Abstract
Rationale
Maternal behavior in laboratory rats requires a network of brain structures including the ventral bed nucleus of the stria terminalis (BSTv) and medial preoptic area (mPOA). Neurotransmitter systems in the BSTv and mPOA influencing maternal behaviors are not well understood, although norepinephrine is an excellent candidate because the BSTv contains the densest noradrenergic fiber plexus in the forebrain and norepinephrine in the mPOA is known to influence other female reproductive functions.
Objectives
We hypothesized that downregulated noradrenergic activity in the BSTv and mPOA is necessary for mothering.
Methods
Postpartum mother-litter interactions were observed after BSTv infusion of yohimbine (an α2 autoreceptor antagonist that increases norepinephrine release), and after BSTv or mPOA infusion of the more selective α2 autoreceptor antagonist idazoxan. Lastly, noradrenergic input to the BSTv/mPOA was selectively lesioned in nulliparous rats with anti-DBH-saporin to determine if this would facilitate mothering.
Results
BSTv yohimbine almost abolished retrieval of pups but did not significantly affect dams’ ability to initiate contact, lick or nurse them. BSTv idazoxan disrupted retrieval somewhat less than yohimbine, but significantly reduced nursing. mPOA idazoxan impaired retrieval more severely than that found after BSTv infusion. Anti-DBH-saporin almost eliminated noradrenergic terminals in the BSTv and reduced them by over 60% in the mPOA, but did not promote maternal responding. It also did not affect females’ anxiety-related behavior.
Conclusions
Downregulated noradrenergic activity in the BSTv and mPOA is necessary for postpartum maternal behavior in rats, but eliminating this system alone is insufficient to promote maternal behaviors in nulliparous females.
Keywords: anxiety, bed nucleus of the stria terminalis, elevated plus maze, lactation, maternal behavior, monoamines, norepinephrine, nursing, preoptic area, saporin
Maternal behavior displayed by female mammals requires the coordinated release of a host of neurochemicals acting on a well-defined network of brain sites. The core of this network is often thought to be the medial preoptic area (mPOA). Estradiol, prolactin, or oxytocin infused into the mPOA facilitates maternal responsiveness in nulliparous rats, while interacting with pups stimulates neural activity (i.e., immediate-early gene expression) in the maternal mPOA (Numan and Numan 1994 1995; Fleming et al. 1994; Kalinichev et al. 2000; Lonstein et al. 1998). In contrast, destroying the mPOA prevents the onset of maternal behavior in nulliparous rats and impairs or completely abolishes oral maternal behaviors including nesting, retrieval, and licking in parous females (Lee et al. 2000; Numan and Insel 2003).
The mPOA may act as a functional unit with the adjacent ventral bed nucleus of the stria terminalis (BSTv) to influence maternal behaviors in rodents (Numan and Numan 1997; Numan and Insel 2003). In support, the BSTv also contains high densities of the steroid and neuropeptide receptors that establish mothering in female rats (Bokowska and Morrell 1997; Freund-Mercier et al. 1987; Pfaff and Keiner 1973), shares many of the same afferent and efferent projections as the mPOA (Dong and Swanson 2006a,b; Simerly and Swanson 1988), has immediate-early gene expression elicited by the same pup stimuli that activate the mPOA (Numan and Numan 1994 1995), and postpartum BSTv lesions disrupt oral maternal behaviors similar to that found after mPOA lesions (Numan and Numan 1997).
The BSTv is critical for maternal care but nothing is known about what neurochemical systems in this site influence nurturant behaviors. An excellent candidate is norepinephrine. The mammalian BSTv contains the densest noradrenergic terminal field in the forebrain, which primarily arises from medullary A1 and A2 cells (Fendt et al. 2005; Phelix et al. 1992; Riche et al. 1990; Roder and Ciriello 1994; Woulfe et al. 1990). In male rats, norepinephrine is tonically released in the BSTv and produces an inhibitory effect by attenuating local glutamate release that otherwise suppresses GABA-mediated inhibitory postsynaptic currents (Forray et al. 1997 1999). Indeed, norepinephrine reduces excitability of 70% of BSTv neurons while increases it in only 2% of them (Casada and Dafny 1993). Norepinephrine’s suppression of BSTv excitability includes glutamatergic cells projecting to the VTA (Dumont and Williams 2004), which may blunt mesolimbic dopamine release necessary for the expression of rewarding behaviors such as mothering (Keer and Stern 1999; Numan et al. 2005).
Given norepinephrine’s inhibitory effect and possible interference with mesolimbic dopamine release, low noradrenergic activity in the BSTv may be characteristic of mother rats and essential for their maternal behavior. There is precedent for noradrenergic downregulation in mothers. Compared to virgin females, the paraventricular nucleus (PVN) of lactating rats has lower basal norepinephrine release and is less responsive to noradrenergic agonists (Toufexis et al. 1996 1998; Windle et al. 1997), and this contributes to the postpartum suppression of the HPA axis (Douglas 2005). Most A1 and A2 noradrenergic projections to the PVN collateralize to the BSTv (Bienkowski and Rinaman 2008; Woulfe et al. 1988), thereby providing a neuroanatomical basis for a simultaneous decrease in norepinephrine release in the postpartum PVN and BSTv.
In the present experiments, we predicted that increasing norepinephrine release in the BSTv would disrupt ongoing maternal behavior in postpartum rats. Conversely, we predicted that decreasing norepinephrine release in the BSTv would facilitate maternal responding in non-parturient females. We tested the first prediction by infusing the α2 autoreceptor antagonist yohimbine, or more selective α2 autoreceptor antagonist idazoxan (each increases BST norepinephrine release - Forray et al., 1995, 1997; Herr et al., 2012; Palij and Stamford 1993; Park et al. 2009), into the BSTv of postpartum rats and evaluated their effects on maternal behaviors. We then examined idazoxan’s effects on mothering after its infusion into the mPOA. The mPOA contains moderate norepinephrine concentrations and receptor expression (Bruning et al. 1987; Rainbow et al. 1984; Simerly et al. 1986), but mPOA α2 autoreceptor antagonism may have a similar effect in maternal care as it does in the BSTv because: 1) both sites receive most of their noradrenergic input from the A1 and A2 groups and probably from the same collateralizing cells (Fernandez-Galaz et al. 1994; Schlitz and Sawchenko 2007), 2) similar to the BSTv, norepinephrine inhibits mPOA neurons by increasing GABA release (Herbison et al. 1989 1990; Leung et al. 1981), 3) noradrenergic activity in the mPOA contributes to other aspects of female reproductive physiology and behavior (Caldwell and Clemens 1986; Etgen 2006).
We tested the second prediction that loss of noradrenergic input to the BSTv and mPOA would facilitate maternal responding in nulliparous rats by selectively destroying the noradrenergic innervation of these sites with the neurotoxin saporin conjugated to an antiserum targeting dopamine-β-hydroxylase (Anti-DBH-SAP). Females then underwent a maternal sensitization paradigm that elicits maternal responding in virgin rats (Rosenblatt 1967). Because high maternal responsiveness may require blunted emotional reactivity (see Lonstein 2007), we also examined virgin females’ anxiety-related behavior.
Methods
Experiments 1 and 2: Effects of α2 autoreceptor antagonism in the BSTv with yohimbine or idazoxan on postpartum maternal behavior
Subjects
Subjects were adult Long-Evans female rats born in our colony and housed as previously described (Smith and Lonstein, 2008). Females were monitored daily with a vaginal impedance meter (Fine Science Tools, Foster City, CA) and on a day of proestrus placed overnight with a sexually experienced Long-Evans male rat. Pregnant females were housed 2–3/cage until being implanted with intracranial cannula between days 15–18 of pregnancy (see below), after which they were singly housed. The day of parturition = day 0 postpartum. Litters were culled soon after birth to contain 4 males and 4 females. The principles of laboratory animal care were followed and procedures approved by the Animal Care and Use Committee at Michigan State University.
Stereotaxic Surgery
Subjects received permanent intracranial cannulae using methods we have previously described (Miller and Lonstein 2005; Miller et al. 2010). Briefly, females were anesthetized with ketamine (90 mg/kg IP; Butler, Dublin, OH) and xylazine (4 mg/kg IM; Butler, Dublin, OH), and holes were drilled bilaterally above the BSTv (A/P 0.0, M/L ± 1.2; n = 23 for yohimbine, n = 34 for idaxozan) or mPOA (A/P 0.0, M/L ± 0.75; n = 21). A 5-mm long, 22-gauge bilateral stainless steel guide cannula (Plastics One, Roanoke, VA) was permanently implanted and kept patent with a dummy stylet extending 1 mm beyond the end of the cannulae. A dust cap covered the entire apparatus. After surgery, subjects received buprenorphine (0.015 mg/kg IP; Reckitt Benckiser, Parsippany NJ) and were placed on a heating pad until recovery.
