Summary
The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) regulates activation of the hypothalamic-pituitary-adrenal (HPA) axis and the adrenal gland in response to various stressors. We previously found that in response to acute psychological stress (restraint), elevated corticotrophin-releasing hormone (CRH) mRNA levels in the hypothalamic paraventricular nucleus (PVN) as well as elevated plasma corticosterone (CORT) were profoundly attenuated in PACAP-deficient mice. To determine whether HPA axis responses and stress-induced depressive-like behaviors in a chronic stress paradigm are affected by PACAP deficiency, we subjected mice to 14 days of social defeat stress. Defeat-exposed PACAP−/− mice showed a marked attenuation of stress-induced increases in serum CORT levels, cellular PVN ΔFosB immunostaining, and depressive-like behaviors (social interaction and forced swim tests) compared to wild-type control mice. The PACAP−/− mice showed reduced PVN FosB-positive cell numbers, but relatively elevated cell counts in several forebrain areas including the medial prefrontal cortex, after social stress. PACAP appears to be specific for mediating HPA activation only in psychological stress because marked elevations in plasma CORT after a systemic stressor (lipopolysaccharide administration) occurred regardless of genotype. We conclude that chronically elevated CORT is a key component of depressive effects of social defeat, and that attenuation of the CORT response at the level of the PVN, as well as extrahypothalamic forebrain regions, in PACAP-deficient mice protects from development of depressive behavior.
Keywords: Psychological stress, Social defeat, Depression, Stress resiliency, Forced swim test, HPA axis, Pituitary adenylate cyclase-activating polypeptide, Neuropeptide, Hypercortisolemia
1. Introduction
Psychological stressors are key contributors to affective disorders in humans. These stressors trigger neuronal activity in multiple sensory modalities in telecephalic brain regions that is integrated through interactions with stress-sensitive regions of the forebrain, hypothalamus, and para-median limbic brainstem (McEwen, 2008). The stress response activates the hypothalamic-pituitary-adrenal (HPA) axis and the hypothalamo-sympathoadrenal (HSA) axis with secretion of adrenocorticotropic hormone (ACTH) and glucocorticoids from the HPA axis and norepinephrine and epinephrine from the adrenal medulla (Goldstein and Kopin, 2008). The transduction and cellular processing of stress signals as they propagate through the stress axes are now known to be regulated, at several key anatomical loci, by neuropeptide transmitters. These molecules are typically released during chronic stimulation and, by regulating second messenger systems, have enduring effects on both gene expression levels and cell secretion patterns in response to stressors, and in response to subsequent stressful stimuli (Hokfelt et al., 2003; Stroth et al., 2011a,b). Thus neuropeptides provide a mechanism through which cells in the nervous system transduce chronic stress stimulation. Because of their role in stress transduction, neuropeptides are a viable target for pharmacological treatments that modulate the stress response.
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a pleiotropic neuropeptide that is an integral regulator in neuroendocrine stress circuits and stress response pathways. PACAP and its receptor PAC1 are highly expressed in hypothalamus, notably the paraventricular nucleus (PVN) (Hannibal, 2002). PACAP appears to be an important regulator of corticotrophin-releasing hormone (CRH) secretion from the PVN leading to ACTH secretion from the pituitary, and in turn adrenocortical secretion of corticosterone (CORT) from the adrenal cortex (Stroth et al., 2011a,b). Thus, PACAP ergic nerve terminals densely innervate the PVN (Legradi et al., 1998; Hannibal, 2002) and i.c.v. injection of PACAP near this region causes elevation of CRH mRNA (Grinevich et al., 1997). At the behavioral level, PACAP−/− and PAC1−/− mice show reduced anxiety-like behaviors (Hashimoto et al., 2001; Otto et al., 2001) whereas intra-hypothalamic infusions of PACAP have anxiogenic effects in non-stressed animals and also augment behavioral responses following emotional stress exposure (Agarwal et al., 2005; Norrholm et al., 2005). Interestingly, PACAP appears to play a more pivotal role in regulating CORT responses to prolonged than to brief stressors (Stroth and Eiden, 2010; Stroth et al., 2011a,b). Accordingly, we placed normal and PACAP−/− mice into a naturalistic chronic stress paradigm —social defeat — and measured sustained HPA axis activation and depressive-like behaviors that result in this paradigm.
Due to its ethological relevance, social defeat is a chronic stress model with high translational value. In humans, psychosocial stress is thought to play an important role in the onset of a variety of psychopathologies including depression, anxiety, and post-traumatic stress disorder (Blanchard et al., 1995, 2001; Huhman, 2006; Bremner et al., 2008). We and others have shown that as mice accumulate social defeats, profound and stable behavioral changes reminiscent of anxiety and depression occur (Kudryavtseva et al., 1991, 1999; Huhman, 2006; Krishnan et al., 2007; Schloesser et al., 2010). Such stress-related diseases are extensively associated with dysregulation of the HPA axis, including disturbances in adrenal glucocorticoid secretion and disruption of hormone feedback loops (Holsboer et al., 1984; Nemeroff and Evans, 1984; Gold and Chrousos, 1985; Pariante and Lightman, 2008; Handwerger, 2009; Wagner et al., 2011).
Since stress responses precipitated by emotional stressors appear to be PACAP-dependent, we hypothesized that PACAP−/− mice would show behavioral resiliency to repeated social defeat. We further utilized FosB/ΔFosB immunohistochemistry to map functional alterations in medial prefrontal cortex (mPFC), hypothalamus, and associated circuits to disclose how PACAP alters the brain response to chronic stress exposure and to correlate changes within these neuroanatomical circuits with changes in behavior. The long half-life of ΔFosB, a truncated splice variant of FosB that progressively accumulates in repeatedly activated neurons (Chen et al., 1997; McClung et al., 2004; Perrotti et al., 2004), allows examination of neuronal activation resulting from chronic social defeat (Berton et al., 2007; Lehmann and Herkenham, 2011). Our data reveal a linkage between PACAP’s role in sustaining prolonged HPA axis responses, activating neuronal circuits associated with emotional control, and permitting the emergence of depressive behaviors following chronic social stress.
2. Materials and methods
2.1. Animals
Male mice homozygous for deletion of PACAP (−/−) from the PrP/PACAP gene locus (Hamelink et al., 2002) were back-crossed into the C57BL/6N strain for 12 generations. At each generation, a wild-type (WT) (+/+) or hemizygous knock-out (+/−) mouse male or female mouse was bred to a commercially available C57Bl/6N, and either +/+ or +/− offspring were bred to commercially available C57Bl/6N mice. Henceforth, −/− mice were obtained by +/− × +/− breeding, followed by −/− × −/− propagation of the −/− strain. The PACAP−/− and WT mice were singly housed in a temperature- and humidity-controlled facility with 12 h light/dark cycle (lights OFF at 0900) with access to standard chow and water ad libitum. Experiments were conducted on mice 2.5—3.5 months of age.
2.2. Lipopolysaccharide (LPS) administration, restraint stress, and social defeat
Restraint and LPS challenges were as previously described (Ait-Ali et al., 2010; Stroth and Eiden, 2010). Briefly, mice were transferred from their home cages into single housing in the treatment room during the light phase of the previous day. LPS and restraint experiments were begun approx. 3 h after the next dark phase had begun. For LPS challenge, mice received 4 μl/g body weight (1 mg/kg) by i.p. injection of a 0.25 mg/ml solution in saline, of LPS from E. coli serotype 0.111: B4 (Sigma—Aldrich, St. Louis, MO), or an equivalent volume of saline (i.p.). Animals were killed by rapid decapitation 3 h later, and blood was collected.
For restraint stress, mice were individually placed in a DecapiCone (Braintree Scientific, Braintree, MA), which were slitted for ventilation, secured with tape, and placed into their homecages for 3 h prior to decapitation and blood collection. Control mice were left in their homecages.
