Abstract
Objective
To review the expression of the glucocorticoid receptor (GR) in anterior pituitary and adrenocortical cells and tumors derived from these tissues as well as factors that may influence its expression.
Methods
We present an overview of the relevant literature, with a focus on data generated from our studies.
Results
The expression of the GR is an essential element of the negative feedback that closes the loop formed by corticotropin-releasing hormone, adrenocorticotropic hormone, and cortisol in the context of the hypothalamic-pituitary-adrenal (HPA) axis. Although the GR expression in anterior pituitary cells—and in particular the corticotrophs—was first demonstrated several years ago, it was not known until relatively recently where, by what cells, and in what form the GR is expressed in the adrenal cortex. The variability in the expression of the GR in pituitary and adrenocortical cells may underlie the substantial differences in HPA axis function across individuals, especially when testing for tumors associated with hypercortisolemia. This expression is influenced by a multitude of tissue-specific factors, which may explain why it is so difficult to interpret (or reproduce) studies that are based on GR functional polymorphisms on different cohorts of patients or even different sets of laboratory animals.
Conclusion
This review highlights the variability in expression and function of the GR in pituitary and adrenocortical cells as one of the reasons for the appreciable differences in HPA axis function across individuals. Particular attention was paid to interactions that may affect the interpretation of diagnostic testing of the HPA axis in patients with pituitary adenomas (Cushing disease) or adrenocortical tumors (Cushing syndrome).
INTRODUCTION
All diagnostic testing for Cushing syndrome (CS) relies on the negative feedback exerted by cortisol and the glucocorticoid receptor (GR), primarily at the corticotropin-releasing hormone-producing neurons of the hypothalamus and secondarily at the adrenocorticotropic hormone (ACTH)-producing cells (corticotrophs) of the anterior pituitary (1). These 2 sites, along with the cortisol-producing zona fasciculata of the adrenal cortex, form the hypothalamic-pituitary-adrenal (HPA) axis.
After binding glucocorticoids, the GR translocates into the nucleus and binds the glucocorticoid response element on the target gene promoters. Although considerable numbers of genes are responsive to glucocorticoids in certain tissues, they may be resistant in others (2); this response may also fluctuate in pathologic states or even during normal physiologic processes (3). Accordingly, several mechanisms have been postulated for tissue-specific regulation of glucocorticoid actions, from different metabolism of the ligands (4) to HEXIM1-mediated repression of GR (5). Binding of receptors to target DNA is followed by recruitment of mediators and coactivators to the proximity, resulting in RNA polymerase II recruitment and activation of transcription (6).
Although the GR expression in pituitary corticotrophs and ACTH-producing tumors was documented early on in several studies (7,8), documentation of the presence of GRs in human fetal and adult adrenal cortex and in adrenocortical tumors (ADTs) was not shown until recently (9-12). Relative GR insensitivity appears to be an early, if not primary, event for the pathogenesis of ACTH-producing pituitary tumors, which are always more resistant to cortisol and other glucocorticoids (for example, dexamethasone) than normal corticotrophs. Rarely, GR resistance in these cells is caused by GR-inactivating mutations (8). Despite the rarity of mutations, GR insensitivity is so widespread in ACTH-producing tumors that we take advantage of this phenomenon in diagnostic testing for CS by using various dexamethasone doses (and administration regimens) to distinguish among normal HPA axis function, pituitary and ACTH-dependent causes (Cushing disease [CD]), and ACTH-independent causes of CS (13-15).
Every study reporting on dexamethasone testing in large cohorts of patients with CD and other forms of CS, however, has documented a large variation between individual responses. For example, in a large cohort of pediatric patients with surgically proven CD who were not exposed to any other medications and had no other comorbidities (unlike most adult patients with CS), individual responses to an 8-mg overnight dexamethasone test varied as much as 30% (16). Interindividual variability in corticotroph GR sensitivity may account for a large proportion of these differences in dexamethasone responses (8), but the molecular mechanisms that are responsible remain unknown.
Likewise, in recent years, variable responses to dexamethasone testing have been noted in patients with ACTH-independent causes of CS, and an attempt has been made to use these responses diagnostically. “Paradoxical” responses to dexamethasone that were associated with an increase in urinary cortisol excretion were first described in 1999 as a diagnostic test in primary pigmented nodular adrenocortical disease (PPNAD) (17), but individual cases with similar results had been reported previously in patients with adrenocortical cancer and other ADTs, especially virilizing adenomas (18,19). Again, the molecular mechanism for this phenomenon remains unknown.
In this review, we present recent data that may offer explanations for at least part of this variability in GR responses of pituitary corticotroph and adrenocortical cells, and we attempt a synthesis with older results rarely cited today. The picture that emerges is that of complex tissue-specific factors that may account for most of the GR sensitivity and function. The implications are wide, from tumorigenesis in the pituitary gland and the adrenal gland, to diagnostic testing in CS and other hypercortisolemic states (for example, depression), to medical treatments that use glucocorticoids or seek to inhibit GR function.
THE GR IN THE ANTERIOR PITUITARY
Shortly after the discovery of the first mutations of the GR in humans with glucocorticoid resistance (20-22), we described the first patient with somatic inactivation of the GR in a large pituitary corticotroph adenoma that was associated with severe Nelson syndrome (23). To date, few other patients with CD have been found to have either somatic or germline GR-inactivating mutations (8,24). The important role of an intact HPA axis function and the normal expression of the GR in preventing corticotroph adenoma expansion (if not formation) is demonstrated by the cases of ACTH-producing tumors in patients with familial glucocorticoid deficiency (25), as well as the relatively frequent loss of heterozygosity, leading to hemizygosity and, thus, haploinsufficiency of the GR gene (NR3C1) (26).
