Abstract
A 9-month-old p53-null female mouse was found dead in its cage. At necropsy, a large thymic mass encompassed the heart. Microscopically, the mass was composed of numerous varying-sized cysts lined with simple squamous epithelial cells to columnar ciliated cells. Also present within this mass was a large aggregate of loosely arranged fusiform-shaped cells. These cells also were found in smaller numbers in the connective tissue surrounding the cysts. The larger aggregate of fusiform cells was positive for desmin and S-100 and negative for smooth muscle actin. Electron microscopy revealed well-formed Z lines and I bands of skeletal muscle phenotype. A diagnosis of rhabdomyoma within a congenital multilocular thymic cyst was made. The thymus contains a small population of myoid cells, which should be taken in consideration when evaluating thymic tumors.
Keywords: genetically engineered mouse, multilocular cyst, myoid cell, p53 gene, thymus, ultrastructure
The thymus in the mouse is similar to that of other mammalian species in respect to anatomy, cell populations, and involutionary changes. In mice, the thymus undergoes involutionary changes at around 35 to 80 days of age,31 with the ability to repopulate.32 Cystic cavities within the thymus are usually seen as incidental findings in adult mice. These cysts are usually small, isolated, and within the corticomedullary junction.14 Besides containing lymphoid cells and epithelial components (Hassall corpuscles), the thymus contains few to rare numbers of myoid cells.23 This article describes a multilocular thymic cyst, an uncommon finding in mice, associated with a myoid cell rhabdomyoma.
A 9-month-old female black p53-null mouse (B6.129S2-Trp53tm1Tyj/J) was housed at the National Institutes of Health (NIH; Bethesda, MD). The care of the animal followed the NIH's Guide to the Care and Use of Laboratory Animals. The mouse was found dead during daily observations by the animal caretakers. The animal was submitted for diagnostic necropsy to the Diagnostic and Research Services Branch of the Division of Veterinary Resources at the NIH. On examination, the animal was obese with mild alopecia of the muzzle. Upon opening of the pleural cavity, a multilobulated reddish-purple mass was found (approximately 2 cm in diameter), which encompassed the heart and compressed the lungs (Fig. 1). On cut surface of the mass, serosanguineous fluid exuded from what appeared to be cystic structures. No other lesions were observed in the remainder of the animal.
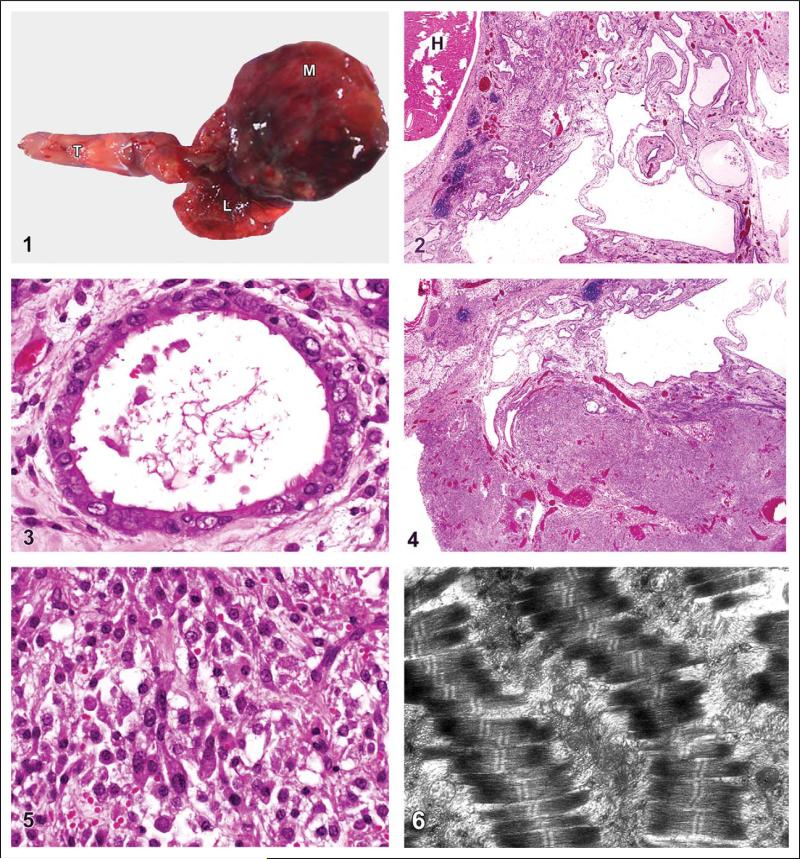
Figure 1.