Drug Infusions
On day 1 postpartum, dams were habituated to the handling and testing procedures as described elsewhere (Miller and Lonstein 2005). The next day, subjects and litters were carried in their home cages to the testing room. Litters were weighed and placed in an incubator set at nest temperature (34° C). Two hr later, dams were brought in a clean cage to the infusion room. In Experiment 1, yohimbine hydrochloride (Sigma USA; 2 or 6 μg, divided in half and delivered in 125 nl 2:1 propylene glycol:saline per hemisphere), idazoxan hydrochloride (Sigma USA; 10 or 20 μg, divided in half and delivered in 125 nl saline per hemisphere), or 125 nl/hemisphere physiological saline was slowly infused into the BSTv over 1 min through a 16-mm-long bilateral 28-gauge injector connected to a 0.5 μl Hamilton syringe (Hamilton, Reno, NV). In Experiment 2, idazoxan hydrochloride (20 μg, divided in half and delivered in 125 nl saline per hemisphere) or 125 nl/hemisphere of saline were infused into the mPOA with a 17-mm-long bilateral injector. Doses were chosen by their effects on other behaviors when infused into the rat brain (Cervo et al. 1990; Dodge and Badura 2002; Gulia et al. 2002; Guo et al. 1996). After infusion, the injectors remained for an additional 60–90 sec and then slowly removed. Subjects were returned to their home cage and left undisturbed for 10 min until reunion with the litter.
Maternal Behavior Testing
Pups were removed from the incubator, expressed of urine and feces, weighed, and scattered in the home cage opposite the nest. Numerous maternal and non-maternal behaviors were continuously recorded on a laptop for 45 min as detailed elsewhere (Miller and Lonstein 2005). If a subject did not retrieve all pups within 10 min, the observation was briefly paused and the remaining pups gently placed in the nest to better assess nursing in response to a full litter. After testing, litters were weighed to assess milk ingestion.
Verification of Infusion Sites
Within a week after testing, subjects were overdosed with sodium pentobarbital and perfused with 100 ml of 0.9% saline. Brains were removed and postfixed overnight in 10% formalin and then submerged in 20% sucrose for at least 2 days. Brains were cut into 40 μm sections and stained with Neutral Red. Sites of infusion were analyzed at 100x magnification and mapped onto plates from Swanson’s (1998) atlas of the rat brain. Only dams with accurately placed infusions were included in the data analyses. An infusion was considered a BSTv hit if it terminated in the subcommissural BST between ~0.0 and −0.51 mm from bregma, where the densest noradrenergic fibers and concentration of norepinephrine are found (Fendt et al. 2005). Infusions were considered an mPOA hit if terminated within this site between ~−0.11 and −0.88 mm from bregma.
Data Analyses
In Experiment 1, behavior latencies and durations were analyzed with one-way ANOVAs. The number of pups retrieved was not normally distributed so was analyzed with Kruskall-Wallis tests. In cases of significant effects (p ≤ 0.05), pair-wise posthoc comparisons were performed using p-corrected Fisher’s LSD or Mann-Whitney U tests, with significant differences indicated by p ≤ 0.017. In Experiment 2, unpaired t-tests were used to compare idazoxan-treated and vehicle-treated dams, with the number of pups retrieved not normally distributed so analyzed with Mann-Whitney U tests. Statistical significance was indicated by p ≤ 0.05. Total time with pups was the summed durations of hovering over the litter plus nursing. Because drug-treated dams often did not retrieve, contact with pups was converted into a percentage of observation time beginning after mothers first hovered over the full litter. Similarly, the percentage of time dams spent nursing was calculated from the time mothers retrieved all pups to the nest or had remaining pups placed there by the experimenter.
Experiment 3: Effects of anti-DBH-SAP on maternal behavior of nulliparous virgin female rats
Anti-DBH-SAP infusion and ovariectomy
25 female Long-Evans rats from our colony were anesthetized as described above. Holes were drilled over the plexus of noradrenergic fibers in the BSTv (A/P = 0.0, M/L = 1.5 from bregma) and 250 nl of anti-DBH-SAP (55 ng; n = 15) or a control conjugate (n = 9; both from Advanced Targeting Systems, San Diego, CA) was slowly injected over 5 min into each hemisphere through a 1.0 μl Hamilton syringe lowered 6.7 mm ventral from the skull. Pilot work revealed this was lowest volume/dose of anti-DBH-SAP that would almost eliminate DBH-ir fibers in the BSTv and also affect the mPOA. The needle remained for 10 min and then slowly retracted. The scalp was closed with surgical sutures and subjects then ovariectomized through two dorsolateral incisions. Because some neural systems inhibiting maternal behavior are revealed only in the presence of low circulating estradiol (Bridges et al. 1999), subjects were subcuanteously implanted with a 1-cm long silastic capsule (0.062 inner diameter) containing 2-mm of crystallized estradiol (Sigma, USA). This provides ~30 pg/ml plasma estradiol, which is well below the levels inducing maternal behavior (Bridges 1984). After surgery, subjects received 1.25 mg ketoprofen and were singly housed until testing.
Behavioral Testing
Beginning 17–20 days after surgery, subjects underwent a maternal sensitization procedure (e.g., Lonstein et al. 1999). Each morning for up to 9 days, 3 recently fed young pups were placed in the subjects’ home cages. Observers recorded subjects’ behaviors every ten seconds for fifteen min and the pups then remained until the next morning. Criteria for maternal behavior were retrieving all 3 pups to a single location and huddling over them during two consecutive observations, after which testing was terminated. The sensitization latency was the first day of the two consecutive days of full maternal behavior. Testing was terminated if a subject attacked pups on two consecutive days. Aggressive and non-sensitizing females were given the maximum latency of 8 days. Because the emergence of maternal responding may involve suppressed emotional reactivity (Fleming and Leubke 1981; Lonstein 2007), 3 days before maternal sensitization began, anxiety-related behavior was assessed during a 10-min test in an elevated plus maze as described elsewhere (Smith and Lonstein 2008; Miller et al. 2010).
Tissue collection, DBH immunohistochemistry and fiber analysis
Within 4 days after maternal sensitization testing, females were perfused with 150 ml of saline followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.6). Brains were harvested and postfixed overnight in 4% paraformaldehyde and then stored in 20% sucrose until sectioning into 40 μm sections. Sections were stored in a sucrose-based cryoprotectant at −20° C. Immunohistochemistry to visualize DBH fibers was performed using methods often used in our laboratory (Smith and Lonstein 2008) using a mouse monoclonal primary antiserum raised against DBH (1:2500; MAB308 Millipore, Billerica, MA) and a biotinylated goat-anti-mouse secondary antiserum (1:500; Vector Laboratories, Burlingame, CA).
DBH immunoreactivity was assessed by obtaining optical density measurements from 3 consecutive sections in the 1-in-2 series of sections through the BSTv and mPOA of each subject. Because noradrenergic projections to the BSTv collateralize to the PVN, density of DBH-ir fibers in the PVN was also determined from 3 sections in the series. Images of each site were captured at 100x and the average light level adjusted for each image so it would be constant across all sections. Boxes of a standardized size for each site covering the area of interest were superimposed upon the images and the area covered by pixels above an established light threshold optimized to capture foreground immunoreactivity was calculated using NIS-Elements.
Statistical Analyses
Maternal behavior latencies were analyzed with Mann-Whitney U and t-test tests. Plus-maze behaviors and DBH immunoreactivity were compared between groups using t-tests. The relationships between females’ DBH immunoreactivity and behavior were evaluated using Pearson’s r. Statistical significance was indicated by p ≤ 0.05.
Results
Experiment 1: Effects of α2 autoreceptor antagonism in the BSTv with yohimbine or idazoxan on postpartum maternal behavior
Yohimbine
Four females with misplaced infusions were excluded from the study. Accurately placed infusions are represented in Fig. 1. All dams rapidly made contact with the litter at reunion (Table 1), but dams receiving yohimbine showed severely disrupted retrieval of pups thereafter. All vehicle-infused rats retrieved the entire litter within 10 min, but only 3/7 dams receiving the low dose and 2/6 dams receiving the high dose of yohimbine retrieved any pups (χ2 = 13.14, p = 0.038). Of the yohimbine-treated dams that retrieved any pups, two low-dose dams and none of the high-dose dams retrieved the entire litter (χ2 = 6.50, p = 0.0014). Accordingly, groups differed in the number of pups retrieved (H2 = 9.27, p < 0.01; Fig. 2). In contrast, yohimbine did not significantly affect time spent licking the pups or nesting/digging. Exploring the cage and self-grooming were each significantly increased by yohimbine (Table 1).
Figure 1.
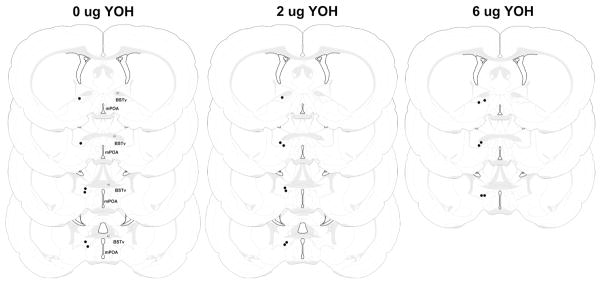
Schematic representation of infusion sites for dams receiving 0, 2, or 6 μg of yohimbine into the BSTv (black circles). The bilateral infusion sites are depicted unilaterally. Figure modified from Swanson (1998). AC – anterior commissure, BSTv – ventral bed nucleus of the stria terminalis, mPOA – medial preoptic area )
Table 1.