The social defeat experiments were conducted in conflict housing as previously described (Lehmann and Herkenham, 2011). Briefly, repeated social defeat was used to induce a depressive-like phenotype in a resident-intruder paradigm. Aggressor CD-1 male mice were singly housed in a large poly-carbonate cage (24 cm × 46 cm × 15.5 cm; Lab products) for 2—4 weeks with bedding incompletely refreshed once per week. Experimental intruder C57BL/6 mice were subsequently placed into the resident CD-1 mouse’s home cage in which a perforated transparent polycarbonate partition separated the pair from tactile but not olfactory, visual, or auditory communication. After a 1-day accommodation period, the partition was removed for 5 min/day (or for 5—15 complete aggressive interactions leading to retreat by the experimental mouse) for 14 consecutive days. After each interaction period, the partition was replaced with each mouse returning to its previous side of the cage. Interaction periods were scored by direct observation under red light, with videotaping for later confirmatory analysis. Following any session in which a CD-1 mouse was not clearly (more than 60%) dominant, it was immediately replaced with a fresh resident CD-1 mouse that was housed as residents in reserve conflict cages. This invariably led to the defeat of the experimental mouse on subsequent testing days. Beginning on day 14, following the last agonist interaction session, behavioral tests (one per day) were carried out in the order listed below, starting from the light/dark box test. Mice remained in their respective housing environments during behavioral testing; although defeated mice were co-housed with the dominant CD-1, they were not exposed to agonist interactions during behavioral testing.
2.3. Behavioral procedures
Homecage Scan
PACAP−/− and WT mice were initially phenotyped in a homecage setting using Homecage Scan (CleverSys Inc., Reston, VA) for a 24 h period. Homecage monitoring consisted in placement of the individual mouse’s homecage into the HomeCageScan monitor system (CleverSys Inc) with videotaping from the side. Activities monitored included time spent grooming, time inactive, time active, locomotor activity, feeding, drinking, and jumping (single and repetitive jumping).
Following Homecage Scan, mice either continued homecage housing or were placed into conflict housing. After 14 days of conflict or homecage housing, mice were examined for anxiety-like and depressive-like behaviors. All behavioral procedures were conducted during the dark phase, between 1100 and 1500. Mice remained in their respective housing conditions during the behavioral testing period. Mice housed in conflict cages were not exposed to agonist interactions during the behavioral testing period.
Light/dark (L/D) box test
The L/D box test was conducted using a 50 cm × 25 cm × 30 cm high Plexiglas box divided into a dark (one-third of total area) and a light (40 lux illuminated) compartment separated by an open door. Time spent in the dark compartment was measured as an indicator of anxiety-like behavior, 10 min after placement in the light compartment. The floor and walls were cleaned with 70% ethanol and dried after each session.
Elevated zero maze (EZM)
The EZM test for anxiety was conducted by placing the mouse into an open quadrant of a continuous circular 5.5 cm-wide track elevated 65 cm above the floor and divided into alternating walled and open quadrants in a dimly lit room, for 6 min. Video recording from above in the last 5 min was automatically scored for time spent in each quadrant (Top Scan software suite, Clever Sys Inc.). The track and walls were cleaned with 70% ethanol and dried after each session (mouse).
Open field test (OFT)
Mice were placed into an open field box, 50 cm × 50 cm × 50 cm high, in a dimly lit room for 30 min and video recorded from above. Time spent in the center of the open field (middle 50% of the arena, 12.5 cm from walls) was measured using Top Scan software (Clever Systems) as an indicator of anxiety-like behavior. The floor and walls were cleaned with 70% ethanol and dried after each session.
Tail Suspension test (TST)
The TST consisted of securing the mouse by the tip of its tail using adhesive tape 60 cm above the floor for 6 min. The last 5 min was videotaped from the side and automatically scored for time spent immobile (no movement of hindlimbs) (Tail Suspension Scan, CleverSys Inc.)
Forced Swim test (FST)
For the FST, mice were placed in a transparent cylindrical tank (30 cm high, 10.2 cm diameter) filled to a depth of 18—22 cm with water at 28—30 °C. The last 5 min of a 6 min session was videotaped and time spent immobile automatically calculated (Forced Swim Scan, CleverSys Inc).
Social Interaction (SI) test
Social interaction was tested by placing mice in an open field 100 cm × 100 cm × 50 cm high with two equally spaced approx. 10 cm-diameter wire mesh cages, one empty and one containing the CD-1 resident mouse encountered in social defeat, or a CD-1 mouse not previously encountered (for testing of home caged control subjects). The experimental mouse can see, smell and explore the animal under the enclosure, but agonistic encounters are prevented. After placement of the subject mouse in the center of the open field, a 30 min session in which the subject mouse explored freely was videotaped and automatically scored for differential proximity to the CD-1-mouse-containing or the empty cage. To assess social interaction, we measured the time the experimental mouse spent interacting with a wire mesh enclosure containing the dominant aggressor CD1 mouse as opposed to an empty wire mesh enclosure. Sessions were videotaped and later analyzed using automated behavioral tracking software (TopScan/ObjectScan, Cleversystems, Leesburg, VA, USA). The software reliably scores the frequency and duration of sniffing on either object or mouse through identification of the animals shape including nose, body and tail. Interaction ratios were calculated using the interaction quotient (interaction duration on mouse/interaction duration on empty cup). Animals were killed by decapitation at the end of the interaction period.
2.4. Serum collection and radioimmunoassay for corticosterone
Following decapitation, trunk blood was collected into 1.5 ml tubes, allowed to coagulate at room temperature for 1 h, and then chilled on ice. Samples were subsequently centrifuged (10 min at 10,000 × g), and the supernatant was transferred to fresh tubes and re-spun, and stored at −80° C until assay. To measure the acute effects of social defeat on CORT responses, tail blood was sampled 30 min after defeat exposure on the first, seventh and fourteenth exposure. Control mice were sampled on the first, seventh, and fourteenth day of non-defeat homecage housing. Tail blood was collected into heparinized capillary tubes after snipping approximately 2 mm from the tail in unanesthetized mice. Following centrifugation, serum was stored at −80° C until assay. CORT levels were determined by radioimmunoassay (Corticosterone double antibody 125I RIA Kit, #07-120-103, MP Biochemicals, Costa Mesa, CA) according to the manufacturer’s instructions.
2.5. Histological examination
For analysis of PVN FosB/ΔFosB cellular immunostaining, a fresh cohort of PACAP−/− and wild-type mice was exposed to 14 days of chronic social defeat or homecage housing, subsequently anesthetized with an overdose of Chloropent i.p., and perfused transcardially with 15 ml of 0.9% saline, followed by 20 ml of ice-cold 4% paraformaldehyde (PFA) containing 0.1 M phosphate buffer (PB), pH 7.4. After perfusion, brains were removed immediately and postfixed for 24 h in 4% PFA at 4 °C and then submerged in 25% sucrose at 4 °C for 48 h prior to sectioning. Brains were sectioned at 30 μm on a sliding microtome. FosB/ΔFosB protein was visualized using a standard avidin—biotin horseradish peroxidase immunohistochemical method. Briefly, and as previously described (Lehmann and Herkenham, 2011), free-floating sections were washed in PBS, treated with 0.9% H2O2, blocked with 4% normal goat serum, and incubated with primary FosB antibody (1:1000; sc-48, Santa Cruz) overnight followed by application of a secondary biotinylated anti-rabbit IgG at 1:500 (Vector Labs Inc., Burlingame, CA, USA) and avidin—biotin—peroxidase complex. Color was developed using a nickel-enhanced DAB peroxidase substrate (Vector Laboratories).