Thymic mass. Mouse. A large fleshy mass (M) surrounds the heart and compresses the lungs (L). Tongue (T). Figure 2. Thymic mass. Mouse. Numerous varying-sized cystic spaces make up a large portion of the mass. Notice how the thymic mass surrounds but does not attach to the heart (H). HE. Figure 3. Thymic mass. Mouse. Smaller cysts contain simple columnar to cuboidal ciliated epithelium. HE. Figure 4. Thymic mass. Mouse. Large proliferations of myoid cells. HE. Figure 5. Thymic mass. Mouse. Myoid cells arranged haphazardly. HE. Figure 6. Myoid cells. Ultrastructure of parallel or stacked myofibrils with prominent I bands and Z line with A bands on either side.
The entire mass with associated heart, lungs, trachea, and tongue was fixed in 10% buffered formalin, processed, and embedded in paraffin; 5-μm sections were then cut and stained with hematoxylin and eosin (HE), Masson trichrome, and periodic acid–Schiff (PAS).
Immunohistochemistry for desmin, S-100, smooth muscle actin, and F4/80 (mouse macrophage) was performed on the mass. Briefly, 5-μm sections were deparaffinized in xylene, placed in absolute alcohol, and processed in a Dako Autostainer Universal Staining System (Dako, Carpinteria, CA). Endogenous peroxidase was inhibited by 5-minute incubation in 3% hydrogen peroxide. Antigen retrieval (desmin, S-100) was accomplished by pressure cooker heating for 20 minutes at 37° C in citrate buffer or with proteinase K for 5 minutes (F4/80) at room temperature. Sections were blocked with a commercially available protein block (Dako) for 5 minutes at room temperature. The primary antibodies were rabbit anti-desmin (1:3000; Thermo-Scientific, Fremont, CA), rabbit anti-S-100 (1:10,000; Dako), mouse anti–smooth muscle actin (1:100; Biocare Medical, Concord, CA), and rat anti-mouse F4/80 (1:400; AbD Serotec, Raleigh, NC). The detection system utilized a biotinylated goat–antirat antibody with streptavidin–horseradish peroxidase label for F4/80, polymerized horseradish peroxidase antirabbit secondary for desmin and S-100, and a mouse on mouse kit for smooth muscle actin. DAB chromogen (Dako) was added, and the slides were counterstained with Mayer hematoxylin. Positive control slides consisted of known positive mammalian tissue incubated with the relevant monoclonal antibody. Negative control slides consisted of the test tissue incubated with an isotype matched immunoglobulin.
An area of interest within the mass was trimmed from the paraffin block and processed for electron microscopy. The tissue was washed 3 times in xylene for 1 hour each and placed into 10% OsO4 in xylene overnight. Subsequently, the tissue was washed 3 times in xylene for 30 minutes each, changed to 2:1 propylene oxide:Epon and then 1:2 propylene oxide:Epon, and embedded in EMBed 812 resin (Electron Microscopy Sciences, Hatfield, PA). Thin sections, approximately 80 nm, were obtained by utilizing the Leica Ultracut-UCT Ultramicrotome (Leica, Deerfield, IL), placed onto 300 mesh copper grids, and stained with saturated uranyl acetate in 50% methanol and then with lead citrate. The grids were viewed in the JEM-1200EXII electron microscope (JEOL Ltd., Tokyo, Japan) at 80 kV, and images were recorded on the XR611M midmounted 10.5-Mpixel CCD (charge-coupled device) camera (Advanced Microscopy Techniques Corp., Danvers, MA).
