Abstract
Breathing emerges through complex network interactions involving neurons distributed throughout the nervous system. The respiratory rhythm generating network is composed of micro networks functioning within larger networks to generate distinct rhythms and patterns that characterize breathing. The pre-Bötzinger complex, a rhythm generating network located within the ventrolateral medulla assumes a core function without which respiratory rhythm generation and breathing cease altogether. It contains subnetworks with distinct synaptic and intrinsic membrane properties that give rise to different types of respiratory rhythmic activities including eupneic, sigh, and gasping activities. While critical aspects of these rhythmic activities are preserved when isolated in in vitro preparations, the pre-Bötzinger complex functions in the behaving animal as part of a larger network that receives important inputs from areas such as the pons and parafacial nucleus. The respiratory network is also an integrator of modulatory and sensory inputs that imbue the network with the important ability to adapt to changes in the behavioral, metabolic, and developmental conditions of the organism. This review summarizes our current understanding of these interactions and relates the emerging concepts to insights gained in other rhythm generating networks.
Keywords: Breathing, Respiratory rhythm generation, Pre-Botzinger complex and interactions
Introduction
Behaviors are continuously adapted to changes in an organism's internal and external environment. Not surprisingly, a large degree of plasticity characterizes all levels of neuronal integration from the molecular, cellular to the network and ultimately behavioral level. Common principles of behavioral plasticity have been described in numerous invertebrates (Harris et al., 2010; Marder and Goaillard, 2006; Nadim et al., 2008; Nusbaum, 2002; Ramirez and Pearson, 1993) and mammalian model systems including humans (Lee et al., 2009; Macfarlane and Mitchell, 2009; Millhorn et al., 1980; Peng et al., 2003). In this review, we will focus on modulatory processes that are critical for the neuronal control of mammalian breathing, but we will also describe how insights gained in the respiratory system relate to other networks and behaviors. Breathing is well integrated with many other behaviors and needs to adapt continuously to changes in the metabolic and behavioral demands of an organism. Both the respiratory frequency and amplitude of breathing are adapted to behavioral conditions like posture, physical activity, sleep, or speech. In fact, breathing is so sensitive to an organism's internal state that the characteristics of breathing can reveal whether someone is calm, agitated, or scared. As in most animal behaviors, plasticity in the respiratory system seems to depend on a variety of amines, steroids, and peptides that exert their modulatory actions by acting on a large number of ion channels, receptors, and second messenger systems (Doi and Ramirez, 2008). Neuromodulatory processes play equally important roles during well-oxygenated and hypoxic conditions, and when disturbed neuromodulation has been associated with a number of pathophysiological conditions ranging from Rett syndrome (Viemari et al., 2005a) to Sudden Infant Death Syndrome (SIDS) (Paterson et al., 2009).
The mammalian respiratory network and the nuclei controlling neuromodulation are widely distributed along the neural axis. Important for breathing are the neuronal networks located in the ventral respiratory column (VRC) (Alheid et al., 2002; Feldman and Del Negro, 2006; Feldman et al., 2003; McCrimmon et al., 2004). These networks (Fig. 1) include, from rostral to caudal, the retrotrapezoid nucleus/parafacial respiratory group complex (RTN/pFRG), the Bötzinger complex, the pre-Bötzinger complex (pre-BötC), the rostral ventral respiratory group (rVRG), and the caudal VRG (cVRG) (Alheid et al., 2002; Feldman and Del Negro, 2006; Feldman et al., 2003; McCrimmon et al., 2004; Onimaru and Homma, 2003; Onimaru et al., 2006; Smith et al., 1991; Thoby-Brisson et al., 2009). The distinction between these networks is based on a histological characterization (Alheid et al., 2002; Gray et al., 1999; Guyenet et al., 2002), and their distinct functional properties (Gray et al., 1999, 2001; Janczewski and Feldman, 2006; Janczewski et al., 2002; McCrimmon et al., 2004; Onimaru and Homma, 2003; Onimaru et al., 2006; Thoby-Brisson et al., 2005, 2009). An important role in respiratory rhythm generation and modulation has also been described for the Kölliker-Fuse nucleus and the parabrachial complex; both are located in the dorsal pons (Alheid et al., 2004; Dick et al., 1994; Kobayashi et al., 2005; Milsom et al., 2004). The role of these neurons in the modulation and generation of eupnea (Chamberlin and Saper, 1994; Dutschmann and Herbert, 1996; St-John and Paton, 2004; Von Euler and Trippenbach, 1976) and in the transition phase between inspiration and expiration (Cohen, 1971; Morschel and Dutschmann, 2009; Von Euler and Trippenbach, 1976) has received considerable attention. Other areas involved in breathing include the cerebellum (Harper, 2000a; Harper et al., 2000b), the neocortex (Davenport et al., 2010; Von Leupoldt et al., 2010), as well as the periaqueductal gray, which is particularly important for the integration of speech and breathing (Subramanian and Holstege, 2010).
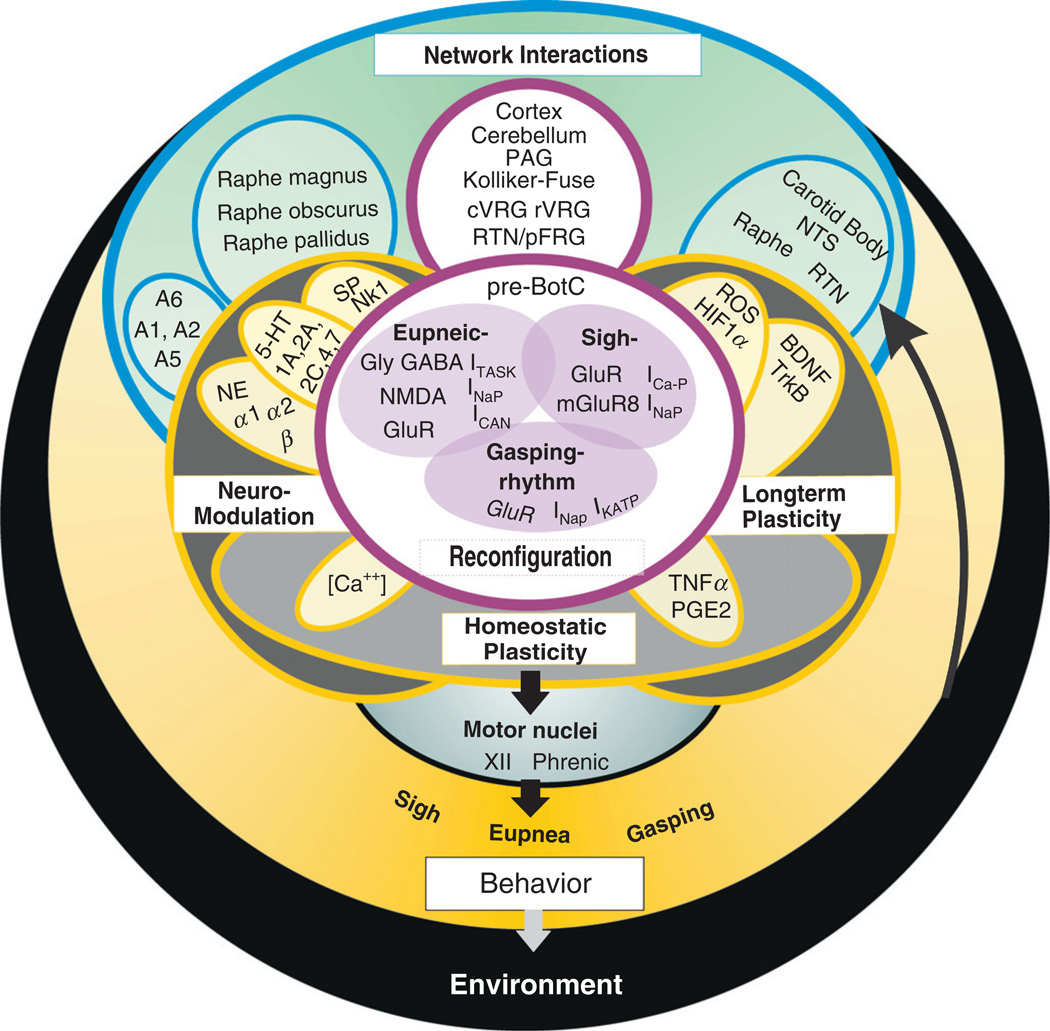
Fig. 1. Networks within networks.