Boundaries for the areas of interest were outlined using the Atlas of Franklin and Paxinos (Franklin and Paxinos, 1997) as a guide and FosB-positive cells counted using NIH Image as described previously (Stone et al., 2006a,b, 2007). The PL and IL regions of the prefrontal cortex were counted for FosB-positive nuclei bilaterally in three to four sections closest to +1.70 mm from bregma. The CG and piriform (PIR) cortices were counted for FosB-positive nuclei bilaterally in the three to four sections closest to 0.98 mm from bregma. The nucleus accumbens (Acb), including both the core and shell, was counted in the sections closest to +1.34 mm from bregma. The dorsal and ventral subdivisions of the bed nucleus of the stria terminalis (dBST and vBST) were demarcated by the anterior commisure, and FosB-positive cells were counted in the three sections closest to 0.14 mm from bregma. The PVN was counted between −0.70 and −0.94 mm from bregma. The dorsal medial hypothalamus (DMH) and ventral medial hypothalamus (VMH) was counted between −1.46 and −1.70 mm from bregma. The basolateral amygdala (BLA) was counted in sections between −0.94 and −1.22 mm from bregma. FosB-positive nuclei were counted from coronal views of the dorsal raphe (DR) in the three to four sections closest to −4.36 mm from bregma. Similarly, subdivisions of the periaqueductal gray including the dorsomedial (dm), dorsolateral (dl), lateral (l) and ventrolateral (vl) subdivisions were counted between −4.24 and −4.84 mm relative to bregma. The locus coeruleus (LC) was counted in the three to four sections closest to −5.34 mm from bregma. For nuclei counting, the Image J (NIH) program was set to accept black areas between 10 and 100 pixels at a magnification of 100 × using a common background. These parameters produced a close agreement between manual and computer-counted FosB-positive nuclei in the present sections. A blinded observer made counts for all sections.
2.6. Statistical analysis
Data for all experiments were analyzed using parametric statistics with Student’s t test or multiple factors ANOVA, as appropriate. ANOVA analysis was followed by Bonferroni multiple comparison post test. For radioimmunoassay results, differences between PACAP genotypes at each time point were analyzed by two-way ANOVA with Bonferroni posttest.
3. Results
3.1. Requirement for PACAP in HPA axis activation is stressor-specific
We examined CORT responses of WT and PACAP−/− mice exposed to either systemic or psychogenic stress (Fig. 1). Following LPS administration, both WT and PACAP−/− mice showed robust CORT responses at 3 h (F1,13 = 53.23, p < 0.0001). However, after 3 h psychogenic restraint stress exposure, significant elevation plasma CORT occurred in WT mice, but this response was significantly attenuated in PACAP−/− mice (Interaction effect F1,22 = 4.874, p < 0.04, Gene effect F1,22 = 8.66, p < 0.01, and stress effect F1,22 = 37.75, p < 0.0001). These data are consistent with previous results that CORT elevation after cold and pain stressors, generally considered to be systemic, are not PACAP-dependent, while CORT elevation after restraint stress is PACAP-dependent (Stroth and Eiden, 2010; Stroth et al., 2011a,b; Tsukiyama et al., 2011).
Figure 1.
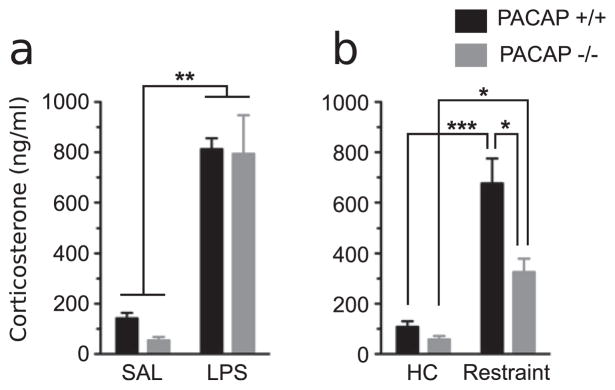
Plasma CORT 3 h after LPS administration as a model for sepsis (a), or the onset of restraint stress (b). Restraint stress significantly elevated CORT levels in PACAP −/− and WT mice but the response is significantly attenuated in PACAP −/− mice, whereas no gene effects are detected in LPS treated mice. Statistically significant differences among groups using 2-way ANOVA and a Bonferroni post-test. N = 6—8 for each group.
3.2. PACAP-deficient mice show normal appetitive behaviors and pronounced hyperactivity in a homecage environment
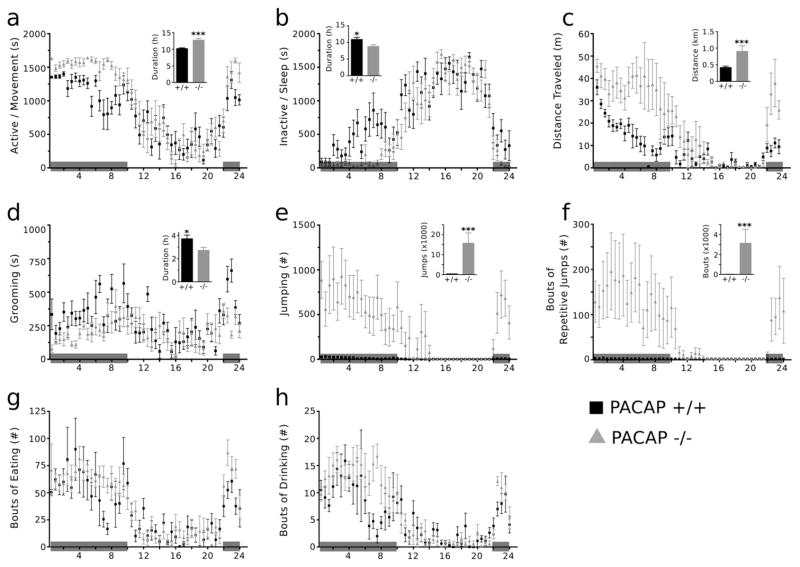
Homecage activity was examined using an automated homecage monitoring apparatus (Fig. 2) in order to better characterize the effects of PACAP expression on natural behaviors. Both mutant and WT mice displayed normal phasic responses represented by an increase in activity during the dark phase and decrease in activity during the light phase (a). PACAP−/− mice displayed a substantial increase in time spent moving (a) (t = 4.25, p < 0.0005) and distance traveled (c) (t = 4.15, p < 0.0005) during a 24 h period and a significant decrease in time spent sleeping (b) (t = 2.55, p < 0.05). PACAP−/− mice spent less time sleeping during the active (dark) phase, and that may account for differences in total time active. PACAP−/− mice also spend significantly less time grooming (d) (t = 2.45, p < 0.03) over a 24 h period compared to WT mice. No differences in total bouts of eating (g) (p > 0.4) or drinking (h) (p > 0.5) were observed between genotypes. PACAP−/− mice display robust jumping behavior (e and f) (jumps: t = 2.53, p < 0.0001; repetitive jumps: t = 2.72, p < 0.0001). Interestingly, there was a striking range of jumping behaviors (250 to >10,000 jumps/day) in PACAP−/− mice, as reflected by the large standard errors observed for jumping behaviors. Analysis of an hour-by-hour activity plot revealed that each animal displayed relatively constant levels of jumping behavior during the active dark phase. Increased locomotor activity has been reported in PACAP−/− animals, and the analysis of homecage behavior was used to balance groups by jumping behavior prior to social defeat exposure. Subsequent correlational analysis of jumping behavior and depressive behaviors revealed no significant correlations.
Figure 2.
PACAP−/− mice exhibit increased spontaneous activity in a homecage setting. PACAP −/− and WT mice were phenotyped in a homecage environment using Homecage Scan. Both mutant and WT mice displayed normal phasic responses represented by an increase in activity (a) during the dark phase and an increase in sleep behavior (b) during the light phase. PACAP−/− mice displayed marked hyperactivity shown by significant increases in total activity (a), distance traveled (c), jumping behavior (e), and bouts of repetitive jumping (f) during a 24 h period. (d) Grooming behavior is significantly reduced in PACAP−/− mice. No effects of genotype on eating (g) or drinking (h) behaviors were observed. Results are expressed as mean ± SEM (n = 12 per group). Paired t test, *p < 0.05; **p < 0.01; ***p < 0.005.
