7.2 and 37°C. The phosphate content of each polymer is indicated within parentheses. Each data point is the mean (±SD) of duplicate determinations from three separate independent experiments.
Discussion
The tooth enamel is composed of calcium phosphate mineral and possesses a charged ionic surface, whereas a PMMA denture surface is hydrophobic without any surface ionic charge. Salivary antimicrobials including histatins and defensins are strongly adsorbed onto the enamel surface primarily by ionic interactions, thereby serving as receptors to mediate microbial interaction and their clearance.4,5,17,19 Natural salivary defense functions mediated by the tooth enamel surface will be hampered when the tooth surface is replaced by a PMMA denture that has no ionic surface to adsorb antimicrobials. Thus, the PMMA denture surface becomes vulnerable to microbial adhesion and colonization. Surface modification of PMMA resulting in the exposure of carboxylate ions, has been reported to inhibit C. albicans adhesion to the polymer.1–3 These studies have suggested the importance of surface anionic charge to inhibit C. albicans adhesion and colonization.2,3 However, mere surface modification of PMMA might not be efficient, since much of the surface ionic charge will be removed during the fabrication and polishing of the PMMA denture. Hence, the ionic charge should be incorporated within the polymer to retain the anionic surface charge after the fabrication process.
The tooth enamel surface and the healthy oral tissues exhibit phosphate anions. Saliva is supersaturated with calcium phosphate and salivary proteins form a pellicle by effective coordination with the phosphate anions.20 Hence, it is biologically relevant to incorporate phosphate anions rather than any other anions into PMMA. In this research study, phosphated PMMA polymers have been found to efficiently inhibit HSMSL and whole saliva-mediated C. albicans adhesion to the polymer surface in a phosphate dependent manner (Figure 1). This could be attributable to the increased amounts of adsorbed salivary cationic antimicrobials onto the polymer surface. The adsorption of histatin 5 on the polymer surface increased with the phosphate content (Figure 2) that could be primarily due to the electrostatic interaction between the cationic histatin 5 and the phosphate anions on the polymer surface. The absence of anionic surface on PMMA polymers may account for the absence of salivary cationic antimicrobials such as histatins and defensins in denture pellicles.1,4 The candidacidal activity of the histatin 5-adsorbed polymers also followed the same phosphate-density dependent trend (Figure 3) presumably due to the increased amounts of adsorbed histatin 5 on the polymer surface. These findings strongly support the existence of the relationship between the phosphate content of the polymer with their efficacy to inhibit C. albicans adhesion to denture polymer surface, and to adsorb the antimicrobial histatin 5. Further, the results provide evidence for the correlation between the phosphate content of the polymer and the candidacidal activity of the histatin 5-bound polymers.
Differences in the bound amounts of salivary antimicrobial components have been reported to influence the microbial colonization of denture bases.4 The absence of phosphate anions in PMMA dental materials could minimize the selective adsorption of cationic salivary antimicrobials that could regulate and control microbial colonization. As a consequence, the protective function of the pellicle-bound denture surface may become far less efficient than it is on the normal oral surfaces that have phosphate anions. Mucins present in whole saliva and palatal saliva that underline the denture bases, have been reported to play a major role in promoting C. albicans adhesion to these surfaces that may often lead to denture-induced infection.4,15 Antimicrobial histatin 5 adsorption and the candidacidal properties observed for the phosphated PMMAs emphasize that such anionic PMMA polymers could control and minimize the C. albicans colonization of denture surface. The results suggest that the anionic phosphated polymeric materials could alter the kind and composition of the adsorbed salivary components on the denture pellicle in such a way to enhance the antimicrobial activity to control and prevent microbial colonization of denture surface.
Incorporation of phosphate anions to the extent of ≤15% in PMMA has been found to substantially inhibit C. albicans adhesion and facilitate the adsorption of histatin 5 as compared with those of the unmodified PMMA. However, it is important to make sure that the phosphated PMMA polymers maintain the physical and mechanical properties suitable for denture prostheses. As part of our continuing studies, these phosphated polymers were examined for their physical and mechanical properties and the data were published previously.21,22 These studies have shown that the incorporation of phosphate into an acrylic resin denture material by monomer substitution did not affect its impact strength, fracture toughness, and resin bonding ability to artificial teeth.22 They also suggest that physical properties such as water sorption and solubility of the phosphate incorporated polymers could remain acceptable up to the addition of 10%–20% phosphate anions.21,22 More comprehensive microbiological, mechanical, and cytotoxicity assessments and the thermal stability of the polymers are in progress to determine the potential in vivo activity and to explore the clinical utility of these materials.
Conclusion
In vitro studies provide evidence that phosphated PMMA polymers could inhibit the colonization of C. albicans and enhance the adsorption of histatin 5 and the candidacidal activity of the histatin 5-bound polymers. These phosphated PMMA polymers may serve as denture base resins that could prevent C. albicans induced denture infection. However, further studies will be required to correlate the results with the in vivo environment.