A schematic illustrating some of the interactions that are involved in the neuronal control of breathing. As complex as this schematic may appear, it still represents only a fraction of the known interactions. The interactions depicted in this figure are clustered according to their function in the neuronal control of breathing. Network interactions that are important for the generation of the respiratory rhythm include the interactions between the cortex, cerebellum, PAG (Periaqueductal Gray), Kölliker-Fuse, cVRG (caudal Ventral Respiratory Group), rVRG (rostral Ventral Respiratory Group), RTN (retrotrapezoid nucleus)/pFRG (parafacial respiratory group), and the pre-Bötzinger complex (pre-BötC). While the pre-BötC is capable of generating three distinct rhythmic activities via network reconfiguration (eupneic-, sigh-, and gasping-rhythm) even in isolation (see also Fig. 2), in the intact animal other networks will also contribute to the reconfiguration and the shaping of the respiratory rhythms that are transmitted to the motor nuclei. The extent and significance of these contributions to the overall motor output and ultimately the behavior is a topic of intense investigations. Respiratory rhythm generation is also the target of three types of modulatory processes: Neuromodulation characterizes the modulatory processes occurring on a moment-to-moment basis and it is mediated via numerous aminergic and peptidergic substances acting on various receptor subtypes. For simplicity, the schematic illustrates only three neuromodulators: NE (norepinephrine) acting on α1, α2, and β noradrenergic receptors; 5-HT (serotonin) acting on 5-HT1A, 5-HT2A, 5-HT2C, 5-HT4, and 5-HT7 receptors; and SP (substance P) acting on the NK1 receptor. These neuromodulators are released by nuclei that include the Raphe magnus, obscurus, and pallidus and the noradrenergic regions: A1, A2, A5, and A6. For further explanations see text. Long-term plasticity characterizes the modulatory processes that lead to long-term changes in respiratory activity, which includes, for example, long-term facilitation of the respiratory frequency and amplitude which is evoked by intermittent hypoxia (see text for details). Long-term plasticity is mediated among other molecules by BDNF acting on the TrkB receptor and reactive oxygen species (ROS) as well as HIF1α. Critical areas involved in long-term plasticity are the carotid body and the pre-BötC as well as a variety of motor nuclei. Important chemosensory areas include the NTS (nucleus tractus solitarius), the RTN, and the Raphe nuclei besides the carotid body. These chemosensitive areas are important sensors for inputs from the environment, which is in part affected by the breathing behavior itself. Homeostatic plasticity characterizes regulatory processes that are critical for stabilizing network activity in the context of respiratory rhythm generation as well as neuromodulation and long-term plasticity. Unfortunately, little is known about the homeostatic mechanisms that are specifically relevant for the neuronal control of breathing, but much is already known in other networks in particular in the networks located in the neocortex and hippocampus.
An area that is both essential and sufficient for generating the respiratory rhythm is the pre-BötC, a network located within the ventrolateral medulla (Smith et al., 1991). Disruption of rhythmic activity in the pre-BötC causes irreversible loss or major disruption of breathing in vivo (Gray et al., 2001; McKay et al., 2005; Ramirez et al., 1998c; Tan et al., 2008). Isolated in a brainstem slice preparation, the pre-BötC continues to generate respiratory activity (Fig. 2) (Ramirez et al., 1996; Smith et al., 1991). In these slices, respiratory rhythmic activity can be recorded directly from the surface of pre-BötC (Lieske et al., 2000) or from the hypoglossal motor nucleus, which receives respiratory rhythmic input via an interneuronal population located outside the hypoglossal nucleus (Koizumi et al., 2008).This in vitro approach has greatly facilitated our understanding of the cellular mechanisms of respiratory rhythm generation and neuromodulation. Although we still lack a deep understanding of how the different areas of the respiratory network interact and how they are altered by neuromodulation, the pre-BötC itself has provided an important avenue to study not only the fundamental elements of respiratory rhythm generation, but also how a single neuronal network can generate multiple rhythmic activity patterns as previously demonstrated in invertebrate neuronal networks (Harris-Warrick and Johnson, 2010; Marder and Goaillard, 2006; Meyrand et al., 1991; Ramirez, 1998a). This review will focus on our current understanding of respiratory rhythm generation in the pre-BötC and its alteration by reconfiguration, neuromodulation, and plasticity (Fig. 1). We will also consider the possible roles of these mechanisms in physiological and pathophysiological conditions.
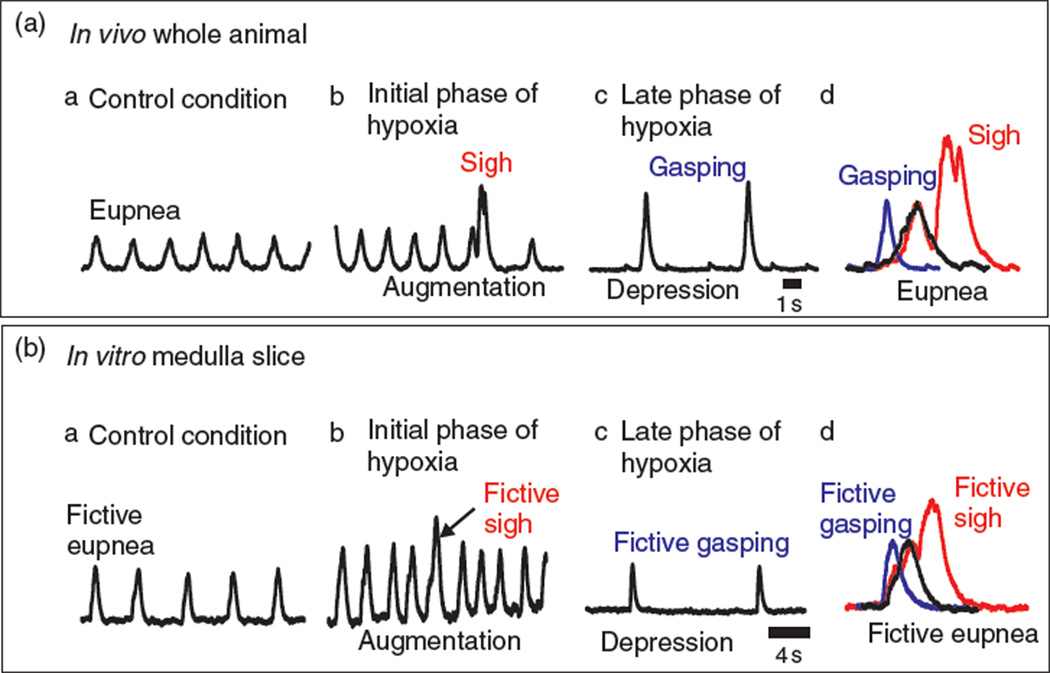
Fig. 2.
Isolating the pre-Bötzinger complex in a single medullary brainstem slice preserves multiple rhythms that reflect breathing rhythms found in vivo and illustrates reconfiguration of a multifunctional network. (A) Integrated traces from an in vivo electromyogram (∫EMG) recorded from respiratory muscles show multiple rhythmic behaviors for breathing. (a) In control conditions, eupnea, “the normal breathing rhythm” conditions, is characterized by augmented bell-shaped waveform. (b) During the transition to hypoxia (initial phase of hypoxia), the frequency of eupnea and sigh rhythms become faster (i.e., augmentation). The sigh rhythm consists of large amplitude complex waveforms. While slower in frequency, sigh rhythms are generated together with the eupnea rhythm (for detail see text). (c) During hypoxia, the frequency of the breathing rhythm is slow (late phase of hypoxia) (i.e., depression) and the waveforms can be clearly distinguished from the eupneic bursts based on their fast rise time. Collectively, these waveforms, during the depression, are described as the gasping rhythm. (d) Overlay of representative eupneic (black line), sigh (red line), and gasping (blue line) waveforms found in the respiratory rhythm in vivo. (B) Integrated activity (∫pre-BötC) of extracellular recordings obtained from the surface of the pre-Bötzinger complex within the medullary brainstem slice illustrates that this neuronal network generates several patterns of neuronal activity that are reminiscent to breathing behaviors found in vivo. (a) In control conditions, fictive eupnea is generated. The waveforms in fictive eupnea are similar to that of eupnea, having an augmented bell-shaped waveform. (b) Large amplitude, complex bursts, described as resembling sighs in both frequency and waveform. Similar to that in vivo, the fictive sigh rhythm is commonly increased during the augmentation phase during the transition to hypoxia (initial phase of hypoxia). (c) During hypoxia, the frequency of the fictive respiratory rhythm slows (late phase of hypoxia, i.e., depression) and the waveforms of the population rhythm changes from the augmented bell-shaped waveform of fictive eupnea to a fictive gasping waveform. Similar to the gasping rhythm in vivo, fictive gasping possess waveforms that have fast rise times. (d) Overlay of representative fictive eupneic (black line), fictive sighs (red line), and fictive gasping (blue line) waveforms illustrates the ability of pre-Bötzinger complex to generate multiple rhythms that likely contribute to in vivo breathing rhythms. Moreover, these waveform patterns represent the summation of different, yet overlapping mechanisms involving network reconfiguration and neuromodulation of the pre-Bötzinger complex (see text for full discussion).
Network reconfiguration
The notion that the respiratory network can reconfigure to generate different forms of breathing such as eupnea, sighs, and gasps was introduced approximately a decade ago (Lieske et al., 2000). Since that time, much has been learned not only about the mechanisms of reconfiguration within the pre-BötC (Pena et al., 2004a), but also about the role of the pons and other areas in reconfiguring the network from the eupneic into the gasping state (Paton et al., 2006).
Under well-oxygenated conditions, the pre-BötC generates two distinct rhythms: a faster small amplitude rhythm (“fictive eupnea”) and a much slower large amplitude rhythm (Fig. 2; “fictive sighs”). Several studies have shown that these activities originate from a multifunctional network located within the pre-BötC that can be partly preserved in the in vitro transverse medullary slice (Lieske and Ramirez, 2006a; Lieske et al., 2000; Ruangkittisakul et al., 2008; Tryba et al., 2008). Although the majority of neurons are activated during both eupneic and sigh rhythmic activities, pharmacological manipulations suggest that both activities emerge through distinct mechanisms. Fictive sigh, but not eupneic activity, is critically dependent on synaptic mechanisms involving the P/Q type calcium channels (Cav2.1). Interestingly, only a relatively small subpopulation of respiratory neurons receive glutamatergic inputs that depend on P/Q type calcium currents (Lieske and Ramirez, 2006a), suggesting that the pool of respiratory neurons contains a subset of neurons with specialized synapses that are critical for sigh rhythm generation. These synapses depend also on the activation of mGluR8 receptors (Lieske and Ramirez, 2006b), while NMDA-dependent mechanisms play an important role in the generation of eupneic activity (Lieske and Ramirez, 2006b; McCrimmon et al., 1997). Thus, in the respiratory network an overlapping pool of neurons generates different rhythmic activities using different behavior-specific types of synaptic mechanisms. This is a common principle that has also been described in the spinal cord (Berkowitz et al., 2010), the neocortex (Kramer et al., 2008; Wulff et al., 2009), and various invertebrate species (Haque et al., 2006; Jing and Gillette, 2003; Wood et al., 2000).