3.3. PACAP deficiency confers anxiolytic and anti-depressive effects to social defeat stress
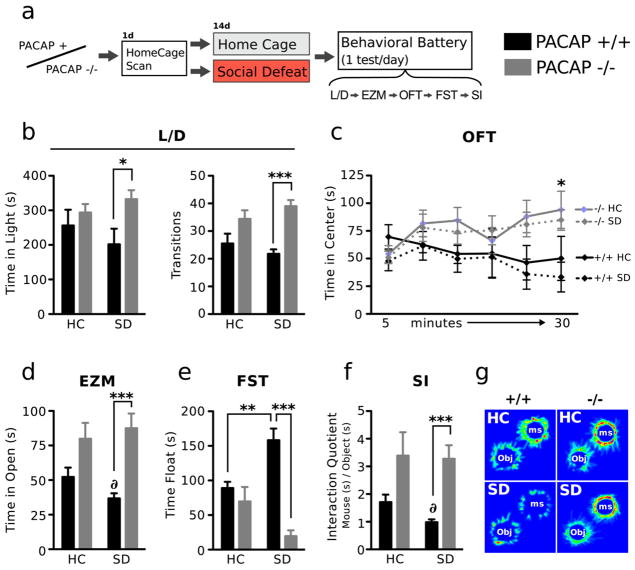
Dysregulated HPA axis activity during prolonged emotional stressors may be a causative factor in the etiology of mood disorders (McEwen, 2008). We therefore examined the impact of PACAP deficiency on the occurrence of emotional disturbances induced by chronic exposure to social defeat. PACAP−/− and WT mice were exposed to 14 days of social defeat stress or control home cage housing (experimental design is shown in Fig. 3). The effects of genotype (WT versus PACAP−/−) and stress (chronic social defeat versus single homecage housing) on a battery of behaviors were examined. In the L/D and EZM tests for anxiety two-way ANOVA (genotype × stress) revealed significant genotype effects. Genetic knockout of PACAP conferred an anxiolytic effect that was observed after social stress exposure (Bonferroni comparisons). In the L/D test, two-way ANOVA revealed a significant genotype effect for time in light compartment (F1,27 = 5.43, p < 0.03) and number of transitions between light and dark compartments (F1,38 = 22.41, p < 0.0001). In both measures defeated PACAP−/− mice showed a significant decrease in anxiety-like behaviors compared to defeated WT mice (Bonferroni comparison). The anxiolytic phenotype of PACAP−/− mice was further confirmed in the EZM (genotype effect, F1,39 = 20.20, p < 0.0001), in which defeated PACAP−/− mice spent significantly more time in the open arms compared to defeated WT mice (Bonferroni comparison). Exposure to social defeat significantly decreased open arm exploration in WT mice (t = 2.192, p < 0.05) while no effect of defeat was observed in PACAP−/− mice. In the OFT, a two-way ANOVA detected a significant genotype effect at the 30 min interval (F1,28 = 9.82, p < 0.005), and homecage housed PACAP−/− mice spent significantly more time in the open field center compared to defeated WT mice during the last 5 min of the OFT (Bonferroni comparisons). Because changes in locomotor activity can confound behavioral interpretations for the OFT we further analyzed distance traveled during the entire 30 min of the OFT, and during the last 5 min, where a significant effect of time in center was detected. Both defeated and non-defeated PACAP−/− mice showed significantly increased locomotor activity during 30 min of the OFT (Genotype effect; F1,28 = 17.2, p < 0.001, two-way ANOVA) (total distance traveled in cm; nondefeated WT 4724 ± 228.8, defeated WT 5319 ± 779.4, nondefeated PACAP−/− 6759 ± 580.1, defeated PACAP −/− 7634 ± 321.8). However this hyperactive response was not evident during the last 5 min of the OFT (two-way ANOVA for genotype, F1,28 = 1.97, p = 0.27); both PACAP−/− and WT mice displayed similar levels of locomotor activity at a time where PACAP−/− mice spent significantly more time in the center of the arena (total distance traveled in cm during the last 5 min; nondefeated WT 690 ± 129.6, defeated WT 650 ± 174.1, nondefeated PACAP−/− 771 ± 139.4, defeated PACAP −/− 853 ± 80.7). The TST and FST were used to measure behavioral despair and depressive-like behavior. We observed numerous instances of tail climbing behavior during the tail-suspension test. Indeed, mouse strains with a C57BL/6 genetic background have a tendency to climb their tail during the TST (Cryan et al., 2005). Consequently, we excluded this test from phenotypic analysis. In the FST, a robust genotype and interaction effect was detected. Defeated WT mice showed a marked increase in immobility compared to home cage housed WT and to defeated PACAP−/− mice (genotype effect, F1,39 = 26.77, p < 0.001 and genotype × stress interaction, F1,39 = 15.22, p < 0.005). Defeated PACAP−/− mice showed significantly decreased immobility time, suggesting an antidepressive effect of the gene deletion.
Figure 3.
PACAP−/− mice show enhanced behavioral resiliency to repeated social defeat. Diagram depicting experimental groups and study design (a). The anxiogenic and depressogenic qualities of SD stress were tested in the light/dark box (L/D, b), elevated zero maze (EZM, c), open field test (OFT, d), forced-swim test (FST, e), and social interaction task (SI, f, g). WT mice showed increased anxiety- and depressive-like behaviors after SD. Defeated WT mice spent more time in the dark compartment and were less likely to cross between compartments compared to defeated KO mice (b), spent significantly less time in the open arms compared to defeated WT mice (c), and showed increased immobility in the forced-swim test (e) compared with all other groups. Compared to defeated WT mice, defeated KO mice spent significantly more time and showed a significant increase in preference for interacting with the aggressor mouse (f, g). HC, homecage; SD, social defeat; Ms, Mouse; Obj, object. Results are expressed as mean ± SEM (n = 11—12 per group). For b—f, two-way ANOVA was followed by Bonferroni’s post hoc test. Bonferroni’s test: *p < 0.05; **p < 0.01; ***p < 0.005.
SI tests are proven reliable measures of social phobia and depression-like behaviors, and defeated mice typically display a pronounced avoidance of the area containing the dominant mouse. In the SI test, PACAP−/− mice displayed a striking preference for the dominant mouse, regardless of stress exposure (genotype effect, F1,40 = 18.07, p < 0.0001), whereas defeated WT mice showed a robust avoidance of the dominant mouse (t = 2.983, p < 0.005, compared to WT home cage mice).
3.4. PACAP−/− mice show attenuated CORT responses during social defeat
We characterized CORT responses to repeated social defeat in WT and PACAP−/− mice (Fig. 4). In non-stressed groups, no significant differences in homecage basal CORT were detected between genotypes. In stressed groups, a robust CORT response from both genotypes was detected 30 min after the first social defeat (stress effect, F1,38 = 23.91, p < 0.0001). A significant effect of stress was also detected on day 7 (F1,38 = 17.92, p < 0.0001) and day 14 (F1,38 = 35.43, p < 0.0001; however, the stress-induced increase was limited to defeated WT mice (Bonferroni comparison). On day 14, the CORT response to defeat in WT mice was significantly elevated compared to non-stressed controls and to stressed PACAP−/− mice (genotype × stress interaction, F1,40 = 9.82, p < 0.005).
Figure 4.