Maternal and non-maternal behaviors of postpartum rats after BSTv infusion of vehicle or yohimbine. Groups with different superscript letters are statistically different from one another (post-hoc p ≤.017).
| Yohimbine | |||||
|---|---|---|---|---|---|
| 0 μg (n = 6) | 2 μg (n = 7) | 6 μg (n = 6) | F (2,16) | p | |
| Latency (s) | |||||
| Contact pups | 1 ± 0 | 5 ± 2 | 9 ± 4 | 2.44 | 0.119 |
| Retrieve first pup* | 8 ± 2a | 344 ± 121ab | 442 ± 105b | 5.34 | 0.017 |
| Hover over pups after all in nest | 23 ± 8a | 35 ± 9a | 837 ± 356b | 5.62 | 0.014 |
| Nurse pups after all in nest | 536 ± 138 | 706 ± 247 | 1187 ± 308 | 1.84 | 0.191 |
| Duration (s) | |||||
| Lick pups | 260 ± 145 | 123 ± 68 | 116 ± 87 | 0.61 | 0.555 |
| Nest/burrow | 7 ± 5 | 4 ± 2 | 20 ± 10 | 1.83 | 0.193 |
| Self-grooming | 191 ± 39a | 364 ± 58ab | 453 ± 58b | 5.94 | 0.012 |
| Explore cage | 227 ± 128a | 272 ± 62a | 714 ± 156b | 5.16 | 0.019 |
| Hover over pups | 649 ± 155 | 563 ± 156 | 189 ± 64 | 3.11 | 0.072 |
| Nursing | 1486 ± 190 | 1207 ± 242 | 887 ± 265 | 1.53 | 0.247 |
| Total time spent with pups | 2135 ± 206 | 1770 ± 345 | 1076 ± 289 | 3.17 | 0.069 |
| % Time nursing after pups in nest | 59 ± 9 | 50 ± 10 | 42 ± 13 | 0.57 | 0.577 |
| Litter Weight Gain (g) | 2.97 ± 0.35a | 1.54 ± 0.42b | 0.83 ± 0.27b | 8.55 | 0.003 |
non-responders assigned latency of 600 sec.
Figure 2.
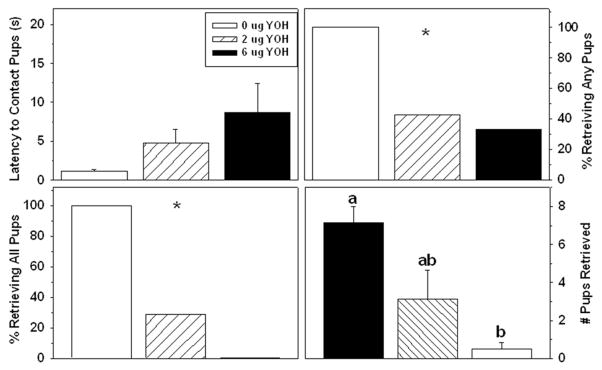
Effects of yohimbine infused into the BSTv on postpartum A) latency to contact pups (Mean ± SEM), B) percentage of dams retrieving any pups, C) percentage of dams retrieving all pups, D) number of pups retrieved (Mean ± SEM). * & significant three&group χ2. Significant differences between any two groups in panel D indicated by different lower&case letters above bars, post&hoc p < 0.05.
The latency for dams to begin hovering over the litter after all pups were in the nest (retrieved by the subject or placed there by the experimenter) was significantly longer in dams receiving the high dose of yohimbine. However, the latency to begin nursing after all pups were in the nest was only non-significantly lengthened by yohimbine (Table 1). The raw duration and percentage of time dams spent nursing did not significantly differ among groups, although these measures were somewhat reduced in the yohimbine-treated groups. Pups of both groups of yohimbine-treated dams gained less weight by the end of testing compared to controls (Table 1).
Idazoxan
Nine females with misplaced infusions were excluded from analysis. Accurately placed idaxozan infusions were very similar in location to the yohimbine infusions detailed above. One control dam was an outlier for the latency to retrieve, and one 20 μg idazoxan dam was an outlier on nesting/burrowing duration (Dixon’s test, p < 0.05), so each was removed from analysis of those variables.
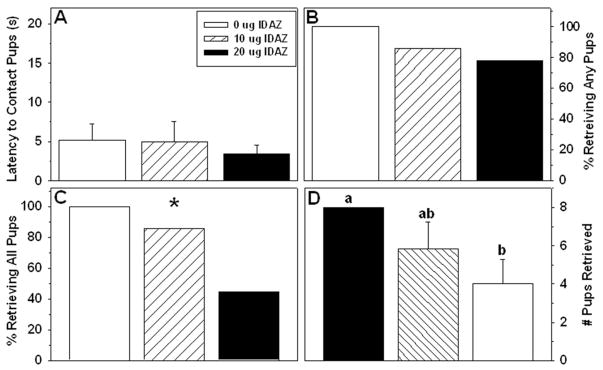
Intra-BSTv infusion of idazoxan disrupted maternal behavior. Similar to yohimbine, idazoxan did not significantly affect the latency for dams to make contact with the litter (Table 2). Idazoxan did disrupt subsequent retrieval, but less dramatically than yohimbine. All vehicle-treated dams, 6/7 low-dose dams, and 7/9 high-dose dams retrieved at least one pup within 10 min (χ2 = 2.15, p = 0.34). The entire litter, however, was retrieved by only 4/7 responding high-dose females (χ2 = 6.89, p = 0.03). Dams given the high dose of idaxozan retrieved significantly fewer pups than did the vehicle-infused controls (H2 = 6.23, p < 0.05; Fig. 3). Time spent licking, nesting/burrowing, and exploring did not significantly differ among the groups. Self-grooming was greater in the high-dose dams compared to the other two groups (Table 2).
Table 2.
Maternal and non-maternal behaviors of postpartum rats after BSTv infusion of idazoxan. Groups with different superscript letters are statistically different from one another (post-hoc p ≤.017).
| Idazoxan Dose | |||||
|---|---|---|---|---|---|
| 0 μg (n = 9) | 10 μg (n = 7) | 20 μg (n = 9) | F (2,22) | p | |
| Latency (s) | |||||
| Contact pups | 5 ± 2 | 5 ± 3 | 3 ± 1 | 0.26 | 0.771 |
| Retrieve first pup* | 9 ± 3 | 119 ± 82 | 170 ± 83 | 1.54 | 0.237 |
| Hover over pups after all in nest | 73 ± 61 | 344 ± 267 | 521 ± 299 | 1.08 | 0.358 |
| Nurse pups after all in nest | 419 ± 112a | 863 ± 241ab | 1156 ± 245b | 3.55 | 0.046 |
| Duration (s) | |||||
| Lick pups | 192 ± 70 | 177 ± 72 | 130 ± 49 | 0.27 | 0.764 |
| Nest/burrow | 81 ± 49 | 28 ± 14 | 89 ± 74 | 0.33 | 0.734 |
| Self-grooming | 111 ± 37a | 196 ± 42a | 432 ± 103b | 5.79 | 0.096 |
| Explore cage | 137 ± 48 | 326 ± 94 | 467 ± 151 | 2.50 | 0.106 |
| Hover over pups | 546 ± 114 | 508 ± 195 | 554 ± 149 | 0.03 | 0.976 |
| Nursing | 1767 ± 167a | 1166 ± 311ab | 787 ± 214b | 5.15 | 0.015 |
| Total time spent with pups | 2313 ± 122a | 1673 ± 311ab | 1340 ± 306b | 4.05 | 0.032 |
| % Time nursing after pups in nest | 68 ± 6a | 47 ± 12ab | 33 ± 10b | 4.12 | 0.030 |
| Litter Weight Gain (g) | 2.33 ± 0.37a | 1.37 ± 0.58ab | 0.82 ± 0.24b | 4.08 | 0.031 |
non-responders assigned latency of 600 sec.
Figure 3.
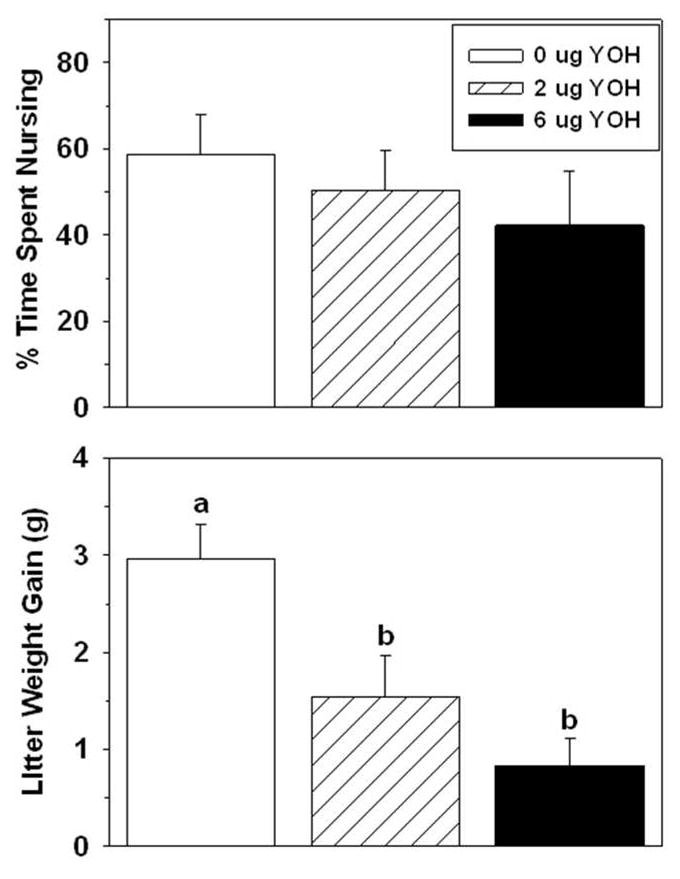
Effects of yohimbine infused into the BSTv on postpartum percentage of time spent nursing (top) and litter weight gain (Mean ± SEM) (bottom). Significant differences between any two groups indicated by different lower-case letters above bars, post-hoc p < 0.05.