Histopathology of the mass revealed numerous varying-sized cystic structures (Fig. 2). The largest cysts were primarily lined by a simple squamous epithelium with occasional plump ciliated epithelial cells. Most of the smaller cysts had simple cuboidal to columnar, most ciliated, epithelial cells (Fig. 3), whereas other cysts had stratified squamous epithelial cells. PAS-positive goblet cells were admixed with the ciliated epithelial cells. The lumina of many of these cysts were partially filled with a wispy fibrillar material admixed with PAS-positive proteinaceous fluid, plump macrophages, lymphocytes, and neutrophils. Multifocal to coalescing cords and islands of squamous epithelial cells (occasionally admixed with a mild to moderate neutrophilic and lymphocytic infiltrate) or forming small incomplete lumina were observed. Many of these lumina contain necrotic debris admixed with a few neutrophils and/or PAS-positive proteinaceous fluid. All cysts were within a mild to moderately edematous fibrous connective tissue matrix as demonstrated on Masson trichrome stain. Thymic lymphocytic remnants were observed at the periphery of the cysts.
Within an extension of the connective tissue that contained the cysts, and near the base of the heart, was a large circumscribed, nonencapsulated mass of mild to moderately anisocytotic and anisokaryotic round to fusiform cells admixed with large areas of necrosis and hemorrhage (Fig. 4). The cells contained round centrally located nuclei, with finely stippled chromatin and fibrillar eosinophilic cytoplasm. There were a few foci where cells had a spider web–like appearance primarily due to contraction of cells. Small clusters of cells were aligned haphazardly with each other (Fig. 5). Similar cells were also present in smaller numbers within the connective tissue containing the cysts. To establish if these cells were myoid in origin, immunohistochemistry was performed for desmin, S-100, smooth muscle actin, and F4/80 (macrophage). Results of the immunohistochemistry revealed a desmin-positive, S-100-positive, smooth muscle actin–negative, F4/80-negative population of cells that were identified as immature striated skeletal myoid cells. To confirm the striated muscle phenotype, electron microscopy of the mass was performed. Within these cells were multiple parallel myofibrils, each containing a single I band and Z line with A bands on either side (Fig. 6). Given the large population of myoid cells along with the numerous varying-sized cysts, a diagnosis of rhabdomyoma within a multilocular thymic cyst was made.
The thymus is a dynamic organ that involutes over time. The primary cell components consist of lymphoid cells and epithelial cells (Hassall corpuscles). Another cell type is the myoid cell, which occurs in small numbers in humans and mice but is quite common in reptilian and avian species.1,10,23,27 Myoid cells are often isolated to the medulla. It is believed that they have an embryological origin from the neural crest and hence are S-100 positive.24 Ultrastructurally, myoid cell myofibrils are parallel bundles; however, they are sometimes irregularly arranged, forming angles in various orientations within the cytoplasm, a consistent finding for an immature myoid cell.23 These cells have been described in healthy humans during fetal and perinatal periods. Their exact role within the thymus is not known. The thymus contains significant autoantigens, and the presence of myoid cells may be necessary for the induction of tolerance in lymphocytes. Myoid cells may also have a role as myogenic precursors or stem cells in muscle.33 Myoid cells play a role in sensitization and subsequent formation of anti-muscle antibodies present in humans with myasthenia gravis.15 Proliferation of myoid cells within the thymus contributing to tumor formation has been documented in humans.11,22 Myoid cells have also been observed in, and have contributed to, human diseases such as thymomas.7,20 A few case reports of rhabdomyomatous and rhabdomyosarcomatous neoplasms of the thymus, as well as thymic carcinomas with skeletal muscle differentiation, have been described in humans.6,9,25,30 Only one case report of a rhabdomyomatous multilocular thymic cyst in human has been documented.2