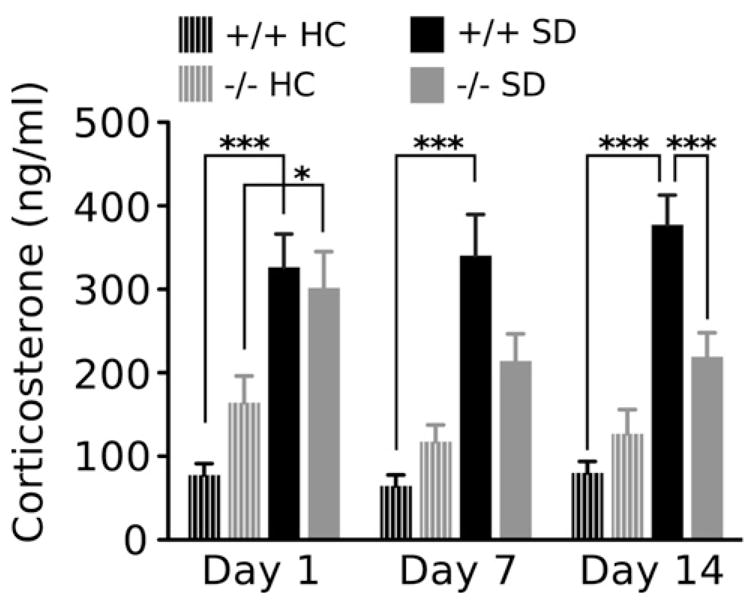
Plasma CORT responses to repeated social defeat are attenuated in PACAP −/− mice. Plasma CORT was measured in wild-type and PACAP-deficient mice exposed to single housing or chronic social defeat. CORT was measured 30 min after one, seven, and fourteen defeat exposures, and on the concurrent day of homecage housing. Results are expressed as mean ± SEM (n = 8 per HC group; n = 14 per SD group). Two-way ANOVA was followed by Bonferroni’s post hoc test. Bonferroni’s test: *p < 0.05; ***p < 0.005.
3.5. PACAP−/− mice show enhanced FosB/ΔFosB immunohistochemical expression in the medial prefrontal cortex during social defeat
The differential hormonal responses to social defeat led us to examine FosB/ΔFosB expression in stress-sensitive brain regions of WT and PACAP−/− mice housed in homecage environments or exposed to social defeat.
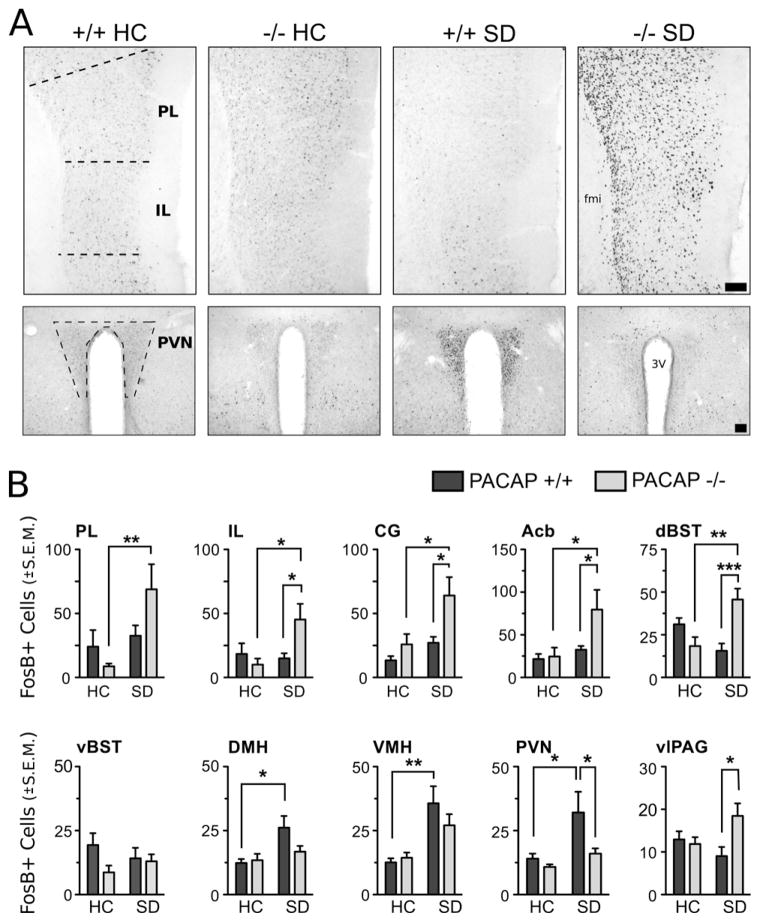
As shown in the photomicrographs and the cell-count analysis in Fig. 5 and Table 1, social defeat stress was a source of major alterations in cellular activity in all examined mPFC, striatal, and hypothalamic areas (F scores shown in Table 1). PACAP-deficient mice exhibit an increase in FosB expression, after chronic stress, in prefrontal cortex, accumbens, vlPAG and dBST. Wild-type mice exhibit no change in FosB expression in these areas after chronic stress, suggesting that endogenous PACAP is normally required to temper activity in forebrain stress-responsive circuits during chronic stress. No significant genotype or stress effects were observed in the anterior piriform cortex (PIR), suggesting that effects of SD exposure were limited to the mPFC.
Figure 5.
PACAP−/− mice show enhanced FosB/ΔFosB expression in the mPFC and attenuated expression in the PVN after social defeat. (A) Representative photomicrographs of FosB/ΔFosB immunoreactivity within mPFC and PVN showing comparative differences between non-defeated WT or PACAP−/− homecage mice and defeated WT and −/− mice. Dashed lines indicate templates within which counts were made. fmi, forceps minor corpus callosum; 3 V third ventricle. Scale bars: 100 μm. (B) Cell count analysis of FosB/ΔFosB expression. In PACAP−/− mice, SD significantly enhanced FosB/ΔFosB expression in the prelimbic (PL), infralimbic (IL), and anterior cingulate (CG) cortices, nucleus accumbens (Acb) dorsal BNST (dBST) and ventrolateral PAG (vlPAG). SD stress significantly increased FosB/ΔFosB in the dorsomedial (DMH), ventromedial (VMH), and paraventricular hypothalamic nuclei (PVN) of WT mice. Results are expressed as mean ± SEM (n = 6 per group). Two-way ANOVA followed by Bonferroni’s post hoc test. Bonferroni’s test: *p < 0.05; **p < 0.01; ***p < 0.001.
Table 1.
The expression of FosB/ΔFosB in examined brain regions of WT PACAP +/+ mice and PACAP −/− mice after 14 day exposure to homecage (HC) housing or social defeat (SD). Values are expressed as mean ± SEM (n = 6 per group). Blue UP arrows denote significant increases in FosB/ΔFosB + cells compared to within genotype HC group. F values obtained from the two-way ANOVAs for FosB/ΔFosB expression in each brain area.