The latency to hover over the pups did not differ among groups, but the latency to begin nursing after all the pups were in the nest was prolonged in dams that received the high dose compared to the control dams. The high dose of idazoxan also significantly reduced both the raw duration and percentage (Table 2) of time spent nursing. Litters interacting with dams infused with the high dose of idazoxan gained less weight compared to litters of control dams (Table 2).
Experiment 2: Effects of α2 autoreceptor antagonism in the mPOA with idazoxan on postpartum maternal behavior
Six dams infused with idazoxan were excluded from analysis and another dam’s data were lost during a computer malfunction. Locations of the correctly placed infusions in the BSTv are shown in Fig. 4. Compared to dams receiving vehicle, dams that received mPOA idazoxan were significantly slower to make contact with and begin retrieving pups (Table 3). Every control dam successfully retrieved all pups back to the nest, 4/7 idaxozan-treated dams retrieved any pups (χ2 = 5.33, p = 0.021) and only two of those retrieved all eight pups (χ2 = 9.60, p = 0.002). The number of pups retrieved was lower for the idazoxan-treated group compared to controls (U = 8, p < 0.006; Fig. 5). Time spent licking pups and nesting/burrowing did not differ between groups, but the duration of time exploring and self-grooming were both higher for the idazoxan-treated group compared to controls (Table 3).
Figure 4.
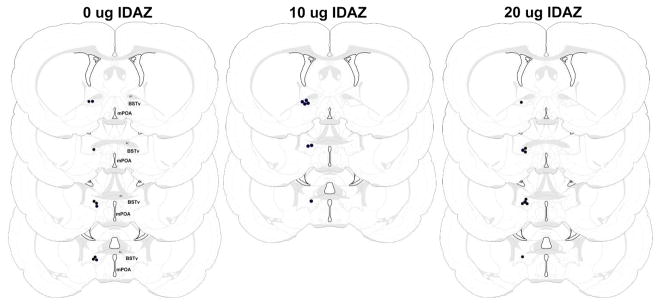
Schematic representation of infusion sites for dams receiving 0, 10, or 20 μg idazoxan into the BSTv (black circles). The bilateral infusion sites are depicted unilaterally. Figure modified from Swanson (1998). AC – anterior commissure, BSTv – ventral bed nucleus of the stria terminalis, mPOA – medial preoptic area
Table 3.
Maternal and non-maternal behaviors of postpartum rats after mPOA infusion of idazoxan.
| Idazoxan Dose | ||||
|---|---|---|---|---|
| 0 μg (n = 8) | 20 μg (n = 7) | t13 | p | |
| Latency (s) | ||||
| Contact pups | 1 ± 1 | 11 ± 4 | 2.35 | 0.035 |
| Retrieve first pup* | 3 ± 1 | 313 ± 104 | 3.19 | 0.007 |
| Hover over pups after all in nest | 77 ± 42 | 858 ± 386 | 2.16 | 0.050 |
| Nurse pups after all in nest | 529 ± 57 | 1458 ± 239 | 4.03 | 0.001 |
| Duration (s) | ||||
| Lick pups | 68 ± 26 | 48 ± 29 | 0.51 | 0.619 |
| Nest/burrow | 1 ± 1 | 5 ± 3 | 1.36 | 0.196 |
| Self-grooming | 85 ± 14 | 442 ± 73 | 5.15 | 0.0002 |
| Explore cage | 88 ± 21 | 621 ± 136 | 4.16 | 0.001 |
| Hover over pups | 349 ± 42 | 155 ± 53 | 2.89 | 0.013 |
| Nurse pups | 1996 ± 86 | 615 ± 228 | 5.98 | 0.0001 |
| Total time spent with pups | 2345 ± 88 | 770 ± 246 | 6.35 | 0.0001 |
| % Time nursing after pups in nest | 74 ± 3 | 29 ± 11 | 4.35 | 0.0008 |
| Litter Weight Gain (g) | 3.01 ± 0.30 | 0.97 ± 0.01 | 4.70 | 0.0004 |
non-responders assigned latency of 600 sec.
Figure 5.
Effects of idazoxan infused into the BSTv on postpartum A) latency to contact pups (Mean ± SEM), B) percentage of dams retrieving any pups, C) percentage of dams retrieving all pups, D) number of pups retrieved (Mean ± SEM). * - significant three(group χ2 Significant differences between any two groups in panel D indicated by different lower-case letters above bars, post-hoc p < 0.05.
Idazoxan-infused dams took longer than controls to begin hovering over and then nurse the full litters (Table 3). The raw duration and percentage of time spent nursing the pups after all were in the nest were significantly lower in the idazoxan-infused group compared to controls, as was the amount of weight litters gained while interacting with idazoxan-treated dams (Table 3).
Experiment 3: Effects of anti-DBH-SAP on maternal behavior of nulliparous virgin female rats
Six lesioned subjects sustained unilateral loss of DBH immunoreactivity in the BSTv and mPOA and were removed from the study. In addition, the brain of one control dam was lost. The remaining anti-DBH-SAP subjects had bilateral noradrenergic lesions involving a >90% loss of DBH-ir fibers in the BSTv (t15 = 3.39, p = 0.00001; Fig. 6). Anti-DBH-SAP also decreased DBH fibers by ~60% in the mPOA (t15 = 2.48, p=0.026) and by ~35% in the PVN (t15=3.08, p=0.008).
Figure 6.
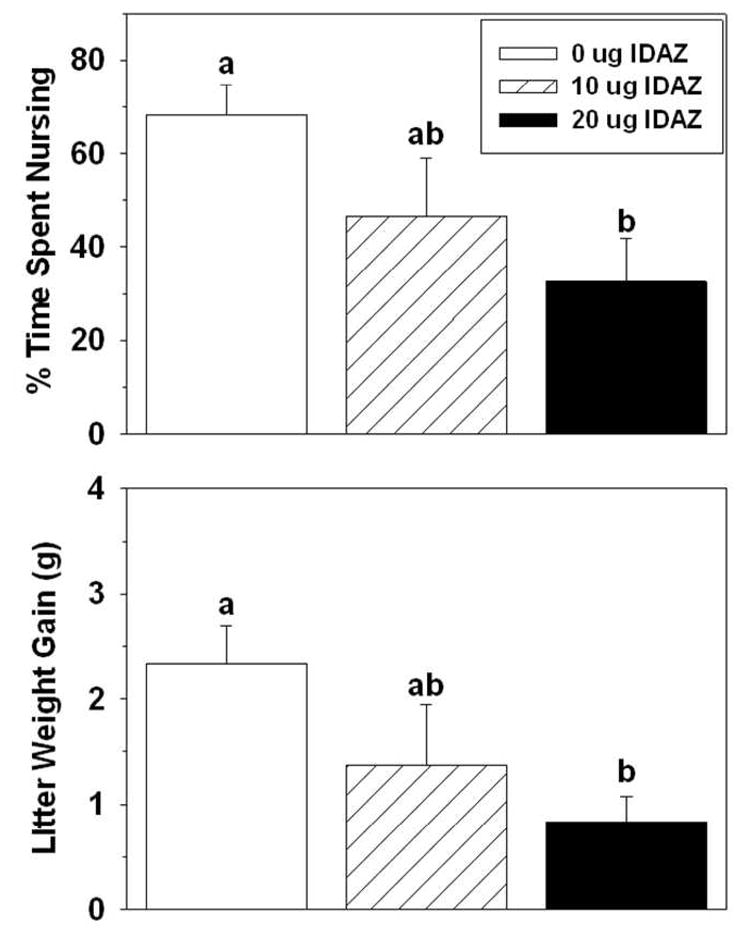
Effects of idazoxan infused into the BSTv on postpartum percentage of time spent nursing (top) and litter weight gain (Mean ± SEM) (bottom). Significant differences between any two groups indicated by different lower-case letters above bars, post-hoc p < 0.05.