Could polymorphisms of the GR gene (NR3C1) that confer partial inactivation in specific functions of the molecule (without causing generalized resistance), such as transcriptional repression of the pro-opiomelanocortin gene or protein-protein and DNA interactions, predispose to ACTH-producing tumors? The GR gene (NR3C1) is sufficiently and frequently polymorphic (27), and this question has been asked but the response is not generally affirmative (8,26,28,29).
Is the GR gene (NR3C1) downregulated at the message or protein level in ACTH-producing adenomas? Several studies have investigated this possibility, inasmuch as loss-of-heterozygosity studies suggested that haploinsufficiency of the GR could be associated with partially defective negative feedback by cortisol and could thereby lead to abnormal growth and proliferation of corticotrophs. It appears that, indeed, in at least those adenomas with deficient responses to dexamethasone, the GR is downregulated (30,31), although this is not a generalized phenomenon and, in fact, in some ACTH-producing adenomas there might even be upregulation of the receptor (32,33).
At least 3 new players in the regulation of GR expression and function in the pituitary corticotrophs add to the variability of GR responses beyond NR3C1 genetic variants and haploinsufficiency. The first is the expression of 11β-hydroxysteroid dehydrogenase (11βHSD) type 2 in pituitary cells, and the corticotrophs in particular (33). Variable expression of this enzyme could modulate cortisol levels that are available for occupying the GR in corticotrophs (33-35); upregulation of 11βHSD type 2 and consequently conversion of cortisol to inactive cortisone may, in at least some ACTH-producing tumors, lead to decreased GR responses and functional resistance without any direct defect of the GR (34,36).
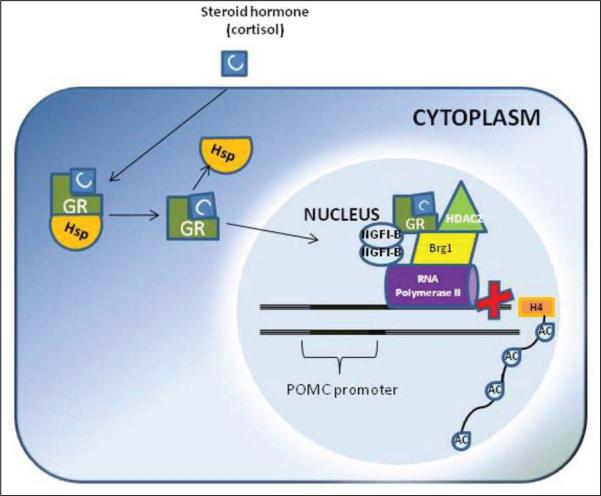
The second factor of variation in GR response is the expression of the Brg1 molecule in pituitary corticotrophs. Negative feedback regulation of the pro-opiomelanocortin gene by the GR is in part exerted by transrepression mediated by the GR and Brg1, the adenosinetriphosphatase subunit of the Swi-Snf complex (37). Brg1 is constitutively present at the pro-opiomelanocortin promoter, whereas recruitment of GR (activated by the ligand) and HDAC2 results in histone H4 deacetylation of the pro-opiomelanocortin locus and inhibition of the promoter clearance by RNA polymerase II (37,38) (Fig. 1). Therefore, loss of Brg1 or HDAC2 should produce resistance to the action of the GR in the pituitary corticotrophs; indeed, it has been shown that approximately 50% of GR-resistant corticotroph adenomas were deficient in nuclear expression of either protein (38).
Fig. 1.
Brg1-dependent transrepression. Ligand (C) activation of glucocorticoid receptor (GR) results in formation of a Brg1-dependent protein complex, which also contains NGFI-B and HDAC2. This scenario results in deacetylation of histone H4, block of polymerase II at the promoter, and inhibition of transcription initiation. Hsp = heat shock protein; POMC = pro-opiomelanocortin.
A third possible player in GR function in the anterior pituitary is the newly identified aryl hydrocarbon receptor (AHR) interacting protein (AIP). AIP, originally called XAP2 (39), is a molecule that not only binds to AHR (as its ligand) but appears to have several other protein-protein interactions, including those with Hsp-90 (39) and the GR (40,41). Recently, patients with familial pituitary tumors were found to harbor germline mutations in the AIP gene (42), which appears to act as a tumor suppressor gene because overexpression of the normal but not the mutated AIP reduces cell proliferation (40,41). The AIP molecule is highly polymorphic in the general population, and how these genetic polymorphisms affect GR function directly or through its interactions with SRC-1 or Hsp-90 (39-41) remains unknown. Although most mutant AIP-caused pituitary tumors are growth hormone- or prolactin-producing lesions, about 2% or more of the ACTH-producing tumors seem to be caused by germline AIP mutations (41,43). In a previous cohort of our patients with CD, a child with a corticotropinoma had an extremely GR-resistant and aggressive tumor; this lesion was eventually cured after 2 surgical procedures and irradiation (43).
