Abstract
Insulin signalling is uniquely required for storing energy as fat in humans. While de novo synthesis of fatty acids and triacylglycerol occurs mostly in liver, adipose tissue is the primary site for triacylglycerol storage. Insulin signalling mechanisms in adipose tissue that stimulate hydrolysis of circulating triacylglycerol, uptake of the released fatty acids and their conversion to triacylglycerol are poorly understood. New findings include (1) activation of DNA-dependent protein kinase to stimulate upstream stimulatory factor (USF)1/USF2 heterodimers, enhancing the lipogenic transcription factor sterol regulatory element binding protein 1c (SREBP1c); (2) stimulation of fatty acid synthase through AMP kinase modulation; (3) mobilisation of lipid droplet proteins to promote retention of triacylglycerol; and (4) upregulation of a novel carbohydrate response element binding protein β isoform that potently stimulates transcription of lipogenic enzymes. Additionally, insulin signalling through mammalian target of rapamycin to activate transcription and processing of SREBP1c described in liver may apply to adipose tissue. Paradoxically, insulin resistance in obesity and type 2 diabetes is associated with increased triacylglycerol synthesis in liver, while it is decreased in adipose tissue. This and other mysteries about insulin signalling and insulin resistance in adipose tissue make this topic especially fertile for future research.
Keywords: Adipose, Fatty acids, Insulin resistance, Lipogenesis, Lipolysis, Obesity, Review, Triacylglycerol
Introduction
Insulin signalling and its impairment in obesity and type 2 diabetes is a vast field that commands the full attention of many hundreds of laboratories worldwide. Scientific output has been extremely prolific, making it unlikely that any investigator has actually read all the relevant literature, which includes 25,082 publications listed in PubMed under the topic ‘insulin signaling’ and 67,013 papers listed under the heading ‘insulin resistance’. Review articles that discuss insulin resistance number 15,711, as of August 2012. Also, for many of the most interesting findings, separating fact from fiction will take years for confirmatory studies to be reported and controversies resolved. These realities create a huge challenge for scientists trying to understand insulin signalling mechanisms and their dysfunctions in metabolic disease, especially for those who are now just entering the field.
One approach to this scientific challenge is to focus on a small but critical corner of the puzzle. Such a niche area in the study of insulin signalling is lipid storage, dramatically highlighted in the images of the first diabetic patients treated with insulin in 1922, who were transformed from emaciated to plump within a few weeks [1]. At the cellular and molecular levels, insulin’s actions indeed coordinately enhance the synthesis of triacylglycerol, the central currency of stored lipid in humans. While hepatocytes are a principal site of de novo lipogenesis (synthesis of fatty acid) as well as overall lipogenesis (esterification of fatty acid with glycerol 3-phosphate to form triacylglycerol) [2], adipocytes are the principal site of triacylglycerol storage in healthy individuals [3]. Importantly, deficits in adipocyte capacity for increasing triacylglycerol deposition, for example in human lipodystrophies and obesity, contribute to systemic lipid overload and ‘lipotoxicity’, which in turn are thought to disrupt whole body glucose tolerance [4]. Thus, we focus in this review on insulin signalling and its dysfunctions specifically in relation to adipocyte triacylglycerol sequestration, recognising this topic’s broader implications for understanding the pathophysiology of obesity and type 2 diabetes.
Adipose lipid storage capacity modulates systemic insulin sensitivity
Increased circulating fatty acids and triacylglycerol are strongly correlated with impaired insulin signalling and glucose intolerance in obesity and type 2 diabetes [5, 6]. Indeed, dysfunctional lipid metabolism has been highlighted as the primary defect in the aetiology of metabolic disease [7]. Furthermore, the accumulation of fat in non-adipose tissue (e.g. liver and muscle) has been described as a strong predictor of type 2 diabetes mellitus, although the molecular mechanisms by which lipids contribute to insulin resistance in these tissues is still unclear (for review, see [8, 9]). Systemic insulin resistance includes impaired suppression of hepatic gluconeogenesis by insulin and attenuated insulin stimulation of skeletal muscle and adipocyte glucose uptake. Inducing hypertriacylglycerolaemia or high circulating NEFA levels in human volunteers using lipid emulsion infusions can cause systemic insulin resistance as measured by hyperinsulinaemic clamps [10–13]. In addition, incubating cells with high concentrations of lipids, primarily palmitate, demonstrates negative effects of increased circulating lipids on insulin action in peripheral tissues [14–16]. However, a contrary viewpoint is that circulating NEFA levels are minimally elevated in human obesity, and more work is required in this area [17].
Evidence that adipose triacylglycerol storage capacity is a critical factor in elevated circulating triacylglycerol and insulin resistance is provided by experimentally promoting adipogenesis in mice [18, 19], which improves systemic insulin sensitivity. This likely occurs in part through sequestration of lipid away from other insulin target tissues, as well as by providing beneficial adipokines [20–22]. Specifically promoting adipocyte triacylglycerol synthesis in transgenic mice through increasing levels of critical lipogenic enzymes in adipocytes, exemplified by acyl-CoA:diacylglycerol acyltransferase [20], indeed produces increased adiposity but also improves glucose tolerance. Furthermore, promoting adipose glucose uptake and lipogenesis by transgenic enhancement of glucose transporter 4 (GLUT4) levels reverses diabetes in mice [21, 23].
Insulin signalling mechanisms to attenuate adipocyte lipolysis
Insulin signalling enhances lipid storage in adipocytes by both stimulating triacylglycerol synthesis and inhibiting its breakdown. Triacylglycerol is stored in lipid droplets, which also contain lipid droplet proteins, including perilipin 1, adipophilin/adipocyte differentiation-related protein, tail-interacting protein of 47 kDa and fat-specific protein of 27 kDa (FSP27)/Cidec [24–27]. Active hydrolysis of stored triacylglycerol into its constituent fatty acids and glycerol occurs in starvation and exercise, through the actions of lipases and their regulators localised on the droplets (Fig. 1). Three key lipases control lipolysis: adipose tissue triglyceride lipase (ATGL) primarily catalyses triacylglycerol into diacylglycerol (DAG), hormone-sensitive lipase (HSL) has a higher affinity toward DAG than triacylglycerol, and monoacylglycerol lipase completes the last step in the process [28–31]. Two lipolytic pathways are highlighted in Fig. 1. The most studied pathway involves β-adrenergic stimulation by catecholamines, leading to increased cAMP levels and protein kinase A (PKA) activation, which increases access of lipases to the triacylglycerol droplet. In the fed state, perilipin binds the ATGL co-activator known as comparative gene identification-58 (CGI-58). Upon activation, PKA phosphorylates perilipin 1, releasing CGI-58 to activate ATGL [32, 33]. In contrast, HSL activation arises through direct phosphorylation by PKA on multiple residues, inducing HSL translocation to the lipid droplet surface via interaction with the NH2-terminal of phosphorylated perilipin-1. This coordinated activation of both HSL and ATGL results in a powerful lipolytic stimulation [34, 35].
Fig. 1.
Insulin signalling attenuates cAMP-mediated lipolysis at multiple steps in adipocytes. (a) NPs signal through NP receptor A to increase cGMP levels and activate PKG. (b) Stimulation of the β-adrenergic receptor increases cAMP levels, which activates PKA. (c) The lipolytic actions of PKA and PKG converge through phosphorylating perilipin-1 (Plin1), releasing CGI-58 to bind and activate ATGL, thereby stimulating hydrolysis of triacylglycerol to DAG. (d) Both PKA and PKG also phosphorylate HSL, inducing its translocation to lipid droplets, where it interacts with phosphorylated perilipin and acts primarily to convert DAG to MAG. (e) Activation of the insulin receptor–IRS signalling pathway inhibits lipolysis through activation of adipose-specific phospholipase A2 (AdPLA2), which inhibits adenylate cyclase via prostaglandin E2 synthesis, while activation of Akt leads to phosphodiesterase 3B (PDE-3B) activation to lower cAMP levels. Lipid droplet protein FSP27 is also upregulated by insulin signalling. (f) mTORC1 acts as a critical node in the control of adipocyte lipid metabolism, through reducing Atgl mRNA levels and (g) stimulating lipogenesis via SREBP1-c. Alternatively, mTORC1 stimulates a negative feedback loop through activation of (h) S6K and growth factor receptor-bound protein 10 (GRB10). (i) PKA can also regulate adipocyte lipid handling by modulating its own activity by phosphorylating and activating PDE-3B, while PKA has also been shown to (j) inhibit mTORC1
A second lipolytic pathway depicted in Fig. 1 is stimulated by natriuretic peptide (NP). A recent study demonstrated that this pathway is activated following cold exposure, increasing the thermogenic activity of white adipose tissue via a p38 mitogen-activated protein kinase (MAPK)-dependent pathway [36]. Binding of NPs to the active A isoform of the NP receptor results in increased cGMP levels and activation of protein kinase G (PKG), which phosphorylates the same targets as PKA, namely HSL and perilipin 1, independently of β-adrenergic stimulation [37–39]. Its role in enhancing adipose tissue thermogenesis, together with the fact that it is downregulated in obesity [36, 40], makes this NP receptor A signalling pathway a potential therapeutic target.
Insulin’s potent inhibition of lipolysis not only favours lipid storage but also markedly decreases circulating fatty acid levels. Insulin signalling is initiated through its receptor tyrosine kinase, which phosphorylates insulin receptor proteins (IRS) leading to phosphatidylinositol 3-kinase activation (PI3K), phosphatidylinositol 3,4,5-triphosphate generation and Akt activation (see [41, 42] for comprehensive reviews). This signalling markedly inhibits PKA- but not PKG-mediated lipolysis. At least four sites of negative regulation by insulin signalling can be identified on the β-adrenergic receptor-mediated lipolytic pathway (Fig. 1). First, insulin inhibits lipolysis through phosphorylation of adipose-specific phospholipase A2, which via arachidonic acid production increases prostaglandin E2 levels and in a paracrine/autocrine manner reduces cAMP levels through inhibition of adenylate cyclase [43, 44]. The exact mechanism of this pathway remains to be elucidated. Second, activation of Akt phosphorylates and activates phosphodiesterase, thereby reducing cAMP levels and PKA activity [45–47]. Third, the downstream target of Akt, mammalian target of rapamycin complex 1 (mTORC1), attenuates β-adrenergic stimulated lipolysis through inhibiting ATGL mRNA levels, while mTORC1 itself is inhibited by PKA [48]. Fourth, insulin upregulates the levels of the lipid droplet protein FSP27 through increasing its transcription, which dampens lipolysis [27, 49]. Thus, insulin action to inhibit lipolysis in this multifaceted mode provides a powerful restraint on the release of fatty acids from triacylglycerol within adipocyte lipid droplets.
