Abstract
There is little doubt that the discovery of ischemic preconditioning (PC) has been one of the fundamental milestones in the field of ischemic biology in the past 20 years. The purpose of this article is to review the pathophysiology and molecular basis of the late phase of myocardial PC. The exploitation of late PC for the development of novel gene therapy strategies aimed at inducing a permanently preconditioned cardiac phenotype (prophylactic cardioprotection) will also be discussed. Deciphering the mechanism of late PC has not only conceptual interest but also a considerable therapeutic implications, since transfer of the genes that underlie late PC would be expected to replicate the salubrious effects of this response of the heart to stress.
Keywords: Nitric oxide synthase, Protection, Cyclooxygenase-2, Late preconditioning
Introduction
Ischemic preconditioning (PC) is an endogenous protective mechanism activated by a mild ischemic stress that enhances the ability of the heart to cope with another similar or greater stress. In this sense, ischemic PC can be viewed as a “vaccination” of sort against myocardial ischemia/reperfusion injury. The important conceptual implication of the discovery of PC has been the realization that the heart can sense and adapt to the environment: when exposed to a stress, it can change its phenotype in a manner that promotes self-preservation. This innate plasticity of the heart was not appreciated until the discovery of PC, and has major pathophysiological and therapeutic implications.
Preconditioning was described in 1986 by Murry, Jennings, and Reimer in a landmark study [1] that has been cited more than 2750 times as of January 31, 2007. These authors made what at that time appeared to be a paradoxical observation. They exposed open-chest dogs to a sequence of four brief ischemic episodes (5-min coronary occlusions interspersed with 5-min reperfusion periods) and then subjected them to a prolonged, more severe ischemic insult (a 40-min coronary occlusion followed by 4 days of reperfusion). Control dogs underwent the 40-min occlusion with no preceding exposure to ischemia. According to conventional wisdom at that time, one would have expected that the dogs that received the four brief coronary occlusions would exhibit greater infarct size, because they had been exposed to more ischemia (an additional 20 min). Surprisingly, Murry et al. found that in this group of dogs infarct size was markedly reduced compared with controls, and that this effect was independent of differences in coronary collateral blood flow. They coined the term “ischemic PC” to describe this phenomenon, opening the floodgates to what has become one of the major themes of research in cardiovascular medicine. Following the seminal observation of Murry et al., the number of studies dealing with PC has escalated dramatically, so that in 2006 over 500 papers were published on this subject. Although initially almost all studies were experimental, clinical studies soon appeared and their number have continued to increase—a testimony to the growing level of interest among clinicians in the phenomenon of PC.
There is a very good reason for this extraordinary explosion of interest in PC. During the past 35 years, hundreds of direct cardioprotective therapies have been claimed to reduce myocardial infarct size in experimental animals. However, a few of these results have been reproducible, and none has been translated into clinical therapies [2]. As a result, after all the enormous amount of time, money, and resources that have been invested into the search for cardioprotective therapies for 35 years, after the endless claims that we have found a drug that will limit infarct size, we are faced with the sobering (and somewhat embarrassing) reality that we still do not have a therapy that has been specifically approved for the reduction of infarct size in patients with acute myocardial infarction. (The reason(s) for this colossal failure are complex and beyond the scope of the present discussion; the reader interested in this topic is referred to an article that has examined this problem in detail [2]). In the midst of this frustration with the failure to identify clinically effective cardioprotective therapies came the discovery of ischemic PC. It became quickly apparent that ischemic PC was quite different from anything that had been tested before. First, it was clear that this cardioprotective phenomenon was extremely powerful. In many experimental models, ischemic PC can reduce infarct size by as much as 80–90%—a degree of infarct size limitation that is rarely, if ever, observed with other interventions. Second, and more important, ischemic PC was found to be remarkably reproducible. Indeed, it has been consistently observed in every experimental model, in every laboratory, and in every species examined. In fact, it is very difficult, if not impossible, to find a study in which the protective effects of ischemic PC have not been observed. This is in stark contrast to other cardioprotective therapies, which are eminently non-reproducible, often being found to work in some models but not in others, in some laboratories but not in others, and in some species but not in others. Importantly, ischemic PC has been observed in humans as well [3, 4]. Given these facts, the enthusiasm of the scientific community for ischemic PC is easy to understand: Investigators interested in cardioprotection had finally found an intervention that was not only highly effective but also totally reproducible.
Naturally, if ischemic PC is to become a clinical tool, a stimulus less noxious and unpleasant than ischemia needs to be found. This has indeed happened. Although PC was initially described as a response of the myocardium to ischemia, it soon became apparent that a similar phenotype could be elicited by a host of stimuli, some of which are clinically relevant. For example, a number of pharmacological agents, including agonists of G-protein coupled receptors (adenosine A1 or A3, bradykinin B2, α1 adrenergic, muscarinic M2, angiotensin AT1, endothelin, δ1 opioid, etc.), nitric oxide (NO) donors, phosphodiesterase inhibitors, and various noxious stimuli (such as endotoxin and endotoxin derivatives, various cytokines, reactive oxygen species, etc.) have all been found to elicit a PC-like phenotype [3, 4]. Indeed, it appears that almost anything that is potentially harmful to the heart can elicit a preconditioned state when applied in small quantities. PC can also be induced by non-pharmacological stimuli, such as physical exercise (even short-term exercise for a few days is sufficient to induce a PC state [5]), heat stress, rapid pacing, and other maneuvers. The importance of these findings is that one need not use ischemia to induce a cardioprotected phenotype; stimuli that are less harmful or unpleasant (and thus more clinically relevant) can also do this.
After the initial description of PC in 1986 [1], the next major discovery was in 1993, when it was found that this phenomenon is not monolithic but rather consist of two chronologically and pathophysiologically distinct phases: an early phase, which develops very quickly (within few minutes from the exposure to the stimulus) but is rather ephemeral, lasting only 1–2 h (this is the phenomenon originally described by Murry et al. [1]), and a late phase, which develops more slowly (requiring 6–12 h) but lasts much longer (3–4 days) [6, 7]. The mechanism for these two phases is completely different. The early phase is caused by rapid post translational modification of preexisting proteins, whereas the late phase is caused by the synthesis of new cardioprotective proteins (which explains the time-course of this phenomenon). The range of protection is also different. The early phase is very effective in limiting lethal ischemia/reperfusion injury (i.e., infarction) but does not protect against reversible postischemic contractile dysfunction (myocardial “stunning”). The late phase protects against both against infarction and stunning, although it is less powerful than the early phase in limiting infarct size. The ability of either phase to prevent arrhythmias associated with ischemia/reperfusion injury remains unclear.