| Effect of PACAP on FosB expression during SD
| |||||||
|---|---|---|---|---|---|---|---|
| Brain area | Treatment groups
|
Source of variance
|
|||||
| +/+ HC | −/− HC | +/+ SD | −/− SD | Gene (G) ANOVA F(1,20) |
Defeat stress (S) | Interaction (G × S) | |
| PL | 24.15 ± 12.89 | 8.7 ± 2.19 | 22.6 ± 8.06 |
 68.87 ± 19.61a 68.87 ± 19.61a
|
— | 6.84, p<0.02 | 4.08, p<0.05 |
| IL | 18.35 ± 8.38 | 10.08 ± 4.7 | 14.91 ± 3.98 |
 45.26 ± 12.29a 45.26 ± 12.29a
|
— | 4.03, p<0.05 | 5.72, p<0.02 |
| CG | 13.33 ± 3.26 | 25.92 ± 8.03 | 27.16 ± 4.63 |
 64.06 ± 14.31a 64.06 ± 14.31a
|
7.44, p<0.01 | 8.21, p<0.001 | — |
| Acb | 21.55 ± 5.97 | 24.61 ± 10.45 | 32.56 ± 4.36 |
 79.52 ± 23.22a 79.52 ± 23.22a
|
— | 5.69, p<0.03 | — |
| Pir | 126.12 ± 23.63 | 130.11 ± 15.6 | 127.91 ± 13.68 | 148.53 ± 11.68 | — | — | — |
| dBST | 29.21 ± 5.11 | 18.42 ± 5.22 | 17.5 ± 4.14 |
 45.63 ± 6.41a 45.63 ± 6.41a
|
— | — | 13.57, p<0.001 |
| vBST | 19.33 ± 4.64 | 8.66 ± 2.71 | 14.16 ± 4.08 | 13.00 ± 2.73 | — | — | — |
| PVN | 14.08 ± 1.93 | 10.83 ± 0.98 |
 36.11 ± 8.09a 36.11 ± 8.09a
|
16.04 ± 2.02 | 5.02, p<0.05 | 7.27, p<0.01 | — |
| DMH | 12.35 ± 1.55 | 13.42 ± 2.53 |
 26.14 ± 4.59b 26.14 ± 4.59b
|
16.79 ± 2.23 | — | 7.82, p<0.01 | — |
| VMH | 12.62 ± 1.58 | 14.38 ± 2.05 |
 35.71 ± 6.65b 35.71 ± 6.65b
|
18.08 ± 4.34 | — | 16.83, p<0.0005 | — |
| BLA | 17.66 ± 3.79 | 50.5 ± 19.46 | 68.46 ± 9.23 | 81.25 ± 13.21 | — | 9.63, p<0.005 | — |
| DR | 27.87 ± 4.88 | 15.5 ± 5.21 | 15.92 ± 4.13 | 29.67 ± 6.01 | — | — | 6.08, p<0.05 |
| LC | 19.30 ± 2.48 | 31.00 ± 7.06 | 10.75 ± 2.39 | 19.05 ± 4.42 | — | 4.69, p<0.05 | — |
| dmPAG | 13.43 ± 5.57 | 16.0 ± 6.02 | 13.31 ± 5.03 | 17.22 ± 2.47 | — | — | — |
| dlPAG | 14.42 ± 3.89 | 13.11 ± 3.88 | 12.81 ± 0.99 | 6.53 ± 2.24 | — | — | — |
| LPAG | 23.77 ± 7.59 | 18.57 ± 3.53 | 13.53 ± 3.17 | 19.92 ± 2.28 | — | — | — |
| vlPAG | 12.95 ± 1.89 | 11.86 ± 1.61 | 9.04 ± 2.13 |
 18.46 ± 2.92c 18.46 ± 2.92c
|
— | — | 5.54, p<0.03 |
Significant vs all other groups.
Significant vs hc.
Significant vs +/+ sd.
The mPFC provides predominately inhibitory control of the PVN response to stress through indirect projections that are relayed through the BNST. Significant increases FosB/ΔFosB within the dorsal BNST were observed only in PACAP−/− mice exposed to SD, an expression pattern that closely mirrors that of the mPFC.
We examined FosB/ΔFosB expression in the PVN, a major effector of the HPA-stress response pathway, and in the DMH and VMH. Social defeat stress significantly altered cellular activity in all three hypothalamic nuclei. However, and in striking contrast to the excitatory effects of social defeat on FosB/ΔFosB expression in the mPFC of PACAP−/− mice, the effects of social defeat on FosB expression were observed only in WT mice. Remarkably, and in the opposite direction from its effects on the limbic cortical areas, defeated PACAP−/− mice showed no significant increase in FosB expression within the hypothalamus and instead showed levels of activity comparable to non-defeated control groups.
We further examined FosB/ΔFosB expression in limbic and brainstem regions shown to be responsive to social stress (Berton et al., 2007). A strong stress effect was observed in the BLA. Whereas SD significantly increased FosB/ΔFosB expression in wild-type mice, no effect was observed in PACAP−/− mice. Non-defeated PACAP−/− mice show an increased, but not significant rise, in FosB/ΔFosB compared to WT mice that may explain the lack of stress effect in the knockouts. In the DR, a strong interaction effect was observed, but Bonferroni post hoc tests revealed no significance difference between groups. In the more rostral portions of the DR, an area corresponding to the ventrolateral periaqueductal gray (vlPAG), a robust increase in FosB/ΔFosB expression was observed in defeated PACAP−/− mice only. This effect was highly specific to the vlPAG — no significant effects of stress or genotype were detected in other regions of the PAG. Lastly, a significant gene effect was detected in the LC, but post hoc tests revealed no significance between groups.
4. Discussion
The present experiments provide evidence for PACAP’s involvement in modulating HPA responses to psychological but not physiological stressors by showing that PACAP deficiency blunts the CORT response and promotes resilience to both acute restraint and chronic defeat stress but does not protect against HPA axis activation induced by LPS. The data also show that changes in levels of FosB-positive neurons in the forebrain and hypothalamus and of plasma CORT after social defeat are concomitant with changes in mood, and all measures are affected in parallel by PACAP deletion.
PACAP is extensively distributed in stress-related centers of the rodent brain (Hannibal, 2002) including the PVN, BNST, and amygdala, and it has been implicated in the pathophysiology of stress adaptation (Hashimoto et al., 2009; Pinhasov et al., 2011; Ressler et al., 2011). These observations have led to numerous studies characterizing phenotypic alterations conferred by PACAP gene deletion, and work from several laboratories has demonstrated an involvement of PACAP in the manifestation of affective behavior using PACAP−/− mice bred on a CD-1 background. Mice from these studies display a strong anxiolytic phenotype (Hashimoto et al., 2001; Girard et al., 2006; Gaszner et al., 2012), and in fact deletion of PACAP has been reported to result in a depressive phenotype in some mouse backgrounds (Hashimoto et al., 2009; Gaszner et al., 2012). In contrast to these studies, our observations show that PACAP−/− mice bred on a C57BL/6 background exhibit no significant alterations in affective behaviors after homecage housing. These rather striking phenotypic differences may be due to the different background strains that were utilized: this critical point will require resolution if the C57Bl/6N PACAP knockout mouse is to be used widely as an acute/chronic stress model (Kudryavtseva et al., 1991). A wealth of information has demonstrated that genotypic differences in the inbred strains used to generate targeted mutant mouse lines can affect the behavioral phenotype of the mutant line, including anxiety profiles. Indeed, strain differences in murine anxiety paradigms affect not only the outcome of experiments with mouse mutants but also pharmacological and lesion studies in all animals (Bouwknecht and Paylor, 2008).
PACAP-deficient mice exhibit prominent behavioral abnormalities, including hyperactivity and robust jumping behavior (Hashimoto et al., 2001) that could potentially affect the outcomes of other behavioral tests for either anxiety or depression. However, a complete phenotypic analysis of PACAP−/− mice in a natural non-stressful habitat has not been done. Therefore, homecage activity was examined in an unbiased manner using a home cage monitoring system, in order to better characterize the effects of PACAP expression on natural behaviors. PACAP-deficient C57Bl/6N mice exhibited repetitive jumping and hyperactivity as monitored in a homecage environment, similar to hyperactivity and jumping observed in test cage environments by others (Hashimoto et al., 2001; Girard et al., 2006; Gaszner et al., 2012), but did not show any other significantly altered home cage behaviors. Thus it appears unlikely that the major findings of resilience to depressogenic effects of social stress reported here can be interpreted as secondary to differences in locomotor or jumping behavior between wild-type and PACAP-deficient mice.
Importantly, PACAP−/− mice displayed a robust resiliency to the deleterious effects of social defeat. This is the first study demonstrating that PACAP gene deletion confers behavioral protection to chronic social defeat. Social defeat induces prolonged maladaptive changes in rodent HPA axis activity (Ruis et al., 1999; Buwalda et al., 2001; Keeney et al., 2006) and dysregulation of this axis is extensively described in the pathophysiology of stress-related disorders (Gold and Chrousos, 2002). We hypothesize that PACAP−/− mice fail to develop maladaptive behaviors after social defeat because they fail to maintain elevated CORT during the two weeks of social stress. This hypothesis is consistent with known effects of PACAP on CRH regulation in PVN and it is consistent with previously reported depressive effects of chronic elevation of CORT in rodents. For instance, chronic exogenous CORT administration is sufficient to induce depressive-like changes in behavior (Zhao et al., 2008, 2009), whereas the glucocorticoid receptor antagonist mifepristone (RU-486) (Wu et al., 2007) or adrenalectomy (Goshen et al., 2008) block behavioral alterations induced by chronic mild stressors. These reports are complemented by recent studies that utilize genetic manipulation of HPA axis regulatory feedback mechanisms to reduce CORT secretion after social defeat, which results in protection from deleterious behavioral effects of stress (Wagner et al., 2011; Hartmann et al., 2012).