THE GR IN THE ADRENAL CORTEX
As mentioned in the Introduction, expression of the GR in human adrenal cortex (and ADTs) was documented relatively recently (9-12), although there had been evidence—from ligand studies—that glucocorticoids had a direct effect on the rat adrenal glands as early as the late 1970s (44). The earlier findings, however, that glucocorticoids have a suppressive effect on adrenocortical function in hypophysectomized rats (44), could not be confirmed in humans (45). As early as in the mid-1980s, there was a suspicion that physiologic (replacement) doses or even moderately higher doses of dexamethasone or cortisol do not suppress zona fasciculata function. Evidence for this was derived from experiments that had been conducted in the mid-1960s: intravenous infusion of ACTH was shown to stimulate steroid production even after high doses of glucocorticoids (45). Investigators who could not confirm this finding had used extraordinarily high doses of glucocorticoids (46-48), as pointed out by Kontula et al (45).
Of note, the affinity (dissociation constant) and specificity of the GR binding in glucocorticoid-exposed adrenal tissue were compatible with the data obtained from human leukocytic GR assays (45), an indication that the adrenal gland GR is no less functional than in other tissues. Its presence in both cortical and medullary tumors (that is, pheochromocytomas) was also shown in the 1980s (49-51) and subsequently confirmed (12,52). More recently, a familial case of GR haploinsufficiency in conjunction with macronodular adrenal hyperplasia was reported; this finding suggested that glucocorticoid-regulated adrenocortical cell signaling may have a role in the regulation of adrenal growth (53). Alternatively, affected patients in this family also presented with inappropriate plasma levels of ACTH, which may explain gradual hyperplasia of the adrenal cortex (53).
Kontula et al (45) were the first to show avid dexamethasone binding by cells derived from human adrenocortical hyperplasia. As mentioned in the Introduction, it was recently shown that patients with particular forms of adrenocortical hyperplasia exhibit an increase in cortisol excretion in response to the administration of dexamethasone during the Liddle test (11,17). In fact, an increase of 50% or higher of the basal level of urinary free cortisol or 17-hydroxysteroid excretion on the last day after administration of high-dose dexamethasone is diagnostic of PPNAD (17) and other micronodular adrenocortical hyperplasias (54). This response, however, is not pathognomonic of PPNAD or micronodular adrenal hyperplasia; patients with cortisol-producing adrenocortical adenomas (55) have this type of response in as many as a fifth of the cases (17,55). In this latter group of patients, the response to dexamethasone has been attributed, at least in part, to the occurrence of somatic PRKAR1A mutations (55). Germline mutations of PRKAR1A, the gene that codes for the type 1A regulatory subunit of the cyclic adenosine monophosphate (cAMP)-dependent kinase or protein kinase A (PKA), are responsible for PPNAD (56,57).
The molecular mechanisms involved in dexamethasone-induced cortisol secretion from PPNAD have not been fully elucidated. A recent study has shown that the plasma cortisol response to administration of dexamethasone observed in vivo in patients with PPNAD (17) can be reproduced in vitro (11). Therefore, the occurrence of a direct stimulatory action of the drug on hyperplastic adrenocortical tissues was confirmed (17). In contrast, dexamethasone was found to have no effect on cortisol release by tissue explants derived from ACTH-independent macronodular adrenal hyperplasias (11). Immunohistochemical studies revealed the presence of the GR in the nodules of PPNAD, whereas limited or no immunoreactivity was detected in the internodular, mostly atrophic, cortex. In addition, the GR appears to be overexpressed in PPNAD tissues in comparison with normal adrenal gland tissues. This observation suggests that the abnormal stimulatory action of dexamethasone on cortisol release is a GR-mediated phenomenon (11). Because it was known that the GR can interact with the cAMP-dependent PKA through a protein-protein interaction (58), the paradoxical increase in cortisol secretion induced by dexamethasone in PPNAD could possibly be the consequence of a GR-mediated action of dexamethasone on PKA that was constitutively active because of the PRKAR1A mutations (59,60). In a recent study, we investigated this hypothesis on cultured adrenocortical cells derived from PPNAD (61). Dexamethasone stimulated in vitro cortisol secretion from cultured dispersed PPNAD cells, and this effect was not reduced by the adenylate cyclase inhibitor SQ22536 or potentiated by the phosphodiesterase inhibitor IMBX and the cAMP analogue 8Br-cAMP. In contrast, the PKA inhibitor H89 and the GR antagonist RU-486 (mifepris-tone) did inhibit the cortisol response to dexamethasone. Interestingly, dexamethasone had no effect on cortisol production from normal human adrenocortical cells but stimulated the production of corticosteroids in the presence of RU-486 (61).
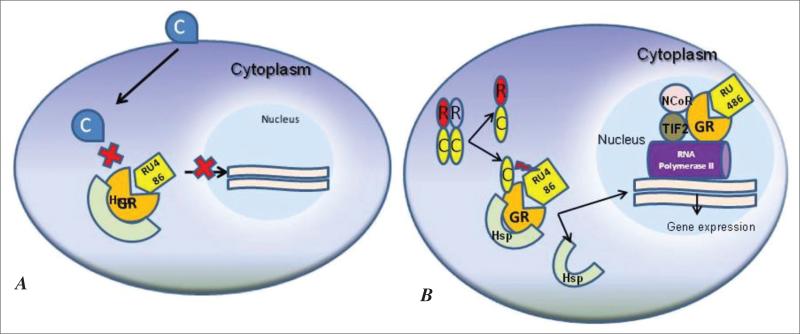
These results are in accordance with the data showing the dual role of mifepristone as an agonist or active antagonist of the GR. As a GR antagonist, mifepristone binds to the receptor and hinders the GR from releasing from the associated heat shock proteins; thus, the translocation of the RU-486/receptor complex to the nucleus is prevented (62) (Fig. 2 A). This action should result in glucocorticoid resistance. Nevertheless, some of the complexes manage to reach the target DNA. This happens because of the agonistic activity of mifepristone. The complex RU-486/receptor translocates to the nucleus, where it can actively recruit nuclear coregulator proteins such as TIF2 and nuclear receptor corepressor and thus regulate gene expression (63) (Fig. 2 B). It seems that the concentration of GR in the cell increases the agonistic activity of mifepristone by driving the formation of receptor:DNA:coactivator complexes, which can then be converted to transcriptionally active complexes (64). Thus, the decrease of basal cortisol and corticosterone levels found in PPNAD cultures (61) might be the consequence of an agonistic effect of mifepristone, which is potentiated by the constitutively active PKA (65). It has been shown that the activated GR mediates negative feedback in adrenocortical steroidogenesis (66). It is possible that there is a local GR resistance in mediating cortisol negative feedback in steroidogenesis of PPNAD cells, which is overcome by administration of mifepristone.