For the remainder of this discussion, it is important to distinguish ischemic PC from direct cardioprotection. Both of these processes enhance the resistance of the heart to ischemia/reperfusion injury; however, they are fundamentally different. With PC, the protection persists after the therapeutic intervention has dissipated, implying that the heart has a “memory” (i.e., the heart “remembers” that it has been exposed to a PC stimulus and maintains a preconditioned phenotype even after the stimulus has been withdrawn). In contrast, with direct cardioprotection the enhanced resistance to ischemia/reperfusion injury occurs only while the therapeutic intervention is being applied and requires the continued presence of this intervention; when the intervention is withdrawn, the protection disappears (i.e., the heart does not keep a “memory” of the therapeutic intervention). Thus, PC is an after-effect that becomes manifest following exposure to a stimulus, whereas direct cardioprotection is the effect that occurs during the stimulus. It is easy to confuse these phenomena when one examines the effects of pharmacologic agents. For example, when chronic treatment with a drug is associated with cardioprotection, this is sometimes referred to as “PC”. However, a drug can be said to precondition only if the protection continues after the drug has disappeared from the cardiac tissue; if the protection fades as soon as the drug is gone, then one is dealing with a direct cardioprotective effect, not a PC effect.
Late phase of PC
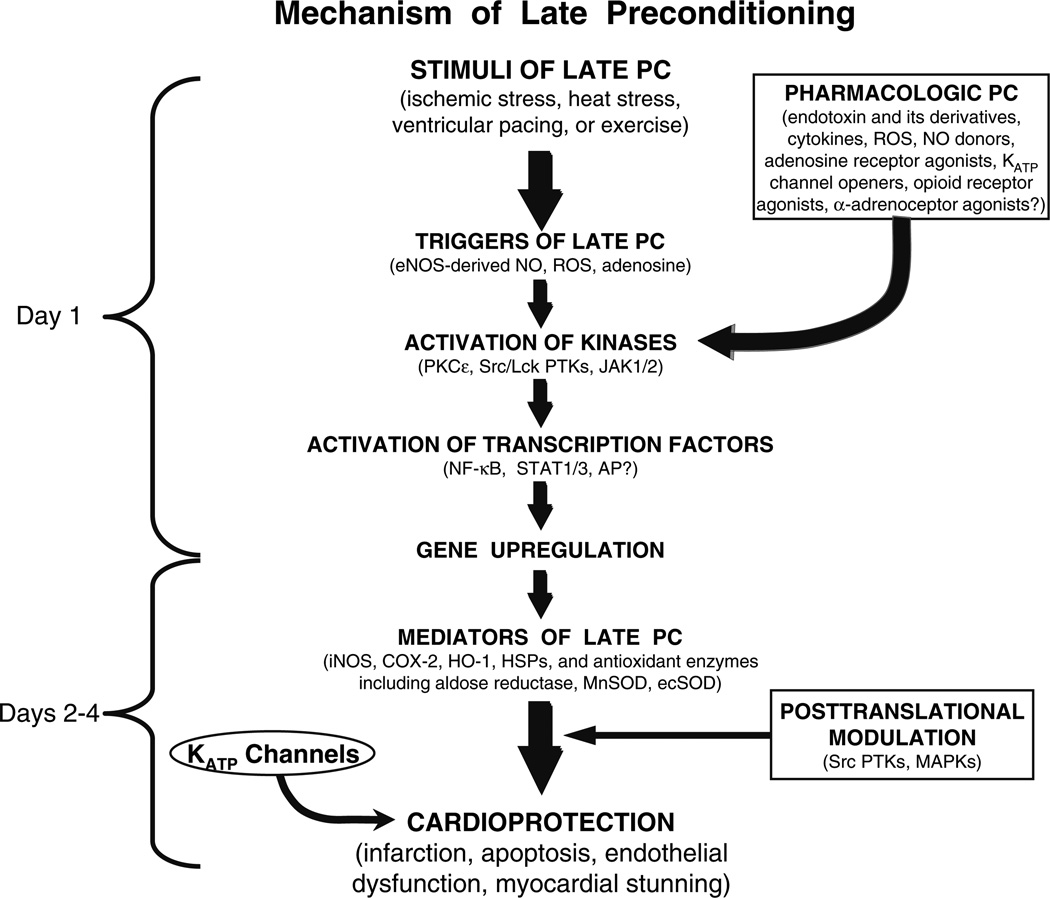
The late phase of PC is likely to have greater clinical relevance, because it has a broader range of protection, and because its duration is 30–40 times greater than that of the early phase. Accordingly, this article will focus on the late phase of PC. The fundamental question to be addressed here pertains to the mechanism of the late phase of PC, because if this issue is not resolved, therapeutic exploitation of this phenomenon will be difficult if not impossible. Figure 1 summarizes our current understanding of the mechanism of late PC. As shown in this figure, a mild, sub-lethal ischemic stress causes the release of chemical signals that are referred to as “triggers” of late PC. These signals can be viewed as “alarm bells” that go off in the presence of a threat, telling the heart that there is trouble looming on the horizon and that it needs to get ready for the impending threat. Numerous triggers have been identified for the late phase of ischemic PC; among them, NO (generated by endothelial NO synthase [eNOS]), reactive oxygen species, opioid agonists, and adenosine play a prominent role, but many other stimuli are likely to be involved as well. The release of these chemicals causes the activation of a complex (and yet incompletely understood) signal transduction cascade that includes the epsilon isoform of protein kinase C (PKC), the Src/Lck isoforms of tyrosine kinases, Janus kinases 1 and 2 (JAK1/ 2), and probably other kinases as well, resulting in the activation of cytoplasmic and normally dormant stress-responsive transcription factors, such as NF-κB, STAT 1 and STAT 3, and almost certainly many other transcriptional regulators. This process culminates in the upregulation of cardioprotective genes, leading to the synthesis of new proteins that mediate the protection afforded by late PC.
Figure 1.