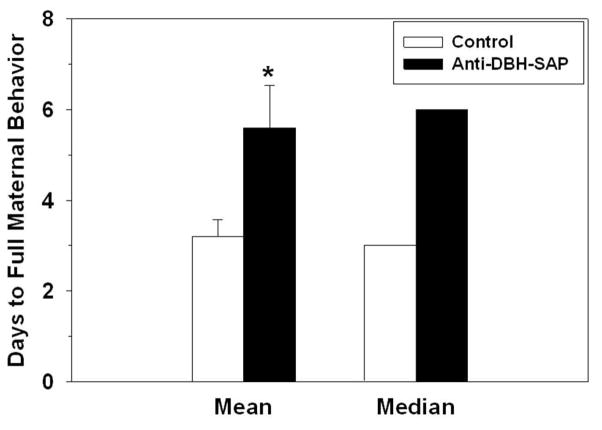
The latency to show two consecutive days of maternal behavior was not significantly affected by anti-DBH-SAP. If anything, lesioned females took longer than controls in their mean (6.7 ± 0.6 vs. 5.3 ± 0.9 days; t16 = 1.23, p = 0.24) and median (8 vs. 4 days; U = 30.0, p = 0.35) latencies to become maternal (Fig. 7). Latencies to display maternal behavior were not significantly correlated with DBH-ir in any of the sites analyzed (rs < −0.2, ps > 0.51).
Figure 7.
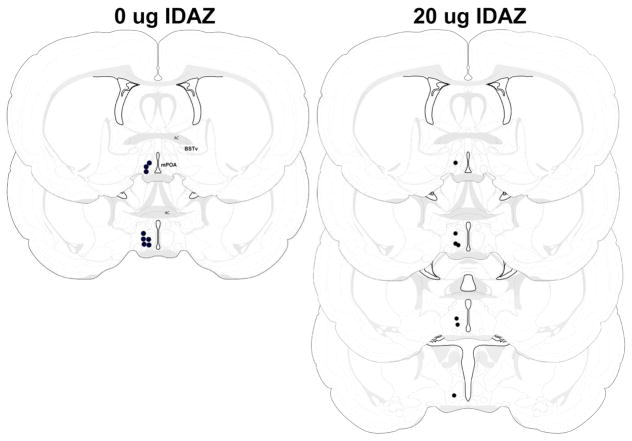
Schematic representation of infusion sites for dams receiving 0 or 20 μg idazoxan into the mPOA (black circles). The bilateral infusion sites are depicted unilaterally. Figure modified from Swanson (1998). AC – anterior commissure, BSTv – ventral bed nucleus of the stria terminalis, mPOA – medial preoptic area
Lesioned females did not significantly differ from controls for any variable measured in the elevated plus maze (Table 4). Further, the latency to display maternal behavior was not significantly correlated with the percentage of time subjects had spent in open arms (r = 0.02, p = 0.93) or the percentage of entries they made into the open arms (r = 0.10, p = 0.68).
Table 4.
Elevated plus-maze behaviors (Mean ± SEM) of ovariectomized, estradiol-treated nulliparous rats that received control or anti-DBH-SAP infusion into the BSTv/mPOA.
| Control (n = 9) | Anti-DBH-SAP (n = 9) | t16 | p | |
|---|---|---|---|---|
| % Entries made into open arms | 37 ± 4 | 38 ± 5 | 0.17 | 0.865 |
| % Time spent in open arms | 17 ± 4 | 20 ± 4 | 0.57 | 0.574 |
| # Closed-arm entries | 17 ± 3 | 17 ± 2 | 0.04 | 0.972 |
| Total arm entries | 27 ± 5 | 29 ± 3 | 0.28 | 0.785 |
Discussion
Early studies of norepinephrine and maternal behavior in laboratory rats reported increased norepinephrine turnover in hypothalamic homogenates (probably including the mPOA and BSTv; Steele et al. 1979) 8–15 hours after parturition (Moltz et al. 1975). A similar increase is seen in the hypothalamus of maternally sensitized rats (Rosenberg et al. 1976). However, three later studies of maternal behavior in rats after forebrain norepinephrine depletion are difficult to reconcile. Intracerebroventricular 6-OHDA given before parturition, but not after parturition, impaired later nest building, nursing, and litter weight gain, but not retrieval (Rosenberg et al. 1977). A second study by this group (Steele et al. 1979) severed the dorsal noradrenergic bundle during early pregnancy, which decreased norepinephrine in the hippocampus and cortex and resulted in almost no females consistently showing all maternal behaviors. In contrast, Bridges et al. (1982) severed both dorsal and ventral noradrenergic pathways during late pregnancy and found very little effect on postpartum maternal behaviors, with the exception of a temporary decrement in nesting. Lactation was impaired, probably due to a lack of noradrenergic stimulation of magnocellular oxytocin cells (Bealer et al. 2010).
The present experiments are the first to study the effects of α2 autoreceptor antagonism (which increases norepinephrine release - Forray et al., 1995 1997; Herr et al., 2012; Palij and Stamford 1993; Park et al. 2009) in the BSTv and mPOA on maternal behavior in postpartum rats, as well as determine if norepinephrine depletion in these sites influences maternal responsiveness in nulliparous rats. We hypothesized that α2 autoreceptor antagonism in the BSTv and mPOA, acting together as a “common functional system” necessary for maternal motivation and behavior (Lonstein and Morrell 2007; Numan and Insel 2003), would impede caregiving behaviors. Norepinephrine tonically inhibits these sites by promoting local release of GABA, which interferes with maternal behavior (Arrati et al. 2006). We also hypothesized that removing noradrenergic inhibition of the BSTv and mPOA would be permissive for mothering. Noradrenergic activity is downregulated in the postpartum PVN (Toufexis et al. 1996 1998; Windle et al. 1997) and we posited this also occurs in the collateralized BSTv and mPOA. This downregulation would be consistent with the lack of behavioral effects of noradrenergic lesions performed after parturition. It would also be consistent with the very minor effects seen when all ascending noradrenergic fibers are severed even before females give birth (Bridges et al 1982). That is, postpartum behavior would not be expected to be altered by removing a neurochemical system that otherwise inhibits maternal caregiving. We found support from postpartum females for the first hypothesis, but our results from the nulliparous females do not support the second hypothesis.
Our data strongly suggest that low noradrenergic activity in the BSTv and mPOA is required for postpartum maternal behavior. Acutely blocking α2 autoreceptor activity in the BSTv with yohimbine impaired retrieval of pups while leaving other behaviors intact. Mostly similar effects were found after BSTv infusion of the more selective α2 antagonist idazoxan. Impaired retrieval was not due to any general locomotor deficit in drug-treated dams because their pup licking and nesting were unaffected, and their durations of other behaviors including cage exploration were sometimes increased. Yohimbine and idazoxan differed in how severely they impaired retrieval. Yohimbine partially or completely prevented retrieval in most dams, but idazoxan did so in less than half. This may be due to idazoxan’s much greater selectivity for the rat α2 receptor when compared to the 5HT1a receptor (51-fold vs. 4-fold) (Newman-Trancridi et al. 1998; Winter and Rabin 1992). Thus, while retrieval is disrupted by putatively reversing the low norepinephrine release of the postpartum BSTv with an α2 autoreceptor antagonist, perhaps only in association with the 5HT1a receptor activity by yohimbine is retrieval almost abolished. One might instead argue that idaxozan would have been expected to be more deleterious to retrieval because α2 receptors exist on serotonergic terminals (Maura et al. 1982; Trendelenburg et al. 1994), thereby allowing α2 antagonists to increase serotonin release (Cheng et al. 1993; Weikop et al. 2004). Like norepinephrine, serotonin also decreases BSTv cell excitability (Hammack et al. 2009). Yohimbine and idazoxan may, therefore, impair retrieval by promoting release of two neurotransmitters that inhibit BSTv activity. Future studies using selective postsynaptic receptor modulators will help clarify the respective contributions of these two neurotransmitter systems in the BSTv for maternal care.
Nursing in mammals requires sufficient offspring suckling, which induces slow-wave sleep and behavioral quiescence that further maintains pup nipple attachment and suckling (Stern 1996). The BSTv and mPOA are activated by offspring sensory cues (Fleming et al. 1994; Kalinichev et al. 2000; Lonstein et al. 1998) and can influence behavioral inhibition or activation via projections to sites involved in locomotion (Numan and Numan 1997). After pups were returned to the nest, BSTv yohimbine reduced some aspects of nursing while idazoxan significantly did so. Peripheral administration of yohimbine or idazoxan can increase locomotor activity (Dickinson et al. 1988; Haapalinna et al. 1997) and norepinephrine in the mPOA elicits wakefulness (Berridge and O’Neill 2001). Increased locomotion may have both contributed to and been a consequence of drug-treated dams’ lower nursing and litter weight gains. Drug-treated females were often delayed in hovering over the pups, thus preventing suckling, and even when pups eventually did suckle it may have been incapable of inducing the quiescence required for continued nursing and milk letdown. Because norepinephrine can enhance oxytocin release (Bealer et al. 2010), litter weight gain deficits may also have been due to impaired milk letdown.