Rapid insulin signalling mechanisms stimulate adipocyte lipogenesis
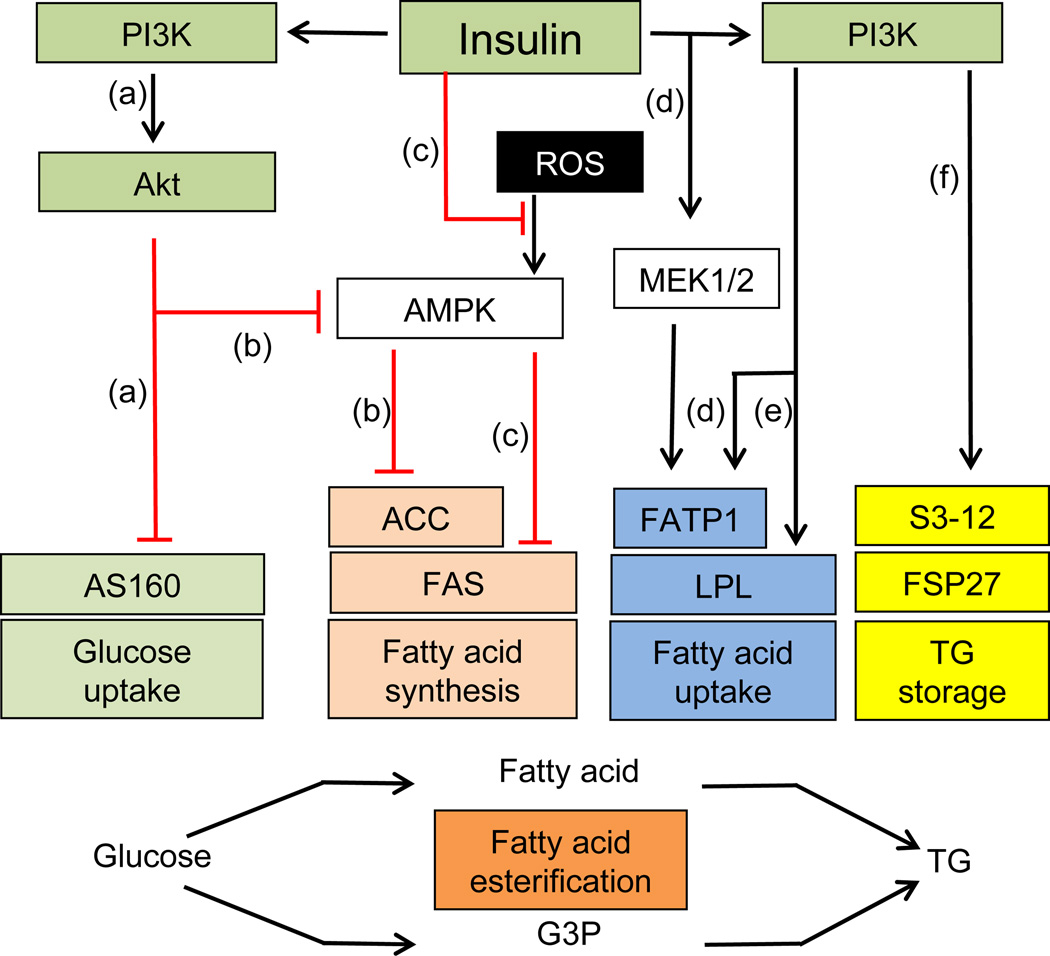
The actions of insulin to stimulate synthesis of triacylglycerol in adipocytes can be divided into two categories based on the time frame of their stimulatory effects. A summary of rapid insulin effects that occur within minutes to an hour or two is presented in Fig. 2. The major acute insulin effect is a several-fold stimulation of glucose transport into cells, mediated by increased translocation of GLUT4 to the plasma membrane through signalling by PI3K [50–54]. In adipocytes, this stimulation of glucose entry (Fig. 2) acts in concert with the process of glyceroneogenesis, [55] to provide intracellular substrate for synthesis of glycerol 3-phosphate needed for esterification of fatty acids into triacylglycerol. It is important to note that the process of glyceroneogenesis, whereby pyruvate is converted to glycerol 3-phosphate, can be the major contributor to this esterification step as well, and is discussed in detail elsewhere (55). The signals downstream of insulin-stimulated PI3K may include atypical protein kinase C (PKC) isoforms [56, 57], but it seems that the predominant signals derive from Akt activation [58, 59]. The RabGAP (Rab GTPase-activating protein) AS160/ TCB1D4 has been identified as an Akt substrate that regulates GLUT4 translocation [60–62], but studies on manipulating AS160 levels suggest that other, as yet unidentified, Akt substrates are involved [63, 64]. In spite of decades of work, the detailed mechanisms of insulin stimulation of glucose transport remain largely unresolved.
Fig. 2.
Insulin signalling exerts rapid stimulation of glucose transport as well as fatty acid uptake, synthesis and esterification to triacylglycerol (TG). (a) Stimulation of the PI3K/Akt pathway by insulin leads to inhibition of AS160 and GLUT4 translocation. Glucose is converted to glycerol 3-phosphaate and fatty acids. (b) Insulin-stimulated Akt may inhibit AMPK by phosphorylation. This in turn would lead to dephosphorylation and activation of ACC, increasing malonyl CoA production and de novo lipogenesis. (c) Reactive oxygen species (ROS) are reported to activate AMPK, which then phosphorylates and inhibits FAS. Insulin treatment decreases the inhibition of FAS induced by ROS. (d) Insulin increases fatty acid uptake by stimulating the translocation of FATP1 from intracellular vesicles to the plasma membrane mediated by PI3K or the MAPK pathway. (e) Insulin increases fatty acid uptake by stimulating LPL levels and activity through PI3K. (f) Insulin triacylglycerol synthesis or retention in adipocytes can be altered through regulation of lipid droplet protein S3-12 redistribution or FSP27 levels
Two pathways stimulated by insulin contribute to the pool of fatty acids that is esterified into triacylglycerol in adipocytes: fatty acid uptake from circulating triacylglycerol and de novo fatty acid synthesis. The former is the major pathway and is mediated in part through insulin stimulation of mRNA and protein levels of lipoprotein lipase (LPL), as well as the activity of LPL, which hydrolyses circulating triacylglycerols in lipoproteins into glycerol and fatty acids (Fig. 2) [65, 66]. Adipocyte-derived LPL is required for efficient fatty acid uptake and storage [67], and insulin infusion in humans increases adipose tissue LPL activity within a few hours [68, 69]. LPL activity is modulated through both post-transcriptional and post-translational mechanisms [66, 70]. In isolated rat adipocytes, inhibition of PI3K completely blocks the stimulation of LPL activity by insulin, while inhibition of mTOR partially inhibits insulin-stimulated LPL activity [71]. Fatty acids enter the adipocyte by diffusion and by capture mediated by fatty acid transporter (FAT/CD36) and fatty acid transporter protein 1 (FATP1), which catalyses the conversion of fatty acids into fatty acyl-CoA [70]. Wu et al showed that insulin-stimulated fatty acid uptake is completely abolished in FATP1-null adipocytes and greatly reduced in the skeletal muscle of FATP1-knockout animals, while basal fatty acid uptake by both tissues was unaffected [72]. Insulin appears to increase fatty acid uptake in adipocytes by stimulating the translocation of FATP1 from intracellular vesicles to the plasma membrane [73]. Blocking PI3K or the MAPK pathway inhibits insulin-stimulated translocation of FATP1 to the cell surface [73].
As mentioned above, insulin also increases the pool of adipocyte fatty acids for esterification through its acute stimulation of de novo lipogenesis, which mostly occurs in the liver and to a smaller extent in adipocytes. The inactivation by phosphorylation of a key enzyme in fatty acid synthesis, acetyl-CoA carboxylase (ACC), by AMP-activated protein kinase (AMPK) has been well established in isolated adipocytes [74–76]. Berggreen et al reported that, in adipocytes, insulin-stimulated Akt might regulate ACC by direct phosphorylation of AMPK, resulting in AMPK inhibition [77]. This in turn would lead to the dephosphorylation and activation of ACC, increasing malonyl CoA production and de novo lipogenesis (Fig. 2). Interestingly, fatty acid synthase (FAS), the last enzyme in the synthesis of fatty acids [78, 79], may also be regulated by AMPK under certain conditions [80].
Insulin might acutely stimulate triacylglycerol synthesis or retention in adipocytes through regulation of S3-12, an adipocyte-specific lipid droplet protein [81]. The formation of S3-12-coated lipid droplets in adipocytes apparently requires active triacylglycerol synthesis, which is insulin-dependent though PI3K activation. Treatment of 3T3-L1 adipocytes for 30 min with insulin was sufficient to redistribute S3-12 protein to lipid droplets [78], whereas a period of about 4 h was required for the upregulation of the lipid droplet protein FSP27 level by insulin [49]. Insulin may also facilitate the reformation of macro lipid droplets during recovery from lipolysis stimulation [82].
Insulin-stimulated transcription of genes encoding lipogenic enzymes
Although long-term insulin stimulation of lipogenesis involves major increases in the expression of genes encoding hepatic lipogenic enzymes [83–86], as depicted in Fig. 3 within the orange background, remarkably little is known about these mechanisms in adipocytes. Sterol regulatory element binding protein (SREBP) was originally identified as a transcription factor that binds to sterol regulatory elements in the promoter of the genes required for cholesterol regulation and adipocyte differentiation [87, 88]. Nuclear entry of SREBP requires proteolytic cleavage of the cytoplasmic N-terminal domain [89, 90], which is stimulated by insulin in hepatocytes [88, 89]. Of the three members of the SREBP family—SREBP-1a, SREBP-1c and SREBP-2—SREBP-1c is abundant in lipogenic tissues, and its own transcription is induced by insulin [91]. SREBP-1c can stimulate the transcription of Glut4 [92] and many lipogenic genes, including, Fas, Lpl, Acc, Elovl6, Acl, Gpat, Dgat, Scd-1, and Scd-2 [87, 93], and strongly promotes de novo lipogenesis in liver. However, adipose-specific knockout of this factor had little or no phenotype in mice [94, 95]. Deficiency of SREBP-1c in mouse tissues can be accompanied by an elevation of SREBP-2 levels, which may be partially functionally redundant, so interpretation of these results is unclear and more work is needed to resolve this issue.