Acknowledgments
This work was supported by the United States Public Health Services Research Grants DE016925 and R21DE014565 and Marquette University ORSP Grants 19760 and 19890. We would like to acknowledge Dr. T. Karunakaran, formerly in the Department of Oral Biology, and Dr. Dinesh K. Sukumaran in the Department of Chemistry, State University of New York at Buffalo, NY, for their assistance with the microbiological and 31P-NMR data, respectively.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Edgerton M, Raj PA, Levine MJ. Surface-modified poly methyl methacrylate enhances adsorption and retains anticandidal activities of salivary histatin 5. J Biomed Mat Res. 1995;29:1277–1286. doi: 10.1002/jbm.820291015. [DOI] [PubMed] [Google Scholar]
- 2.Park SE, Periathamby AR, Loza JC. Effect of surface-charged poly(methyl methacrylate) on the adhesion of Candida albicans. J Prosthodont. 2003;12:249–254. doi: 10.1016/s1059-941x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 3.Park SE, Chao M, Raj PA. Mechanical properties of surface-charged poly(methyl methacrylate) as denture resins. Int J Dent. 2009;2009:1–6. doi: 10.1155/2009/841431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gocke R, Gerath F, von Schwanewede H. Quantitative determination of salivary components in the pellicle on PMMA denture base material. Clin Oral Invest. 2002;6:227–235. doi: 10.1007/s00784-002-0176-1. [DOI] [PubMed] [Google Scholar]
- 5.Raj PA, Dentino AR. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol Lett. 2002;206:9–18. doi: 10.1111/j.1574-6968.2002.tb10979.x. [DOI] [PubMed] [Google Scholar]
- 6.Raj PA, Dentino AR. Intraoral delivery of antimicrobials. Drug News Perspectives. 2005;18:362–374. doi: 10.1358/dnp.2005.18.6.927928. [DOI] [PubMed] [Google Scholar]
- 7.Iacopino AM, Wathen WF. Oral candidal infection and denture stomatitis: a comprehensive review. J Am Dent Assoc. 1992;23:46–51. doi: 10.14219/jada.archive.1992.0023. [DOI] [PubMed] [Google Scholar]
- 8.Ramage G, Tomsett K, Wickes BL, Lopez-Ribot JL, Redding SW. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont. 2004;98:53–59. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Samaranayake LP, Leung WK, Jin L. Oral mucosal fungal infection. Periodontol 2000. 2009;49:29–59. doi: 10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 10.Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: a review. Part 3. Treatment of oral candidosis. Aust Dent J. 1998;43:244–249. doi: 10.1111/j.1834-7819.1998.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 11.Raj PA, Venkataraman G. New and novel denture-base polymers resistant to microbial infection. J Dent Res. 2001;80:51. [Google Scholar]
- 12.Raj PA, Edgerton M, Levine MJ. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem. 1990;265:3898–3905. [PubMed] [Google Scholar]
- 13.Raj PA, Johnsson M, Levine MJ, Nancollas GH. Salivary statherin. Dependence on sequence, charge, hydrogen bonding potency, and helical conformation for adsorption to hydroxyapatite and inhibition of mineralization. J Biol Chem. 1992;267:5968–5976. [PubMed] [Google Scholar]
- 14.Phoenix RD. Denture base resins: technical considerations and processing techniques. In: Anusavice KJ, editor. Phillip’s Science of Dental Materials. Philadelphia, PA: Saunders WB Company; 1996. pp. 237–271. [Google Scholar]
- 15.Edgerton M, Scannapieco FA, Reddy MS, Levine MJ. Human submandibular-sublingual saliva promotes adhesion of Candida albicans to polymethylmethacrylate. Infect Immun. 1993;61:2644–2652. doi: 10.1128/iai.61.6.2644-2652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwood FC, Hunter WM, Glover JS. The preparation of 131I-labeled human growth hormone of high specific radioactivity. Biochem J. 1963;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raj PA, Soni S-D, Levine MJ. Membrane-induced helical conformation of an active candidacidal fragment of salivary histatins. J Biol Chem. 1994;269:9610–9619. [PubMed] [Google Scholar]
- 18.Raj PA, Marcus E, Edgerton M. Delineation of an active fragment and poly-L-proline II conformation for candidacidal activity of bactenecin 5. Biochemistry. 1996;35:4314–4325. doi: 10.1021/bi951681r. [DOI] [PubMed] [Google Scholar]
- 19.Edgerton M, Koshlukova SE, Lo TE, Chrzan BG, Straubinger RM, Raj PA. Candidacidal activity of histatins: identification of an histatin 5 binding protein on Candida albicans. J Biol Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- 20.Scannapieco FA, Levine MJ. Saliva and dental pellicle. In: Genco RJ, Goldman HM, Cohen DW, editors. Contemporary Periodontics. St. Louis, MO: Mosby Company; 1990. pp. 117–125. [Google Scholar]
- 21.Dhir G, Berzins DW, Dhuru VB, Periathamby AR, Dentino AR. Physical properties of denture base resins potentially resistant to Candida adhesion. J Prosthodont. 2007;16:465–472. doi: 10.1111/j.1532-849X.2007.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri G, Berzins DW, Dhuru VB, et al. Effect of phosphate group addition on the properties of denture base resins. J Prosthet Dent. 2008;100:302–308. doi: 10.1016/S0022-3913(08)60210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]