Norepinephrine in the mPOA coordinates sexual behavior with ovulation in female rats (Caldwell and Clemens 1986; Etgen 2006), so the mPOA was a plausible locus of noradrenergic control of maternal behaviors. Indeed, we found that idazoxan greatly interrupted retrieval after mPOA infusion. The absence of any significant effect on licking, another prominent oral maternal behavior, is salient because mPOA lesioning or infusion of a D1 receptor antagonist disrupts both behaviors (Lee et al. 2000; Miller and Lonstein 2005). GABA receptor agonism in the mPOA also impairs retrieval but not licking (Arrati et al. 2006), suggesting that idazoxan’s effects may be mediated by increased mPOA GABA release in response to norepinephrine (Forray et al. 1997 1999; Herbison et al. 1989, 1990; Leung et al. 1981).
Differentiating the roles of the mPOA and BSTv for maternal behavior is difficult because of their close proximity. An alternative explanation for the mostly similar effects of idazoxan infused into the BSTv or mPOA is that the drug diffused between the sites. Our mPOA target site was almost 1.5 mm ventromedial to our BSTv target. Fluid diffusion in the brain depends on many factors, but numerous studies reveal that 200–500 nl of various dyes or drugs infused into the BST or other sites spreads only ~0.05–1 mm after a 30–90 min survival (Liu and Liang 2009; Lohman et al., 2005; Sandkuhler et al. 1987; Myers and Hoch 1978). Our experiments are some of the first to attempt distinguishing between the BSTv and mPOA in their neurochemical control of maternal behavior, and we delivered our drugs in a very small volume (125 nl/hemisphere) that is ¼–⅛ of what those typically used in similar studies. Further, the effects on retrieval were observable within 10–15 min. An autoradiographic analysis of the extent of idazoxan binding after BSTv or mPOA infusion could help clarify its unique actions on each site, but even given these consideration it was notable that idazoxan impaired retrieval more severely after mPOA infusion than after BSTv infusion. The greater effect after mPOA infusion is reminiscent of reports that damage or inactivation of the mPOA has a greater impact on mothering than do manipulations of the BSTv (Numan and Numan 1996; Perrin et al. 2007). Differences in the mPOA and BSTv projections activated when mothers interact with pups may underlie these subtle differences in their behavioral function (Numan and Numan 1997).
Even without the hormonal fluctuations of pregnancy, nulliparous rats will often exhibit caregiving behaviors if repeatedly exposed to young pups (Rosenblatt 1967). This maternal sensitization involves acceptance of the initially aversive pup olfactory cues and can be hastened by peripheral anosmia or olfactory bulbectomy (Fleming and Rosenblatt 1974a,b). Aversion to pup cues is partly driven by the neural networks processing emotionally relevant stimuli, such as the BSTv (Davis et al. 2010). Norepinephrine release in the BSTv increases when male rats are exposed to aversive stimuli and reducing BSTv NE release with an α2 autoreceptor agonist prevents their aversion-related behaviors (Fendt et al. 2005; Park et al. 2012; Schweimer et al. 2005). Thus, the BSTv is a site of interface between the brain networks necessary for maternal and aversive responding, and we have found that BSTv yohimbine not only disrupts mothering but also increases dams’ anxiety (Smith et al. in preparation). By destroying the noradrenergic innervation of the BSTv in nulliparous females, we attempted to remove part of the neural systems processing aversive stimuli. This probably would not alone induce maternal behavior, as simultaneous upregulation of maternal motivation would also be necessary (Lonstein and Morrell 2007), but upregulated maternal motivation also could have been achieved by anti-DBH-SAP because lesions removed the tonic noradrenergic inhibition of BSTv excitatory projections to the VTA (Dumont and Williams 2004; Georges and Aston-Jones 2002) and tonic inhibition of many mPOA cells (Herbison et al. 1989 1990; Kim et al. 1988; Leung et al. 1981). By mimicking the naturally suppressed noradrenergic system of postpartum rats, anti-DBH-SAP lesions could have produced a two-pronged effect promoting maternal responding in nulliparae.
Anti-DBH-SAP did almost eliminate DBH fibers in the BSTv, as well as most in the mPOA and some in the PVN, consistent with other studies (Banihashemi and Rinaman 2006; Bienkowski and Rinaman 2008; Schlitz and Sawchenko 2007). Even this extensive noradrenergic lesion was incapable of hastening maternal sensitization, though. Clearly, noradrenergic input to these sites does not alone inhibit mothering. It is possible that instead of a complete withdrawal, a complex pattern of noradrenergic input promotes maternal care. Rats do show a brief periparturitional increase in noradrenergic neurotransmission that may “prime” the brain to then respond to the decline in norepinephrine with a permissive effect on mothering.
Anxiety-related behavior was unaffected by anti-DBH-SAP, which was surprising, although elevated zero maze behavior is similarly unaffected in male rats with anti-DBH-SAP lesions of the BSTv (Carvalho et al. 2010). This negative result is worth further investigation considering the extensive literature on BST norepinephrine and anxiety behaviors. Perhaps anti-DBH-SAP would have blunted anxiety in our nulliparous females only under particular circumstances, such as after exposure to an acute stressor (Morilak et al. 2005).
In sum, acute α2 autoreceptor antagonism in the BSTv/mPOA disrupts postpartum maternal behaviors, but removing the noradrenergic innervation of these sites does not stimulate mothering in nulliparous females. A more complex pattern of noradrenergic activity, perhaps requiring the presence of stimulatory factors such as the estradiol and prolactin receptor activity characteristic of late pregnancy, may be required for the peripartum onset of maternal behaviors.
Figure 8.
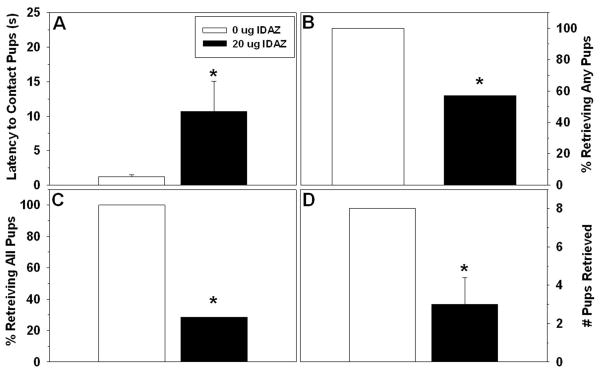
Effects of idazoxan infused into the mPOA on postpartum A) latency to contact pups (Mean ± SEM), B) percentage of dams retrieving any pups, C) percentage of dams retrieving all pups, D) number of pups retrieved (Mean ± SEM). * p < 0.05.
Figure 9.
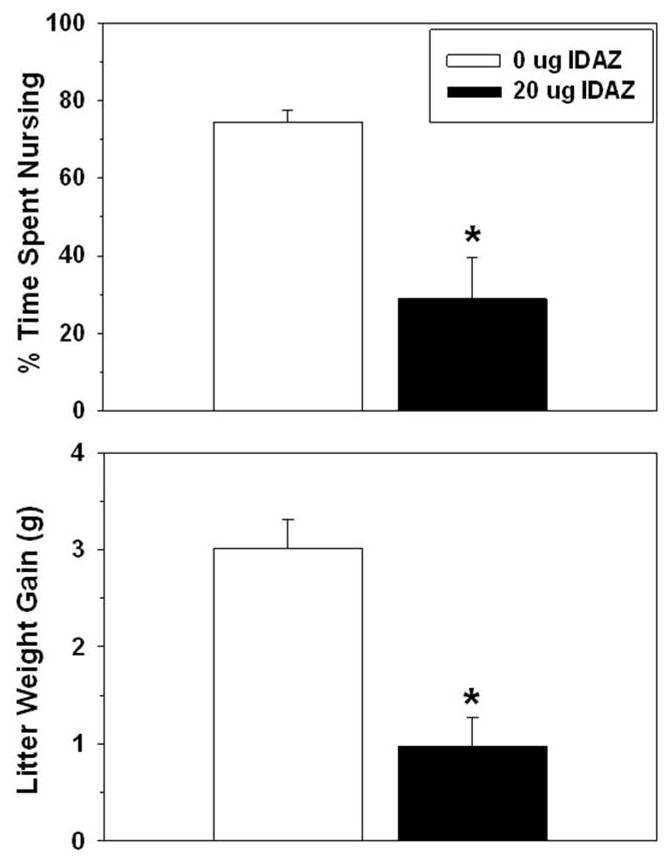
Effects of idazoxan infused into the mPOA on postpartum percentage of time spent nursing (top) and litter weight gain (Mean ± SEM) (bottom).
Figure 10.
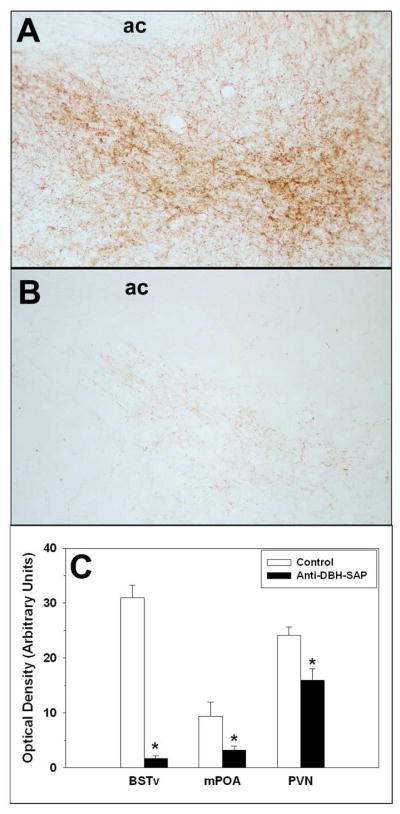
Photomicrographs of DBH immunoreactivity in the BSTv of representative nulliparous females receiving A) control or B) anti-DBH-SAP into the BSTv/mPOA. C) Optical density (Mean ± SEM) of DBH immunoreactivity in the BSTv, mPOA and PVN of control and anti-DBH-SAP nulliparous females. * p < 0.05.