Fig. 2.
A, Mifepristone (RU-486) can act as a glucocorticoid receptor (GR) antagonist; when mifepristone binds to the GR, it prevents the release of the GR from the associated heat shock protein (Hsp) and prevents the translocation of the RU-486/GR complex to the nucleus. B, In primary pigmented nodular adrenocortical disease cells, mifepristone probably acts as an agonist or antagonist, depending on the protein kinase A (PKA) status. Through a GR-PKA interaction, the constitutively active PKA in these cells may potentiate the agonist activity of mifepristone. The complex RU-486/GR translocates to the nucleus, where it recruits nuclear coactivator TIF2 and nuclear receptor corepressor (NCoR) and regulates gene expression.
Overall, these data indicated that dexamethasone stimulates PKA catalytic subunits in PPNAD cells through a GR-mediated mechanism that may involve direct GR-PKA catalytic subunit interaction (61,67). From these studies, we concluded that “the GR-mediated stimulatory effect of glucocorticoids on cortisol synthesis, together with overexpression of GR and PKA constitutive activation, is likely to form a local amplification loop that may explain the high secretory activity of PPNAD cells” (61).
Dissimilar responses by human and rodent adrenocortical cells to administration of dexamethasone may explain the discrepancies between the data obtained by Kontula et al (45) and earlier studies (44) (in addition to dose differences). In our study, we also found that dexamethasone inhibited the production of corticosterone by wild-type mouse adrenocortical cells, similar to that observed in earlier studies in mice and rats (44,68), whereas dexamethasone had no effect on release of cortisol from normal human adrenocortical cells (61). In addition, incubation of both normal human and mouse adrenocortical cells with RU-486 unmasked a stimulatory effect of dexamethasone on release of cortisol and corticosterone (61) that corresponds, therefore, to a GR-independent and likely nongenomic action, as recently observed in an ectopic dexamethasone-stimulated adrenocortical adenoma responsible for hypercortisolism (69). It is possible that this unexpected effect may be mediated by an unknown membrane receptor and may involve second messengers, as has been proposed in other tissue models (70). Because dexamethasone alone has no influence on production of cortisol from normal human adrenocortical tissues, we postulate that, in physiologic conditions, the GR-independent stimulatory action of dexamethasone on production of glucocorticoid is counteracted by an inhibitory GR-mediated effect of the drug through an unidentified cross-talk at the PKA level. Apparently, the GR-mediated inhibitory action of dexamethasone is predominant in mouse adrenocortical tissues.
In human PPNAD, the increase in PKA catalytic subunit intracellular content that results from PRKAR1A-inactivating mutations seems to favor a GR-dependent stimulatory action of dexamethasone. In a transgenic mouse model of PPNAD (71-73), no effect of dexamethasone was observed in vitro (61) or in vivo (72,73); this finding suggests that inactivation of the PRKAR1A gene reduces the GR-dependent inhibitory action of dexamethasone on mouse cells. In contrast, the observation that dexamethasone stimulated the release of corticosterone in the presence of RU-486 in this transgenic mouse as efficiently as it did in wild-type mouse adrenocortical tissues (61) indicates that PRKAR1A (partial) inactivation does not modify the GR-independent stimulatory effect on glucocorticoid production.
Collectively, these data suggested that the effects of glucocorticoids on adrenocortical cells are complex, are tissue-specific and possibly context-specific, and may involve nongenomic, in addition to GR-mediated, actions. Most likely, genomic effects of glucocorticoids mediate negative feedback in the adrenocortical steroidogenesis, whereas nongenomic effects serve to enhance it under specific circumstances.
This complexity in adrenocortical GR responses should be considered whenever dexamethasone is administered to humans for diagnostic or therapeutic purposes, beyond the known differences in dexamethasone absorption and metabolism (16,74). The fact that the GR responses to dexamethasone of each human are controlled by so many factors also explains the old observation that despite the wide interperson variability (probably attributable to many genetic variants of the molecules that participate in the foregoing phenomena), there is relative intra-person stability in HPA axis testing results (75). Some of this variation can be ascribed to functional GR polymorphisms, which may also predispose to adrenocortical tumor formation (76), but clearly GR genetic differences are not the only source of HPA axis functional variance.
CONCLUSION
This review emphasizes the variability in expression and function of the GR in pituitary and adrenocortical cells as one of the reasons for the considerable differences in HPA axis function across individuals. There are other phenomena, especially clinical observations, such as those of CS in a patient with normal secretion of ACTH and cortisol but high GR numbers (77) and the absence of CS in a patient with high levels of ACTH and cortisol and low 11βHSD type 1 activity (78). The molecular cause of these observations remains largely unexplained; therefore, much more must be learned about the HPA axis and its regulation.