Schematic representation of the cellular mechanisms underlying late PC. A nonlethal cellular stress (eg, reversible ischemia, heat stress, ventricular pacing, or exercise) causes release of chemical signals (NO, ROS, adenosine, and possibly opioid receptor agonists) that trigger the development of late PC (and thus are referred to as “triggers”). These substances activate a complex signal transduction cascade that includes PKC (specifically, the ε isoform), protein tyrosine kinases (specifically, Src and/or Lck), and probably other as-yet-unknown kinases. A similar activation of PKC and downstream kinases can be elicited pharmacologically by a wide variety of agents, including naturally occurring—and often noxious—substances (eg, endotoxin, interleukin-1, TNF-α, TNF-β, leukemia inhibitor factor, or ROS), as well as clinically applicable drugs (NO donors, adenosine A1 or A3 receptor agonists, endotoxin derivatives, or δ1-opioid receptor agonists). The recruitment of PKC and distal kinases leads to activation of NF-κB, STAT1/3, and almost certainly other transcription factors, resulting in increased transcription of multiple cardioprotective genes and synthesis of multiple cardioprotective proteins that serve as comediators of protection 2–4 days after the PC stimulus. The mediators of late PC identified thus far include iNOS, COX-2, HO-1, aldose reductase, ecSOD, and Mn SOD. Among the products of COX-2, PGE2 and/or PGI2 appear to be the most likely effectors of COX-2-dependent protection. Increased synthesis of HSPs is unlikely to be a mechanism of late PC, although the role of posttranslational modification of preexisting HSPs remains to be determined. In addition, the occurrence of cardioprotection on days 2 to 4 requires the activity of protein tyrosine kinases and possibly p38 MAPKs, potentially because iNOS and other mediators need to undergo posttranslational modulation to confer protection against ischemia. Opening of KATP channels is also essential for the protection against infarction (but not against stunning) to become manifest. Although solid evidence indicates that COX-2 is downstream of iNOS (i.e., COX-2 is activated by NO) and that NO induces HO-1, the exact interrelationships among iNOS, COX-2, HO-1, aldose reductase, ecSOD, Mn SOD, and KATP channels remain to be elucidated
The importance of NOS in PC
The first gene to be identified as a mediator of late PC was the inducible isoform of NOS (iNOS) [8, 9]; subsequently, other genes have been discovered, including cyclooxy-genase-2 (COX-2), heme oxygenase-1 (HO-1), and antioxidant enzymes such as extracellular SOD (ecSOD), aldose reductase, manganese SOD, etc. [3, 10–12]. Heat-stress proteins have been suggested to contribute to late PC but this remains to be confirmed. Thus, late PC is a genetic reprogramming of the heart that is elicited by the exposure to potentially threatening stimuli and is mediated by the activation of stress-responsive and protective genes.
It is instructive to discuss the mediators of late PC in the chronological order in which they were discovered. As mentioned above, iNOS was the first protein to be identified as a mediator of late PC [8, 9]. Approximately 10 years ago, we postulated that iNOS could participate in late PC, because this enzyme is known to be induced by stress and since NO possesses a host of cardioprotective actions that would be expected to be beneficial during myocardial ischemia/reperfusion [11]. Indeed, the literature is replete with studies documenting the salubrious actions of NO during myocardial ischemia/reperfusion injury [11]. For example, in the decade 1990–2000 a total of 92 full-length articles addressed the role of NO in modulating myocardial ischemia/reperfusion injury [11]. Of these, 67 concluded that NO is beneficial and only 11 that NO is detrimental; there were, however, methodological problems with many of these 11 “negative” studies. The precise mechanism whereby NO induces cardioprotection is beyond the scope of this review; the reader is referred to a recent review on this topic [12]. Briefly, plausible mechanisms whereby NO enhances resistance to cell death following ischemia/reperfusion include inhibition of calcium influx, antagonism of beta-adrenergic stimulation, reduction in myocardial oxygen demands, opening of sar-colemmal and/or mitochondrial KATP channels, activation of COX-2 leading to the synthesis of cardioprotective prostanoids, and possibly direct antioxidant actions, such as inhibition of the effects of superoxide anion () and peroxynitrite (ONOO−).
To determine, whether iNOS mediates the late phase of ischemic PC, we undertook a study in mice [9]. First, we asked whether iNOS is upregulated by ischemic PC. We found that 24 h after exposure of the heart to an ischemic PC protocol (6 cycles of 4-min occlusion/4-min reperfu-sion), there was a significant, albeit modest, increase in the total content of iNOS in the ischemic/reperfused region (+40%). The small magnitude of this increase is important because iNOS is known to be toxic when expressed at very high levels; we propose that a mild upregulation of iNOS is beneficial whereas a severe upregulation can be detrimental. The increased protein expression was associated with increased activity (as determined by the L-citrulline assay), documenting that the protein is functionally competent. Of course, just because iNOS is upregulated 24 h after ischemic PC does not mean that the protein is involved in the genesis of this phenomenon. Myriad proteins are up-regulated following ischemic PC, and most of them have nothing to do with the protection (i.e., they represent epi-phenomena). To determine whether the upregulation of iNOS was causally related to protection, we performed the second phase of the study in which either wild-type (WT) or iNOS−/− mice that were subjected to myocardial infarction (30-min coronary occlusion followed by 24 h of rep-erfusion) [9]. We found that under control (unstressed) conditions, infarct size was similar in WT and iNOS−/− mice, implying that iNOS does not modulate myocardial ischemia/reperfusion injury in the absence of stress. This was not surprising, as myocardial iNOS is barely detectable under control conditions. Subjecting mice to a sham PC protocol (open-chest state for 1 h with no coronary occlusion) had no detectable effect on infarct size in WT or iNOS−/− mice. When WT mice were subjected to ischemic PC 24 h earlier (six 4-min occlusion/4-min reperfusion cycles), infarct size was markedly reduced compared with sham-operated controls, indicating a robust late PC effect. However, when iNOS−/− mice were subjected to the same ischemic PC protocol, infarct size was similar to that observed in sham-operated mice. Thus, in the absence of iNOS, the late phase of ischemic PC failed to develop [9]. To determine whether this was due to an inherent inability of the myocardium of iNOS−/− mice to be preconditioned, we also studied the early phase of ischemic PC. WT and iNOS−/− mice were subjected to 6 cycles of 4-min occlusion/4-min reperfusion and then, 10 min later were subjected to a 30-min coronary occlusion followed by 24 h reperfusion. Both WT and iNOS−/− mice exhibited a pronounced reduction in infarct size, of comparable magnitude, demonstrating that iNOS−/− myocardium can indeed be protected and that iNOS is not required for the early phase of ischemic PC. Collectively, these results provided conclusive molecular genetic evidence that iNOS is necessary for the protective effects of late PC to occur [11].
An important concept stemming from our studies of iNOS is that the cellular type that expresses this protein is crucial in determining its effects on ischemia/reperfusion injury. Indeed, we found that, following ischemic PC, iNOS is upregulated in cardiac myocytes, as demonstrated both by in situ hybridization at 3 h after the PC stimulus and by iNOS immunohistochemistry at 24 h after the PC stimulus [13]. In contrast, in the setting of an infarction (permanent coronary occlusion), we found, using both in situ hybridization and immunohistochemistry, that iNOS was expressed in inflammatory cells (at 48–66 h after occlusion), but not in surviving myocytes. We propose that the expression of iNOS in cells such as myocytes that do not produce large quantities of is protective, whereas its expression in cells that generate large quantities of (e.g., macrophages, neutrophils, etc.) is detrimental, because of the formation of ONOO−. Another important difference between the upregulation of iNOS in ischemic PC and that observed in other pathophysiological situations is the magnitude of protein expression. As mentioned above, the increase in cardiac iNOS following ischemic PC is mild (approximately 40%), whereas after application of endotoxin (lipopolysaccharide), it is massive (>30-fold) [13].