As the respiratory network responds to hypoxia, the breathing frequency in vivo transitions into an augmentation followed by depression (Fig. 2A; England et al., 1995; Haddad and Mellins, 1984; Neubauer et al., 1990), a sequence that is also seen in the isolated pre-BötC (Telgkamp and Ramirez, 1999). During the depression phase, the inspiratory burst changes from an augmenting, bell-shaped burst to a decrementing burst which is one of the characteristic features of gasping (Fig. 2A and B; Lieske et al., 2000; Pena et al., 2004a). The transition into the gasping activity pattern can be gradual, both in vitro and in vivo. During the transition, bursts with varying rise time and burst duration are typical, a characteristic that was referred to as pre-gasping in vivo (Wang et al., 1993). Hypoxia causes also a characteristic decrease in synaptic inhibition, which has been observed under both in vivo and in vitro conditions (England et al., 1995; Ramirez et al., 1998b; Richter et al., 1991; Thoby-Brisson and Ramirez, 2000; Völker et al., 1995). The depression of synaptic inhibition contributes to the reconfiguration of the respiratory network by altering the discharge pattern of a variety of neurons. Late-inspiratory neurons discharge earlier during inspiration (England et al., 1995). Many expiratory and inspiratory neurons in the ventrolateral medulla become inactive before cessation of phrenic and/or hypoglossal (XII) activity (Ballanyi et al., 1994; England et al., 1995; Ramirez, 1998a; Richter et al., 1991; Telgkamp and Ramirez, 1999; Thoby-Brisson and Ramirez, 2000). While XII neurons exhibit a massive potentiation of the rhythmic bursts both in vivo and in vitro (Ramirez et al., 1997; Telgkamp and Ramirez, 1999), respiratory neurons in the ventrolateral medulla respond inconsistently and become either weakly de- or hyperpolarized (Ramirez et al., 1998b; Richter et al., 1991; Thoby-Brisson and Ramirez, 2000).
Within the pre-BötC, neurons can be differentiated into nonpacemaker and pacemaker neurons dependent on their ability to intrinsically generate bursting activity. Since nonpacemaker and pacemaker neurons are differentially affected by hypoxia and neuromodulators, they will be considered in more detail in this review. In isolation from fast synaptic transmission, nonpacemaker neurons either enter a tonic firing state or become quiescent while pacemaker neurons retain spontaneous bursting properties (Pena et al., 2004a). Pacemaker neurons can be further identified into cadmium sensitive (CS) and cadmium insensitive (CI) pacemaker neurons. Bursting in CS pacemakers seems to depend on a nonspecific cation current (ICAN) while burst properties of CI pacemaker appear to be mediated via the persistent sodium current (INaP) (Pena et al., 2004a). Inhibition of these currents in the respective pacemaker subtypes eliminates their ability to spontaneously burst in synaptic isolation (Chevalier et al., 2008; Del Negro et al., 2002a, 2005; Pena et al., 2004a; Tryba and Ramirez, 2004). However, it is important to emphasize that neither ICAN nor INaP are exclusive currents mediating pacemaker properties. These inward currents play also critical roles in amplifying synaptic inputs not only in pacemaker neurons, but also nonpacemaker neurons (Del Negro et al., 2002b, 2005; Ramirez et al., 1996; Rubin et al., 2009). Indeed, the role of bursting properties in amplifying synaptic inputs has been described in many neuronal systems, such as the locust flight system in which sensory synaptic inputs are amplified in a nonlinear manner by intrinsic bursting mechanisms (Ramirez and Pearson, 1991, 1993). As will be discussed later in this review, bursting properties can be induced and suppressed by neuromodulators, which imbues neuronal networks with the ability to amplify specific synaptic pathways in a state and behavioral dependent manner. In the locust flight system, bursting properties are induced at the onset of flight to nonlinearly amplify sensory inputs (Ramirez and Pearson, 1993). During locomotion intrinsic membrane properties boost synaptic input also in the spinal cord (Brownstone et al., 1992). These inward currents play important roles also in a variety of other mammalian systems, such as in networks involved in mastication (Kolta et al., 2007). In the neocortex, these currents depolarize neurons toward their firing threshold (Pennartz et al., 1997) and boost synaptic input (Schwindt and Crill, 1999; Stuart and Sakmann, 1995). Pharmacological blockade of INaP has been shown to modulate locomotion generated in the spinal cord (Darbon et al., 2004; Tazerart et al., 2007) and it can suppress slow oscillations generated in cortical networks (van Drongelen et al., 2006).
It is impossible to negate the fact that a substantial portion of neurons that are activated during respiration possess bursting properties. Moreover, the majority of neurons possess INaP or ICAN or both, and the balance between these two major inward currents and a set of outward currents determines whether a neuron is a pacemaker or nonpacemaker (Koizumi et al., 2010). This conclusion has important implications for rhythm generation in general (Hudson and Prinz, 2010), which makes it difficult to differentiate between pacemakers and nonpacemakers as the balance between inward and outward currents is gradual. Nonpacemakers without any bursting properties and pacemakers with strong bursting properties are the extremes of a gradient of neurons possessing different degrees of bursting properties. Consequently, it is probably impossible to unambiguously dissect the relative contribution of pacemaker versus nonpacemakers. The ratio between pacemakers and nonpacemakers is not fixed, but dependent on the metabolic and modulatory state of the network, since neuromodulators, such as NE, SP, or 5-HT, can induce bursting in nonpacemakers (Pena and Ramirez, 2002, 2004b; Viemari and Ramirez, 2006). In an attempt to determine the relative contribution of these inward currents to respiratory rhythm generation, various laboratories have employed substances that are known to block these currents. Blocking either INaP with Riluzole or the ICAN by Flufenamic acid alone does not block fictive eupnea (Pena et al., 2004a), but both substances together lead to the cessation of respiratory rhythmic activity indicating that these conductances are critical for respiratory rhythm generation. A recent study extended these observations by demonstrating that applying Substance P or low doses of AMPA in the presence of Riluzole and Flufenamic acid could restimulate rhythmogenesis (Del Negro et al., 2005). Hence, the relative importance of pacemaker neurons in well-oxygenated conditions is the matter of an ongoing debate.
It is, however, clear that the relative contribution of these currents changes considerably during the transition from eupneic to gasping activity (Fig. 1). While eupneic activity involves the activation of neurons possessing INaP and ICAN-dependent bursting mechanisms, pacemaker neurons that depend on ICAN selectively hyperpo-larize during hypoxia rendering the network more dependent on INaP during gasping, a finding that has been confirmed in vitro, in situ, and in vivo (Paton et al., 2006; Pena and Aguileta, 2007; Pena et al., 2004a, 2008).
Pacemaker neurons may also differentially contribute to the generation of eupneic versus sigh activity, because the generation of sighs is more sensitive to the manipulation of the INaP current (Tryba et al., 2008). Thus, lessons learned from the respiratory network indicate that the different network states are characterized by differential contribution of different types of bursting mechanisms.
Neuromodulation and rhythm generation
In the respiratory network, the same neuromodulator differentially acts on a variety of receptor subtypes, thereby exerting specific and sometimes even diverging effects on different parameters of the network output. Perhaps, best understood in the neuronal control of breathing is the role of catecholaminergic neurons that are found in the brainstem and project onto neurons of the respiratory network (Doi and Ramirez, 2008; Hilaire, 2006; Hokfelt et al., 1984; VanderHorst and Ulfhake, 2006; Viemari and Hilaire, 2002; Viemari and Ramirez, 2006). These neurons are clustered in noradrenergic (Fig. 1; A1, A2, A5, A6, and A7) and adrenergic (C1 to C3) nuclei. In the pons, the activity of A6 (i.e., the locus coeruleus) neurons is modulated by hypoxia both in vivo (Guyenet et al., 1993) and in vitro (Nieber et al., 1995; Yang et al., 1997). Although some A6 neurons are excited while others inhibited during hypoxia, A6 exerts an overall stimulatory effect on breathing. Electrical stimulation of the locus coeruleus increases breathing frequency (Doi and Ramirez, 2010), whereas genetic alteration of A6 decreases respiratory activity (Viemari et al., 2004; Viemari et al., 2005a). Unlike the A6 nucleus, all neurons in A5 appear to be activated by hypoxia in vivo (Guyenet et al., 1993) and inhibit breathing. Thus, lesions of A5 increase the respiratory frequency in vitro (Viemari and Hilaire, 2002) and reduce the posthypoxic frequency depression (Coles and Dick, 1996). Thus, while both A5 and A6 contain noradrenergic neurons that modulate respiratory network activity, their effects are very diverse even though they are modulated under similar conditions. In mutants where A5 or A6 neurons are altered (Viemari et al., 2004, 2005a), the hypoxic response is blunted supporting a role of both groups in oxygen sensing. A5 and A6 are not the only noradrenergic nuclei modulating respiration: In the medulla, endogenous release of NE from A1/C1 neurons stimulates the respiratory rhythm in vitro (Zanella et al., 2006). Exogenously applied NE on medullary slices stimulates the respiratory rhythm by a direct effect on inspiratory neurons (Viemari and Ramirez, 2006). Because hypoxia increases TH expression (Peyronnet et al., 2003; Roux et al., 2000, 2003) and Fos-like immunoreactivity (Erickson and Millhorn, 1994; Teppema et al., 1997), it is likely that neurons in the A1/C1 and A2/C2 region are activated by low oxygen levels. This modulatory role may be disturbed in certain neurological disorders. In Mecp2 mutant mice, a model for Rett syndrome, the number of tyrosine hydroxylase positive neurons in A1/C1 and A2/C2 and the level of norepinephrine in the medulla is decreased (Viemari et al., 2005b), yet these mice show an increased ventilatory response to hypoxia (Roux et al., 2008; Voituron et al., 2009).