Thymic cysts have been documented in multiple animal species (mice, cow, sheep, dog, cat, dolphin, chicken, human)4,14,17 and may be of diverse pathogenesis (congenital, infective, inflammatory, neoplastic).29 Single small cysts are a frequent finding in the thymus and are thought to be congenital and to arise from the endodermal vestiges of third and fourth fetal branchial arches. Cysts can be lined by squamous, cuboidal, or columnar epithelial cells. Cysts can be lined by cilia and associated with goblet cells, similar in appearance to respiratory epithelium, which also arises from the branchial arches.14 Small numbers of thymic cysts, primarily in the corticomedullary junction, are common in mice and are vital structures that may be engaged in active transportation of lymphocytes and secretions into the circulation.14 Numerous thymic cysts in mice are uncommon for most strains, except for nude mice, whose bulk of the thymus is composed of small cysts.3 In humans, multilocular cysts are thought to be an acquired reactive process due to some inflammatory condition that leads to cystic formation.29 Although this mouse did not have much in the way of inflammation, the term multilocular cyst was used to identify the numerous varying-sized and lined cysts that were present.
The cysts were most likely congenital in origin and became enlarged by the presence of the mass of myoid cells. In humans, multilocular thymic cysts have been associated with thymoma,21 carcinoma,19 follicular hyperplasia,12 and pseudoepitheliomatous hyperplasia.28
Although the cysts presented in this case are presumed to have arisen from the thymus, one cannot rule out other origins. Cysts arising from the mediastinum are rare in humans and animals. Pericardial and bronchogenic cysts are the most commonly described cysts in humans.16,26 In the veterinary literature, 1 dog had a bronchogenic cyst,5 9 cats had a solitary mediastinal cyst of unknown origin (histology was not performed),34 and 2 cats had cysts of thymic origin.18
The p53 gene regulates the cell cycle. Alterations in this gene (mutations and loss) can lead to formation of numerous types of neoplasms, including lymphosarcoma, osteosarcoma, hemangiosarcoma, and rhabdomyosarcoma.8,13 The most common thymic tumor in p53-null mice is lymphoma. The myoid cell rhabdomyoma in the thymus of this p53-null mouse may have the same fundamental growth pattern as that of rhabdomyosarcoma. Loss of the p53 gene may have allowed uncontrollable growth of myoid cells in the thymus.
We believe this to be the first report of an animal with a rhabdomyoma composed of myoid cells within a multilocular thymic cyst. Myoid cells are a normal yet rare cell type found in the thymus of animals and humans and should be considered a possible cell type in the formation of tumors within the thymus.
Acknowledgements
We would like to thank Jorge Chavez and Annie Merriweather for technical assistance. This work was supported in part by the Intramural Research Program of the National Institutes of Health, Office of Research Services (MFS), and in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health intramural project Z01-HD-000642-04 (CAS).
Financial Disclosure/Funding
The authors declared that they received no financial support for their research and/or authorship of this article.
Footnotes
Declaration of Conflict of Interest
The authors declared that they had no conflicts of interests with respect to their authorship or the publication of this article.