Fig. 3.
Transcriptional regulation of lipogenic enzymes by insulin and glucose studied in hepatocytes (orange background) and adipocytes (yellow background). The degree to which the mechanisms discovered in liver apply to adipocytes is likely to be high, but this has not yet been established. (a) Insulin may increase the levels of active SREBP-1c through the atypical PKC PKCλ/ζ and (b) PI3K. (c) Activation of PI3K by insulin leads to increased SREBP-1c levels through mTORC1. (d) Insulin stimulates processing of SREBP-1c through the mTORC1 substrate S6K. (e) Insulin negatively regulates levels of Insig-2a, which inhibits SREBP processing. (f) Lipin-1 is a direct substrate of mTORC1 and a negative regulator of nuclear SREBP activity. Once active, SREBP can induce the transcription of lipogenic genes. (g) Insulin-stimulated protein phosphatase-1 (PP1) dephosphorylates and activates DNA-PK, which in turn phosphorylates USF1/2. By interacting with SREBP, USF1/2 increases expression of Fas and de novo lipogenesis. (h) Insulin-stimulated glucose uptake in adipocytes activates ChREBP-α, which stimulates production of its isoform ChREBP-β. The target genes of SREBP1c and ChREBP-α and ChREBP-β are involved in adipogenesis, glucose uptake, glycolysis, lipogenesis, and triacylglycerol storage. FA, fatty acid; SCAP, SREBP cleavage-activating protein
Insulin signalling to increase both SREBP-1c levels and processing is robust in liver (orange background in Fig. 3). Mechanisms may include a role of atypical PKC since a constitutively active PKCλ/ζ in liver upregulated SREBP-1c while an active Akt construct did not [96]. These data complement previously published studies on mouse knockouts indicating that atypical PKC activity is necessary for insulin stimulation of SREBP-1c levels [97]. In contrast, hepatic SREBP1c processing appears to be controlled by insulin through the Akt–mTOR pathway [98–100]. It was reported that mTORC1 mediates insulin-stimulated processing of SREBP-1c through its substrate protein kinase S6K, while the insulin signalling to stimulate SREBP-1c levels is less clear [101, 102]. Lipin-1, a phosphatidic acid phosphatase and a transcriptional co-activator, is a direct substrate of mTORC1 and a negative regulator of nuclear SREBP activity [98]. In mouse liver, nuclear transport of SREBP may be regulated by Akt signalling through control of INSIG-2 levels [100, 103]. The MAPK pathways, c-Jun N-terminal kinase, extracellular-signal-regulated kinase and p38 MAPK have been shown to phosphorylate SREBP-1a in liver [104, 105], and mutation of the SREBP-1a sites phosphorylated by the MAPKs in vivo abolished the transcriptional activation by SREBP1-a and protected mice from fatty liver and visceral obesity (not shown in Fig. 3) [105]. Thus, insulin acts at multiple regulatory steps to control the activity and levels of SREBPs in hepatocytes but, remarkably, none of these pathways has yet been carefully evaluated in adipocytes.
Two pathways that have been demonstrated to regulate expression of lipogenic genes downstream of insulin signalling in adipocytes are also depicted in Fig. 3 within the yellow background section. First, insulin regulates the binding of heterodimers of basic helix–loop–helix leucine zipper transcription factors upstream stimulatory factor-1 and -2 (USF1/USF2) to the Fas promoter in cultured adipocytes [86]. Consistent with these findings, USF null mice exhibit significantly impaired lipogenic gene induction in liver [106]. USF and SREBP-1c also interact in vitro and in vivo, while co-transfection of USF and SREBP-1c result in highly synergistic activation of the Fas promoter [84]. Insulin signalling through protein phosphatase-1 has been proposed to dephosphorylate and activate the protein kinase DNA-dependent protein kinase (DNA-PK), which in turn phosphorylates USF1 and increases transcriptional activation of FAS and de novo lipogenesis [107]. In DNA-PK-deficient severe combined immune deficiency (SCID) mice, feeding-induced USF1 and FAS activation are impaired, resulting in decreased circulating triacylglycerol levels and reduced adiposity [108].
A second insulin-regulated lipogenic transcription factor in adipocytes is the carbohydrate response element–binding protein (ChREBP), also known to enhance lipogenesis in liver. Insulin-stimulated glucose uptake in adipocytes activates ChREBP, which upregulates de novo lipogenesis in adipose tissue [21]. The target genes of ChREBP are involved in glycolysis, lipogenesis and gluconeogenesis [109–112]. During fasting, PKA and AMPK phosphorylate and inhibit ChREBP function [113]. Intermediates of glucose metabolism (xylulose 5-phosphate or glucose 6-phosphate) may be essential for both ChREBP nuclear translocation and transcriptional activity in response to glucose in liver cells [113, 114]. ChREBP−/− (also known as Mlxipl) mice display significantly reduced adipose tissue and are insulin resistant [108]; this is potentially due to dysfunctional adipocyte lipogenesis. Interestingly, glucose-induced ChREBPα transcriptional activity increases the levels of a novel isoform, ChREBPβ (Fig. 3), which plays an even more active role in the regulation of lipogenic genes in adipose tissue [21]. While transgenic mice producing high levels of GLUT4 in adipose tissue improves insulin sensitivity, elevating adipocyte GLUT4 levels in ChREBP−/− mice does not [21]. These data are consistent with the hypothesis that adipocyte lipogenesis stimulated by insulin is important in regulating whole body metabolism, perhaps by generating beneficial lipids that can affect whole body insulin sensitivity [115]. That insulin signalling is important for adipocyte lipogenesis is reinforced by data showing that adipose-specific knockout of the insulin receptor leads to lower adipose mass [116, 117].
Adipose tissue immune cells and their bioactive factors that effect lipogenesis
Expansion of fat mass in obesity is accompanied by infiltration of cells of innate and adaptive immunity, including macrophages, T cells, B cells, natural killer T cells, neutrophils, eosinophils and mast cells [118–123]. Macrophages are the most abundant immune cell population in adipose tissue in obesity [120], and are the main source of pro-inflammatory molecules (e.g. TNF-α, IL1β) secreted in adipose tissue in the obese state [124, 125]. Macrophages may be attracted into adipose tissue by increased fatty acid release from either viable or dying adipocytes in obesity [126, 127], which may shift their phenotype from M2 towards the M1 pro-inflammatory phenotype [128]. Adipose tissue macrophages can also accumulate lipids and become ‘foam cell like-cells’ [129], which may complement adipocyte lipid sequestration in adipose tissue. While much of the relevant literature suggests macrophages in adipose tissue inhibit adipose function [125, 130], some data indicate a beneficial role, for example, in attenuating lipolysis [128].
T lymphocytes represent the second most abundant immune cell population in adipose tissue and might accelerate adipose tissue macrophage recruitment and activation [131, 132]. Levels of pro-inflammatory CD8+ and IFNγ+ T helper (Th) type 1 cells appear to increase with obesity, while levels of anti-inflammatory IL4+Th2 and regulatory T cells appear to decrease in adipose tissue from obese mice and humans [133–137]. Thus, T cells may mediate inhibitory effects on insulin action and lipogenic genes [135, 136]. Th17 cells producing IL17 may impair adipose tissue metabolism via inhibition of adipogenesis and decreased glucose uptake [137, 138]. IL17-deficient mice gained weight but sustained glucose tolerance under high-fat diet (HFD) conditions [139]. In addition, natural killer T lymphocytes in adipose tissue are activated by binding to lipids or glycolipids associated with the protein CD1d. Although these and other immune cells represent a very low percentage of the adipose tissue total, a role in obesity-induced insulin resistance is suggested by their experimental depletion in mice [119, 121, 122, 140]. Macrophages and lymphocytes can also become insulin resistant [141]. Indeed, depletion of insulin receptor in myeloid cells protected mice from HFD-induced insulin resistance and adipose tissue inflammation [142, 143].
Table 1 summarises the major factors produced by infiltrating immune cells in adipose tissue of obese rodents and humans and their reported effects on adipose tissue metabolism and insulin signalling. These include a broad range of pro-inflammatory cytokines, including TNF-α, IL1β, IL6, and IFNγ, whose negative effect on insulin signalling is indicated in several studies using knockout animals and other approaches [125, 144, 145]. Furthermore, TNF-α injection or adipocyte-specific production of the active transmembrane form of TNF-α exacerbates systemic and local insulin resistance, respectively [146–148]. IFNγ , produced by Th1 and CD8+ lymphocytes, also downregulates insulin signalling and adipogenesis, while increasing lipolysis [149]. Moreover, depletion of IFNγ protects mice from HFD-induced insulin resistance [150]. IL17 secreted by Th17 cells in adipose tissue also negatively regulates adipocyte lipid metabolism [139]. The production of inflammatory lipid mediators such as 12/15-lipoxygenase products (leukotrienes, hydroxyeicosatetraenoic acids) may add to the detrimental effect on lipid metabolism in adipocytes by inducing the secretion of pro-inflammatory cytokines [151, 152]. Studies in humans and rodents continue to reveal novel inflammatory molecules from the chemokine or interleukin families that influence adipose tissue insulin signalling and lipid storage, exemplified by CCL7, CXCL5, IL7 and IL33 [153–155].
Table 1.