Mechanistically, NE stimulates inspiratory nonpacemaker and pacemaker neurons contained within the pre-BötC acting presumably via α1, α2, and β-noradrenergic mechanisms (Viemari and Ramirez, 2006). NE induces ICAN-dependent bursting properties in active nonpacemaker neurons, and it depolarizes CI pacemakers and increases their burst frequency. In CS pacemakers, NE increases only the amplitude of the depolarizing drive potential and the number of action potentials during the burst. However, in contrast to the situation in CI pacemakers NE does not affect the burst frequency in CS pacemakers. This differential effect is preserved at the network level, since only the modulation of the burst amplitude but not the frequency depends on the activation of ICAN (Viemari and Ramirez, 2006). This leads to the important conclusion that different network parameters are differentially modulated by the same neuromodulator acting on different cellular targets.
NE is not the only bioamine acting on the respiratory network. Like other catecholaminergic neurons, serotonergic neurons are also found in the brainstem and project to neurons involved in breathing (Dahlstrom and Fuxe, 1964; Fuxe, 1965; Holtman et al., 1990; VanderHorst and Ulfhake, 2006). Serotonergic neurons are contained in nuclei numbered from B1 to B9, from the caudal to the rostral axis (Dahlstrom and Fuxe, 1964). The nuclei can also be referred to as raphe pallidus (B1), raphe obscurus (B2), and raphe magnus (B3). The action of these groups on breathing is diverse and often data are contradictory probably due to species differences, state of the animals (awake or sleep), or type and level of anesthesia (Besnard et al., 2009; Doi and Ramirez, 2010; Holtman et al., 1986; Lalley, 1986; Sessle et al., 1981). For instance, in rats under volatile anesthesia (Besnard et al., 2009) electric stimulation of raphe magnus and obscurus induced apnea, whereas stimulation of the raphe pallidus induced tachypnea. On the other hand, in mice anesthetized with urethane (i.p.) electric stimulation of the raphe magnus increases respiratory frequency (Doi and Ramirez, 2010). In cats, stimulation of raphe pallidus and obscurus (Holtman et al., 1986; Lalley, 1986) increases phrenic discharge amplitude and frequency, whereas they decrease during stimulation of the raphe magnus (Lalley, 1986; Sessle et al., 1981). Similarly, exogenous application of serotonergic agents in vitro has various actions on respiratory activity (Di Pasquale et al., 1992, 1994; Hilaire et al., 1997; Morin et al., 1990; Schwarzacher et al., 2002). In brainstem slices containing the pre-BötC, exogenous application of 5HT2A agonist or blockade of serotonergic reuptake increases inspiratory frequency (Pena and Ramirez, 2002). Consistent with these results, blockade of the activation of 5HT2A receptors by endogenous release of serotonin decreases respiratory frequency. This has been recently confirmed in rats using transverse brainstem slices and in situ preparations and extended to 5HT2C and 5HT4 receptors (Ptak et al., 2009). Indeed, neurons from the raphe obscurus show a tonic activity and emit projections to the pre-BötC, which innervates the raphe reciprocally.
Neuromodulation cannot be discussed without also emphasizing the importance of peptidergic modulation. Perhaps, the most studied peptide in the respiratory network is substance P (Gray et al., 2001; Hayes and Del Negro, 2007; Morgado-Valle and Feldman, 2004; Pena and Ramirez, 2004b). Neurons releasing substance P are localized in the nucleus of solitary tract (NTS), the nucleus ambiguous (NA), the raphe, the dorsal motor nucleus of the vagus (X), and the hypoglossal nucleus (Ribeiro-da-Silva and Hokfelt, 2000). Substance P is often coreleased with other neurotransmitter. For example, neurons in the raphe contain 5-HT and substance P (Kachidian et al., 1991; Ptak et al., 2009) and they project directly on the pre-BötC. NK-1 receptors are strongly expressed on pre-BötC neurons as well as serotonergic neurons of the raphe (Alheid and McCrimmon, 2008; Gray et al., 2001; Stornetta et al., 2003; Wang et al., 2001). Substance P activates the inspiratory frequency at the network and behavioral level (Del Negro et al., 2005; Doi and Ramirez, 2010; Gray et al., 2001; Pena and Ramirez, 2004b; Thoby-Brisson et al., 2005). At the cellular level, substance P slowly depolarizes nonpacemaker neurons, leading to an increase of the firing rate of action potentials. Substance P also dramatically activates CS pacemakers and to a lesser extent CI pacemakers, causing an increase in burst amplitude, frequency, and duration (Pena and Ramirez, 2004b).
This discussion leads to the important conclusion that every parameter in rhythm generation is controlled by multiple modulators. It must be emphasized that for simplicity we focused in this review only on three neuromodulators, even though there are many additional modulators that play equally important roles in the neuronal control of breathing (Doi and Ramirez, 2008). Another important conclusion is that the same modulator can exert many different effects on rhythmic activity. Thus, the influence of any given neuromodulator occurs in concert with many other aminergic and peptidergic substances (Fig. 1). The complexity of neuromodulation as described here for the respiratory network is reminiscent to the complexity that is well documented in invertebrate neuronal networks (Nusbaum, 2002; Thirumalai and Marder, 2002) and has important implications for all neuronal networks. Different sets of modulators with diverse, convergent, and divergent actions will define different states of a rhythm generating network that may change dependent on the metabolic, developmental or behavioral conditions of an animal. These network states involve a complex orchestration of large sets of different ion-channels, multiple receptors, and numerous neuropeptides and biogenic amines in addition to those described here (Doi and Ramirez, 2008; Grashow et al., 2009; Thoby-Brisson and Simmers, 1998). A modulatory state will, therefore, not only determine how a rhythm is generated, but will also determine the responsiveness of neuronal networks to inputs. Many recent experimental and computational studies suggest that neuronal networks respond differently to inputs if their state is altered by modulators (Destexhe and Contreras, 2006; MacLean et al., 2005; Nadim et al., 2008; Prescott and De Koninck, 2003). In the respiratory network, numerous excitatory and inhibitory inputs that arise from multiple anatomical regions of the brain to modulate breathing in amplitude, frequency, and regularity have been described (Doi and Ramirez, 2008).
Unfortunately, only little is known how different competing inputs interact in the context of different neuromodulators. In the respiratory network, excitatory inputs mediated by substance P are only critical for respiratory rhythm generation when levels of serotonin or NE are low, that is, under conditions that resemble more closely the sleep state (Jones, 2005). By contrast, when 5-HT2a receptors and α-1 receptors are fully activated, that is in a state when serotonin and norepinephrine levels are high, inputs mediated by substance P are not critical for respiratory rhythm generation (Doi and Ramirez, 2010). Thus, the convergence of different neuromodulatory inputs (Fig. 1) provides a safety net in case a given modulator or receptor is disturbed. It may also explain the state-dependency of a variety of disorders. In SIDS, there is mounting evidence for a role for 5-HT (Broadbelt et al., 2009; Duncan et al., 2010; Kinney et al., 2009; Paterson et al., 2006; Rand et al., 2007). SIDS (and also sleep apnea) occurs during sleep, when aminergic and presumably also SP levels are reduced. Thus, it seems that the respiratory network is critically dependent on an individual neuromodulator only in a reduced “modulatory state.” By contrast, in the awake animal, it is unlikely that a disruption of a single neuromodulator will have much of a critical effect, since the level of other converging modulators is relatively high. Thus, understanding the convergence of different neuromodulators is not only an interesting basic scientific issue, but will also be important for gaining insights into the neuropathology of breathing disorders.