References
- 1.Chan AS. Ultrastructure of myoid cells in the chick thymus. Br Poult Sci. 1995;36:197–203. doi: 10.1080/00071669508417768. [DOI] [PubMed] [Google Scholar]
- 2.Chetty R, Reddi A. Rhabdomyomatous multilocular thymic cyst. Am J Clin Pathol. 2003;119:816–821. doi: 10.1309/QDJC-A1HX-QLHL-CFTM. [DOI] [PubMed] [Google Scholar]
- 3.Cordier AC. Ultrastructure of the cilia of thymic cysts in nude mice. Anat Rec. 1974;181:227–250. doi: 10.1002/ar.1091810206. [DOI] [PubMed] [Google Scholar]
- 4.Cowan DF. Involution and cystic transformation of the thymus in the bottle-nose dolphin, Tursiops truncatus. Vet Pathol. 1994;31:648–653. doi: 10.1177/030098589403100603. [DOI] [PubMed] [Google Scholar]
- 5.Dahl K, Rorvik AM, Lanageland M. Bronchogenic cyst in a german shepherd. J Small Anim Pract. 2002;43:456–458. doi: 10.1111/j.1748-5827.2002.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 6.de Queiroga EM, Chikota H, Bacchi CE, Moran CA, Suster S. Rhabdomyomatous carcinoma of the thymus. Am J Surg Pathol. 2004;28:1245–1250. doi: 10.1097/01.pas.0000126056.41540.f4. [DOI] [PubMed] [Google Scholar]
- 7.Deveci MS, Ceyhan K, Deveci G, Finci R. Pericardial rhabdomyomatous spindle cell thymoma with mucinous cystic degeneration. Histopathology. 2001;38:479–481. doi: 10.1046/j.1365-2559.2001.1163b.x. [DOI] [PubMed] [Google Scholar]
- 8.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 9.Eimoto Tm Kitaoka M, Ogawa H, Niwa H, Murase T, Tateyama H, Inagaki H, Soji T, Wang HJ. Thymic sarcomatoid carcinoma with skeletal muscle differentiation: report of two cases, one with cytogenetic analysis. Histopathology. 2002;40:46–57. doi: 10.1046/j.1365-2559.2002.01310.x. [DOI] [PubMed] [Google Scholar]
- 10.Feltkamp-Vroom T. Myoid cells in human thymus. Lancet. 1966;287:1320–1321. [Google Scholar]
- 11.Henry K. An unusual thymic tumour with a striated muscle (myoid) component (with a brief review of the literature on myoid cells). Brit J Dis Chest. 1972;66:291–299. doi: 10.1016/0007-0971(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 12.Izumi H, Nobukawa B, Takahashi K, Kumasaka T, Miyamoto H, Yamazaki A, Sonobe S, Uekusa T, Suda K. Multilocular thymic cyst associated with follicular hyperplasia: clinopathologic study of 4 resected cases. Hum Pathol. 2005;36:841–844. doi: 10.1016/j.humpath.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 14.Khosla S, Ovalle WK. Morphology and distribution of cystic cavities in thenormal murine thymus. Cell Tissue Res. 1986;246:531–542. doi: 10.1007/BF00215193. [DOI] [PubMed] [Google Scholar]
- 15.Leite MI, Jones M, Strobel P, Marx A, Gold R, Niks E, Verschuuren JJ, Berrih-Aknin S, scaravilli F, Canelhas A, Morgan BP, Vincent A, Willcox N. Myasthenia gravis thymus: complement vulnerability of epithelial and myoid cells, complement attack on them, and correlations with autoantibody status. Am J Pathol. 2007;171:893–905. doi: 10.2353/ajpath.2007.070240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limaiem A, Ayadi-Kaddour A, Djilani H, Kilani T, El Mezni F. Pulmonary and medistinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung. 2007;186:55–61. doi: 10.1007/s00408-007-9056-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu S-K, Patnaik AK, Burk RL. Thymic branchial cysts in the dog and cat. J Am Vet Med Assoc. 1983;182:1095–1098. [PubMed] [Google Scholar]