In summary, we propose that upregulation of iNOS has a pronounced dose-dependent effect; at low levels, iNOS is beneficial if it increases in cells that do not produce , whereas at high levels iNOS could be detrimental, particularly if it is upregulated in an environment or a cell type that is associated with increased ROS generation.
It is apparent from these data that NO plays a pivotal role both as a trigger of late PC, at the beginning of the cascade, and as a mediator of late PC, at the end of the cascade [10] (Fig. 1). This was somewhat unexpected; conceptually, there is no a priori reason why the same molecule should serve as a trigger as well as a mediator. Nevertheless, these observations emphasize the fundamental role of NO in late PC: that is, NO plays a dual role in the late phase of this phenomenon, acting initially as its trigger and subsequently as its mediator. (Interestingly, in contrast to late PC, eNOS is not necessary for the early phase of ischemic PC; in recent studies we have found that ischemia fully induces the early phase of PC in eNOS null mice, thereby refuting the theory that eNOS is a necessary component of the mechanism of early PC.)
When we discovered the role of iNOS in late PC, we assumed that the problem of identifying the mediator of late PC had been solved. It soon became apparent, however, that late PC is not mediated by just one protein, but rather by a battery of cardioprotective proteins, all of which are necessary for the cardioprotected phenotype to appear [3, 11, 14]. Thus, late PC should be viewed as a polygenic adaptation of the heart to stress that is underlain by the concerted activation of a number of cardioprotective genes that act in a coordinated fashion to confer resistance to ischemia/reperfusion injury.
COX-2 Involvement in PC
The second protein (after iNOS) to be identified as a mediator of late PC was COX-2, which is known to be frequently co-induced with iNOS in response to stress. It has been known for many years that prostanoids (such as prostacyclin [PGI2] and PGE2) are protective during myocardial ischemia/reperfusion [14]. The exact mechanism for this beneficial effect has not been pinpointed, but it probably involves a combination of actions, including antagonism of adenylyl cyclase, opening of KATP channels, inhibition of calcium influx, anti-adrenergic actions, and possibly attenuation of neutrophil infiltration. Some of these actions are reminiscent of those that underlie the protective actions of NO [14]. On the basis of these facts, we postulated that COX-2 could be involved as a co-mediator of late PC together with iNOS. To test this hypothesis, we utilized a conscious rabbit model of late PC and first asked the question of whether ischemic PC upregulates COX-2 expression [15]. We found that following a standard ischemic PC protocol (six 4-min occlusion/4-min reperfusion cycles), the COX-2 protein content in the ischemic/reperfused region was robustly increased 24 h later [15]. This was associated with an increased content of the byproducts of COX-2 activity, mainly PGE2 and 6-keto-PGF1α (the stable byproduct of PGI2). As in the case of iNOS, however, the mere fact that COX-2 is upregulated does not signify that this protein participates in the genesis of the protection. To establish, whether the increased activity and expression of COX-2 are causally linked to the protection of late PC, we subjected rabbits to a 30-min coronary occlusion followed by 72 h of reperfusion in the presence or absence of ischemic PC 24 h earlier. Treated rabbits received a selective COX-2 inhibitor (either NS-398 or celecoxib) on day 2, just prior to the 30-min occlusion, in order to inhibit COX-2 activity during the development of infarction (when it would be expected to exert protection). We reasoned that if the increased production of COX-2 metabolites plays a necessary role in the protection of late PC, pretreatment with COX-2 inhibitors ought to abolish the protection; conversely, if the increased activity of COX-2 is not responsible for protection, the administration of COX-2 inhibitors should have no effect. First, we documented that the doses of NS-398 and celecoxib that we used were able to prevent the increase in the myocardial levels of PGE2 and 6-keto-PGF1α, i.e., that they completely inhibited COX-2 activity 24 h after ischemic PC. Next, we tested the effect of these same doses on protection (infarct size). Our results demonstrated that following pretreatment with either NS-398 or celecoxib, the reduction in infarct size observed during late PC in control rabbits was completely abrogated, indicating that COX-2 activity is necessary [15]. These studies were the first to demonstrate that COX-2 is an obligatory mediator of the cardioprotective effects of late PC.
The recognition that COX-2 is a necessary mediator of the salubrious effects of late PC [14] came as a surprise, because this protein was commonly thought to exert nefarious effects on the cardiovascular system, largely because of its postulated involvement in atherosclerosis. It may well be that the role of COX-2 in vascular lesions is detrimental; however, our results demonstrate that this concept does not apply when it comes to myocardial ischemia/reperfusion injury. Our results [15] impelled a reassessment of current views regarding COX-2, revealing a heretofore unappreciated role of this enzyme as a fundamental cardioprotectant. Indeed, the demonstration that COX-2 is a major cardioprotective protein that has been an important paradigm shift in cardiovascular biology. It should be noted that this discovery [15] occurred several years before the highly publicized controversy on the cardiovascular effects of COX-2 inhibitors erupted. Although most of the publicity has focused on the pro-thrombotic effects of COX-2 inhibitors, we suggest that a fundamental reason for their adverse cardiovascular profile is that they deprive the heart of its innate ability to shift to a preconditioned (protected) phenotype in response to stress [16].
The discovery that both iNOS and COX-2 mediate late PC (Fig. 1) begged the obvious question of whether these two proteins act in concert or independently. To address this issue, we conducted a series of studies in mice and rabbits, using both pharmacologic and genetic inhibition of COX-2 [17, 18]. When COX-2−/− mice were subjected to ischemic PC, there was, 24 h later, an increase in iNOS protein expression and activity, as expected [18]. When iNOS−/− mice were subjected to ischemic PC, COX-2 protein expression was increased 24 h later, as expected; however, the increase COX-2 protein content was not accompanied by increased myocardial content of COX-2 byproducts (PGF2 and 6-keto-PGF1α), indicating that COX-2 was not active. Thus, the absence of COX-2 did not affect iNOS expression or activity following ischemic PC; in contrast, the absence of iNOS prevented the activity of the induced COX-2 protein following ischemic PC [18]. We also found that iNOS and COX-2 coprecipitated 24 h after ischemic PC in homogenates of preconditioned myocardium [18], indicating a physical interaction between these two proteins. A recent study has confirmed the co-precipitation of iNOS and COX-2 in non-myocytes [19]; however, it was the study by Xuan et al. [18] that provided the first evidence for this phenomenon. In keeping with these mouse studies, we have obtained similar results in conscious rabbits, in which we found that pharmacologic inhibition of COX-2 had no effect on iNOS activity whereas pharmacologic inhibition of iNOS with 1400W blocked the increased COX-2 activity [17]. In this latter study, administration of the soluble guanylate cyclase inhibitor ODQ did not block COX-2 activity, indicating that NO activates COX-2 via cGMP-independent mechanisms [17]. Together, these studies [17, 18] demonstrate that, in late PC, COX-2 activity requires iNOS-derived NO whereas iNOS activity is independent of COX-2-derived prostanoids, implying that COX-2 is located downstream of iNOS in the protective pathway of late PC. Thus, these two proteins act in series, with iNOS being the protein that drives COX-2 activity.