The data summarized above indicate that it is not possible to associate specific network states with the action of individual neuromodulators. Instead, a network state is defined by a complex set of different modulators with highly diverse, convergent, and divergent actions. A particular neuromodulator can differentially act on a variety of receptor subtypes, thereby exerting specific, yet sometimes diverging effects on different parameters of the respiratory network (e.g., frequency vs. amplitude). Conversely, different neuromodulators, such as serotonin, SP, or NE, can exert similar effects via different cellular mechanisms. The specificity and types of these modulatory effects are not necessarily preserved across different animal species even if compared between related rodent species and strains. These conclusions are reminiscent of findings in invertebrate systems. Like in the respiratory system, modulatory responses can vary between different species and even across individuals of the same species at different developmental stages (Newcomb and Katz, 2007, 2009; Rehm et al., 2008). Also similar to the situation in the respiratory network is the observation that different neuromodulatory inputs can exert distinct or comparable activity patterns from the same neuronal ensemble (Saideman et al., 2007). Like in these invertebrate networks, it is remarkable that despite the large number of concurrent and rather diverse modulatory processes the various parameters of rhythm generating networks are relatively tightly maintained even when exposed to extreme conditions. This can be illustrated for the control of the respiratory frequency in humans. The breathing frequency of children under normal conditions is 45±13 breaths/min. Acute exposure to 3109 m increases the frequency range on average by only 86 breaths/min to 51.9±15 breaths/min (Yaron et al., 2003). Under extreme altitudes, such as climbing to the Everest (8000 m), only one individual of the American Medical Research Expedition raised the breathing rate to up to 86 breaths/min (West, 2010). Thus, despite the presence of numerous modulatory systems affecting breathing frequency, the range over which this particular parameter is modulated is relatively narrow under normal as well as extreme conditions. For the invertebrate model system, it has been suggested that homeostatic mechanisms play critical roles in maintaining different parameters in a tightly controlled range (Rehm et al., 2008). Although little is known about how these mechanisms regulate the various parameters of the mammalian respiratory rhythm, they must play critical roles in the homeostasis of breathing (Fig. 1) as will be discussed below.
Homeostatic plasticity and other forms of long-term plasticity
Homeostatic plasticity is a fundamental mechanism that has been demonstrated in a variety of neural networks. It is crucial to maintain network stability and it affects nearly every aspect of circuit development and function (Fig. 1). One principle mechanism is the activity dependent scaling of neuronal receptors to maintain neurons in a certain firing range (Turrigiano et al., 1998). But, multiple forms of homeostatic plasticity have been described in a variety of brain areas (Aizenman et al., 2003; Desai et al., 1999; Ibata et al., 2008; Koch et al., 2010; Marder and Goaillard, 2006; Stellwagen and Malenka, 2006). Homeostatic plasticity has been shown to regulate pre- and postsynaptic scaling of excitation and inhibition. Such changes involve alterations in ion-channel composition which tune and anchor intrinsic neuronal excitability to a defined range of activity. The mechanisms that define and regulate these setpoints of cellular activity are not well understood. Alterations in internal calcium levels [Ca2+]i have been discussed as a possible mechanism balancing synaptic homeostasis in neuronal networks (Turrigiano, 2008). In fact, since calcium fluctuations are directly related to the activity of the cells, it has been hypothesized that calcium plays important roles in regulating many forms of plasticity (Grubb and Burrone, 2010; Lisman et al., 2002; Malenka and Bear, 2004; Zhang and Linden, 2003). Consistent with this hypothesis, blockade of calcium channels can trigger upscaling of excitatory synaptic terminals (Ibata et al., 2008). Moreover, pharmacological blockade of calcium dependent kinases (i.e., the CaMK- family) prevents the effects of activity deprivation on excitatory synaptic transmission (Thiagarajan et al., 2002). Besides intracellular calcium as an activity sensor, multiple other molecules have been implicated to be involved to mediate homeostatic synaptic plasticity (Aoto et al., 2008; Koch et al., 2010; Rutherford et al., 1998; Stellwagen and Malenka, 2006). Interestingly, some of these molecules are part of the inflammatory pathway and include prostaglandin-E2 (PGE2) and tumor necrosis factor-α (TNF-α) (Koch et al., 2010; Stellwagen and Malenka, 2006). Activation of glia cells and TNF-α signaling are necessary for synaptic upscaling in cortical neurons, since blocking the activation of TNF-α receptors prevents the upscaling (Stellwagen and Malenka, 2006). Recently PGE2, the major reaction product of the Cyclooxygenase-2 enzymes (COX-2) was reported to acutely inhibit network activity in the neocortex, but if chronically applied led to a predominantly presynaptic increase in excitatory synaptic transmission (Koch et al., 2010). The COX-2 pathway is directly activated by hypoxia through the hypoxia induced factor-1α (HIF-1α) and PGE2 (Fig. 1) has been reported to be an important regulator of the respiratory rhythm generator (Hofstetter et al., 2007). Thus, the link of activity-dependent scaling and inflammation could potentially be important for understanding pathophysiological changes that occur in the cardiorespiratory control following chronic intermittent hypoxia (IH).
In the context of cardio-respiratory homeostasis, several studies described long-term regulatory processes for the NTS, an area that is critical for sensory integration not only in the context of breathing but also other autonomic functions (Greenberg et al., 1999a, 1999b; Kline et al., 2007; Zhou et al., 1997). Activity-dependent long-term depression (LTD) can be elicited in a subset of NTS neurons (Zhou et al., 1997), and chronic exposure to IH causes an activity dependent synaptic downscaling of excitatory synaptic transmission (Kline et al., 2007).
Perhaps, the best studied form of long-term plasticity (Fig. 1) in the respiratory system is the response to intermittent hypoxia. Investigating the neuronal consequences of repetitive episodes of hypoxia (Acute Intermittent Hypoxia, AIH) is clinically very relevant, as this condition is associated with a variety of breathing disorders including Rett syndrome and obstructive sleep apnea (Lee et al., 2009; Weese-Mayer et al., 2006). AIH causes persistent increases in respiratory frequency and amplitude of integrated motor neuronal bursts in vivo (Baker and Mitchell, 2000; Hayashi et al., 1993; Millhorn et al., 1980; Turner and Mitchell, 1997). These changes persisting for ≤90 min are collectively referred to as long-term facilitation (LTF) (Fuller et al., 2000; Powell et al., 1998). The degree of influence varies with preparation (Bach and Mitchell, 1996; Turner and Mitchell, 1997), animal strain (Fuller et al., 2000), gender (Zabka et al., 2006), and experimental conditions (Baker and Mitchell, 2000). AIH causes changes at multiple levels of the respiratory system, including the carotid body (Dogas et al., 1995; Powell et al., 1998), and motor nuclei (Kinkead et al., 1998). The likely site for the long-term frequency modulation is the pre-BötC, since intermittent hypoxia causes a long-lasting frequency increase within the pre-BötC that persists for ≤90 min after repetitive hypoxic episodes (Blitz and Ramirez, 2002). AIH causes immediate changes in the modulatory milieu and long-term changes involving gene expression. For this to occur, the intermittent pattern and not duration of hypoxia is critical, as even prolonged hypoxic exposure does not evoke LTF (Baker and Mitchell, 2000). AIH causes the intermittent production of reactive oxygen species (MacFarlane and Mitchell, 2009; Pawar et al., 2009) and release of aminergic neuromodulators. Blockade of serotonin receptors abolishes both motor amplitude and frequency LTF in vivo (Bach and Mitchell, 1996; Kinkead et al., 1998). It has been hypothesized (Bach and Mitchell, 1996; Fuller et al., 2000), that LTF is induced by chemoreceptor activation of serotonergic raphe neurons (Erickson and Millhorn, 1994; McCrimmon et al., 1997; Pawar et al., 2009). However, not only serotonin, but also repeated exposure to norepinephrine elicits LTF in vitro (Bocchiaro and Feldman, 2004; Neverova et al., 2007). Both modulatory systems seem to interact (Kinkead et al., 2001). There is accumulating evidence indicating that long-lasting changes in intracellular signaling molecules involving a reactive oxygen species activated PKC pathway cause new synthesis of BDNF acting on TrkB receptors (Fig. 1), which in turn will increase postsynaptic glutamate receptor density and thereby increases glutamatergic synaptic transmission (Baker-Herman et al., 2004). Thus, taken together these studies suggest that neuronal networks involved in respiratory control are regulated by multiple forms of long-term synaptic plasticity.
Conclusion
The convergence of reconfiguration, neuromodulation, state dependency, and homeostatic plasticity (Fig. 1) provides multiple mechanisms by which the pre-BötC is capable of generating multiple, dynamically responsive yet stable sets of respiratory rhythmic activity. As discussed, the pre-BötC retains its ability to generate stable rhythmicity throughout changes in the oxygen environment using both network reconfiguration and neuromodulation. The ability of the pre-BötC to retain stable rhythmicity and transmit this activity to a variety of motor outputs is not a trivial property, as many neuronal networks within the mammalian nervous system shut down during hypoxia in an attempt to conserve the energy during oxygen limiting conditions. This is readily seen in the hippocampus (Krnjevic and Leblond, 1987; Garcia et al., 2010), neocortex (Jiang and Haddad, 1992), and striatum (Calabresi et al., 1995). Hence, sustaining stable rhythmicity in the pre-BötC even during severe levels of hypoxia underscores the importance of this network, as loss of the respiratory rhythmic activity would ultimately lead to death. This robustness may be one reason why the pre-BötC is capable of generating behaviorally relevant rhythmicity even when isolated from the rest of the nervous system (Fig. 2), thus facilitating a rigorous cellular and network analysis. In the intact animal, the pre-BötC constitutes a core network which operates within a larger network of interconnected nuclei that contribute not only to the generation of the respiratory rhythm, but also to the plasticity and state-dependency that characterizes breathing (Fig. 1). The principles gained in the respiratory network apply to all neuronal network functions not only to the mammalian nervous system, but also to the networks of invertebrates. The emerging conclusions gained by studying the respiratory network are surprisingly complex, and we are clearly only touching the surface of our understanding. Yet, this degree of complexity is obviously necessary to guarantee that the respiratory network is very adaptive and at the same time sufficiently stable to maintain regular network activity even under adverse environmental and behavioral conditions.
Acknowledgment
We acknowledge the financial support from various grants awarded by the National Institute of Health to JMR.