Effects of cytokines on insulin signalling, glucose uptake, lipogenesis and lipolysis in adipocytes and on hepatic lipogenesis
| Molecule | Effect on in vitro adipocyte insulin signalling |
Effect on in vitro adipocyte glucose uptake |
Effect on in vitro adipocyte lipogenesis |
Effect on in vitro adipocyte lipolysis |
Insulin sensitivity |
Triacylglycerol synthesis |
Ref. |
|---|---|---|---|---|---|---|---|
| KO mice |
KO mice |
||||||
| TNF-α | ↓IRS1, Akt | ↓GLUT4 | ↓PPARγ, SREBP1c, ACS, SCD1, LPL, aP2 | ↑ATGL, HSL | ↑ | ↓ (liver) | [125, 144, 148, 202, 232–235] |
| IL1β | ↓IRS1, Akt | ↓GLUT4 | ↓PPARγ, SREBP1c, ACC, FAS | ↑HSL | ↑ | ↓ (liver) | [236–238] |
| IL6 | ↓IRS1, Akt | ↓GLUT4 | ↓PPARγ, FAS, aP2 | ↑ | ↓ | ↑ (liver) | [145, 239–241] |
| IFNγ | ↓IRS1, IR, Akt | ↓GLUT4 | ↓LPL, FAS, PPARγ | ↑ | ↑ | [134, 149, 150, 242] | |
| IL17 | ↓GLUT4 | ↓PPARγ, PLIN | ↑ | [139] | |||
| Lipoxygenase products | ↓IRS1, Akt | ↑HSL | ↑ | [150, 151] | |||
| IL4 | ↑Akt ↓GSK-3β | ↓ | ↓ | ↑ (liver) | [156, 160] | ||
| IL10 | ↑Akt | ↑ | ↔ | [128, 158, 159, 243] | |||
| IL1Ra | ↑LPL activity, PPARγ | ↑ | [244–246] |
First four columns: major bioactive molecules produced by immune cells that can modulate adipocyte insulin signalling elements, GLUT4 and glucose uptake, conversion of precursors to triacylglycerol (lipogenesis), and glycerol and fatty acid release (lipolysis) in vitro (↓ inhibitory effect, ↑ stimulatory effect)
Last two columns: effects of gene knockout of indicated cytokines on whole body insulin sensitivity or triacylglycerol synthesis in indicated tissue GSK-3β, glycogen synthase kinase-3β; IR, insulin receptor; LPL, lipoprotein lipase; SCD, stearoyl-CoA desaturase
Anti-inflammatory cytokines IL4, IL10 and IL1 receptor antagonist (IL1Ra), which blunt the actions of pro-inflammatory cytokines on insulin signalling can also be measured in adipose tissue of obese individuals [128, 156–158]. For example, IL10 inhibits TNF-α production in macrophages [158], while transgenic mice producing elevated levels of IL10 show improved insulin sensitivity under HFD conditions [159]. Recent studies suggest that IL4 enhances insulin action in adipose tissue, promoting activation of Akt and inhibition of glycogen synthase kinase-3β, attenuating adipose tissue inflammation [156, 160]. A key point from these studies is that immune cells can secrete both beneficial and deleterious factors within adipose depots, and the overall effect under a given physiological condition represents the integration of the effects of these multiple factors in real time.
Mechanisms of insulin resistance of adipocyte lipogenesis
Prior to discoveries starting in the 1980s that uncovered the molecules of insulin signalling, the problem of insulin resistance was viewed as simply whether obesity mostly impaired insulin binding to its receptor vs ‘post-receptor’ signalling events. Insulin binding to adipocytes was indeed found to be inhibited in obesity, reflecting a decrease in the number of adipocyte surface receptors [161, 162] following induction of receptor endocytosis in response to insulin binding [163, 164]. However, the remaining receptors were calculated to be sufficient to mediate a full response at high insulin concentrations, which is at odds with the greatly decreased maximum response of lipogenesis that was observed [165, 166].
We now know that tyrosine phosphorylation of IRS proteins and activation of Akt2, the major insulin signalling pathway leading to glucose transport stimulation [53, 58], are also blunted in adipocytes from obese mice [167, 168] and obese, insulin-resistant humans [169–171]. Mechanisms for this impaired signalling could include inhibition of IRS function by the negative feedback loop from S6 kinase (S6K) [172] and inhibition of mTORC2-mediated Akt2 phosphorylation and activation, also through negative regulation by S6K [173] (Fig. 1). This concept is consistent with the hypothesis that the hyperinsulinaemia associated with obesity may actually be the cause, rather than simply the consequence, of insulin resistance via such feedback inhibition [165]. Other major candidates for obesity-mediated attenuation of insulin signalling to Akt2 in adipocytes in obesity include (1) factors secreted from immune cells in adipose tissue, as described in the section above; (2) factors secreted from endothelial cells [174, 175]; (3) factors from neuronal innervation of adipose tissue [176–178]; and (4) increased influx of fatty acids into adipocytes, particularly palmitate [179, 180]. It should be noted that insulin itself may modulate the production of cytokines [142, 181–186]. Moreover, hyperinsulinaemic clamp conditions in humans cause increased cytokine secretion by adipose tissue [187, 188]. Thus, hyperinsulinaemia may act synergistically with adipose tissue immune cells in producing insulin resistance.
The potential mechanisms for inhibition of insulin signalling to Akt by the above factors have been extensively reviewed [189, 190]. Much experimental emphasis has been placed on Ser/Thr phosphorylation of IRS proteins by various protein kinases activated by the factors listed above, leading to inhibition of IRS tyrosine phosphorylation and its signalling through PI3K [191]. However, an underappreciated aspect of insulin resistance in adipose tissue is the fact that inhibition of insulin receptor signalling to Akt is not sufficient to explain the degree to which insulin-stimulated lipogenesis is suppressed in adipocytes under physiological glucose concentrations [192–195]. Stimulated Akt activity is in large excess over that needed to fully upregulate glucose transport in adipocytes, and even large inhibitions of its activity, as occurs in obesity, are unlikely to fully account for the great diminutions of acute glucose conversion to triacylglycerol observed [195]. Thus, a key concept is that attenuation of the upstream insulin signalling pathway to Akt in obesity is only one contributor, and possibly a minor one, to what is measured as resistance of lipogenesis to insulin in adipocytes.
It is well established that, in human obesity, adipocyte enzymes involved in fatty acid esterification and de novo fatty acid synthesis pathways are markedly reduced [196]. These include diacylglycerol acyltransferase (DGAT) in the former pathway and ATP citrate lyase, ACC and FAS in the latter [197]. The decreased level of lipogenic enzymes is also observed in most rodent models of obesity [198, 199]. Furthermore, it has been shown that large adipocytes from old obese rats exhibit higher rates of glucose transport per cell than small adipocytes from lean animals, even though insulin-stimulated conversion of radiolabelled glucose to triacylglycerol, glycerol and fatty acids is completely blocked in the former [192]. Taken together, these data indicate that the uptake of glucose into the adipocyte may not be the major rate-limiting step in obesity but, rather, the enzymatic capacity to convert it to triacylglycerol is limited. Thus, in this sense, the term ‘insulin resistance’ may be a misnomer in that the major physiological impairment is in the end target of insulin action (lipogenic enzyme deficit) rather than solely in the initial insulin signalling pathway.
It is confounding that hepatic lipogenesis is greatly enhanced in response to hyperinsulinaemia in obesity, while adipocyte lipogenic capacity is attenuated under these same conditions. One explanation for the increased hepatic lipogenesis in obesity appears to be activation of mTORC1 by the excess nutrient amino acids, causing stimulation of one of its known downstream targets, the lipogenic transcription factor SREBP1 [98–100]. In contrast, SREBP1 abundance and processing to its active, truncated form in adipocytes is decreased in obesity [198–202], even though mTOR and S6K activities are apparently actually increased in the adipose tissue of obese mice [172]. One possible answer to this paradox relates to the selective negative influence on lipogenesis of certain immune cell factors and cytokines elevated in adipose tissue in obesity (Fig. 4). In obesity, immune cell infiltration in adipose tissue is more severe than that observed in liver [203, 204]. Strikingly, TNF-α greatly decreases adipose tissue levels of peroxisome proliferator-activated receptor γ (PPARγ) and its target SREBP1 [191, 202, 205], while it increases SREBP1 levels and processing in liver through mechanisms independent of PPARγ [206, 207]. The negative regulation of PPARγ in adipocytes by TNF-α appears to include decreases in PPARγ transcription, PPARγ mRNA stability and protein turnover [191, 208, 209]. Phosphorylation of PPARγ by protein kinases upregulated in obesity may be part of this inhibitory pattern [210]. PPARγ, in turn, stimulates transcription and activation of SREBP1c, as well as acting directly on the promoters of many genes encoding lipogenic enzymes and factors [211–213]. The latter include lipid droplet proteins that help to sequester triacylglycerol in the droplets, protected from lipolytic enzymes [214–216]. Other cytokines such as IL1, which are released by macrophages in adipose tissue of obese individuals [124, 217], mediate similar attenuations of PPARγ activity or levels [210]. Additionally, since insulin signalling through Akt and mTORC1 causes processing and activation of SREBP1c [98–100] (Fig. 3), the attenuated activation of Akt in obesity may contribute to decreased SREBP1c activity.
Fig. 4.
PPARγ as a key regulator of lipogenesis in adipocytes and hypothetical major target for inhibitors of insulin-stimulated lipogenesis in obesity. Adipocytes from obese rodents and humans display decreased levels of lipogenic transcription factors (SREBP1c and ChREBP) and lipogenic enzymes (FAS, SCD1, ELOVL, DGAT) compared with lean controls. (a) Attenuation of PPARγ by candidate inhibitors generated in obesity would explain the downregulation of all of these proteins. Direct inhibition of the lipogenic transcription factors and enzymes by such inhibitors is also possible. (b) Inhibition of early steps in insulin signalling is also evident in adipocytes in obesity, through attenuation of IRS tyrosine phosphorylation and other inhibitory mechanisms, as shown in Fig. 1. While the factors listed as candidate inhibitors in the top box can be shown in vitro to exert such effects, the extent to which they contribute to insulin resistance in adipocytes in vivo remains to be fully elucidated. It should be noted that PPARγ also stimulates production of the enzyme phosphoenolpyruvate carboxykinase (not shown in the Figure), which in turn stimulates lipogenesis through the pathway of glyceroneogenesis [55]. Thus PPARγ disruption will also lead to attenuation of this pathway. DGAT, Diglyceride acyltransferase; ELOVL, Elongation of long-chain fatty acids; SCD1, stearoyl-CoA desaturase
It is remarkable that key experiments have not yet been performed to verify even the most popular hypotheses related to adipose dysfunction in obesity. For example, testing the concept that adipose inflammation by immune cells is a cause of systemic glucose intolerance would require immune cells to be experimentally depleted in adipose tissue while being maintained in other tissues. This critical experiment is a technically difficult one, and has not been accomplished to date. Furthermore, several anti-inflammatory strategies have been tested in metabolic disease with little success [218–220], indicating more research is necessary to unravel the role of adipose tissue inflammation in diabetes.