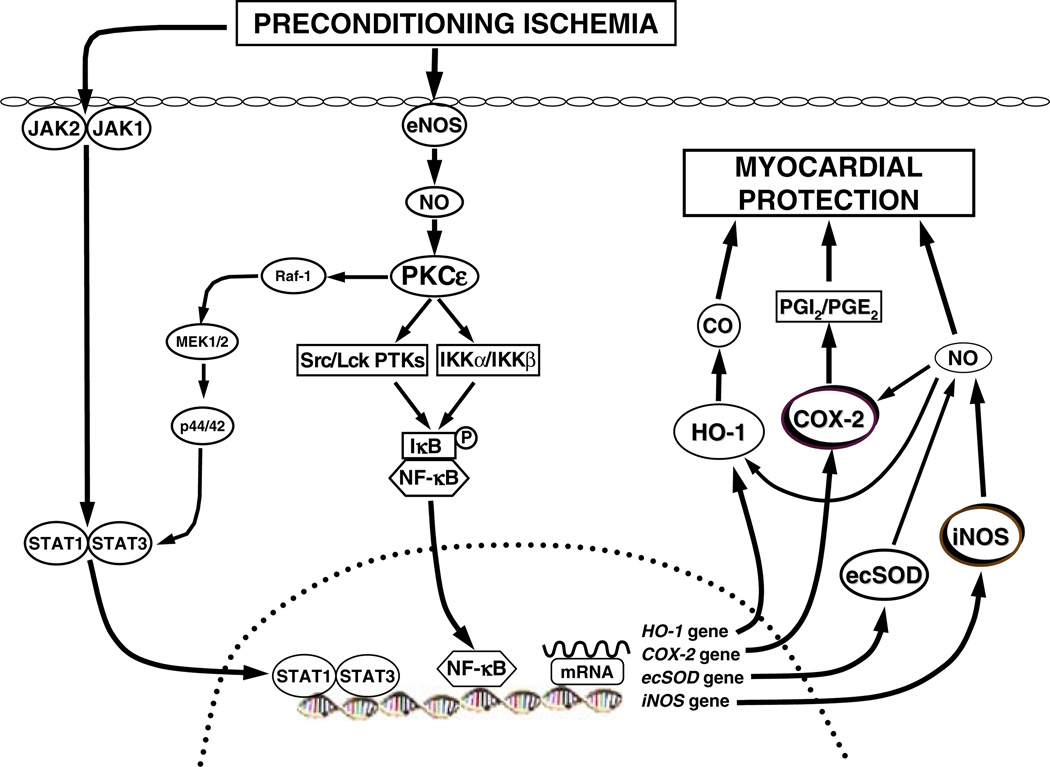
Figure 2 summarizes our view on the mechanism of late PC, based upon the past 15 years of research. As can be seen, the initial PC ischemia activates at least two signaling pathways that act in parallel: the PKCε-Src/Lck-NF-κB pathway and the JAK1/JAK2-STAT1/STAT3 pathway. These pathways lead to nuclear translocation of NF-κB and STAT1/STAT3, which bind to the cognate promoter sequences of the iNOS and COX-2 genes, resulting in increased synthesis of the respective proteins. iNOS produces NO, which is in itself cardioprotective but also acts by activating COX-2, leading to the synthesis of the cytoprotective prostanoids PGI2 and PGE2. The combined (and possibly synergistic) actions NO and PGI2/PGE2 result in myocardial protection.
Figure 2.
Schematic representation of the mechanism of late PC. The PC stimulus activates at least two signaling pathways: The PKCε-Src/Lck-NF-κB pathway and the JAK1/JAK2-STAT1/STAT3 pathway. PKCs activates the Src and Lck tyrosine kinases leading to tyrosine phosphorylation of IκBα (the inhibitor of NF-κB); in addition, PKCε directly activates IKKα/IKKβ, leading to inhibition of IκBα. NF-κB translocates to the nucleus where it binds to its cognate sequence on the promoter region of target genes, including iNOS and COX-2. The PC ischemia also activates JAK1 and JAK2, which then tyrosine phosphorylate and activate STAT1 and STAT3. In addition to tyrosine phosphorylation, full activation of STAT1 and STAT3 requires also serine phosphorylation via a PKCε-Raf-1-MEK1/2-p44/42 MAPK signaling pathway. The activated STAT1/STAT3 hetero-dimer translocates to the nucleus where it binds to the promoter of target genes. The combinatorial actions of NF-κB and STAT1/STAT3, and almost certainly other transcription factors, result in transcriptional activation of iNOS, COX-2, HO-1, and ecSOD, leading to the synthesis of the respective proteins. (A multitude of transcription factors are likely to be mobilized during late PC and to lead to the recruitment of cardioprotective genes, acting in concert.) iNOS produces NO which directly protects the myocardium; in addition, NO activates COX-2, leading to the synthesis of cardioprotective prostanoids, mainly PGI2 and PGE2. It follows from this scheme that the activity of newly-synthesized COX-2 protein requires iNOS-dependent NO generation whereas the activity of iNOS does not require COX-2-dependent prostanoid generation; thus, COX-2 is downstream of iNOS in the pathophysiological cascade of late PC. In addition, NO can also induce HO-1. Thus, iNOS-derived NO can protect the myocardium from recurrent ischemia via direct actions, via activation of COX-2-dependent synthesis of cardioprotective prostanoids, and via generation of HO-1 byproducts (CO and biliverdin). Among the products of COX-2, PGE2 and/or PGI2 appear to be the most likely effectors of cytoprotection. A similar upregulation of COX-2 can be elicited pharmacologically by δ-opioid receptor agonists but not by adenosine A1 or A3 receptor agonists
Before leaving this topic, an important caveat must be mentioned, i.e., that virtually all of the studies of PC described above were performed in relatively young, healthy animals. Whether the results of these studies apply to animals (or humans) that are old or have risk factors for cardiovascular disease (e.g., hypercholesterolemia, diabetes, hypertension) is an important issue that needs to be thoroughly investigated. Considerable evidence indicates that hypercholesterolemia abrogates both early [20] and late PC [21, 22], although the effects on early PC remain controversial [23]. In addition, it appears that early and late PC are either attenuated or absent in the presence of diabetes [24–26]. No information is available regarding the impact of hypertension on PC. The effect of aging on PC is unclear. Some studies [27, 28, 29] but not others [30] support the concept that the early PC response is absent in old animals. Aging abolishes the late phase of ischemia-induced PC in mice [31] but not the late phase of opioid-induced late PC in rats [32]. Since most patients with ischemic heart disease are old and/or have risk factors, defining the impact of these conditions on PC and its mechanism will be of the utmost importance in future studies.