References
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. doi: 10.1016/s0896-6273(03)00527-0. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. Journal of Neurocytology. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respiratory Physiology & Neurobiology. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: An overview. Respiratory Physiology & Neurobiology. 2004;143:105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respiration Physiology. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. The Journal of Physiology. 2000;52(91):215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nature Neuroscience. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Volker A, Richter D. Anoxia induced functionalinactivation of neonatal respiratory neurons in vitro. Neuroreport. 1994;6:165–168. doi: 10.1097/00001756-199412300-00042. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Roberts A, Soffe SR. Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Frontiers in Behavioral Neuroscience. 2010;4:36. doi: 10.3389/fnbeh.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard S, Denise P, Cappelin B, Dutschmann M, Gestreau C. Stimulation of the rat medullary raphe nuclei induces differential responses in respiratory muscle activity. Respiratory Physiology & Neurobiology. 2009;165:208–214. doi: 10.1016/j.resp.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. Journal of Neurophysiology. 2002;87:2964–2971. doi: 10.1152/jn.2002.87.6.2964. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbelt KG, Barger MA, Paterson DS, Holm IA, Haas EA, Krous HF, et al. Serotonin-related FEV gene variant in the sudden infant death syndrome is a common polymorphism in the African-American population. Pediatric Research. 2009;66:631–635. doi: 10.1203/PDR.0b013e3181bd5a31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk SJ. On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Experimental Brain Research. 1992;90:441–455. doi: 10.1007/BF00230927. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Hypoxia-induced electrical changes in striatal neurons. Journal of Cerebral Blood Flow and Metabolism. 1995;15:1141–1145. doi: 10.1038/jcbfm.1995.142. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. The Journal of Neuroscience. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier M, Ben-Mabrouk F, Tryba AK. Background sodium current underlying respiratory rhythm regularity. The European Journal of Neuroscience. 2008;28:2423–2433. doi: 10.1111/j.1460-9568.2008.06537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI. Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. The Journal of Physiology. 1971;217:133–158. doi: 10.1113/jphysiol.1971.sp009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SK, Dick TE. Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. The Journal of Physiology. 1996;497(1):79–94. doi: 10.1113/jphysiol.1996.sp021751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Darbon P, Yvon C, Legrand JC, Streit J. INaP underlies intrinsic spiking and rhythm generation in networks of cultured rat spinal cord neurons. The European Journal of Neuroscience. 2004;20:976–988. doi: 10.1111/j.1460-9568.2004.03565.x. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Reep RL, Thompson FJ. Phrenic nerve afferent activation of neurons in the cat SI cerebral cortex. The Journal of Physiology. 2010;588:873–886. doi: 10.1113/jphysiol.2009.181735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr., Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Botzinger complex inspiratory neurons in vitro. Journal of Neurophysiology. 2002a;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Feldman JL. Respiratory rhythm: An emergent network property? Neuron. 2002b;34:821–830. doi: 10.1016/s0896-6273(02)00712-2. [DOI] [PubMed] [Google Scholar]
- Del Negro C, Morgado-Valle C, Hayes J, MacKay D, Pace R, Crowder E, et al. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. The Journal of Neuroscience. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nature Neuroscience. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D. Neuronal computations with stochastic network states. Science. 2006;314:85–90. doi: 10.1126/science.1127241. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G. Endogenous serotonin modulates the fetal respiratory rhythm: An in vitro study in the rat. Brain Research. Developmental Brain Research. 1994;80:222–232. doi: 10.1016/0165-3806(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: An in vitro study in the rat. Neuroscience Letters. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats. Brain Res. 1994;636:259–269. doi: 10.1016/0006-8993(94)91025-1. [DOI] [PubMed] [Google Scholar]
- Dogas Z, Stuth EA, Hopp FA, McCrimmon DR, Zuperku EJ. NMDA receptor-mediated transmission of carotid body chemoreceptor input to expiratory bulbospinal neurones in dogs. The Journal of Physiology. 1995;487(3):639–651. doi: 10.1113/jphysiol.1995.sp020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respiratory Physiology & Neurobiology. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez J. State-Dependent Interactions between Excitatory Neuromodulators in the Control of Breathing. The Journal of Neuroscience. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, et al. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kolliker-Fuse nucleus mediates the trigeminally induced apnoea in the rat. Neuroreport. 1996;7:1432–1436. doi: 10.1097/00001756-199605310-00022. [DOI] [PubMed] [Google Scholar]
- England S, Melton J, Douse M, Duffin J. Activity of respiratory neurons during hypoxia in the chemodenervated cat. Journal of Applied Physiology. 1995;78:856–861. doi: 10.1152/jappl.1995.78.3.856. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. The Journal of Comparative Neurology. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: New perspectives on respiratory rhythm. Nature Reviews. Neuroscience. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, plasticity, chemosensitivity. Annual Review of Neuroscience. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respiration Physiology. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. IV. Distribution of monoamine nerve terminals in the central nervous system. Acta physiologica Scandinavica. Supplementum. 1965;247:37. [PubMed] [Google Scholar]
- Garcia AJ, 3rd, Putnam RW, Dean JB. Hyperbaric hyperoxia and normobaric reoxygenation increase excitability and activate oxygen-induced potentiation (OxIP) in CA1 hippocampal neurons. Journal of Applied Physiology. 2010;109:804–819. doi: 10.1152/japplphysiol.91429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashow R, Brookings T, Marder E. Reliable neuromodulation from circuits with variable underlying structure. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11742–11746. doi: 10.1073/pnas.0905614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires pre-Botzinger complex neurokinin-1 receptor-expressing neurons. Nature Neuroscience. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the pre-Botzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. Journal of Applied Physiology. 1999a;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- Greenberg H, Sica A, Scharf S, Ruggiero D. Expression of c-fos in the rat brainstem after chronic inttermittent hypoxia. Brain Research. 1999b;816:638–645. doi: 10.1016/s0006-8993(98)01222-0. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Koshiya N, Huangfu D, Verberne AJ, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. The American Journal of Physiology. 1993;264:R1035–R1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. The Journal of Neuroscience. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad G, Mellins R. Hypoxia and respiratory control in early life. Annual Review of Physiology. 1984;46:629–643. doi: 10.1146/annurev.ph.46.030184.003213. [DOI] [PubMed] [Google Scholar]
- Haque Z, Lee TK, Inoue T, Luk C, Hasan SU, Lukowiak K, et al. An identified central patterngenerating neuron co-ordinates sensory-motor components of respiratory behavior in Lymnaea. The European Journal of Neuroscience. 2006;23:94–104. doi: 10.1111/j.1460-9568.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- Harper RM. Sudden infant death syndrome: A failure of compensatory cerebellar mechanisms? Pediatric Research. 2000a;48:140–142. doi: 10.1203/00006450-200008000-00004. [DOI] [PubMed] [Google Scholar]
- Harper RM, Woo MA, Alger JR. Visualization of sleep influences on cerebellar and brainstem cardiac and respiratory control mechanisms. Brain Research Bulletin. 2000b;53:125–131. doi: 10.1016/s0361-9230(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Harris G, Mills H, Wragg R, Hapiak V, Castelletto M, Korchnak A, et al. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. The Journal of Neuroscience. 2010;30:7889–7899. doi: 10.1523/JNEUROSCI.0497-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick R, Johnson B. Checks and balances in neuromodulation. Frontiers in Behavioral Neuroscience. 2010;4:47. doi: 10.3389/fnbeh.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: Intact vs. decerebellate rats. The American Journal of Physiology. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Hayes JA, Del Negro CA. Neurokinin receptorexpressing pre-Botzinger complex neurons in neonatal mice studied in vitro. Journal of Neurophysiology. 2007;97:4215–4224. doi: 10.1152/jn.00228.2007. [DOI] [PubMed] [Google Scholar]
- Hilaire G. Endogenous noradrenaline affects the maturation and function of the respiratory network: Possible implication for SIDS. Autonomic Neuroscience. 2006;126–127:320–331. doi: 10.1016/j.autneu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Bou C, Monteau R. Serotonergic modulation of central respiratory activity in the neonatal mouse: An in vitro study. European Journal of Pharmacology. 1997;329:115–120. [PubMed] [Google Scholar]
- Hofstetter AO, Saha S, Siljehav V, Jakobsson PJ, Herlenius E. The induced prostaglandin E2 pathway is a key regulator of the respiratory response to infection and hypoxia in neonates. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9894–9899. doi: 10.1073/pnas.0611468104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Goldstein M. Chemical anatomy of the brain. Science. 1984;225:1326–1334. doi: 10.1126/science.6147896. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr., Anastasi NC, Norman WP, Dretchen KL. Effect of electrical and chemical stimulation of the raphe obscurus on phrenic nerve activity in the cat. Brain Research. 1986;362:214–220. doi: 10.1016/0006-8993(86)90446-4. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr., Marion LJ, Speck DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience. 1990;37:541–552. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- Hudson AE, Prinz AA. Conductance ratios and cellular identity. PLoS Computational Biology. 2010;6:e1000838. doi: 10.1371/journal.pcbi.1000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. The Journal of Physiology. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: In vivo and in vitro study in the newborn rat. The Journal of Physiology. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Haddad GG. Differential responses of neocortical neurons to glucose and/or O2 deprivation in the human and rat. Journal of Neurophysiology. 1992;68:2165–2173. doi: 10.1152/jn.1992.68.6.2165. [DOI] [PubMed] [Google Scholar]