Another major regulatory pathway of triacylglycerol synthesis involves the ability of glucose to increase the activity of ChREBPα, which promotes the expression of lipogenic genes [21]. Similar to SREBP1c, hepatic ChREBPα levels are increased in obesity, while adipocyte ChREBPα is decreased [221–223]. In adipose tissue, unlike liver, insulin stimulates glucose uptake thus enhancing ChREBPα function. Levels of the GLUT4 protein itself are substantially decreased in adipocytes of obese mice and humans [224, 225]. Recent work revealed that ChREBPβ levels are increased by ChREBPα, and is even more potent in stimulating transcription of lipogenic genes [21]. Adipose-specific depletion of GLUT4 decreases abundance of ChREBPα and ChREBPβ, while increased GLUT4 elevates their levels [21], indicating that the deficit in GLUT4 in obesity may contribute to the observed downregulation of these lipogenic factors. Furthermore, ChREBPα is also a downstream target of PPARγ [222], which is decreased in adipocytes in obesity as discussed above. Taken together, these results indicate that dysfunctions in the ChREBP pathway contribute to adipose lipogenic deficiency in obesity.
Since the transcription factors SREBP1c and ChREBP, along with the many genes that encode lipogenic enzymes themselves, are all downstream of PPARγ, direct obstruction of this master regulator in obesity is expected to decrease adipocyte lipogenesis capacity (Fig. 4). Such effects of PPARγ inhibition are further magnified by the simultaneous disruption of downstream feed-forward cycles. For example, the PPARγ target enzyme FAS catalyses synthesis of palmitate, which can apparently be converted to derivatives that act as PPARγ ligands [226–228]. Thus, decrements in FAS due to PPARγ attenuation, reduces the extent to which the remaining PPARγ can be activated. Furthermore, PPARγ upregulates elements of the insulin signalling pathway such as IRS-1 [229–231], suggesting that suppression of the upstream insulin signalling pathway to Akt in obesity is also in part secondary to PPARγ inhibition in obesity.
Perspectives
The role of adipose tissue as the primary depot for robust triacylglycerol storage in humans appears to be an important factor in preventing systemic insulin resistance and diabetes in response to obesity. Insulin signalling maintains this high capacity for triacylglycerol synthesis and storage in adipose tissue in healthy humans, but chronic hyperinsulinaemia, increased cytokines and other abnormal secretions from cells within adipose tissue are among the dysfunctions in obesity that may contribute to a decrease in this capacity. Thus, to fully understand the relationships between adipose tissue function and whole body glucose tolerance, it is crucial to unravel the underlying mechanisms of insulin signalling, insulin resistance and control of lipogenesis in adipose. Two basic key questions among many others remain unanswered to date: What are the signals that downregulate adipose lipogenic transcription factors and lipogenic enzymes in obesity? Do the cytokines released in adipose tissue by immune cells in obesity contribute to the downregulation of adipocyte lipogenesis in vivo? Finding truly innovative approaches to answer these questions will surely move this field forward and potentially yield new therapeutic strategies for treating type 2 diabetes.
Acknowledgements
We thank Drs. Adilson Guilherme, Joseph Virbasius and members of our laboratory at University of Massachusetts, Worcester, MA, U.S.A. for excellent discussion of the issues discussed in this review.
Funding
Studies from our laboratory related to this topic are supported by grants to M. P. Czech from the National Institutes of Health (DK085753, DK030898) and from the International Research Alliance at the Novo Nordisk Foundation Center for Metabolic Research.
Abbreviations
- ACC
Acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- ATGL
Adipose tissue triglyceride lipase
- CGI-58
Comparative gene identification-58
- ChREBP
Carbohydrate response element binding protein
- DAG
Diacylglycerol
- DNA-PK
DNA-dependent protein kinase
- FAS
Fatty acid synthase
- FATP1
Fatty acid transporter protein 1
- FSP27
Fat-specific protein of 27 kDa
- HFD
High-fat diet
- HSL
Hormone-sensitive lipase
- LPL
Lipoprotein lipase
- MAPK
Mitogen-activated protein kinase
- mTOR
Mammalian target of rapamycin
- mTORC
Mammalian target of rapamycin complex
- NP
Natriuretic peptide
- PI3K
Phosphatidylinositol 3-kinase
- PKA
Protein kinase A
- PKC
Protein kinase C
- PKG
Protein kinase G
- PPAR
Peroxisome proliferator-activated receptor
- SOCS
Suppressor of cytokine signalling proteins
- SREBP
Sterol regulatory element binding protein
- S6K
S6 kinase
- Th
T helper
- USF
Upstream stimulatory factor
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All authors were responsible for the conception and design of the manuscript, drafting the article and revising it critically for important intellectual content. All authors approved the version to be published.
References
- 1.Tattersall RB. A force of magical activity: the introduction of insulin treatment in Britain 1922–1926. Diabet Med. 1995;12:739–755. doi: 10.1111/j.1464-5491.1995.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 2.Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81–86. doi: 10.1016/j.biochi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Frayn KN. Regulation of fatty acid delivery in vivo. Adv Exp Med Biol. 1998;441:171–179. doi: 10.1007/978-1-4899-1928-1_16. [DOI] [PubMed] [Google Scholar]
- 4.Frayn KN. Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc. 2001;60:375–380. doi: 10.1079/pns200195. [DOI] [PubMed] [Google Scholar]
- 5.Boden G. Free fatty acids-the link between obesity and insulin resistance. Endocr Pract. 2001;7:44–51. doi: 10.4158/EP.7.1.44. [DOI] [PubMed] [Google Scholar]
- 6.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 8.Chavez JA, Summers SA. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim Biophys Acta. 2010;1801:252–265. doi: 10.1016/j.bbalip.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland WL, Bikman BT, Wang LP, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoy AJ, Brandon AE, Turner N, et al. Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1 Akt or AS160 phosphorylation. Am J Physiol Endocrinol Metab. 2009;297:E67–E75. doi: 10.1152/ajpendo.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 15.Sinha S, Perdomo G, Brown NF, O’Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem. 2004;279:41294–41301. doi: 10.1074/jbc.M406514200. [DOI] [PubMed] [Google Scholar]
- 16.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 17.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Fan W, Xu J, et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugii S, Olson P, Sears DD, et al. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci U S A. 2009;106:22504–22509. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HC, Stone SJ, Zhou P, Buhman KK, Farese RV., Jr Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme A:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 2002;51:3189–3195. doi: 10.2337/diabetes.51.11.3189. [DOI] [PubMed] [Google Scholar]
- 21.Herman MA, Peroni OD, Villoria J, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusminski CM, Holland WL, Sun K, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- 24.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Botas J, Anderson JB, Tessier D, et al. Absence of perilipin results in leanness and reverses obesity in Leprdb/db mice. Nat Genet. 2000;26:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 27.Puri V, Konda S, Ranjit S, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 28.Haemmerle G, Zimmermann R, Strauss JG, et al. Hormone-sensitive lipase deficiency in mice changes the plasma lipid profile by affecting the tissue-specific expression pattern of lipoprotein lipase in adipose tissue and muscle. J Biol Chem. 2002;277:12946–12952. doi: 10.1074/jbc.M108640200. [DOI] [PubMed] [Google Scholar]
- 29.Taschler U, Radner FP, Heier C, et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem. 2011;286:17467–17477. doi: 10.1074/jbc.M110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zechner R, Zimmermann R, Eichmann TO, et al. Fat signals – lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann R, Strauss JG, Haemmerle G, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 32.Gruber A, Cornaciu I, Lass A, et al. The N-terminal region of comparative gene identification-58 (CGI-58) is important for lipid droplet binding and activation of adipose triglyceride lipase. J Biol Chem. 2010;285:12289–12298. doi: 10.1074/jbc.M109.064469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lass A, Zimmermann R, Haemmerle G, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Miyoshi H, Perfield JW, 2nd, Souza SC, et al. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem. 2007;282:996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- 35.Shen WJ, Patel S, Miyoshi H, Greenberg AS, Kraemer FB. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res. 2009;50:2306–2313. doi: 10.1194/jlr.M900176-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moro C, Galitzky J, Sengenes C, Crampes F, Lafontan M, Berlan M. Functional and pharmacological characterization of the natriuretic peptide-dependent lipolytic pathway in human fat cells. J Pharmacol Exp Ther. 2004;308:984–992. doi: 10.1124/jpet.103.060913. [DOI] [PubMed] [Google Scholar]
- 38.Sengenes C, Berlan M, de Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 39.Sengenes C, Bouloumie A, Hauner H, et al. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem. 2003;278:48617–48626. doi: 10.1074/jbc.M303713200. [DOI] [PubMed] [Google Scholar]
- 40.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 41.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 43.Duncan RE, Sarkadi-Nagy E, Jaworski K, Ahmadian M, Sul HS. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA) J Biol Chem. 2008;283:25428–25436. doi: 10.1074/jbc.M804146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaworski K, Ahmadian M, Duncan RE, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–168. doi: 10.1038/nm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eriksson H, Ridderstrale M, Degerman E, et al. Evidence for the key role of the adipocyte cGMP-inhibited cAMP phosphodiesterase in the antilipolytic action of insulin. Biochim Biophys Acta. 1995;1266:101–107. doi: 10.1016/0167-4889(94)00237-9. [DOI] [PubMed] [Google Scholar]
- 46.Kitamura T, Kitamura Y, Kuroda S, et al. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol Cell Biol. 1999;19:6286–6296. doi: 10.1128/mcb.19.9.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahn T, Ridderstrale M, Tornqvist H, et al. Essential role of phosphatidylinositol 3-kinase in insulin-induced activation and phosphorylation of the cGMP-inhibited cAMP phosphodiesterase in rat adipocytes. Studies using the selective inhibitor wortmannin. FEBS Lett. 1994;350:314–318. doi: 10.1016/0014-5793(94)00797-7. [DOI] [PubMed] [Google Scholar]
