Abstract
Carney triad, as originally described in 1977, was the association of 3 tumors: gastric epithelioid leiomyosarcoma [later renamed gastrointestinal stromal tumor (GIST)], extra-adrenal paraganglioma, and pulmonary chondroma. The disorder affected mostly young women and was not familial. We studied the clinical and pathologic features of the gastric neoplasm in 104 patients with the syndrome. Most (88%) were young women (mean age, 22 y), and the usual presentation was gastric bleeding. The tumors, commonly antral-based (61%), were multifocal, and ranged from 0.2 to 18.0 cm in dimension. Most (86%) featured round and polygonal (epithelioid) cells. Metastasis occurred in 49 patients (47%): to gastric lymph nodes (29%), liver (25%), and peritoneum (13%). Immunopositivity was detected in the tumors tested as follows: KIT, 100%; CD34, 75%; PKCy, 21%; PDGFRA, 90%; and smooth muscle actin, 6%. Fourteen patients (13%) died of metastatic GIST at a mean age of 45 years (range, 30 to 69 y). Estimated 10 and 40-year survivals were 100% and 73%, respectively. Median survival time was 26.5 years (range, 16 to 60 y). There was no correlation between the National Institutes of Health tumor risk classification and the tumor behavior. Compared with sporadic gastric GISTs, the gastric stromal tumor in Carney triad showed distinctive features: female predilection, young patient age, epithelioid cell predominance, multifocality, frequent lymph node metastasis, serial tumor occurrence, and unpredictable behavior. Thus, the Carney triad gastric stromal tumor is different clinically, pathologically, and behaviorally from sporadic gastric GIST.
Keywords: Carney triad, nonfamilial syndrome, gastric gastrointestinal stromal tumor, pulmonary chondroma, paraganglioma
Carney triad (hereinafter referred to as the triad) is a rare multicentric and multifocal tumor syndrome first described in 1977.58 The components initially recognized were gastric gastrointestinal stromal tumor (GIST) (earlier referred to as epithelioid leiomyosarcoma), functioning extra-adrenal paraganglioma, and pulmonary chondroma.18 Subsequently, 2 other tumors were added to the syndrome, esophageal leiomyoma, and adrenal cortical adenoma.15 The tumor multicentricity and multifocality suggested that the syndrome would be heritable. This proved not to be the case—in a further surprise, the disorder had a strong female predilection.
The commonest and most serious of the neoplasms was the gastric tumor, a lesion that has been successively referred to as leiomyoblastoma,47 epithelioid leiomyosarcoma,14 GIST,37 and gastric stromal sarcoma.15
We reviewed 104 cases of the triad from Mayo Clinic patient records, consultation cases of one of the authors (J.A.C.), and 50 years of medical literature. This article describes the unusual clinical, pathologic, and behavioral features of the gastric tumors in the syndrome and how these differ substantially from those of the sporadic gastric GIST.
PATIENTS AND METHODS
Patients
We identified 104 patients who had gastric GISTs and 1 or more of these neoplasms: pulmonary chondroma, paraganglioma, esophageal leiomyoma, and adrenal adenoma. Patients with 2 of the tumors were considered to have incomplete expression of the triad. Seventy-nine of the patients with follow-up were included in an earlier report.15 This study included 25 new cases, 19 of which had at least 5 years of follow-up after gastric surgery. Clinical, pathologic, and follow-up data from the cases were abstracted from Mayo Clinic patient records, the files of one of the authors (J.A.C.), and literature reports.5,6,10–13,21,23,37,38,40,50,62,65,67–69,74–79
Tumors
Data on the gastric tumors were obtained from clinical, surgical, and pathology reports. For 65 cases (62%), 1 or more sections of formalin-fixed, paraffin-embedded, hematoxylin and eosin-stained gastric tumor were available; a variable number of unstained slides were obtained for 61 of these.
The low-power, intermediate-power, and high-power microscopic findings including tumor location within the stomach wall, encapsulation, architecture, mucosal ulceration, tumor necrosis, and angiolymphatic invasion were recorded. Evaluation of nuclear features included size, shape and chromaticity, intracellular location, and, multinucleation. Mitotic activity in the neoplasms was counted in 50 consecutive contiguous high-power microscopic fields (HPFs) from a randomly selected area of tumor. Tumor cellularity was defined arbitrarily as the ratio of the area of the tumor occupied by neoplastic cells to that of the tumor occupied by stroma. Lesions composed predominantly of cells with scant stroma were recorded as having high cellularity; those with easily identifiable collagen or myxoid stroma were listed as having low or moderate cellularity. The tumor cell type was recorded as epithelioid when more than 90% of the cells were round or polygonal, as spindle when more than 90% of the cells were spindle, and mixed epithelioid and spindle when the minor component exceeded 10% of the tumor cells. The tumors were subclassified according to the scheme of Miettinen et al.54
National Institutes of Health consensus criteria28 were applied to 37 tumors for which histologic sections and tumor size were available. The criteria separated the lesions into 4 categories: (1) very low risk (<2 cm,<5 mitoses/50 HPFs; (2) low risk (2 to 5 cm, <5 mitoses/50 HPFs); (3) intermediate risk (5 to 10 cm, <5 mitoses/50 HPFs); and (4) high risk (5 to 10 cm and >5 mitoses/50 HPFs or >10 cm, regardless of mitotic count, or >10 mitoses/50 HPFs, regardless of tumor size).