Late PC: A template for prophylactic cardioprotection
Besides the intellectual drive to unravel the molecular basis of late PC, our motivation to study the mechanism of this powerful adaptation to stress stemmed from a desire to exploit this phenomenon for therapeutic purposes. From the outset 15 years ago, it seemed clear to us that if we could understand how PC makes the heart resistant to ischemia, it should then be possible to induce this phenomenon in patients at risk for myocardial infarction. One could argue that the use of PC mimetic strategies is not necessary and that protection of ischemic myocardium in patients can be achieved using direct cardioprotective strategies (rather than PC); however, there are several inherent limitations in this approach. Direct cardioprotection in patients can usually be implemented only by treating at the time of reperfusion (when the patient presents to the hospital). Unfortunately, therapies started at reperfusion have limited efficacy, and particularly so in patients, in whom, in most cases, reperfusion cannot be implemented until 4–5 h after the onset of symptoms. By that time, it is likely that a significant part (perhaps most) of the damage has already occurred. Administration of an infarct-sparing drug 4–5 h after the onset of myocardial infarction is too late, and unfortunately this is the predicament in which most patients find themselves. On the other hand, it is usually impossible to pretreat patients because the onset of an acute infarction is unpredictable. In theory one could administer infarct-sparing agents chronically in anticipation of an infarct, but this would be an exceptionally cumbersome and expensive approach to infarct size reduction. Similarly, chronic treatment with a PC-mimetic agent in anticipation of an ischemic event that may not occur for years and may never occur would be quite impractical. Consequently, we reasoned that the most efficacious strategy to limit infarct size might be to induce a chronically protected (defensive) cardiac phenotype analogous to that associated with late PC, by transferring the genes responsible for late PC. We refer to this approach as prophylactic cardioprotection.
We believe that the most important implication of understanding the mechanism of late PC is that this knowledge offers a template for implementing prophylactic cardioprotection in patients, using gene therapy. The rationale is clear. As discussed above, late PC is a genetic reprogramming of the heart that confers a protected (defensive) phenotype, and is underlain by the upregulation of specific cardioprotective genes, some of which have already been identified. Based on this premise, it is plausible to postulate that long-term expression of the proteins that mediate the antiischemic effects of late PC should protect the heart, whenever ischemia occurs and throughout the entire ischemia/reperfusion sequence. The important points of this hypothesis are that the protection afforded by gene therapy would be present at any time when ischemia strikes (which is unpredictable) and would be operative from the very onset of ischemia (unlike direct cardioprotection, which usually is implemented at the onset of reperfusion). Thus, prophylactic gene therapy has the potential for limiting the size of the infarct whenever it occurs and for combating the damage inflicted by both ischemia and reperfusion, not just that inflicted by reperfusion.
As a first step toward the development of prophylactic gene therapy, we sought to determine whether it is possible to induce a chronically protected cardiac phenotype by transferring to the myocardium the genes responsible for the salubrious actions of late PC. We have developed viral vectors encoding several of the genes identified as mediators of late PC, specifically, iNOS, COX-2, HO-1, and ec-SOD. We started by testing the effects of iNOS because, historically, this was the first protein to be identified as a mediator of late PC and because a multitude of studies have demonstrated that iNOS is a necessary mediator of late PC induced by diverse stimuli, including ischemia, physical exercise, NO donors, various GPCR agonists, and endotoxin derivatives, indicating that this enzyme plays a central role in cardioprotection and may well be the final common pathway for any kind of late PC stimulus [10, 11]. It was abundantly clear from these studies that iNOS is necessary for late PC to occur; however, it remained to be determined whether a selective increase in iNOS activity via gene transfer is sufficient to replicate the salubrious affects of late PC. Initially, we employed a second-generation adenoviral vector (Ad5/iNOS) deleted in the El, E2A, and E3 regions [33]. A total of 107 pfu of Ad-iNOS were injected intramyocardially in the soon-to-be ischemic region of mouse hearts. Three days later, the injected region exhibited an increase in iNOS protein content, iNOS activity, and nitroxide (NO2/NO3) levels, indicating effective transduction of myocytes with functionally competent iNOS. Immunohistochemistry confirmed that the transgene was expressed in cardiac myocytes. In another series of experiments, mice received either Ad5/iNOS or Ad5/LacZ and, 3 days later, were subjected to a 30-min coronary occlusion followed by reperfusion. Although the intramyocardial injection of iNOS resulted in transduction of only approximately 20% of the risk region, there was a marked reduction in infarct size in Ad5/iNOS transduced mice compared with Ad5/LacZ transduced mice [33]. These data demonstrate that iNOS gene therapy results in effective limitation of infarct size 3 days later and suggest that paracrine effects of NO secreted by transduced cells amplify the effects of gene transfer. We subsequently extended our follow-up to several weeks after Ad5/iNOS gene transfer and found that, as late as eight weeks after injection of Ad5/iNOS, there was increased iNOS protein expression and activity in the myocardium, associated with persistent limitation of infarct size [34]. Serial echocardiographic assessment of LV function revealed no detrimental effects of iNOS gene therapy on LV function or dimensions up to 8 weeks later. We have recently completed preliminary studies at 1 year after rAAV-mediated LacZ or iNOS gene transfer, which suggest persistent protection even at this late time-point. Together, these studies [33, 34] demonstrate that iNOS gene transfer affords effective cardioprotection, and that the magnitude is equivalent to that afforded by late PC but the duration is much longer (at least several weeks). These initial results encouraged us to pursue this line of investigation; they provide proof of principal for the efficacy of gene therapy against ischemia/reperfusion injury which increases local myocardial NO levels without hemodynamic effects, thereby obviating the need for continuous intravenous infusion of NO donors.