Figure 11.
Maternal sensitization of nulliparous females that received control or anti-DBH-SAP infusion into the BSTv/mPOA. Only responders are included in these measurements of mean (± SEM) and median latencies, n = 5/group. * - p < 0.05
Acknowledgments
This research was supported by NICHD grant R01HD057962 to JSL and a NICHD National Research Service Award to CDS.
Footnotes
These experiments comply with current laws of the United States. The authors claim no conflicts of interest.
References
- Arrati PG, Carmona C, Dominguez G, Beyer C, Rosenblatt JS. GABA receptor agonists in the medial preoptic area and maternal behavior in lactating rats. Physiol Behav. 2006;87:51–65. doi: 10.1016/j.physbeh.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Bakowska JC, Morrell JI. Atlas of the neurons that express mRNA for the long form of the prolactin receptor in the forebrain of the female rat. J Comp Neurol. 1997;386:161–177. doi: 10.1002/(sici)1096-9861(19970922)386:2<161::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J Neurosci. 2006;26:11442–53. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bealer SL, Armstrong WE, Crowley WR. Oxytocin release in magnocellular nuclei: neurochemical mediators and functional significance during gestation. Am J Physiol Regul Integr Comp Physiol. 2010;299:R452–8. doi: 10.1152/ajpregu.00217.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, O’Neill J. Differential sensitivity to the wake-promoting actions of norepinephrine within the medial preoptic area and the substantia innominata. Behav Neurosci. 2001;115:165–74. doi: 10.1037/0735-7044.115.1.165. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156:1093–102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Clifton DK, Sawyer CH. Postpartum luteinizing hormone release and maternal behavior in the rat after late-gestational depletion of hypothalamic norepinephrine. Neuroendocrinology. 1982;34:286–291. doi: 10.1159/000123314. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Mann PE, Coppeta JS. Hypothalamic involvement in the regulation of maternal behaviour in the rat: inhibitory roles for the ventromedial hypothalamus and the dorsal/anterior hypothalamic areas. J Neuroendocrinol. 1999;11:259–266. doi: 10.1046/j.1365-2826.1999.00322.x. [DOI] [PubMed] [Google Scholar]
- Bruning G, Kaulen P, Baumgarten HG. Quantitative autoradiographic localization of alpha 2-antagonist binding sites in rat brain using [3H]idazoxan. Neurosci Lett. 1987;83:333–337. doi: 10.1016/0304-3940(87)90110-8. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Clemens LG. Norepinephrine infusions into the medial preoptic area inhibit lordosis behavior. Pharmacol Biochem Behav. 1986;24:1015–1023. doi: 10.1016/0091-3057(86)90450-8. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Reyes AR, Sterling RC, Unterwald E, Van Bockstaele EJ. Contribution of limbic norepinephrine to cannabinoid-induced aversion. Psychopharmacology. 2010;211:479–91. doi: 10.1007/s00213-010-1923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Responses of neurons in bed nucleus of the stria terminalis to microiontophoretically applied morphine, norepinephrine and acetylcholine. Neuropharmacology. 1993;32:279–284. doi: 10.1016/0028-3908(93)90112-g. [DOI] [PubMed] [Google Scholar]
- Cervo L, Grignaschi G, Samanin R. Alpha 2-adrenoceptor blockade prevents the effect of desipramine in the forced swimming test. Eur J Pharmacol. 1990;175:301–307. doi: 10.1016/0014-2999(90)90568-q. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Costall B, Ge J, Naylor RJ. The profiles of interaction of yohimbine with anxiolytic and putative anxiolytic agents to modify 5-HT release in the frontal cortex of freely-moving rats. Br J Pharmacol. 1993;110:1079–1084. doi: 10.1111/j.1476-5381.1993.tb13924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson SL, Gadie B, Tulloch IF. Alpha 1- and alpha 2-adrenoreceptor antagonists differentially influence locomotor and stereotyped behaviour induced by d-amphetamine and apomorphine in the rat. Psychopharmacology. 1988;96:521–7. doi: 10.1007/BF02180034. [DOI] [PubMed] [Google Scholar]
- Dodge JC, Badura LL. Infusion of alpha-2-adrenergic agents into the paraventricular and arcuate nuclei of the hypothalamus in the Siberian hamster: opposing effects on basal prolactin. Neuroendocrinology. 2002;75:175–184. doi: 10.1159/000048235. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006a;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J Comp Neurol. 2006b;494:108–141. doi: 10.1002/cne.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AJ. Central noradrenergic mechanisms underlying acute stress responses of the Hypothalamo-pituitary-adrenal axis: adaptations through pregnancy and lactation. Stress. 2005;8:5–18. doi: 10.1080/10253890500044380. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, González-Flores O, Todd BJ. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol. 2006;27:363–75. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Galaz C, Dyer RG, Herbison AE. Analysis of brainstem A1 and A2 noradrenergic inputs to the preoptic area using microdialysis in the rat. Brain Res. 1994;636:227–32. doi: 10.1016/0006-8993(94)91021-9. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: emotionality differences between nulliparous and parturient females. Physiol Behav. 1981;27:863–868. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J Comp Physiol Psychol. 1974a;86:221–32. doi: 10.1037/h0035937. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. II. Effects of peripherally induced anosmia and lesions of the lateral olfactory tract in pup-induced virgins. J Comp Physiol Psychol. 1974b;86:233–46. doi: 10.1037/h0035936. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Suh EJ, Korsmit M, Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav Neurosci. 1994;108:724–34. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Regulation of norepinephrine release from the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res. 1997;50:1040–1046. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1040::AID-JNR15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Noradrenaline inhibits glutamate release in the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res. 1999;55:311–320. doi: 10.1002/(SICI)1097-4547(19990201)55:3<311::AID-JNR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Palacios JM, Pazos A, Reichhart JM, Porte A, Richard P. Pharmacological characteristics and anatomical distribution of [3H]oxytocin-binding sites in the Wistar rat brain studied by autoradiography. Neuroscience. 1987;20:599–614. doi: 10.1016/0306-4522(87)90113-8. [DOI] [PubMed] [Google Scholar]
- Fuentealba JA, Forray MI, Gysling K. Chronic morphine treatment and withdrawal increase extracellular levels of norepinephrine in the rat bed nucleus of the stria terminalis. J Neurochem. 2000;75:741–748. doi: 10.1046/j.1471-4159.2000.0750741.x. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia KK, Kumar VM, Mallick HN. Role of the lateral septal noradrenergic system in the elaboration of male sexual behavior in rats. Pharmacol Biochem Behav. 2002;72:817–823. doi: 10.1016/s0091-3057(02)00771-2. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Poree L, Golden W, Stein J, Fujinaga M, Maze M. Antinociceptive response to nitrous oxide is mediated by supraspinal opiate and spinal alpha 2 adrenergic receptors in the rat. Anesthesiology. 1996;85:846–852. doi: 10.1097/00000542-199610000-00020. [DOI] [PubMed] [Google Scholar]
- Haapalinna A, Viitamaa T, MacDonald E, Savola JM, Tuomisto L, Virtanen R, Heinonen E. Evaluation of the effects of a specific alpha 2-adrenoceptor antagonist, atipamezole, on alpha 1- and alpha 2-adrenoceptor subtype binding, brain neurochemistry and behaviour in comparison with yohimbine. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:570–82. doi: 10.1007/pl00005092. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1309–20. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Heavens RP, Dyer RG. Oestrogen and noradrenaline modulate endogenous GABA release from slices of the rat medial preoptic area. Brain Res. 1989;486:195–200. doi: 10.1016/0006-8993(89)91295-x. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Heavens RP, Dyer RG. Oestrogen modulation of excitatory A1 noradrenergic input to rat medial preoptic gamma aminobutyric acid neurones demonstrated by microdialysis. Neuroendocrinology. 1990;52:161–8. doi: 10.1159/000125568. [DOI] [PubMed] [Google Scholar]
- Herr NR, Park J, McElligott ZA, Belle AM, Carelli RM, Wightman RM. In vivo voltammetry monitoring of electrically evoked extracellular norepinephrine in subregions of the bed nucleus of the stria terminalis. J Neurophysiol. 2012;107:1731–7. doi: 10.1152/jn.00620.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Nakabeppu Y, Morrell JI. Induction of c-fos-like and fosB-like immunoreactivity reveals forebrain neuronal populations involved differentially in pup-mediated maternal behavior in juvenile and adult rats. J Comp Neurol. 2000;416:45–78. doi: 10.1002/(sici)1096-9861(20000103)416:1<45::aid-cne5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Kim YI, Dudley CA, Moss RL. A1 noradrenergic action on medial preoptic-medial septal neurons: a neuropharmacological study. Synapse. 1988;2:494–507. doi: 10.1002/syn.890020505. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- Leung PC, Whitmoyer DI, Arendash GW, Sawyer CH. Effects of intraventricular norepinephrine on preoptic-anterior hypothalamic electrical activity in the freely-moving rat: modulation by ovarian steroid hormones. Brain Res. 1981;226:143–54. doi: 10.1016/0006-8993(81)91089-1. [DOI] [PubMed] [Google Scholar]