ACKNOWLEDGMENT
This work was supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA, and, in part, by the Department of Pediatrics, Faculty of Medicine, University of Crete, Heraklion, Crete, Greece.
Abbreviations
- ACTH
adrenocorticotropic hormone
- ADTs
adrenocortical tumors
- AHR
aryl hydrocarbon receptor
- AIP
AHR interacting protein
- cAMP
cyclic adenosine monophosphate
- CD
Cushing disease
- CS
Cushing syndrome
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- 11βHSD
11β-hydroxysteroid dehydrogenase
- PKA
protein kinase A
- PPNAD
primary pigmented nodular adrenocortical disease
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Gomez MT, Magiakou MA, Mastorakos G, Chrousos GP. The pituitary corticotroph is not the rate limiting step in the postoperative recovery of the hypothalamic-pituitary-adrenal axis in patients with Cushing syndrome. J Clin Endocrinol Metab. 1993;77:173–177. doi: 10.1210/jcem.77.1.8392083. [DOI] [PubMed] [Google Scholar]
- 2.So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;3:e94. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu NZ, Cidlowski JA. The origin and functions of multiple human glucocorticoid receptor isoforms. Ann NY Acad Sci. 2004;1024:102–123. doi: 10.1196/annals.1321.008. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson JW, Walker EA, Bujalska IJ, et al. 11beta-Hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu N, Yoshikawa N, Wada T, et al. Tissue- and context-dependent modulation of hormonal sensitivity of glucocorticoid-responsive genes by hexamethylene bisacetamide-inducible protein 1. Mol Endocrinol. 2008;22:2609–2623. doi: 10.1210/me.2008-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadonaga JT. Regulation of RNA polymerase II transcription by sequence-specific DNA binding factors. Cell. 2004;116:247–257. doi: 10.1016/s0092-8674(03)01078-x. [DOI] [PubMed] [Google Scholar]
- 7.Suda T, Tozawa F, Dobashi I, et al. Corticotropin-releasing hormone, proopiomelanocortin, and glucocorticoid receptor gene expression in adrenocorticotropin-producing tumors in vitro. J Clin Invest. 1993;92:2790–2795. doi: 10.1172/JCI116898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamberts SW. Glucocorticoid receptors and Cushing's disease. Mol Cell Endocrinol. 2002;197:69–72. doi: 10.1016/s0303-7207(02)00280-0. [DOI] [PubMed] [Google Scholar]
- 9.Condon J, Gosden C, Gardener D, et al. Expression of type 2 11beta-hydroxysteroid dehydrogenase and corticosteroid hormone receptors in early human fetal life. J Clin Endocrinol Metab. 1998;83:4490–4497. doi: 10.1210/jcem.83.12.5302. [DOI] [PubMed] [Google Scholar]
- 10.Paust HJ, Loeper S, Else T, et al. Expression of the glucocorticoid receptor in the human adrenal cortex. Exp Clin Endocrinol Diabetes. 2006;114:6–10. doi: 10.1055/s-2005-873007. [DOI] [PubMed] [Google Scholar]
- 11.Bourdeau I, Lacroix A, Schürch W, Caron P, Antakly T, Stratakis CA. Primary pigmented nodular adrenocortical disease: paradoxical responses of cortisol secretion to dexamethasone occur in vitro and are associated with increased expression of the glucocorticoid receptor. J Clin Endocrinol Metab. 2003;88:3931–3937. doi: 10.1210/jc.2002-022001. [DOI] [PubMed] [Google Scholar]
- 12.Tacon LJ, Soon PS, Gill AJ, et al. The glucocorticoid receptor is overexpressed in malignant adrenocortical tumors. J Clin Endocrinol Metab. 2009;94:4591–4599. doi: 10.1210/jc.2009-0546. [DOI] [PubMed] [Google Scholar]
- 13.Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endocr Rev. 1998;19:647–672. doi: 10.1210/edrv.19.5.0346. [DOI] [PubMed] [Google Scholar]
- 14.Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Findling JW, Raff H, Aron DC. The low-dose dexamethasone suppression test: a reevaluation in patients with Cushing's syndrome. J Clin Endocrinol Metab. 2004;89:1222–1226. doi: 10.1210/jc.2003-030207. [DOI] [PubMed] [Google Scholar]
- 16.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children referred for the investigation of Cushing syndrome. Pediatrics. 2007;120:e575–e586. doi: 10.1542/peds.2006-2402. [DOI] [PubMed] [Google Scholar]
- 17.Stratakis CA, Sarlis NJ, Kirschner LS, et al. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med. 1999;131:585–591. doi: 10.7326/0003-4819-131-8-199910190-00006. [DOI] [PubMed] [Google Scholar]
- 18.Yotsumoto S, Aizawa T, Kotani M, Yamada T. Virilizing adrenal adenoma stimulated by dexamethasone in a middle-aged woman. J Clin Endocrinol Metab. 1979;48:660–663. doi: 10.1210/jcem-48-4-660. [DOI] [PubMed] [Google Scholar]
- 19.Clouston WM, Cannell GC, Fryar BG, Searle JW, Martin NI, Mortimer RH. Virilizing adrenal adenoma in an adult with the Beckwith-Wiedemann syndrome: paradoxical response to dexamethasone. Clin Endocrinol (Oxf) 1989;31:467–473. doi: 10.1111/j.1365-2265.1989.tb01270.x. [DOI] [PubMed] [Google Scholar]