Since in the setting of ischemic PC the beneficial effects of iNOS upregulation are mediated by COX-2 [17, 18], we then sought to determine the role of COX-2 in the beneficial effects of iNOS gene therapy [33]. We found that, 3 days after Ad5-mediated iNOS gene transfer, there was upregulation of COX-2 in the same myocardial region in which iNOS was upregulated. The increased expression of COX-2 was associated with increased myocardial content of COX-2 byproducts (PGF2 and 6-keto-PGF1α), a pattern reminiscent of that observed following ischemic PC [15]. When iNOS-transduced mice were pretreated with the COX-2 inhibitor NS-398 prior to the 30-min coronary occlusion, the beneficial effects of iNOS gene transfer were completely abrogated [33]. Similarly, when Ad5—mediated iNOS gene transfer was performed in COX-2−/− mice, no reduction in infarct size was observed [35]. Collectively, these studies [33, 35] demonstrate that (1) iNOS gene transfer results in increased myocardial COX-2 protein expression and increased PGE2 and PGI2 levels, and (2) pharmacologic inhibition or genetic ablation of COX-2 abrogates the infarct-sparing effects of iNOS gene transfer, demonstrating that increased biosynthesis of COX-2—derived prostanoids, such as PGE2 and PGI2, represents an essential mechanism of iNOS—dependent cardioprotection. Together with our previous studies of ischemic PC [17, 18], these observations [33, 35] reveal a close and heretofore unappreciated functional coupling between cardiac iNOS and cardiac COX-2; that is, upregulation of iNOS leads to secondary upregulation of COX-2, which in turn mediates the cytoprotective effects of iNOS.
How does iNOS gene transfer lead to upregulation of COX-2? Our first attempt to tackle this issue was to examine NF-κB. We tested the role of NF-κB because (1) this transcription factor is known to be activated by NO [36], (2) the promoter of the COX-2 gene contains NF-κB-binding sequences [37], and (3) NF-κB is known to be critical for the development of the late phase of ischemic PC [36]. We used transgenic mice that express cardiac specifically a mutant of IκBα (IκBαS32A, S36A) that cannot be serine-phosphorylated and thus inhibited; overexpression of this dominant negative IκBα mutant prevents NF-κB activation [38]. We found that in IκBαS32A, S36A mice, gene therapy with Ad5/iNOS failed to increase myocardial COX-2 protein content and to limit infarct size, demonstrating an obligatory role of NF-κB in iNOS-dependent COX-2 upregulation and in iNOS-dependent protection [35].
Given our finding that COX-2 is necessary for the beneficial effects of iNOS gene therapy, we asked the obvious question of whether selective upregulation of COX-2 in itself (without iNOS gene therapy) would be sufficient to confer a protected phenotype. To this end, we used recombinant adeno-associated virus (rAAV) vectors. rAAVs offer a number of important advantages compared with recombinant adenovirus (rAd) vectors. rAAVs are not associated with any known human disease, infect both mitotic and post-mitotic cells, and are smaller than rAds, which may favor extravasations and tissue transduction. Most importantly, rAAVs lead to chromosomal, not episomal, integration of the transgene, and do not induce synthesis of any viral proteins, so that there is virtually no immune/inflammatory reaction after rAAV—mediated gene transfer [39]. As a result of these last two features, rAAV—mediated gene transfer results in long-lasting (at least 1 year in several models), possibly permanent, transgene expression, which enables potentially indefinite production of the therapeutic protein [39]. Thus, the hypothesis driving our study was that the durable transgene expression afforded by rAAV vectors might enable chronic, possibly permanent, prophylactic cardioprotection. We utilized a rAAV helper-free system to prepare rAAV vectors for injection, thereby ensuring that there would be no contamination of the injectate with helper virus. Since of their long-term nature, these studies are laborious and time-intensive, and are still ongoing. Nevertheless, we have obtained preliminary results demonstrating that, 3 months after rAAV—mediated COX-2 gene transfer, there is robust upregulation of COX-2 protein expression in the transduced myocardium in mice, which is associated with protection against myocardial infarction [40]. We are currently conducting studies with other rAAV vectors encoding iNOS, HO-1, and ecSOD. The common goal of all of these studies is to test the concept that rAAV—mediated gene transfer can confer permanent protection against infarction.
Another promising candidate for cardioprotective gene therapy is ecSOD. Our interest in ecSOD gene therapy stems from the fact that in contrast to all other anti-oxidant proteins, which are intracellular, ecSOD is a secreted protein that acts on the extracellular matrix, thereby producing a paracrine effect on non-transduced cells. By intercepting extracellular , ecSOD prevents formation of cytotoxic ONOO− and also protects NO from inactivation, thereby increasing NO bioavailability. Thus, ecSOD limits oxidative and nitrative stress and facilitates diffusion of NO to adjacent cells. We have recently completed preliminary studies with rAAV-mediated ecSOD gene transfer, which suggest that three months after rAAV-mediated ecSOD gene transfer, myocardial ecSOD levels are increased and infarct size is reduced vis-à-vis mice transduced with rAAV/LacZ; even at 12 months, we have found persistent expression of ecSOD in cardiac myocytes and persistent protection against infarction.
Taken together, the studies summarized above [33–35, 40] indicate that cardiac transfer of the genes that mediate late PC (i.e., iNOS, COX-2, ecSOD) confers a cardioprotected phenotype that emulates late PC. rAAV-mediated transfer of these genes dramatically extends the duration of the protection (for at least several months) compared with late PC (which lasts only for 3 days), with no inflammatory reaction. Thus, it is possible to genetically reprogram the heart in a manner that confers prolonged (possibly even permanent) protection against ischemia/reperfusion injury (a state that be referred to as chronic prophylactic cardio-protection). This approach is intriguing and exciting. At present, the major limitation to translating these basic studies to humans is the lack of effective techniques for transducing significant numbers of myocytes in vivo. Nevertheless, as new technologies emerge (e.g., new vectors or delivery methods), it seems likely that techniques for transducing cardiac myocytes will be developed that could be applied to patients with ischemic heart disease. Once such techniques become available, it will be important to test whether transfer of the genes that the heart utilizes to protect itself during late PC can confer long-lasting or permanent protection against infarction in patients. This would be the ultimate goal of studying the phenomenon of cardiac PC.
In conclusion, the study of ischemic PC has had two major implications. First, it has revealed that the heart possesses a remarkable phenotypic plasticity (i.e., it responds to stress by changing its phenotype in a teleologically useful manner). Second, genetic exploitation of ischemic PC may offer unprecedented potential for protecting the ischemic myocardium. About 20 years after its discovery, it is clear that ischemic PC has been a major paradigm shift in the biology of myocardial ischemia, although the therapeutic expectations it raised remain to be fulfilled. Commonly used drugs (e.g., nitrates) have been shown experimentally to mimic the late phase of PC but the feasibility and efficacy of their prophylactic use for infarct size limitation in patients remain unresolved. Perhaps the greatest potential resides in gene therapy, which mimics late PC and offers great promise as a long-term prophylactic measure against ischemia/reperfusion injury, although its clinical application must await the advent of effective and safe gene delivery methods.