- 48.Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59:775–781. doi: 10.2337/db09-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JY, Liu K, Zhou S, Tillison K, Wu Y, Smas CM. Assessment of fat-specific protein 27 in the adipocyte lineage suggests a dual role for FSP27 in adipocyte metabolism and cell death. Am J Physiol Endocrinol Metab. 2008;294:E654–E667. doi: 10.1152/ajpendo.00104.2007. [DOI] [PubMed] [Google Scholar]
- 50.Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- 52.Guilherme A, Czech MP. Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by beta1-integrin signaling in rat adipocytes. J Biol Chem. 1998;273:33119–33122. doi: 10.1074/jbc.273.50.33119. [DOI] [PubMed] [Google Scholar]
- 53.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980;77:2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nye C, Kim J, Kalhan SC, Hanson RW. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol Metab. 2008;19:356–361. doi: 10.1016/j.tem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Farese RV, Sajan MP, Standaert ML. Atypical protein kinase C in insulin action and insulin resistance. Biochem Soc Trans. 2005;33:350–353. doi: 10.1042/BST0330350. [DOI] [PubMed] [Google Scholar]
- 57.Kotani K, Ogawa W, Matsumoto M, et al. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 59.Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci U S A. 2003;100:7569–7574. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eguez L, Lee A, Chavez JA, et al. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Kane S, Sano H, Liu SC, et al. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 62.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 63.Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab. 2011;13:68–79. doi: 10.1016/j.cmet.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Picard F, Naimi N, Richard D, Deshaies Y. Response of adipose tissue lipoprotein lipase to the cephalic phase of insulin secretion. Diabetes. 1999;48:452–459. doi: 10.2337/diabetes.48.3.452. [DOI] [PubMed] [Google Scholar]
- 66.Semenkovich CF, Wims M, Noe L, Etienne J, Chan L. Insulin regulation of lipoprotein lipase activity in 3T3-L1 adipocytes is mediated at posttranscriptional and posttranslational levels. J Biol Chem. 1989;264:9030–9038. [PubMed] [Google Scholar]
- 67.Gonzales AM, Orlando RA. Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr Metab (Lond) 2007;4:22. doi: 10.1186/1743-7075-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sadur CN, Eckel RH. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J Clin Invest. 1982;69:1119–1125. doi: 10.1172/JCI110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yki-Jarvinen H, Taskinen MR, Koivisto VA, Nikkila EA. Response of adipose tissue lipoprotein lipase activity and serum lipoproteins to acute hyperinsulinaemia in man. Diabetologia. 1984;27:364–369. doi: 10.1007/BF00304851. [DOI] [PubMed] [Google Scholar]
- 70.Albalat A, Saera-Vila A, Capilla E, Gutierrez J, Perez-Sanchez J, Navarro I. Insulin regulation of lipoprotein lipase (LPL) activity and expression in gilthead sea bream (Sparus aurata) Comp Biochem Physiol B Biochem Mol Biol. 2007;148:151–159. doi: 10.1016/j.cbpb.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Kraemer FB, Takeda D, Natu V, Sztalryd C. Insulin regulates lipoprotein lipase activity in rat adipose cells via wortmannin- and rapamycin-sensitive pathways. Metabolism. 1998;47:555–559. doi: 10.1016/s0026-0495(98)90239-6. [DOI] [PubMed] [Google Scholar]
- 72.Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol. 2006;26:3455–3467. doi: 10.1128/MCB.26.9.3455-3467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell. 2002;2:477–488. doi: 10.1016/s1534-5807(02)00143-0. [DOI] [PubMed] [Google Scholar]
- 74.Daval M, Diot-Dupuy F, Bazin R, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 75.Orci L, Cook WS, Ravazzola M, et al. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci U S A. 2004;101:2058–2063. doi: 10.1073/pnas.0308258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- 77.Berggreen C, Gormand A, Omar B, Degerman E, Goransson O. Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes. Am J Physiol Endocrinol Metab. 2009;296:E635–E646. doi: 10.1152/ajpendo.90596.2008. [DOI] [PubMed] [Google Scholar]
- 78.Jayakumar A, Tai MH, Huang WY, et al. Human fatty acid synthase: properties and molecular cloning. Proc Natl Acad Sci U S A. 1995;92:8695–8699. doi: 10.1073/pnas.92.19.8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith S, Witkowski A, Joshi AK. Structural and functional organization of the animal fatty acid synthase. Prog Lipid Res. 2003;42:289–317. doi: 10.1016/s0163-7827(02)00067-x. [DOI] [PubMed] [Google Scholar]
- 80.An Z, Wang H, Song P, Zhang M, Geng X, Zou MH. Nicotine-induced activation of AMP-activated protein kinase inhibits fatty acid synthase in 3T3L1 adipocytes: a role for oxidant stress. J Biol Chem. 2007;282:26793–26801. doi: 10.1074/jbc.M703701200. [DOI] [PubMed] [Google Scholar]
- 81.Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE. Adipocyte protein S3-12 coats nascent lipid droplets. J Biol Chem. 2003;278:37713–37721. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- 82.Ariotti N, Murphy S, Hamilton NA, et al. Postlipolytic insulin-dependent remodeling of micro lipid droplets in adipocytes. Mol Biol Cell. 2012;23:1826–1837. doi: 10.1091/mbc.E11-10-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Assimacopoulos-Jeannet F, Brichard S, Rencurel F, Cusin I, Jeanrenaud B. In vivo effects of hyperinsulinemia on lipogenic enzymes and glucose transporter expression in rat liver and adipose tissues. Metabolism. 1995;44:228–233. doi: 10.1016/0026-0495(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 84.Griffin MJ, Wong RH, Pandya N, Sul HS. Direct interaction between USF and SREBP-1c mediates synergistic activation of the fatty-acid synthase promoter. J Biol Chem. 2007;282:5453–5467. doi: 10.1074/jbc.M610566200. [DOI] [PubMed] [Google Scholar]
- 85.Kim JB, Sarraf P, Wright M, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sul HS, Latasa MJ, Moon Y, Kim KH. Regulation of the fatty acid synthase promoter by insulin. J Nutr. 2000;130:315S–320S. doi: 10.1093/jn/130.2.315S. [DOI] [PubMed] [Google Scholar]
- 87.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 88.Shimomura I, Shimano H, Korn BS, Bashmakov Y, Horton JD. Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J Biol Chem. 1998;273:35299–35306. doi: 10.1074/jbc.273.52.35299. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77:53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 90.Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci U S A. 2003;100:3155–3160. doi: 10.1073/pnas.0130116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amemiya-Kudo M, Shimano H, Yoshikawa T, et al. Promoter analysis of the mouse sterol regulatory element-binding protein-1c gene. J Biol Chem. 2000;275:31078–31085. doi: 10.1074/jbc.M005353200. [DOI] [PubMed] [Google Scholar]
- 92.Im SS, Kwon SK, Kang SY, et al. Regulation of GLUT4 gene expression by SREBP-1c in adipocytes. Biochem J. 2006;399:131–139. doi: 10.1042/BJ20060696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tabor DE, Kim JB, Spiegelman BM, Edwards PA. Identification of conserved cis-elements and transcription factors required for sterol-regulated transcription of stearoyl-CoA desaturase 1 and 2. J Biol Chem. 1999;274:20603–20610. doi: 10.1074/jbc.274.29.20603. [DOI] [PubMed] [Google Scholar]
- 94.Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99:846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimano H, Shimomura I, Hammer RE, et al. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J Clin Invest. 1997;100:2115–2124. doi: 10.1172/JCI119746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taniguchi CM, Kondo T, Sajan M, et al. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 97.Matsumoto M, Ogawa W, Akimoto K, et al. PKClambda in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peterson TR, Sengupta SS, Harris TE, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quinn WJ, 3rd, Birnbaum MJ. Distinct mTORC1 pathways for transcription and cleavage of SREBP-1c. Proc Natl Acad Sci U S A. 2012;109:15974–15975. doi: 10.1073/pnas.1214113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yecies JL, Zhang HH, Menon S, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bae EJ, Xu J, Oh da Y, et al. Liver-specific p70 S6 kinase depletion protects against hepatic steatosis and systemic insulin resistance. J Biol Chem. 2012;287:18769–18780. doi: 10.1074/jbc.M112.365544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li S, Ogawa W, Emi A, et al. Role of S6K1 in regulation of SREBP1c expression in the liver. Biochem Biophys Res Commun. 2011;412:197–202. doi: 10.1016/j.bbrc.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 103.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 104.Kotzka J, Knebel B, Haas J, et al. Preventing phosphorylation of sterol regulatory element-binding protein 1a by MAP-kinases protects mice from fatty liver and visceral obesity. PLoS One. 2012;7:e32609. doi: 10.1371/journal.pone.0032609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roth G, Kotzka J, Kremer L, et al. MAP kinases Erk1/2 phosphorylate sterol regulatory element-binding protein (SREBP)-1a at serine 117 in vitro. J Biol Chem. 2000;275:33302–33307. doi: 10.1074/jbc.M005425200. [DOI] [PubMed] [Google Scholar]
- 106.Casado M, Vallet VS, Kahn A, Vaulont S. Essential role in vivo of upstream stimulatory factors for a normal dietary response of the fatty acid synthase gene in the liver. J Biol Chem. 1999;274:2009–2013. doi: 10.1074/jbc.274.4.2009. [DOI] [PubMed] [Google Scholar]
- 107.Brady MJ, Saltiel AR. The role of protein phosphatase-1 in insulin action. Recent Prog Horm Res. 2001;56:157–173. doi: 10.1210/rp.56.1.157. [DOI] [PubMed] [Google Scholar]
- 108.Wong RH, Chang I, Hudak CS, Hyun S, Kwan HY, Sul HS. A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell. 2009;136:1056–1072. doi: 10.1016/j.cell.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281:28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 111.Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem. 2004;279:15662–15669. doi: 10.1074/jbc.M311301200. [DOI] [PubMed] [Google Scholar]