- 20.Hurley DM, Accili D, Stratakis CA, et al. Point mutation causing a single amino acid substitution in the hormone binding domain of the glucocorticoid receptor in familial glucocorticoid resistance. J Clin Invest. 1991;87:680–686. doi: 10.1172/JCI115046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stratakis CA, Karl M, Schulte HM, Chrousos GP. Glucocorticosteroid resistance in humans: elucidation of the molecular mechanisms and implications for pathophysiology [with discussion]. Ann NY Acad Sci. 1994;746:362–376. doi: 10.1111/j.1749-6632.1994.tb39257.x. [DOI] [PubMed] [Google Scholar]
- 22.Karl M, Lamberts SW, Detera-Wadleigh SD, et al. Familial glucocorticoid resistance caused by a splice site deletion in the human glucocorticoid receptor gene. J Clin Endocrinol Metab. 1993;76:683–689. doi: 10.1210/jcem.76.3.8445027. [DOI] [PubMed] [Google Scholar]
- 23.Karl M, Von Wichert G, Kempter E, et al. Nelson's syndrome associated with a somatic frame shift mutation in the glucocorticoid receptor gene. J Clin Endocrinol Metab. 1996;81:124–129. doi: 10.1210/jcem.81.1.8550738. [DOI] [PubMed] [Google Scholar]
- 24.Karl M, Lamberts SW, Koper JW, et al. Cushing's disease preceded by generalized glucocorticoid resistance: clinical consequences of a novel, dominant-negative glucocorticoid receptor mutation. Proc Assoc Am Physicians. 1996;108:296–307. [PubMed] [Google Scholar]
- 25.Benoit I, Drui D, Chaillous L, et al. A corticotroph pituitary adenoma as the initial presentation of familial glucocorticoid deficiency. Eur J Endocrinol. 2009;161:195–199. doi: 10.1530/EJE-09-0100. [DOI] [PubMed] [Google Scholar]
- 26.Huizenga NA, de Lange P, Koper JW, et al. Human adrenocorticotropin-secreting pituitary adenomas show frequent loss of heterozygosity at the glucocorticoid receptor gene locus. J Clin Endocrinol Metab. 1998;83:917–921. doi: 10.1210/jcem.83.3.4648. [DOI] [PubMed] [Google Scholar]
- 27.Bray PJ, Cotton RG. Variations of the human glucocorticoid receptor gene (NR3C1): pathological and in vitro mutations and polymorphisms. Hum Mutat. 2003;21:557–568. doi: 10.1002/humu.10213. [DOI] [PubMed] [Google Scholar]
- 28.Koper JW, Stolk RP, de Lange P, et al. Lack of association between five polymorphisms in the human glucocorticoid receptor gene and glucocorticoid resistance. Hum Genet. 1997;99:663–668. doi: 10.1007/s004390050425. [DOI] [PubMed] [Google Scholar]
- 29.Antonini SR, Latronico AC, Elias LL, et al. Glucocorticoid receptor gene polymorphisms in ACTH-secreting pituitary tumours. Clin Endocrinol (Oxf) 2002;57:657–662. doi: 10.1046/j.1365-2265.2002.01639.x. [DOI] [PubMed] [Google Scholar]
- 30.Mu YM, Takayanagi R, Imasaki K, et al. Low level of glucocorticoid receptor messenger ribonucleic acid in pituitary adenomas manifesting Cushing's disease with resistance to a high dose-dexamethasone suppression test. Clin Endocrinol (Oxf) 1998;49:301–306. doi: 10.1046/j.1365-2265.1998.00520.x. [DOI] [PubMed] [Google Scholar]
- 31.Tateno T, Izumiyama H, Doi M, et al. Differential gene expression in ACTH-secreting and non-functioning pituitary tumors. Eur J Endocrinol. 2007;157:717–724. doi: 10.1530/EJE-07-0428. [DOI] [PubMed] [Google Scholar]
- 32.Raverot G, Wierinckx A, Jouanneau E, et al. Clinical, hormonal and molecular characterization of pituitary ACTH adenomas without (silent corticotroph adenomas) and with Cushing's disease. Eur J Endocrinol. 2010;163:35–43. doi: 10.1530/EJE-10-0076. [DOI] [PubMed] [Google Scholar]
- 33.Ebisawa T, Tojo K, Tajima N, et al. Immunohistochemical analysis of 11-beta-hydroxysteroid dehydrogenase type 2 and glucocorticoid receptor in subclinical Cushing's disease due to pituitary macroadenoma. Endocr Pathol. 2008;19:252–260. doi: 10.1007/s12022-008-9052-0. [DOI] [PubMed] [Google Scholar]
- 34.Korbonits M, Bujalska I, Shimojo M, et al. Expression of 11 beta-hydroxysteroid dehydrogenase isoenzymes in the human pituitary: induction of the type 2 enzyme in corticotropinomas and other pituitary tumors. J Clin Endocrinol Metab. 2001;86:2728–2733. doi: 10.1210/jcem.86.6.7563. [DOI] [PubMed] [Google Scholar]
- 35.Rabbitt EH, Ayuk J, Boelaert K, et al. Abnormal expression of 11 beta-hydroxysteroid dehydrogenase type 2 in human pituitary adenomas: a prereceptor determinant of pituitary cell proliferation. Oncogene. 2003;22:1663–1667. doi: 10.1038/sj.onc.1206293. [DOI] [PubMed] [Google Scholar]
- 36.Rabbitt EH, Gittoes NJ, Stewart PM, Hewison M. 11beta-Hydroxysteroid dehydrogenases, cell proliferation and malignancy. J Steroid Biochem Mol Biol. 2003;85:415–421. doi: 10.1016/s0960-0760(03)00224-3. [DOI] [PubMed] [Google Scholar]