- Liu TL, Liang KC. Posttraining infusion of cholinergic drugs into the ventral subiculum modulated memory in an inhibitory avoidance task: interaction with the bed nucleus of the stria terminalis. Neurobiol Learn Mem. 2009;91:235–42. doi: 10.1016/j.nlm.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Lohman RJ, Liu L, Morris M, O’Brien TJ. Validation of a method for localised microinjection of drugs into thalamic subregions in rats for epilepsy pharmacological studies. J Neurosci Methods. 2005;146:191–7. doi: 10.1016/j.jneumeth.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Horm Behav. 2005;47:241–255. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–141. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Morrell JI. Neuropharmacology and neuroendocrinology of maternal motivation and behavior. In: Blaustein JD, editor. Handbook of Neurochemistry and Molecular Biology. vol 18 - Behavioral Neurobiology. Springer Press; 2007. pp. 195–245. [Google Scholar]
- Lonstein JS, Wagner CK, De Vries GJ. Comparison of the “nursing” and other parental behaviors of nulliparous and lactating female rats. Horm Behav. 1999;36:242–51. doi: 10.1006/hbeh.1999.1544. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–81. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Maura G, Gemignani A, Raiteri M. Noradrenaline inhibits central serotonin release through alpha 2-adrenoceptors located on serotonergic nerve terminals. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:272–4. doi: 10.1007/BF00510140. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopamine D1 and D2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav Neurosci. 2005;119:1072–1083. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- Miller SM, Piasecki CC, Peabody MF, Lonstein JS. GABAA receptor antagonism in the ventrocaudal periaqueductal gray increases anxiety in the anxiety-resistant postpartum rat. Pharmacol Biochem Behav. 2010;95:457–465. doi: 10.1016/j.pbb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Moltz H, Rowland D, Steele M, Halaris A. Hypothalamic norepinephrine: concentration and metabolism during pregnancy and lactation in the rat. Neuroendocrinology. 1975;19:252–258. doi: 10.1159/000122445. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–24. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Myers RD, Hoch DB. 14C-dopamine microinjected into the brain-stem of the rat: dispersion kinetics, site content and functional dose. Brain Res Bull. 1978;3:601–9. doi: 10.1016/0361-9230(78)90006-0. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Nicolas JP, Audinot V, Gavaudan S, Verriele L, Touzard M, Chaput C, Richard N, Millan MJ. Actions of alpha2 adrenoceptor ligands at alpha2A and 5-HT1A receptors: the antagonist, atipamezole, and the agonist, dexmedetomidine, are highly selective for alpha2A adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- Numan M, Numan MJ. Expression of Fos-like immunoreactivity in the preoptic area of maternally behaving virgin and postpartum rats. Behav Neurosci. 1994;108:379–394. doi: 10.1037//0735-7044.108.2.379. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Importance of pup-related sensory inputs and maternal performance for the expression of Fos-like immunoreactivity in the preoptic area and ventral bed nucleus of the stria terminalis of postpartum rats. Behav Neurosci. 1995;109:135–149. doi: 10.1037//0735-7044.109.1.135. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J Neuroendocrinol. 1997;9:369–384. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119:1588–604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Palij P, Stamford JA. Real-time monitoring of endogenous noradrenaline release in rat brain slices using fast cyclic voltammetry. 2. Operational characteristics of the alpha 2 autoreceptor in the bed nucleus of stria terminalis, pars ventralis. Brain Res. 1993;607:134–40. doi: 10.1016/0006-8993(93)91498-h. [DOI] [PubMed] [Google Scholar]
- Park J, Kile BM, Wightman RM. In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Eur J Neurosci. 2009;30:2121–33. doi: 10.1111/j.1460-9568.2009.07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM. Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biol Psychiatry. 2012;71:327–34. doi: 10.1016/j.biopsych.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992;28:949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–9. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riche D, De Pommery J, Menetrey D. Neuropeptides and catecholamines in efferent projections of the nuclei of the solitary tract in the rat. J Comp Neurol. 1990;293:399–424. doi: 10.1002/cne.902930306. [DOI] [PubMed] [Google Scholar]
- Roder S, Ciriello J. Collateral axonal projections to limbic structures from ventrolateral medullary A1 noradrenergic neurons. Brain Res. 1994;638:182–188. doi: 10.1016/0006-8993(94)90648-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg P, Halaris A, Moltz H. Effects of central norepinephrine depletion on the initiation and maintenance of maternal behavior in the rat. Pharmacol Biochem Behav. 1977;6:21–24. doi: 10.1016/0091-3057(77)90155-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg P, Leidahl L, Halaris A, Moltz H. Changes in the metabolism of hypothalamic norepinephrine associated with the onset of maternal behavior in the nulliparous rat. Pharmacol Biochem Behav. 1976;4:647–649. doi: 10.1016/0091-3057(76)90215-x. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1514. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Maisch B, Zimmermann M. The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Exp Brain Res. 1987;68:168–78. doi: 10.1007/BF00255242. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. J Comp Neurol. 2007;502:455–67. doi: 10.1002/cne.21329. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. Eur J Pharmacol. 2005;507:117–124. doi: 10.1016/j.ejphar.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Scotti MA, Lee G, Gammie SC. Maternal defense is modulated by beta adrenergic receptors in lateral septum in mice. Behav Neurosci. 2011;125:434–45. doi: 10.1037/a0023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Gorski RA, Swanson LW. Neurotransmitter specificity of cells and fibers in the medial preoptic nucleus: an immunohistochemical study in the rat. J Comp Neurol. 1986;246:343–363. doi: 10.1002/cne.902460305. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Smith CD, Lonstein JS. Contact with infants modulates anxiety-generated c-fos activity in the brains of postpartum rats. Behav Brain Res. 2008;190:193–200. doi: 10.1016/j.bbr.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele MK, Rowland D, Moltz H. Initiation of maternal behavior in the rat: possible involvement of limbic norepinephrine. Pharmacol Biochem Behav. 1979;11:123–30. doi: 10.1016/0091-3057(79)90001-7. [DOI] [PubMed] [Google Scholar]
- Stern JM. Somatosensation and maternal care in Norway rats. In: Rosenblatt JS, Snowdon CT, editors. Advances in the study of behavior. Vol. 25. San Diego: Academic Press; 1996. pp. 243–294. [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier Science; 1998. [Google Scholar]
- Toufexis DJ, Thrivikraman KV, Plotsky PM, Morilak DA, Huang N, Walker CD. Reduced noradrenergic tone to the hypothalamic paraventricular nucleus contributes to the stress hyporesponsiveness of lactation. J Neuroendocrinol. 1998;10:417–427. doi: 10.1046/j.1365-2826.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Walker CD. Noradrenergic facilitation of the adrenocorticotropin response to stress is absent during lactation in the rat. Brain Res. 1996;737:71–77. doi: 10.1016/0006-8993(96)00627-0. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Trendelenburg M, Starke K, Limberger N. Release-inhibiting alpha 2-adrenoceptors at serotonergic axons in rat and rabbit brain cortex: evidence for pharmacological identity with alpha 2-autoreceptors. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:25–33. doi: 10.1007/BF00178202. [DOI] [PubMed] [Google Scholar]
- Weikop P, Kehr J, Scheel-Krüger J. The role of alpha1- and alpha2-adrenoreceptors on venlafaxine-induced elevation of extracellular serotonin, noradrenaline and dopamine levels in the rat prefrontal cortex and hippocampus. J Psychopharmacol. 2004;18:395–403. doi: 10.1177/026988110401800311. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Brady MM, Kunanandam T, Da Costa AP, Wilson BC, Harbuz M, Lightman SL, Ingram CD. Reduced response of the hypothalamo-pituitary-adrenal axis to alpha1-agonist stimulation during lactation. Endocrinology. 1997;138:3741–8. doi: 10.1210/endo.138.9.5405. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J Pharmacol Exp Ther. 1992;263:682–689. [PubMed] [Google Scholar]
- Woulfe JM, Flumerfelt BA, Hrycyshyn AW. Efferent connections of the A1 noradrenergic cell group: a DBH immunohistochemical and PHA-L anterograde tracing study. Exp Neurol. 1990;109:308–322. doi: 10.1016/s0014-4886(05)80022-6. [DOI] [PubMed] [Google Scholar]
- Woulfe JM, Hrycyshyn AW, Flumerfelt BA. Collateral axonal projections from the A1 noradrenergic cell group to the paraventricular nucleus and bed nucleus of the stria terminalis in the rat. Exp Neurol. 1988;102:121–4. doi: 10.1016/0014-4886(88)90084-2. [DOI] [PubMed] [Google Scholar]