- 112.Yamashita H, Takenoshita M, Sakurai M, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid 'sparing’ effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 114.Dentin R, Tomas-Cobos L, Foufelle F, et al. Glucose 6-phosphate, rather than xylulose 5-phosphate, is required for the activation of ChREBP in response to glucose in the liver. J Hepatol. 2012;56:199–209. doi: 10.1016/j.jhep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 115.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bluher M, Michael MD, Peroni OD, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 117.Boucher J, Mori MA, Lee KY, et al. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat Commun. 2012;3:902. doi: 10.1038/ncomms1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 119.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu L, Parekh VV, Gabriel CL, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci U S A. 2012;109:E1143–E1152. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 125.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–E174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 127.Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Prieur X, Mok CY, Velagapudi VR, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 131.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu H, Ghosh S, Perrard XD, et al. T cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 133.Deiuliis J, Shah Z, Shah N, et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One. 2011;6:e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Duffaut C, Zakaroff-Girard A, Bourlier V, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 135.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 137.Winer S, Paltser G, Chan Y, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 138.Jagannathan-Bogdan M, McDonnell ME, Shin H, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zuniga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ohmura K, Ishimori N, Ohmura Y, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 141.Tabas I, Tall A, Accili D. the impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circulation Research. 2010;106:58–67. doi: 10.1161/CIRCRESAHA.109.208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mauer J, Chaurasia B, Plum L, et al. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 2010;6:e1000938. doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baumgartl J, Baudler S, Scherner M, et al. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab. 2006;3:247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 145.Ji C, Chen X, Gao C, et al. IL-6 induces lipolysis and mitochondrial dysfunction, but does not affect insulin-mediated glucose transport in 3T3-L1 adipocytes. J Bioenerg Biomembr. 2011;43:367–375. doi: 10.1007/s10863-011-9361-8. [DOI] [PubMed] [Google Scholar]
- 146.Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130:43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- 147.Rask-Madsen C, Dominguez H, Ihlemann N, Hermann T, Kober L, Torp-Pedersen C. Tumor necrosis factor-alpha inhibits insulin's stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation. 2003;108:1815–1821. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- 148.Xu H, Hirosumi J, Uysal KT, Guler AD, Hotamisligil GS. Exclusive action of transmembrane TNF alpha in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology. 2002;143:1502–1511. doi: 10.1210/endo.143.4.8715. [DOI] [PubMed] [Google Scholar]
- 149.McGillicuddy FC, Chiquoine EH, Hinkle CC, et al. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem. 2009;284:31936–31944. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rocha VZ, Folco EJ, Sukhova G, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity (Silver Spring) 2009;17:1657–1663. doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Horrillo R, Gonzalez-Periz A, Martinez-Clemente M, et al. 5-Lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol. 2010;184:3978–3987. doi: 10.4049/jimmunol.0901355. [DOI] [PubMed] [Google Scholar]
- 153.Lucas S, Taront S, Magnan C, et al. Interleukin-7 regulates adipose tissue mass and insulin sensitivity in high-fat diet-fed mice through lymphocyte-dependent and independent mechanisms. PLoS One. 2012;7:e40351. doi: 10.1371/journal.pone.0040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miller AM, Asquith DL, Hueber AJ, et al. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650–658. doi: 10.1161/CIRCRESAHA.110.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chavey C, Lazennec G, Lagarrigue S, et al. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 2009;9:339–349. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Levings MK, Schrader JW. IL-4 inhibits the production of TNF-alpha and IL-12 by STAT6-dependent and -independent mechanisms. J Immunol. 1999;162:5224–5229. [PubMed] [Google Scholar]
- 158.Smallie T, Ricchetti G, Horwood NJ, Feldmann M, Clark AR, Williams LM. IL-10 inhibits transcription elongation of the human TNF gene in primary macrophages. J Exp Med. 2010;207:2081–2088. doi: 10.1084/jem.20100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hong EG, Ko HJ, Cho YR, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chang YH, Ho KT, Lu SH, Huang CN, Shiau MY. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int J Obes (Lond) 2012;36:993–998. doi: 10.1038/ijo.2011.168. [DOI] [PubMed] [Google Scholar]
- 161.Olefsky JM. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976;57:1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Olefsky JM, Reaven GM. Effects of age and obesity on insulin binding to isolated adipocytes. Endocrinology. 1975;96:1486–1498. doi: 10.1210/endo-96-6-1486. [DOI] [PubMed] [Google Scholar]
- 163.Grako KA, Olefsky JM, McClain DA. Tyrosine kinase-defective insulin receptors undergo decreased endocytosis but do not affect internalization of normal endogenous insulin receptors. Endocrinology. 1992;130:3441–3452. doi: 10.1210/endo.130.6.1317784. [DOI] [PubMed] [Google Scholar]
- 164.Garvey WT, Olefsky JM, Marshall S. Insulin receptor down-regulation is linked to an insulin-induced postreceptor defect in the glucose transport system in rat adipocytes. J Clin Invest. 1985;76:22–30. doi: 10.1172/JCI111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–S268. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 166.Czech MP. Cellular basis of insulin insensitivity in large rat adipocytes. J Clin Invest. 1976;57:1523–1532. doi: 10.1172/JCI108422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carvalho E, Rondinone C, Smith U. Insulin resistance in fat cells from obese Zucker rats – evidence for an impaired activation and translocation of protein kinase B and glucose transporter 4. Mol Cell Biochem. 2000;206:7–16. doi: 10.1023/a:1007009723616. [DOI] [PubMed] [Google Scholar]
- 168.Shao J, Yamashita H, Qiao L, Friedman JE. Decreased Akt kinase activity and insulin resistance in C57BL/KsJ-Leprdb/db mice. J Endocrinol. 2000;167:107–115. doi: 10.1677/joe.0.1670107. [DOI] [PubMed] [Google Scholar]
- 169.Freidenberg GR, Henry RR, Klein HH, Reichart DR, Olefsky JM. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest. 1987;79:240–250. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Carvalho E, Eliasson B, Wesslau C, Smith U. Impaired phosphorylation and insulin-stimulated translocation to the plasma membrane of protein kinase B/Akt in adipocytes from type II diabetic subjects. Diabetologia. 2000;43:1107–1115. doi: 10.1007/s001250051501. [DOI] [PubMed] [Google Scholar]
- 171.Cleveland-Donovan K, Maile LA, Tsiaras WG, Tchkonia T, Kirkland JL, Boney CM. IGF-I activation of the AKT pathway is impaired in visceral but not subcutaneous preadipocytes from obese subjects. Endocrinology. 2010;151:3752–3763. doi: 10.1210/en.2010-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 173.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ishibashi KI, Imamura T, Sharma PM, Huang J, Ugi S, Olefsky JM. Chronic endothelin-1 treatment leads to heterologous desensitization of insulin signaling in 3T3-L1 adipocytes. J Clin Invest. 2001;107:1193–1202. doi: 10.1172/JCI11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Usui I, Imamura T, Babendure JL, et al. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol Endocrinol. 2005;19:2760–2768. doi: 10.1210/me.2004-0429. [DOI] [PubMed] [Google Scholar]
- 176.Kreier F, Fliers E, Voshol PJ, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. J Clin Invest. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zvonic S, Cornelius P, Stewart WC, Mynatt RL, Stephens JM. The regulation and activation of ciliary neurotrophic factor signaling proteins in adipocytes. J Biol Chem. 2003;278:2228–2235. doi: 10.1074/jbc.M205871200. [DOI] [PubMed] [Google Scholar]
- 178.Scherer T, O’Hare J, Diggs-Andrews K, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J Nutr. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- 180.Hunnicutt JW, Hardy RW, Williford J, McDonald JM. Saturated fatty acid-induced insulin resistance in rat adipocytes. Diabetes. 1994;43:540–545. doi: 10.2337/diab.43.4.540. [DOI] [PubMed] [Google Scholar]
- 181.Dandona P, Aljada A, Mohanty P, et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 182.Dandona P, Chaudhuri A, Mohanty P, Ghanim H. Anti-inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care. 2007;10:511–517. doi: 10.1097/MCO.0b013e3281e38774. [DOI] [PubMed] [Google Scholar]
- 183.Viardot A, Grey ST, Mackay F, Chisholm D. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology. 2007;148:346–353. doi: 10.1210/en.2006-0686. [DOI] [PubMed] [Google Scholar]
- 184.Ghanim H, Korzeniewski K, Sia CL, et al. Suppressive effect of insulin infusion on chemokines and chemokine receptors. Diabetes Care. 2010;33:1103–1108. doi: 10.2337/dc09-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Iida KT, Shimano H, Kawakami Y, et al. Insulin up-regulates tumor necrosis factor-alpha production in macrophages through an extracellular-regulated kinase-dependent pathway. J Biol Chem. 2001;276:32531–32537. doi: 10.1074/jbc.M009894200. [DOI] [PubMed] [Google Scholar]
- 186.McTernan PG, Harte AL, Anderson LA, et al. Insulin and rosiglitazone regulation of lipolysis and lipogenesis in human adipose tissue in vitro. Diabetes. 2002;51:1493–1498. doi: 10.2337/diabetes.51.5.1493. [DOI] [PubMed] [Google Scholar]
- 187.Soop M, Duxbury H, Agwunobi AO, et al. Euglycemic hyperinsulinemia augments the cytokine and endocrine responses to endotoxin in humans. Am J Physiol Endocrinol Metab. 2002;282:E1276–E1285. doi: 10.1152/ajpendo.00535.2001. [DOI] [PubMed] [Google Scholar]
- 188.Siklova-Vitkova M, Polak J, Klimcakova E, et al. Effect of hyperinsulinemia and very-low-calorie diet on interstitial cytokine levels in subcutaneous adipose tissue of obese women. Am J Physiol Endocrinol Metab. 2009;297:E1154–E1161. doi: 10.1152/ajpendo.00086.2009. [DOI] [PubMed] [Google Scholar]
- 189.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–626. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 191.Ye J. Regulation of PPARgamma function by TNF-alpha. Biochem Biophys Res Commun. 2008;374:405–408. doi: 10.1016/j.bbrc.2008.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Czech MP. Regulation of the d-glucose transport system in isolated fat cells. Mol Cell Biochem. 1976;11:51–63. doi: 10.1007/BF01792833. [DOI] [PubMed] [Google Scholar]