- 37.Muchardt C, Yaniv M. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J. 1993;12:4279–4290. doi: 10.1002/j.1460-2075.1993.tb06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bilodeau S, Vallette-Kasic S, Gauthier Y, et al. Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev. 2006;20:2871–2886. doi: 10.1101/gad.1444606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laenger A, Lang-Rollin I, Kozany C, et al. XAP2 inhibits glucocorticoid receptor activity in mammalian cells. FEBS Lett. 2009;583:1493–1498. doi: 10.1016/j.febslet.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 40.Chahal HS, Chapple JP, Frohman LA, Grossman AB, Korbonits M. Clinical, genetic and molecular characterization of patients with familial isolated pituitary adenomas (FIPA). Trends Endocrinol Metab. 2010;21:419–427. doi: 10.1016/j.tem.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Tahir A, Chahal HS, Korbonits M. Molecular genetics of the AIP gene in familial pituitary tumorigenesis. Prog Brain Res. 2010;182:229–253. doi: 10.1016/S0079-6123(10)82010-2. [DOI] [PubMed] [Google Scholar]
- 42.Vierimaa O, Georgitsi M, Lehtonen R, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 43.Stratakis CA, Tichomirowa MA, Boikos S, et al. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. 2010;78:457–463. doi: 10.1111/j.1399-0004.2010.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loose DS, Do YS, Chen TL, Feldman D. Demonstration of glucocorticoid receptors in the adrenal cortex: evidence for a direct dexamethasone suppressive effect on the rat adrenal gland. Endocrinology. 1980;107:137–146. doi: 10.1210/endo-107-1-137. [DOI] [PubMed] [Google Scholar]
- 45.Kontula K, Pomoell UM, Gunsalus GL, Pelkonen R. Glucocorticoid receptors and responsiveness of normal and neoplastic human adrenal cortex. J Clin Endocrinol Metab. 1985;60:283–289. doi: 10.1210/jcem-60-2-283. [DOI] [PubMed] [Google Scholar]
- 46.Novak E, Stubbs SS, Seckman CE, Hearron MS. Effects of a single large intravenous dose of methylprednisolone sodium succinate. Clin Pharmacol Ther. 1970;11:711–717. doi: 10.1002/cpt1970115711. [DOI] [PubMed] [Google Scholar]
- 47.Saito E, Ichikawa Y, Homma Y. Direct inhibitory effect of dexamethasone on steroidogenesis of human adrenal in vivo. J Clin Endocrinol Metab. 1979;48:861–863. doi: 10.1210/jcem-48-5-861. [DOI] [PubMed] [Google Scholar]
- 48.Landon J, Wynn V, James VH, Wood JB. Adrenal response to infused corticotropin in subjects receiving glucocorticoids. J Clin Endocrinol Metab. 1965;25:602–611. doi: 10.1210/jcem-25-5-602. [DOI] [PubMed] [Google Scholar]
- 49.Tischler AS, Perlman RL, Morse GM, Sheard BE. Glucocorticoids increase catecholamine synthesis and storage in PC12 pheochromocytoma cell cultures. J Neurochem. 1983;40:364–370. doi: 10.1111/j.1471-4159.1983.tb11291.x. [DOI] [PubMed] [Google Scholar]
- 50.Kendall JW, Sloop PR., Jr Dexamethasone-suppressible adrenocortical tumor. N Engl J Med. 1968;279:532–535. doi: 10.1056/NEJM196809052791007. [DOI] [PubMed] [Google Scholar]
- 51.Rayfield EJ, Rose LI, Cain JP, Dluhy RG, Williams GH. ACTH-responsive, dexamethasone-suppressible adrenocortical carcinoma. N Engl J Med. 1971;284:591–592. doi: 10.1056/NEJM197103182841108. [DOI] [PubMed] [Google Scholar]
- 52.Boyle B, Butz H, Liko I, et al. Expression of glucocorticoid receptor isoforms in human adrenocortical adenomas. Steroids. 2010;75:695–700. doi: 10.1016/j.steroids.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Bouligand J, Delemer B, Hecart AC, et al. Familial glucocorticoid receptor haploinsufficiency by non-sense mediated mRNA decay, adrenal hyperplasia and apparent mineralocorticoid excess. PLoS One. 2010;5:e13563. doi: 10.1371/journal.pone.0013563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stratakis CA, Boikos SA. Genetics of adrenal tumors associated with Cushing's syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab. 2007;3:748–757. doi: 10.1038/ncpendmet0648. [DOI] [PubMed] [Google Scholar]
- 55.Bertherat J, Groussin L, Sandrini F, et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. 2003;63:5308–5319. [PubMed] [Google Scholar]
- 56.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 57.Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with Carney complex. Hum Mol Genet. 2000;9:3037–3046. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 58.Doucas V, Shi Y, Miyamoto S, West A, Verma I, Evans RM. Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 2000;97:11893–11898. doi: 10.1073/pnas.220413297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson-White A, Meoli E, Stergiopoulos S, et al. PRKAR1A mutations and protein kinase A interactions with other signaling pathways in the adrenal cortex. J Clin Endocrinol Metab. 2006;91:2380–2388. doi: 10.1210/jc.2006-0188. [DOI] [PubMed] [Google Scholar]