- Jing J, Gillette R. Directional avoidance turns encoded by single interneurons and sustained by multifunctional serotonergic cells. The Journal of Neuroscience. 2003;23:3039–3051. doi: 10.1523/JNEUROSCI.23-07-03039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: Neuronal and chemical substrates. Trends in Pharmacological Sciences. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kachidian P, Poulat P, Marlier L, Privat A. Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: A dual labeling in the rat. Journal of Neuroscience Research. 1991;30:521–530. doi: 10.1002/jnr.490300309. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: Intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 2001;130:207–218. doi: 10.1016/s1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. The Journal of Neuroscience. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annual Review of Pathology. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: Evidence for homeostatic plasticity. The Journal of Neuroscience. 2007;27:4663–4673. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Onimaru H, Inoue M, Inoue T, Sasa R. Localization and properties of respiratory neurons in the rostral pons of the newborn rat. Neuroscience. 2005;134:317–325. doi: 10.1016/j.neuroscience.2005.03.049. [DOI] [PubMed] [Google Scholar]
- Koch H, Huh S-E, Elsen FP, Carroll M, Hodge R, Bedogni F, et al. Prostaglandin E2 induced synaptic plasticity in neocortical networks of organotypic slice cultures. The Journal of Neuroscience. 2010;30:11678–11687. doi: 10.1523/JNEUROSCI.4665-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R, Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. The Journal of Neuroscience. 2010;30:4273–4284. doi: 10.1523/JNEUROSCI.4017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Wilson C, Wong S, Yamanishi T, Koshiya N, Smith J. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. The Journal of Neuroscience. 2008;28:2353–2365. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolta A, Brocard F, Verdier D, Lund JP. A review of burst generation by trigeminal main sensory neurons. Archives of Oral Biology. 2007;52:325–328. doi: 10.1016/j.archoralbio.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Roopun AK, Carracedo LM, Traub RD, Whittington MA, Kopell NJ. Rhythm generation through period concatenation in rat somatosensory cortex. PLoS Computational Biology. 2008;4:e1000169. doi: 10.1371/journal.pcbi.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K, Leblond J. Anoxia reversibly suppresses neuronal calcium currents in rat hippocampal slices. Canadian Journal of Physiology and Pharmacology. 1987;65:2157–2161. doi: 10.1139/y87-340. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Responses of phrenic motoneurones of the cat to stimulation of medullary raphe nuclei. The Journal of Physiology. 1986;380:349–371. doi: 10.1113/jphysiol.1986.sp016290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Badr MS, Mateika JH. Progressive augmentation and ventilatory long-term facilitation are enhanced in sleep apnoea patients and are mitigated by antioxidant administration. The Journal of Physiology. 2009;587:5451–5467. doi: 10.1113/jphysiol.2009.178053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength. Journal of Neurophysiology. 2006a;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. II. Intrinsic modulation by metabotropic glutamate receptors. Journal of Neurophysiology. 2006b;95:1334–1344. doi: 10.1152/jn.00506.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: Eupnea, sighs and gasps [see comment] Nature Neuroscience. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Reviews. Neuroscience. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Macfarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. The Journal of Physiology. 2009;587:5469–5481. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nature Reviews. Neuroscience. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Alheid GF, Jiang M, Calandriello T, Topgi A. Converging functional and anatomical evidence for novel brainstem respiratory compartments in the rat. Advances in Experimental Medicine and Biology. 2004;551:101–105. doi: 10.1007/0-387-27023-x_16. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CF, Stuth EA, et al. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respiration Physiology. 1997;110:161–176. doi: 10.1016/s0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleepdisordered breathing after targeted ablation of pre-Botzinger complex neurons. Nature Neuroscience. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrand P, Simmers J, Moulins M. Construction of a pattern-generating circuit with neurons of different networks. Nature. 1991;351:60–63. doi: 10.1038/351060a0. [DOI] [PubMed] [Google Scholar]
- Millhorn D, Eldridge F, Waldrop T. Prolonged stimulation of respiration by a new central neural mechanism. Respiration Physiology. 1980;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Milsom WK, Chatburn J, Zimmer MB. Pontine influences on respiratory control in ectothermic and heterothermic vertebrates. Respiratory Physiology & Neurobiology. 2004;143:263–280. doi: 10.1016/j.resp.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Morgado-Valle C, Feldman JL. Depletion of substance P and glutamate by capsaicin blocks respiratory rhythm in neonatal rat in vitro. The Journal of Physiology. 2004;555:783–792. doi: 10.1113/jphysiol.2003.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin D, Hennequin S, Monteau R, Hilaire G. Serotonergic influences on central respiratory activity: An in vitro study in the newborn rat. Brain Research. 1990;535:281–287. doi: 10.1016/0006-8993(90)91611-j. [DOI] [PubMed] [Google Scholar]
- Morschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2009;364:2517–2526. doi: 10.1098/rstb.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim F, Brezina V, Destexhe A, Linster C. State dependence of network output: Modeling and experiments. The Journal of Neuroscience. 2008;28:11806–11813. doi: 10.1523/JNEUROSCI.3796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer J, Melton J, Edelman N. Modulation of respiration during brain hypoxia. Journal of Applied Physiology. 1990;68:441–451. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of alpha1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. The Journal of Neuroscience. 2007;27:4435–4442. doi: 10.1523/JNEUROSCI.2803-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb JM, Katz PS. Homologues of serotonergic central pattern generator neurons in related nudibranch molluscs with divergent behaviors. Journal of Comparative Physiology A, Neuroethology, Sensory, Neural, and Behavioral Physiology. 2007;193:425–443. doi: 10.1007/s00359-006-0196-4. [DOI] [PubMed] [Google Scholar]
- Newcomb JM, Katz PS. Different functions for homologous serotonergic interneurons and serotonin in species-specific rhythmic behaviours. Proceedings. Biological Sciences. 2009;276:99–108. doi: 10.1098/rspb.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieber K, Sevcik J, Illes P. Hypoxic changes in rat locus coeruleus neurons in vitro. The Journal of Physiology. 1995;486(1):33–46. doi: 10.1113/jphysiol.1995.sp020788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP. Regulating peptidergic modulation of rhythmically active neural circuits. Brain, Behavior and Evolution. 2002;60:378–387. doi: 10.1159/000067791. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. The Journal of Neuroscience. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. Journal of Neurophysiology. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Hilaire G, Weese-Mayer DE. Medullary serotonin defects and respiratory dysfunction in sudden infant death syndrome. Respiratory Physiology & Neurobiology. 2009;168:133–143. doi: 10.1016/j.resp.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, et al. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nature Neuroscience. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, et al. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2009;296:R735–R742. doi: 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Aguileta MA. Effects of riluzole and flufenamic acid on eupnea and gasping of neonatal mice in vivo. Neuroscience Letters. 2007;415:288–293. doi: 10.1016/j.neulet.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Pena F, Meza-Andrade R, Paez-Zayas V, Gonzalez-Marin MC. Gasping generation in developing Swiss-Webster mice in vitro and in vivo. Neurochemical Research. 2008;33:1492–1500. doi: 10.1007/s11064-008-9616-x. [DOI] [PubMed] [Google Scholar]
- Pena F, Parkis M, Tryba A, Ramirez J. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004a;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. The Journal of Neuroscience. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. The Journal of Neuroscience. 2004b;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Overholt J, Kline D, Kumar G, Prabhakar N. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: Implications for recurrent apneas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Bierlaagh MA, Geurtsen AM. Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: Involvement of a slowly inactivating component of sodium current. Journal of Neurophysiology. 1997;78:1811–1825. doi: 10.1152/jn.1997.78.4.1811. [DOI] [PubMed] [Google Scholar]
- Peyronnet J, Roux JC, Perrin D, Pequignot JM, Lagercrantz H, Dalmaz Y. Prenatal hypoxia and early postnatal maturation of the chemoafferent pathway. Advances in Experimental Medicine and Biology. 2003;536:525–533. doi: 10.1007/978-1-4419-9280-2_66. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respiration Physiology. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Gain control of firing rate by shunting inhibition: Roles of synaptic noise and dendritic saturation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2076–2081. doi: 10.1073/pnas.0337591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, et al. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. The Journal of Neuroscience. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J. Reconfiguration of the respiratory network at the onset of locust flight. Journal of Neurophysiology. 1998a;80:3137–3147. doi: 10.1152/jn.1998.80.6.3137. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Pearson KG. Octopamine induces bursting and plateau potentials in insect neurones. Brain Research. 1991;549:332–337. doi: 10.1016/0006-8993(91)90477-d. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Pearson KG. Alteration of bursting properties in interneurons during locust flight. Journal of Neurophysiology. 1993;70:2148–2160. doi: 10.1152/jn.1993.70.5.2148. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. The Journal of Physiology. 1996;491(3):799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J, Quellmalz U, Wilken B, Richter D. The hypoxic response of neurones within the in vitro mammalian respiratory network. The Journal of Physiology. 1998b;507:571–582. doi: 10.1111/j.1469-7793.1998.571bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Botzinger complex in vivo eliminates breathing but not gasping. The Journal of Physiology. 1998c;507(3):895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ, Richter DW. Respiratory rhythm generation in mammals: Synaptic and membrane properties. Respiration Physiology. 1997;110:71–85. doi: 10.1016/s0034-5687(97)00074-1. [DOI] [PubMed] [Google Scholar]