- 193.Hoehn KL, Turner N, Cooney GJ, James DE. Phenotypic discrepancies in acetyl-CoA carboxylase 2-deficient mice. J Biol Chem. 2012;287:15801. doi: 10.1074/jbc.O112.356915. author reply 287:15802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shirakami A, Toyonaga T, Tsuruzoe K, et al. Heterozygous knockout of the IRS-1 gene in mice enhances obesity-linked insulin resistance: a possible model for the development of type 2 diabetes. J Endocrinol. 2002;174:309–319. doi: 10.1677/joe.0.1740309. [DOI] [PubMed] [Google Scholar]
- 195.Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW. Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol. 2007;21:215–228. doi: 10.1210/me.2006-0154. [DOI] [PubMed] [Google Scholar]
- 196.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2002;282:E46–E51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- 197.Ortega FJ, Mayas D, Moreno-Navarrete JM, et al. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring) 2010;18:13–20. doi: 10.1038/oby.2009.202. [DOI] [PubMed] [Google Scholar]
- 198.Lan H, Rabaglia ME, Stoehr JP, et al. Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes. 2003;52:688–700. doi: 10.2337/diabetes.52.3.688. [DOI] [PubMed] [Google Scholar]
- 199.Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci U S A. 2000;97:11371–11376. doi: 10.1073/pnas.97.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kolehmainen M, Vidal H, Alhava E, Uusitupa MI. Sterol regulatory element binding protein 1c (SREBP-1c) expression in human obesity. Obes Res. 2001;9:706–712. doi: 10.1038/oby.2001.95. [DOI] [PubMed] [Google Scholar]
- 201.Ducluzeau PH, Perretti N, Laville M, et al. Regulation by insulin of gene expression in human skeletal muscle and adipose tissue. Evidence for specific defects in type 2 diabetes. Diabetes. 2001;50:1134–1142. doi: 10.2337/diabetes.50.5.1134. [DOI] [PubMed] [Google Scholar]
- 202.Sewter C, Berger D, Considine RV, et al. Human obesity and type 2 diabetes are associated with alterations in SREBP1 isoform expression that are reproduced ex vivo by tumor necrosis factor-alpha. Diabetes. 2002;51:1035–1041. doi: 10.2337/diabetes.51.4.1035. [DOI] [PubMed] [Google Scholar]
- 203.Clementi AH, Gaudy AM, van Rooijen N, Pierce RH, Mooney RA. Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling. Biochim Biophys Acta. 2009;1792:1062–1072. doi: 10.1016/j.bbadis.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Roche HM, Noone E, Sewter C, et al. Isomer-dependent metabolic effects of conjugated linoleic acid: insights from molecular markers sterol regulatory element-binding protein-1c and LXRalpha. Diabetes. 2002;51:2037–2044. doi: 10.2337/diabetes.51.7.2037. [DOI] [PubMed] [Google Scholar]
- 206.Endo M, Masaki T, Seike M, Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c) Exp Biol Med (Maywood) 2007;232:614–621. [PubMed] [Google Scholar]
- 207.Lawler JF, Jr, Yin M, Diehl AM, Roberts E, Chatterjee S. Tumor necrosis factor-alpha stimulates the maturation of sterol regulatory element binding protein-1 in human hepatocytes through the action of neutral sphingomyelinase. J Biol Chem. 1998;273:5053–5059. doi: 10.1074/jbc.273.9.5053. [DOI] [PubMed] [Google Scholar]
- 208.Zhang B, Berger J, Hu E, et al. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol Endocrinol. 1996;10:1457–1466. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- 209.Xing H, Northrop JP, Grove JR, Kilpatrick KE, Su JL, Ringold GM. TNF alpha-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARgamma without effects on Pref-1 expression. Endocrinology. 1997;138:2776–2783. doi: 10.1210/endo.138.7.5242. [DOI] [PubMed] [Google Scholar]
- 210.Suzawa M, Takada I, Yanagisawa J, et al. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- 211.Festuccia WT, Blanchard PG, Turcotte V, et al. Depot-specific effects of the PPARgamma agonist rosiglitazone on adipose tissue glucose uptake and metabolism. J Lipid Res. 2009;50:1185–1194. doi: 10.1194/jlr.M800620-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Festuccia WT, Blanchard PG, Turcotte V, et al. The PPARgamma agonist rosiglitazone enhances rat brown adipose tissue lipogenesis from glucose without altering glucose uptake. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1327–R1335. doi: 10.1152/ajpregu.91012.2008. [DOI] [PubMed] [Google Scholar]
- 213.Laplante M, Sell H, MacNaul KL, Richard D, Berger JP, Deshaies Y. PPAR-gamma activation mediates adipose depot-specific effects on gene expression and lipoprotein lipase activity: mechanisms for modulation of postprandial lipemia and differential adipose accretion. Diabetes. 2003;52:291–299. doi: 10.2337/diabetes.52.2.291. [DOI] [PubMed] [Google Scholar]
- 214.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dalen KT, Schoonjans K, Ulven SM, et al. Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes. 2004;53:1243–1252. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 216.Wolins NE, Quaynor BK, Skinner JR, et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 217.Su D, Coudriet GM, Hyun Kim D, et al. FoxO1 links insulin resistance to proinflammatory cytokine IL-1beta production in macrophages. Diabetes. 2009;58:2624–2633. doi: 10.2337/db09-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1 receptor antagonist-treatment of patients with type 2 diabetes. Ugeskr Laeger. 2007;169:3868–3871. [article in Danish] [PubMed] [Google Scholar]
- 220.Stanley TL, Zanni MV, Johnsen S, et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E146–E150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Letexier D, Peroni O, Pinteur C, Beylot M. In vivo expression of carbohydrate responsive element binding protein in lean and obese rats. Diabetes Metab. 2005;31:558–566. doi: 10.1016/s1262-3636(07)70231-8. [DOI] [PubMed] [Google Scholar]
- 222.He Z, Jiang T, Wang Z, Levi M, Li J. Modulation of carbohydrate response element-binding protein gene expression in 3T3-L1 adipocytes and rat adipose tissue. Am J Physiol Endocrinol Metab. 2004;287:E424–E430. doi: 10.1152/ajpendo.00568.2003. [DOI] [PubMed] [Google Scholar]
- 223.Hurtado del Pozo C, Vesperinas-Garcia G, Rubio MA, et al. ChREBP expression in the liver, adipose tissue and differentiated preadipocytes in human obesity. Biochim Biophys Acta. 2011;1811:1194–1200. doi: 10.1016/j.bbalip.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 224.Koranyi L, James D, Mueckler M, Permutt MA. Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. J Clin Invest. 1990;85:962–967. doi: 10.1172/JCI114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Garvey WT, Maianu L, Huecksteadt TP, Birnbaum MJ, Molina JM, Ciaraldi TP. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest. 1991;87:1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lodhi IJ, Yin L, Jensen-Urstad AP, et al. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab. 2012;16:189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu LH, Wang XK, Hu YD, Kang JL, Wang LL, Li S. Effects of a fatty acid synthase inhibitor on adipocyte differentiation of mouse 3T3-L1 cells. Acta Pharmacol Sin. 2004;25:1052–1057. [PubMed] [Google Scholar]
- 228.Schmid B, Rippmann JF, Tadayyon M, Hamilton BS. Inhibition of fatty acid synthase prevents preadipocyte differentiation. Biochem Biophys Res Commun. 2005;328:1073–1082. doi: 10.1016/j.bbrc.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 229.Jiang G, Dallas-Yang Q, Li Z, et al. Potentiation of insulin signaling in tissues of Zucker obese rats after acute and long-term treatment with PPARgamma agonists. Diabetes. 2002;51:2412–2419. doi: 10.2337/diabetes.51.8.2412. [DOI] [PubMed] [Google Scholar]
- 230.Jiang G, Dallas-Yang Q, Biswas S, Li Z, Zhang BB. Rosiglitazone, an agonist of peroxisome-proliferator-activated receptor gamma (PPARgamma), decreases inhibitory serine phosphorylation of IRS1 in vitro and in vivo. Biochem J. 2004;377:339–346. doi: 10.1042/BJ20031207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Leonardini A, Laviola L, Perrini S, Natalicchio A, Giorgino F. Cross-talk between PPARgamma and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009;2009 doi: 10.1155/2009/818945. 818945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang X, Zhang X, Heckmann BL, Lu X, Liu J. Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-alpha (TNF-alpha)-induced lipolysis in adipocytes. J Biol Chem. 2011;286:40477–40485. doi: 10.1074/jbc.M111.257923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 234.Chen X, Xun K, Chen L, Wang Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27:407–416. doi: 10.1002/cbf.1596. [DOI] [PubMed] [Google Scholar]
- 235.Weiner FR, Smith PJ, Wertheimer S, Rubin CS. Regulation of gene expression by insulin and tumor necrosis factor alpha in 3T3-L1 cells. Modulation of the transcription of genes encoding acyl-CoA synthetase and stearoyl-CoA desaturase-1. J Biol Chem. 1991;266:23525–23528. [PubMed] [Google Scholar]
- 236.Delikat SE, Galvani DW, Zuzel M. The metabolic effects of interleukin 1 beta on human bone marrow adipocytes. Cytokine. 1995;7:338–343. doi: 10.1006/cyto.1995.0043. [DOI] [PubMed] [Google Scholar]
- 237.McGillicuddy FC, Harford KA, Reynolds CM, et al. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 2011;60:1688–1698. doi: 10.2337/db10-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.de Roos B, Rungapamestry V, Ross K, et al. Attenuation of inflammation and cellular stress-related pathways maintains insulin sensitivity in obese type I interleukin-1 receptor knockout mice on a high-fat diet. Proteomics. 2009;9:3244–3256. doi: 10.1002/pmic.200800761. [DOI] [PubMed] [Google Scholar]
- 239.Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun. 2003;311:372–379. doi: 10.1016/j.bbrc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 240.Matthews VB, Allen TL, Risis S, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53:2431–2441. doi: 10.1007/s00125-010-1865-y. [DOI] [PubMed] [Google Scholar]
- 241.Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab. 2004;287:E182–E187. doi: 10.1152/ajpendo.00189.2003. [DOI] [PubMed] [Google Scholar]
- 242.O’Rourke RW, White AE, Metcalf MD, et al. Systemic inflammation and insulin sensitivity in obese IFN-gamma knockout mice. Metabolism. 2012;61:1152–1161. doi: 10.1016/j.metabol.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kowalski GM, Nicholls HT, Risis S, et al. Deficiency of haematopoietic-cell-derived IL-10 does not exacerbate high-fat-diet-induced inflammation or insulin resistance in mice. Diabetologia. 2011;54:888–899. doi: 10.1007/s00125-010-2020-5. [DOI] [PubMed] [Google Scholar]
- 244.Perrier S, Darakhshan F, Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett. 2006;580:6289–6294. doi: 10.1016/j.febslet.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 245.Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–2218. doi: 10.1210/en.2007-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Somm E, Henrichot E, Pernin A, et al. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes. 2005;54:3503–3509. doi: 10.2337/diabetes.54.12.3503. [DOI] [PubMed] [Google Scholar]