Acknowledgments
This study was supported in part by NIH R01 grants HL-55757, HL-68088, HL-70897, HL-76794, HL-78825, and HL-74351.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R, Becker L, Gross G, Mentzer R, Jr., Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 4.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Wu WJ, Zhu XP, Li Q, Tang XL, Bolli R. Exercise-induced late preconditioning is triggered by generation of nitric oxide. J Mol Cell Cardiol. 2001;33:A41. (Abstr.) [Google Scholar]
- 6.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 7.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 8.Bolli R, Manchikalapudi S, Tang XL, Takano H, Qiu Y, Guo Y, Zhang Q, Jadoon AK. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase. Evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischemic preconditioning. Circ Res. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 12.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Guo Y, Zhang SX, Wu WJ, Wang J, Bao W, Bolli R. Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol. 2002;34:5–15. doi: 10.1006/jmcc.2001.1482. [DOI] [PubMed] [Google Scholar]
- 14.Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res. 2002;55:506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinmura K, Bolli R. The risk for myocardial infarction with cyclooxygenase-2 inhibitors. Ann Intern Med. 2005;143:615–618. doi: 10.7326/0003-4819-143-8-200510180-00022. [DOI] [PubMed] [Google Scholar]
- 17.Shinmura K, Xuan YT, Tang XL, Kodani E, Han H, Zhu Y, Bolli R. Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ Res. 2002;90:602–608. doi: 10.1161/01.res.0000012202.52809.40. [DOI] [PubMed] [Google Scholar]
- 18.Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, Bolli R. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol. 2003;35:525–537. doi: 10.1016/s0022-2828(03)00076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 20.Ueda Y, Kitakaze M, Komamura K, Minamino T, Asanuma H, Sato H, Kuzuya T, Takeda H, Hori M. Pravastatin restored the infarct size-limiting effect of ischemic preconditioning blunted by hypercholesterolemia in the rabbit model of myocardial infarction. J Am Coll Cardiol. 1999;34:2120–2125. doi: 10.1016/s0735-1097(99)00440-4. [DOI] [PubMed] [Google Scholar]
- 21.Tang XL, Takano H, Xuan YT, Sato H, Kodani E, Dawn B, Zhu Y, Shirk G, Wu WJ, Bolli R. Hypercholesterolemia abrogates late preconditioning via a tetrahydrobiopterin-dependent mechanism in conscious rabbits. Circulation. 2005;112:2149–2156. doi: 10.1161/CIRCULATIONAHA.105.566190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang XL, Stein AB, Shirk G, Bolli R. Hypercholesterolemia blunts NO donor-induced late preconditioning against myocardial infarction in conscious rabbits. Basic Res Cardiol. 2004;99:395–403. doi: 10.1007/s00395-004-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kremastinos DT, Bofilis E, Karavolias GK, Papalois A, Kaklamanis L, Iliodromitis EK. Preconditioning limits myocardial infarct size in hypercholesterolemic rabbits. Atherosclerosis. 2000;150:81–89. doi: 10.1016/s0021-9150(99)00389-5. [DOI] [PubMed] [Google Scholar]
- 24.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 25.Kristiansen SB, Lofgren B, Stottrup NB, Khatir D, Nielsen-Kudsk JE, Nielsen TT, Botker HE, Flyvbjerg A. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia. 2004;47:1716–1721. doi: 10.1007/s00125-004-1514-4. [DOI] [PubMed] [Google Scholar]
- 26.del Valle HF, Lascano EC, Negroni JA, Crottogini AT. Absence of ischemic preconditioning protection in diabetic sheep hearts: role of sarcolemmal KATP channel dysfunction. Mol Cell Biochem. 2003;249:21–30. [PubMed] [Google Scholar]
- 27.Fenton RA, Dickson EW, Meyer TE, Dobson JG., Jr Aging reduces the cardioprotective effect of ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 2000;32:1371–1375. doi: 10.1006/jmcc.2000.1189. [DOI] [PubMed] [Google Scholar]
- 28.Abete P, Ferrara N, Cioppa A, Ferrara P, Bianco S, Calabrese C, Cacciatore F, Longobardi G, Rengo F. Preconditioning does not prevent postischemic dysfunction in aging heart. J Am Coll Cardiol. 1996;27:1777–1786. doi: 10.1016/0735-1097(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 29.Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, Schulz R. Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.01071.2006. (in press) [DOI] [PubMed] [Google Scholar]
- 30.Przyklenk K, Li G, Whittaker P. No loss in the in vivo efficacy of ischemic preconditioning in middle-aged and old rabbits. J Am Coll Cardiol. 2001;38:1741–1747. doi: 10.1016/s0735-1097(01)01603-5. [DOI] [PubMed] [Google Scholar]
- 31.Wu WJ, Xuan YT, Tan W, Zhu X, Zhu Y, Guo Y. The loss of ischemic preconditioning in the senescent heart is associated with impaired upregulation of inducible nitric oxide synthase and cyclooxygenase-2. Circulation. 2003;108:IV-187. (Abstr.) [Google Scholar]
- 32.Shinmura K, Nagai M, Tamaki K, Bolli R. Gender and aging do not impair opioid-induced late preconditioning in rats. Basic Res Cardiol. 2004;99:46–55. doi: 10.1007/s00395-003-0436-5. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Guo Y, Xuan YT, Lowenstein CJ, Stevenson SC, Prabhu SD, Wu WJ, Zhu Y, Bolli R. Gene therapy with inducible nitric oxide synthase protects against myocardial infarction via a cyclooxygenase-2-dependent mechanism. Circ Res. 2003;92:741–748. doi: 10.1161/01.RES.0000065441.72685.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Guo Y, Tan W, Stein AB, Dawn B, Wu WJ, Zhu X, Lu X, Xu X, Siddiqui T, Tiwari S, Bolli R. Gene therapy with iNOS provides long-term protection against myocardial infarction without adverse functional consequences. Am J Physiol Heart Circ Physiol. 2006;290:H584–H589. doi: 10.1152/ajpheart.00855.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y, Luo C, Dawn B, Tan W, Wu WJ, Hunt G, Zhu X, Li Q. The cardioprotection afforded by iNOS gene therapy is mediated by COX-2 via an NF-kappaB-dependent pathway. Circulation. 2004;110:III-29. doi: 10.1161/CIRCULATIONAHA.107.689810. (Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, Qiu Y, Li JJ, Bolli R. Nuclear factor-kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res. 1999;84:1095–1109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 37.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302(Pt 3):723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dawn B, Xuan YT, Marian M, Flaherty MP, Murphree SS, Smith TL, Bolli R, Jones WK. Cardiac-specific abrogation of NF- kappa B activation in mice by transdominant expression of a mutant I kappa B alpha. J Mol Cell Cardiol. 2001;33:161–173. doi: 10.1006/jmcc.2000.1291. [DOI] [PubMed] [Google Scholar]
- 39.Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Guo Y, Luo C, Tan W, Wu WJ, Xu B, Siddiqui T, Rokosh GD, Bolli R. Long-term protection against myocardial infarction with cyclooxygenase-2 (COX-2) gene therapy via a recombinant adeno-associated viral (rAAV) vector. Circulation. 2004;110:III-107. (Abstr.) [Google Scholar]