- Rand CM, Berry-Kravis EM, Zhou L, Fan W, Weese-Mayer DE. Sudden infant death syndrome: Rare mutation in the serotonin system FEV gene. Pediatric Research. 2007;62:180–182. doi: 10.1203/PDR.0b013e3180a725a0. [DOI] [PubMed] [Google Scholar]
- Rehm KJ, Deeg KE, Marder E. Developmental regulation of neuromodulator function in the stomatogastric ganglion of the lobster, Homarus americanus. The Journal of Neuroscience. 2008;28:9828–9839. doi: 10.1523/JNEUROSCI.2328-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Da-Silva A, Hokfelt T. Neuroanatomical localisation of Substance P in the CNS and sensory neurons. Neuropeptides. 2000;34:256–271. doi: 10.1054/npep.2000.0834. [DOI] [PubMed] [Google Scholar]
- Richter D, Bischoff A, Anders K, Bellingham M, Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. The Journal of Physiology. 1991;443:231–256. doi: 10.1113/jphysiol.1991.sp018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux JC, Dura E, Villard L. Tyrosine hydroxylase deficit in the chemoafferent and the sympathoadrenergic pathways of the Mecp2 deficient mouse. Neuroscience Letters. 2008;447:82–86. doi: 10.1016/j.neulet.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Roux JC, Mamet J, Perrin D, Peyronnet J, Royer C, Cottet-Emard JM, et al. Neurochemical development of the brainstem catecholaminergic cell groups in rat. Journal of Neural Transmission. 2003;110:51–65. doi: 10.1007/s00702-002-0767-7. [DOI] [PubMed] [Google Scholar]
- Roux JC, Pequignot JM, Dumas S, Pascual O, Ghilini G, Pequignot J, et al. O2-sensing after carotid chemodenervation: Hypoxic ventilatory responsiveness and upregulation of tyrosine hydroxylase mRNA in brainstem catecholaminergic cells. The European Journal of Neuroscience. 2000;12:3181–3190. doi: 10.1046/j.1460-9568.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, Poon BY, et al. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. The Journal of Neuroscience. 2008;28:2447–2458. doi: 10.1523/JNEUROSCI.1926-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2939–2944. doi: 10.1073/pnas.0808776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Saideman SR, Blitz DM, Nusbaum MP. Convergent motor patterns from divergent circuits. The Journal of Neuroscience. 2007;27:6664–6674. doi: 10.1523/JNEUROSCI.0315-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Gunther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Schwindt P, Crill W. Mechanisms underlying burst and regular spiking evoked by dendritic depolarization in layer 5 cortical pyramidal neurons. Journal of Neurophysiology. 1999;81:1341–1354. doi: 10.1152/jn.1999.81.3.1341. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Ball GJ, Lucier GE. Suppressive influences from periaqueductal gray and nucleus raphe magnus on respiration and related reflex activities and on solitary tract neurons, and effect of naloxone. Brain Research. 1981;216:145–161. doi: 10.1016/0006-8993(81)91283-x. [DOI] [PubMed] [Google Scholar]
- Smith J, Ellenberger H, Ballanyi K, Richter D, Feldman J. Pre-Bötzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Role of pontile mechanisms in the neurogenesis of eupnea. Respiratory Physiology & Neurobiology. 2004;143:321–332. doi: 10.1016/j.resp.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Botzinger complex. The Journal of Comparative Neurology. 2003;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. Periaqueductal gray control of breathing. Advances in Experimental Medicine and Biology. 2010;669:353–358. doi: 10.1007/978-1-4419-5692-7_72. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski W, Yang P, Shao X, Callaway E, Feldman J. Silencing pre-Bötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nature Neuroscience. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazerart S, Viemari JC, Darbon P, Vinay L, Brocard F. Contribution of persistent sodium current to locomotor pattern generation in neonatal rats. Journal of Neurophysiology. 2007;98:613–628. doi: 10.1152/jn.00316.2007. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Ramirez JM. Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. Journal of Neurophysiology. 1999;82:2163–2170. doi: 10.1152/jn.1999.82.5.2163. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. The Journal of Comparative Neurology. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Thirumalai V, Marder E. Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets. The Journal of Neuroscience. 2002;22:1874–1882. doi: 10.1523/JNEUROSCI.22-05-01874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the pre-Botzinger complex. Nature Neuroscience. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez J. Role of inspiratory pacemaker neurons in mediating the hypoxic response of the respiratory network in vitro. The Journal of Neuroscience. 2000;20:5858–5866. doi: 10.1523/JNEUROSCI.20-15-05858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Simmers J. Neuromodulatory inputs maintain expression of a lobster motor patterngenerating network in a modulation-dependent state: Evidence from long-term decentralization in vitro. The Journal of Neuroscience. 1998;18:2212–2225. doi: 10.1523/JNEUROSCI.18-06-02212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Trinh JB, Champagnat J, Fortin G. Emergence of the pre-Botzinger respiratory rhythm generator in the mouse embryo. The Journal of Neuroscience. 2005;25:4307–4318. doi: 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. Journal of Neurophysiology. 2008;99:2114–2125. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Ramirez JM. Background sodium current stabilizes bursting in respiratory pacemaker neurons. Journal of Neurobiology. 2004;60:481–489. doi: 10.1002/neu.20050. [DOI] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. The Journal of Physiology. 1997;499(2):543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: Synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Van Drongelen W, Koch H, Elsen FP, Lee HC, Mrejeru A, Doren E, et al. Role of persistent sodium current in bursting activity of mouse neocortical networks in vitro. Journal of Neurophysiology. 2006;96:2564–2577. doi: 10.1152/jn.00446.2006. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Ulfhake B. The organization of the brainstem and spinal cord of the mouse: Relationships between monoaminergic, cholinergic, and spinal projection systems. Journal of Chemical Neuroanatomy. 2006;31:2–36. doi: 10.1016/j.jchemneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Bevengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, et al. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. The Journal of Neuroscience. 2004;24:928–937. doi: 10.1523/JNEUROSCI.3065-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari J, Hilaire G. Noradrenergic receptors and in vitro respiratory rhythm: Possible interspecies differences between mouse and rat neonates. Neuroscience Letters. 2002;324:149–153. doi: 10.1016/s0304-3940(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Maussion G, Bevengut M, Burnet H, Pequignot JM, Nepote V, et al. Ret deficiency in mice impairs the development of A5 and A6 neurons and the functional maturation of the respiratory rhythm. The European Journal of Neuroscience. 2005a;22:2403–2412. doi: 10.1111/j.1460-9568.2005.04441.x. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. Journal of Neurophysiology. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, et al. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. The Journal of Neuroscience. 2005b;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respiratory Physiology & Neurobiology. 2009;168:109–118. doi: 10.1016/j.resp.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Völker A, Ballanyi K, Richter D. Anoxic disturbance of the isolated respiratory network of neonatal rats. Experimental Brain Research. 1995;103:9–19. doi: 10.1007/BF00241960. [DOI] [PubMed] [Google Scholar]
- Von Euler C, Trippenbach T. Excitability changes of the inspiratory “off-switch” mechanism tested by electrical stimulation in nucleus parabrachialis in the cat. Acta Physiologica Scandinavica. 1976;97:175–188. doi: 10.1111/j.1748-1716.1976.tb10250.x. [DOI] [PubMed] [Google Scholar]
- Von Leupoldt A, Keil A, Chan PY, Bradley MM, Lang PJ, Davenport PW. Cortical sources of the respiratory-related evoked potential. Respiratory Physiology & Neurobiology. 2010;170:198–201. doi: 10.1016/j.resp.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Fung ML, St John WM. Pontile regulation of ventilatory activity in the adult rat. Journal of Applied Physiology. 1993;74:2801–2811. doi: 10.1152/jappl.1993.74.6.2801. [DOI] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. The Journal of Comparative Neurology. 2001;434:128–146. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, et al. Autonomic nervous system dysregulation: Breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatric Research. 2006;60:443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- West JB. American medical research expedition to everest. High Altitude Medicine & Biology. 2010;11:103–110. doi: 10.1089/ham.2009.1089. [DOI] [PubMed] [Google Scholar]
- Wood DE, Stein W, Nusbaum MP. Projection neurons with shared cotransmitters elicit different motor patterns from the same neural circuit. The Journal of Neuroscience. 2000;20:8943–8953. doi: 10.1523/JNEUROSCI.20-23-08943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bahner F, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JJ, Chou YC, Lin MT, Chiu TH. Hypoxia-induced differential electrophysiological changes in rat locus coeruleus neurons. Life Sciences. 1997;61:1763–1773. doi: 10.1016/s0024-3205(97)00800-x. [DOI] [PubMed] [Google Scholar]
- Yaron M, Niermeyer S, Lindgren KN, Honigman B, Strain JD, Cairns CB. Physiologic response to moderate altitude exposure among infants and young children. High Altitude Medicine & Biology. 2003;4:53–59. doi: 10.1089/152702903321488988. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. The Journal of Physiology. 2006;576:903–912. doi: 10.1113/jphysiol.2006.114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella S, Roux JC, Viemari JC, Hilaire G. Possible modulation of the mouse respiratory rhythm generator by A1/C1 neurones. Respiratory Physiology & Neurobiology. 2006;153:126–138. doi: 10.1016/j.resp.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: Experience-driven changes in neuronal intrinsic excitability. Nature Reviews. Neuroscience. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Champagnat J, Poon CS. Phasic and long-term depression in brainstem nucleus tractus solitarius neurons: Differing roles of AMPA receptor desensitization. The Journal of Neuroscience. 1997;17:5349–5356. doi: 10.1523/JNEUROSCI.17-14-05349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]