- 60.Robinson-White A, Hundley TR, Shiferaw M, Bertherat J, Sandrini F, Stratakis CA. Protein kinase A activity in PRKAR1A-mutant cells, and regulation of mitogen-activated protein kinases ERK1/2. Hum Mol Genet. 2003;12:1475–1484. doi: 10.1093/hmg/ddg160. [DOI] [PubMed] [Google Scholar]
- 61.Louiset E, Stratakis CA, Perraudin V, et al. The paradoxical increase in cortisol secretion induced by dexamethasone in primary pigmented nodular adrenocortical disease involves a glucocorticoid receptor-mediated effect of dexamethasone on protein kinase A catalytic subunits. J Clin Endocrinol Metab. 2009;94:2406–2413. doi: 10.1210/jc.2009-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johanssen S, Allollio B. Mifepristone (RU 486) in Cushing's syndrome. Eur J Endocrinol. 2007;157:561–569. doi: 10.1530/EJE-07-0458. [DOI] [PubMed] [Google Scholar]
- 63.Schoch GA, D'Arcy B, Stihle M, et al. Molecular switch in the glucocorticoid receptor: active and passive antagonist conformations. J Mol Biol. 2010;395:568–577. doi: 10.1016/j.jmb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Zhang S, Jonklaas J, Danielsen M. The glucocorticoid agonist activities of mifepristone (RU486) and progesterone dependent on glucocorticoid receptor levels but not on EC50 values. Steroids. 2007;72:600–608. doi: 10.1016/j.steroids.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Nordeen SK, Bona BJ, Beck CA, Edwards DP, Borror KC, DeFranco DB. The two faces of a steroid antagonist: when an antagonist isn't. Steroids. 1995;60:97–104. doi: 10.1016/0039-128x(94)00001-s. [DOI] [PubMed] [Google Scholar]
- 66.Gummow BM, Scheys JO, Cancelli VR, Hammer GD. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol Endocrinol. 2006;20:2711–2723. doi: 10.1210/me.2005-0461. [DOI] [PubMed] [Google Scholar]
- 67.Wiemann S, Steuer B, Alonso A, Kinzel V, Pyerin W. Promoter of the gene encoding the bovine catalytic subunit of cAMP-dependent protein kinase isoform C beta 2. Biochim Biophys Acta. 1996;1309:211–220. doi: 10.1016/s0167-4781(96)00175-3. [DOI] [PubMed] [Google Scholar]
- 68.Carsia RV, Malamed S. Glucocorticoid control of steroidogenesis in isolated rat adrenocortical cells. Biochim Biophys Acta. 1983;763:83–89. doi: 10.1016/0167-4889(83)90028-9. [DOI] [PubMed] [Google Scholar]
- 69.Louiset E, Gobet F, Libé R, et al. ACTH-independent Cushing's syndrome with bilateral micronodular adrenal hyperplasia and ectopic adrenocortical adenoma. J Clin Endocrinol Metab. 2010;95:18–24. doi: 10.1210/jc.2009-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song IH, Buttgereit F. Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol. 2006;246:142–146. doi: 10.1016/j.mce.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Griffin KJ, Kirschner LS, Matyakhina L, et al. Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res. 2004;64:8811–8815. doi: 10.1158/0008-5472.CAN-04-3620. [DOI] [PubMed] [Google Scholar]
- 72.Griffin KJ, Kirschner LS, Matyakhina L, et al. A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. J Med Genet. 2004;41:923–931. doi: 10.1136/jmg.2004.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griffin KJ, Kirschner LS, Matyakhina L, et al. A mouse model for Carney complex. Endocr Res. 2004;30:903–911. doi: 10.1081/erc-200044145. [DOI] [PubMed] [Google Scholar]
- 74.Yanovski JA, Cutler GB, Jr, Chrousos GP, Nieman LK. The dexamethasone-suppressed corticotropin-releasing hormone stimulation test differentiates mild Cushing's disease from normal physiology. J Clin Endocrinol Metab. 1998;83:348–352. doi: 10.1210/jcem.83.2.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huizenga NA, Koper JW, de Lange P, et al. Interperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitary-adrenal axis to a low dose of dexamethasone in elderly individuals. J Clin Endocrinol Metab. 1998;83:47–54. doi: 10.1210/jcem.83.1.4498. [DOI] [PubMed] [Google Scholar]
- 76.Majnik J, Patocs A, Balogh K, et al. Overrepresentation of the N363S variant of the glucocorticoid receptor gene in patients with bilateral adrenal incidentalomas. J Clin Endocrinol Metab. 2006;91:2796–2799. doi: 10.1210/jc.2006-0066. [DOI] [PubMed] [Google Scholar]
- 77.Newfield RS, Kalaitzoglou G, Licholai T, et al. Normocortisolemic Cushing's syndrome initially presenting with increased glucocorticoid receptor numbers. J Clin Endocrinol Metab. 2000;85:14–21. doi: 10.1210/jcem.85.1.6220. [DOI] [PubMed] [Google Scholar]
- 78.Tomlinson JW, Draper N, Mackie J, et al. Absence of cushingoid phenotype in a patient with Cushing's disease due to defective cortisone to cortisol conversion. J Clin Endocrinol Metab. 2002;87:57–62. doi: 10.1210/jcem.87.1.8189. [DOI] [PubMed] [Google Scholar]