Numerous lines of evidence implicate PACAP as a pivotal regulator of HPA-axis activity following emotional stress exposure. For instance, CRH mRNA and CORT elevation are attenuated in PACAP−/− mice after restraint stress (Stroth and Eiden, 2010; Tsukiyama et al., 2011) whereas i.c.v. PACAP injection stimulates PVN CRH neurons and elevates plasma corticosterone levels (Agarwal et al., 2005). Furthermore, microinjection of PACAP into the PVN has anxiogenic effects in non-stressed animals, and enhances behavioral responses after restraint stress (Norrholm et al., 2005). These findings coupled with the current results suggest an intimate relationship between adrenal secretion and the development of depressive symptoms and implicate PACAP as a pivotal molecule in the induction of HPA-axis dysregulation during chronic psychological stress. PVN responses to systemic stressors on the other hand, such as hypoglycemia, are clearly regulated via non-PACAPergic control mechanisms, most notably noradrenergic innervation of the hypothalamus (Ritter et al., 2003; Khan et al., 2011). The current data linking PACAP to psychogenic stress, and previous work on the critical importance of norepinephrine in systemic stress (Watts, 2010), highlight the existence of two parallel systems for HPA activation in psychological versus systemic stress that converge on the PVN.
Social defeat failed to elevate FosB/ΔFosB expression in the PVN of PACAP−/− mice, consistent with a role for PACAPergic innervation of PVN in mediating psychogenic stress-specific HPA axis response. FosB/ΔFosB expression in certain extrahypothalamic brain regions associated with psychogenic stress processing and regulation, although unaffected by chronic stress in wild-type mice, was actually elevated in PACAP−/− mice. Thus, FosB/ΔFosB activity levels in the mPFC, BNST, and Acb, unaffected by chronic stress in WT mice, were elevated after chronic stress in PACAP−/− mice. It is therefore likely that there is a PACAP ergic contribution to the psychological stress response beyond the hypothalamus, in brain PACAP/PAC1-rich areas such as the mPFC and BNST that are functionally connected with the PVN (Hammack et al., 2009, 2010; Gaszner et al., 2012). Supporting the likelihood of an extra-hypothalamic effect of PACAP on behavior, we show here that PACAP deficiency alters levels of defeat-induced FosB/ΔFosB-marked activity in numerous limbic-associated brain areas. The important role of forebrain activity is underscored by the failure of PACAP to be involved in modulating the effect of a systemic stressor (LPS), which involves ascending pathways to the PVN from visceroceptive areas in the medulla (Li et al., 1996). We previously demonstrated that FosB/ΔFosB expression within the PVN correlates strongly with stress resiliency, and mice resistant to social defeat showed attenuated FosB/ΔFosB expression in the PVN coupled with enhanced expression in the mPFC (Lehmann and Herkenham, 2011). PACAP−/− mice showed the same labeling pattern, suggesting that PACAP-containing circuits normally suppress cortical activation and sustain HPA axis activation and elevated plasma CORT, thereby permitting the emergence of maladaptive behaviors following chronic social stress.
Psychogenic stressors such as social defeat result in adrenocortical and adrenomedullary hormone secretion that are controlled by complex central circuitry in stress-sensitive regions of the limbic forebrain, hypothalamus, and brainstem, and the responses are transmitted through limbic stress pathways (Herman et al., 2003; Stone et al., 2006a,b, 2008a,b, 2012). The mPFC connects with other limbic cortical and brainstem areas associated with stress neuro-circuitry and plays a major role in the inhibition of HPA responses following emotional stressors. For example, direct implants of CORT into the mPFC decrease stress-induced ACTH and corticosterone secretion following repeated restraint stress (Diorio et al., 1993). Lesions of the dorsal mPFC (prelimbic cortex) enhance HPA responses to restraint stress (Radley et al., 2006), and lesions of the ventral mPFC (infralimbic cortex) enhance FosB expression in the PVN during social defeat (Lehmann and Herkenham, 2011). Our current observations show that enhanced FosB expression in both prelimbic and infralimbic cortices is strongly correlated with attenuated FosB accumulation in the PVN and reduced CORT secretion following chronic social defeat. Anatomical studies indicate that the prefrontal cortex does not directly innervate the PVN, but fibers from the prelimbic, infralimbic, and anterior cingulate cortex innervate several predominantly GABAergic PVN-projecting regions, including the BNST (Sesack et al., 1989; Vertes, 2004; Radley, 2012). Indeed FosB labeling in the mPFC strongly parallels that within the dBNST. Thus, our current observations suggest that heightened mPFC activity projects through the dBNST to attenuate PVN activity during social defeat. Interestingly, this expression pattern is manifested only in PACAP−/− mice. The continued activation of the mPFC and dBNST during social defeat may explain the decreased anxiety and depressive-like behaviors observed in PACAP−/− mice. However, due to limitations in study design, it is difficult to interpret whether changes in mPFC FosB activity decrease PVN output or whether reduced PVN output in PACAP−/− mice is responsible for enhanced mPFC activity.
The prelimbic and infralimbic cortices also project to the amygdaloid complex and raphe nuclei and may thereby modulate HPA activation mediated by these structures. The genotype and stress effects on FosB expression were not as strong in these regions as they were in the prefrontal cortex, therefore interpretation is limited. However, a significant interaction effect was detected within a highly specific region of the ventrolateral PAG. The vlPAG is known to receive PACAP-containing fibers (Hannibal, 2002). The vlPAG been characterized previously as an important neural substrate for passive coping responses (Bandler and Shipley, 1994), and FosB/ΔFosB expression within this area is highly correlated with behavioral resiliency to social defeat (Berton et al., 2007). Neurons in the vlPAG are known to send their principal ascending projections to the Acb and the amygdala (Li et al., 1990). These are areas involved with promoting active defense responses to social defeat, an adaptive behavior observed in behaviorally resilient mice (Berton et al., 2006, 2007; Schloesser et al., 2010). The increased FosB expression within these regions coupled with decreased behavioral despair observed in defeated PACAP−/− mice implicate PACAP as a mediator in shifting defensive responses from active to passive.
The results reported here allow the generation of the working hypothesis that a central PACAP-dependent mechanism operating in the forebrain, hypothalamus, and midbrain stimulates PVN CRH release and compensatory biosynthesis in response to social defeat. The lack of emergence of depressive behaviors after social stress in PACAP−/− mice suggests that HPA axis activation may be sufficient for development of depressive-like effects of chronic social defeat. If so, pharmacological antagonism of PACAP’s action during periods of chronic social stress could mitigate the development of such behaviors, and as such might offer a less direct and more psychogenic stress-specific alternative to antagonism of CRH signaling as a therapeutic target in melancholic depression.
5. Contributors
Michael Lehmann contributed to experimental design, performed behavioral and immunohistochemical experiments, analyzed data and wrote the paper. Tomris Mustafa contributed to experimental design, performed LPS and restraint stress experiments, ran assays, analyzed data and wrote the paper. Adrian Eiden performed immunochemical experiments and analyzed data. Miles Herkenham contributed to experimental design, analyzed data, and wrote the paper. Lee Eiden contributed to experimental design, performed behavioral, LPS and restraint stress experiments, analyzed data and wrote the paper.
Acknowledgments
6. Role of funding source
This work was supported as a part of NIMH Projects Z01-MH001090 and Z01-002386.
The authors thank Eric Holaday and Dennisse Jimenez for technical support.
Footnotes
7. Conflict of interest
None declared.