- 18.Malik R, Gabor L, Hunt GB, Church DB, Barrs VR, Churcher R, Dixon RT, Huxley C, Canfield PJ. Benign cranial mediastinal lesions in three cats. Aust Vet J. 1997;75:183–187. doi: 10.1111/j.1751-0813.1997.tb10062.x. [DOI] [PubMed] [Google Scholar]
- 19.Moran CA, Suster S, El-Naggar A, Luna MA. Carcinomas arising in multilocular thymic cysts of the neck: a clinicopathological study of three cases. Histopathology. 2004;44:64–68. doi: 10.1111/j.1365-2559.2004.01767.x. [DOI] [PubMed] [Google Scholar]
- 20.Moran CA, Koss MN. Rhabdomyomatous thymoma. Am J Surg Pathol. 1993;17:633–636. doi: 10.1097/00000478-199306000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Muller-Hermelink HK. Multilocular thymic cysts associated with thymoma: A case report. Pathol Res Pract. 1997;193:531–532. [PubMed] [Google Scholar]
- 22.Murakami S, Shamoto M, Miura K, Takeuchi J. A thymic tumor with massive proliferation of myoid cells. Acta Pathol Jpn. 1984;34:1375–1383. doi: 10.1111/j.1440-1827.1984.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 23.Nabarra B, Andrianarison I. Thymic reticulum of mice III: the connective component (innervation, vascularization, fibrous tissue and myoid cells). Tissue Cell. 1995;27:249–261. doi: 10.1016/s0040-8166(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura H, Lievre CA-L. Neural crest and thymic myoid cells. Curr Top Dev Biol. 1986;20:111–115. doi: 10.1016/s0070-2153(08)60658-4. [DOI] [PubMed] [Google Scholar]
- 25.Okudela K, Nakamura N, Sano J-Y, Ito T, Kitamura H. Thymic carcinosarcoma consisting of squamous cell carcinomatous and embryonal rhabdomyosarcomatous components. Pathol Res Pract. 2001;197:205–210. doi: 10.1078/0344-0338-00035. [DOI] [PubMed] [Google Scholar]
- 26.Raveglia F, Baisi A, Calati AM, Kaiser LR. Bilocular pericardial cyst in an aberrant location. Interact Cardiovasc Thorac Surg. 2008;8:160–161. doi: 10.1510/icvts.2008.180729. [DOI] [PubMed] [Google Scholar]
- 27.Saad AH, Zapata A. Reptilian thymus gland: an ultrastructural overview. Thymus. 1992;20:135–152. [PubMed] [Google Scholar]
- 28.Suster S, Barbuto D, Carlson G, Rosai J. Multilocular thymic cysts with pseudoepitheliomatous hyperplasia. Hum Pathol. 1991;22:455–460. doi: 10.1016/0046-8177(91)90131-8. [DOI] [PubMed] [Google Scholar]
- 29.Suster S, Rosai J. Multilocular thymic cyst: an acquired reactive process. Study of 18 cases. Am J Surg Pathol. 1991;15:388–398. [PubMed] [Google Scholar]
- 30.Toprani TH, Tamboli P, Amin MB, Ordonez NG, Ayala AG, Ro JY. Thymic carcinoma with rhabdoid features. Ann Diagn Pathol. 2003;7:106–111. doi: 10.1053/adpa.2003.50007. [DOI] [PubMed] [Google Scholar]
- 31.Ward JM, Mann PC, Morishima H, Frith CH. Thymus, spleen, and lymph nodes. In: Maronpot RR, editor. Pathology of the Mouse. Cache River Press; Vienna, IL: 1999. pp. 333–360. [Google Scholar]
- 32.Wijnands MVW, Kuper CF, Schuurman H-J, Woutersen RA. Nonneoplastic lesions of the hematopoietic system. In: Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, editors. Pathobiology of the Aging Mouse. Vol. 1. ILSI Press; Washington, DC: 1996. pp. 205–217. [Google Scholar]
- 33.Wong A, Garrett KL, Anderson JE. Myoid cell density in the thymus is reduced during mdx dystrophy and after muscle crush. Biochem Cell Biol. 1999;77:33–40. [PubMed] [Google Scholar]
- 34.Zekas LJ, Adams WM. Cranial mediastinal cysts in nine cats. Vet Radiol Ultrasound. 2002;43:413–418. doi: 10.1111/j.1740-8261.2002.tb01027.x. [DOI] [PubMed] [Google Scholar]