References
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Ali D, Stroth N, Sen JM, Eiden LE. PACAP-cytokine interactions govern adrenal neuropeptide biosynthesis after systemic administration of LPS. Neuropharmacology. 2010;58:208–214. doi: 10.1016/j.neuropharm.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Berton O, Covington HE, III, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Mitnick N, Taylor AE, Loos WR, Buckley TC. The impact of severity of physical injury and perception of life threat in the development of post-traumatic stress disorder in motor vehicle accident victims. Behav Res Ther. 1995;33:529–534. doi: 10.1016/0005-7967(94)00079-y. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Biotechnol Prog. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Pitfalls in the interpretation of genetic and pharmacological effects on anxiety-like behaviour in rodents. Behav Pharmacol. 2008;19:385–402. doi: 10.1097/FBP.0b013e32830c3658. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, Felszeghy K, Horvath KM, Nyakas C, de Boer SF, Bohus B, Koolhaas JM. Temporal and spatial dynamics of corticosteroid receptor down-regulation in rat brain following social defeat. Prog Biotechnol. 2001;72:349–354. doi: 10.1016/s0031-9384(00)00414-5. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- Gaszner B, Kormos V, Kozicz T, Hashimoto H, Reglodi D, Helyes Z. The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger–Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience. 2012;202:283–299. doi: 10.1016/j.neuroscience.2011.11.046. [DOI] [PubMed] [Google Scholar]
- Girard BA, Lelievre V, Braas KM, Razinia T, Vizzard MA, Ioffe Y, El Meskini R, Ronnett GV, Waschek JA, May V. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 2006;99:499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Clinical studies with corticotropin releasing factor: implications for the diagnosis and pathophysiology of depression Cushing’s disease, and adrenal insufficiency. Psychoneuroendocrinology. 1985;10:401–419. doi: 10.1016/0306-4530(85)90080-0. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul. 2008;42:111–119. [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Fournier A, Pelletier G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus. Brain Res. 1997;773:190–196. doi: 10.1016/s0006-8993(97)01011-1. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE. Pituitary adenylate cyclase activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci USA. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci. 2010;42:327–340. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger K. Differential patterns of HPA activity and reactivity in adult posttraumatic stress disorder and major depressive disorder. Harv Rev Psychiatry. 2009;17:184–205. doi: 10.1080/10673220902996775. [DOI] [PubMed] [Google Scholar]
- Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, Hausch F, Rein T, Schmidt U, Touma C, Cheung-Flynn J, Cox MB, Smith DF, Holsboer F, Muller MB, Schmidt MV. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62:332–339. doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Hashimoto R, Shintani N, Tanaka K, Yamamoto A, Hatanaka M, Guo X, Morita Y, Tanida M, Nagai K, Takeda M, Baba A. Depression-like behavior in the forced swimming test in PACAP-deficient mice: amelioration by the atypical antipsychotic risperidone. J Neurochem. 2009;110:595–602. doi: 10.1111/j.1471-4159.2009.06168.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, Sakaue M, Miyazaki J-i, Niwa H, Tashiro F, Yamamoto K, Koga K, Tomimoto S, Kunugi A, Suetake S, Baba A. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proc Natl Acad Sci USA. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Bartfai T, Bloom F. Neuropeptides:opportunities for drug discovery. Lancet Neurology. 2003;2:463–472. doi: 10.1016/s1474-4422(03)00482-4. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Von Bardeleben U, Gerken A, Stalla GK, Muller OA. Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor in depression. N Engl J Med. 1984;311:1127. doi: 10.1056/NEJM198410253111718. [DOI] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Khan AM, Kaminski KL, Sanchez-Watts G, Ponzio TA, Kuzmiski JB, Bains JS, Watts AG. MAP kinases couple hindbrain-derived catecholamine signals to hypothalamic adrenocortical control mechanisms during glycemia-related challenges. J Neurosci. 2011;31:18479–18491. doi: 10.1523/JNEUROSCI.4785-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Lipina TV, Koryakina LA. Effects of haloperidol on communicative and aggressive behavior in male mice with different experiences of aggression. Pharmacol Biochem Behav. 1999;63:229–236. doi: 10.1016/s0091-3057(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Legradi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neurosci Lett. 1998;246:145–148. doi: 10.1016/s0304-3940(98)00255-9. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31:6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci USA. 1996;93:2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Rao ZR, Shi JW. Midbrain periaqueductal gray neurons with substance P- or enkephalin-like immunoreactivity send projection fibers to the nucleus accumbens in the rat. Neurosci Lett. 1990;119:269–271. doi: 10.1016/0304-3940(90)90850-9. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Evans DL. Correlation between the dexa-methasone suppression test in depressed patients and clinical response. Am J Psychiatry. 1984;141:247–249. doi: 10.1176/ajp.141.2.247. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Das M, Legradi G. Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN) Regul Pept. 2005;128:33–41. doi: 10.1016/j.regpep.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhasov A, Nesher E, Gross M, Turgeman G, Kreinin A, Yadid G. The role of the PACAP signaling system in depression. Curr Pharm Des. 2011;17:990–1001. doi: 10.2174/138161211795589328. [DOI] [PubMed] [Google Scholar]
- Radley JJ. Toward a limbic cortical inhibitory network: implications for hypothalamic-pituitary-adrenal responses following chronic stress. Front Behav Neurosci. 2012;6:7. doi: 10.3389/fnbeh.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepi-nephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–1367. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psycho-neuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D. Depressive behavior in mice due to immune stimulation is accompanised by reduced neural activity in brain regions involved in positively motivated behavior. J Biol Psychiatry. 2006a;60:803–811. doi: 10.1016/j.biopsych.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D. Reduced evoked fos expression in activity-related brain regions in animal models of behavioral depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1196–1207. doi: 10.1016/j.pnpbp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Quartermain D. A final common pathway for depression? Progress toward a general conceptual framework. Neurosci Biobehav Rev. 2008;32:508–524. doi: 10.1016/j.neubiorev.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y. Toward the rapid treatment of depression by selective inhibition of central stress circuits. Open Neuropsychopharmacol J. 2012;5:9–17. [Google Scholar]
- Stone EA, Yan L, Ahsan MR, Lehmann ML, Yeretsian J, Quartermain D. Role of CNS alpha1-adrenoceptor activity in central fos responses to novelty. Synapse. 2006b;59:299–307. doi: 10.1002/syn.20243. [DOI] [PubMed] [Google Scholar]
- Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Ann N Y Acad Sci. 2011a;1220:49–59. doi: 10.1111/j.1749-6632.2011.05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Liu Y, Aguilera G, Eiden LE. Pituitary adenylate cyclase-activating polypeptide (PACAP) controls stimulus-transcription coupling in the hypothalamic-pituitary-adrenal axis to mediate sustained hormone secretion during stress. J Neuroendocrinol. 2011b;23:944–955. doi: 10.1111/j.1365-2826.2011.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama N, Saida Y, Kakuda M, Shintani N, Hayata A, Morita Y, Tanida M, Tajiri M, Hazama K, Ogata K, Hashimoto H, Baba A. PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress. 2011;14:368–375. doi: 10.3109/10253890.2010.544345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wagner KV, Wang XD, Liebl C, Scharf SH, Muller MB, Schmidt MV. Pituitary glucocorticoid receptor deletion reduces vulnerability to chronic stress. Psychoneuroendocrinology. 2011;36:579–587. doi: 10.1016/j.psyneuen.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Watts AG. Neuroendocrine Parvocellular Neurons. Encyclopedia of Life Sciences. John Wiley and Sons, Ltd; Chichester: 2010. [Google Scholar]
- Wu LM, Han H, Wang QN, Hou HL, Tong H, Yan XB, Zhou JN. Mifepristone repairs region-dependent alteration of synapsin I in hippocampus in rat model of depression. Neuropsychopharmacology. 2007;32:2500–2510. doi: 10.1038/sj.npp.1301386. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ma R, Shen J, Su H, Xing D, Du L. A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol. 2008;581:113–120. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xie W, Dai J, Wang Z, Huang Y. The varying effects of short-term and long-term corticosterone injections on depression-like behavior in mice. Brain Res. 2009;1261:82–90. doi: 10.1016/j.brainres.2008.12.083. [DOI] [PubMed] [Google Scholar]