Immunohistochemical staining was carried out on a subset of the tumors (the number of unstained slides varied among the cases). After antigen retrieval (steam EDTA), these antibodies were applied to the sections of tumor with standard techniques, including staining of positive and negative control sections: (1) mouse monoclonal antibodies against CD34 (dilution 1/100; Becton Dickinson, Franklin Lakes, NJ), PKCy (dilution 1/200; BD Pharmigen, San Diego, CA), smooth muscle actin (dilution 1/100; Dako, Carpinteria, CA), and desmin (dilution 1/100; Novocastra, Leica Microsystems Inc, Bannockburn, IL), and (2) rabbit polyclonal antibodies against KIT (dilution 1/100; Dako,), S100 (dilution 1/100; Dako), and PDGFRA (dilution 1/50; Fitzgerald Industries International, Concord, MA).
Treatment
The neoplasms were all initially treated surgically. The procedures included 1 or more segmental tumor excisions (17 patients), subtotal gastrectomy (64 patients), and total gastrectomy (17 patients). Fifty patients (48%) had more than 1 gastric operation. One patient had 8 abdominal operations, including second-look procedures. The tumors were found at autopsy in 2 patients. Twenty-seven patients who initially had segmental excision or subtotal gastrectomy later underwent completion gastrectomy for serial tumor development. Thirteen patients received adjuvant treatment (chemotherapy, radiotherapy, hyperthermia, or interferon, alone or in various combinations). Another 16 patients were treated with imatinib mesylate (Gleevec; Novartis, East Hanover, NJ), 200 to 800 mg daily, for up to 4 years. Some liver metastases were treated by excision, hepatic lobectomy, chemoembolization, or radiofrequency ablation; 1 patient underwent hepatic transplant.13
Follow-up
Follow-up was obtained from Mayo Clinic records and physician or patient contact. Length of follow-up was calculated from the date of the first gastric surgery to the date of the latest contact.
RESULTS
Clinical Features
The patients were 92 females (88%) and 12 (12%) males whose ages at their first gastric operation ranged from 6 to 53 years (mean, 22 y; median, 18 y). Of the 5 neoplasms comprising the syndrome, 1 patient had all of 5 tumors identified pathologically or radiographically; 5 patients had 4; 45 patients had 3; and 54 patients had 2 (Table 1). No patient had a similarly affected relative. One patient had an unaffected identical twin. Several patients had other tumors, including mammary carcinoma (4 patients), gastric adeno-carcinoma (2 patients and probably a third), renal cell carcinoma (2 patients), renal angiomyolipoma (1 patient), hyperparathyroidism (2 patients), and colonic adenocarcinoma (1 patient). Other disorders in the group were arthritis (4 patients), nephrolithiasis (3 patients), branchial cleft cyst (3 patients), bony exostosis (2 patients), colonic inertia (2 patients), and bilateral congenital ear deformity (1 patient, the index patient to the triad).
TABLE 1.
Tumor Combinations in 104 Patients*
| Gastric Stromal Tumor | Pulmonary Chondroma | Paraganglioma | Adrenal Adenoma | Esophageal Leiomyoma | Patients (No.) |
|---|---|---|---|---|---|
| + | + | 37 | |||
| + | + | + | 22 | ||
| + | + | 16 | |||
| + | + | + | 10 | ||
| + | + | + | 8 | ||
| + | + | + | + | 4 | |
| + | + | + | 4 | ||
| + | + | + | + | + | 1 |
| + | + | + | + | 1 | |
| + | + | + | 1 |
Plus sign indicates the presence of tumor.
The presenting sign or symptom in 77 patients (74%) was bleeding from ulceration of the gastric tumors that resulted in 1 or more of the following: anemia, melena, hematemesis, and acute abdominal symptoms (owing to tumor rupture and hemoperitoneum). Occasional patients were asymptomatic and had a lesion that was palpated by the patient or a physician (4 patients), found during laparotomy performed for nongastric reasons (1 patient), or discovered at autopsy (2 patients).
One or more calcified pulmonary tumors were the first manifestation of the triad in 10 asymptomatic patients. Hypertension or an indeterminate mass (paraganglioma) was the initial manifestation in another 10.
Gross Pathology
Multiple stomach tumors were found in each patient. In 1 patient who had a single tumor at age 9 years, a second tumor developed at the age of 32 years. The tumors ranged in size from 0.2 to 18.0 cm (mean, 6.3 cm). They were circumscribed and infrequently encapsulated. Often juxtaposed or forming an agglomerated mass, they also occurred separately. Most were antral-based and adjacent to the lesser gastric curvature (63 patients; 61%) (Fig. 1), but they occurred throughout the stomach. The neoplasms were intramural and expanded the stomach wall, creating broad-based polypoid protrusions into the gastric lumen or bulging masses from the serosal surface or both. The intraluminal protrusions were covered by flattened mucosa that was sometimes ulcerated; the protruding serosal masses typically had secondary and small tertiary tumor excrescences. Some serosal tumors, attached to the stomach by a narrow pedicle or a broad base, were not visible endoscopically. There was no direct invasion of extragastric structures by the tumors.
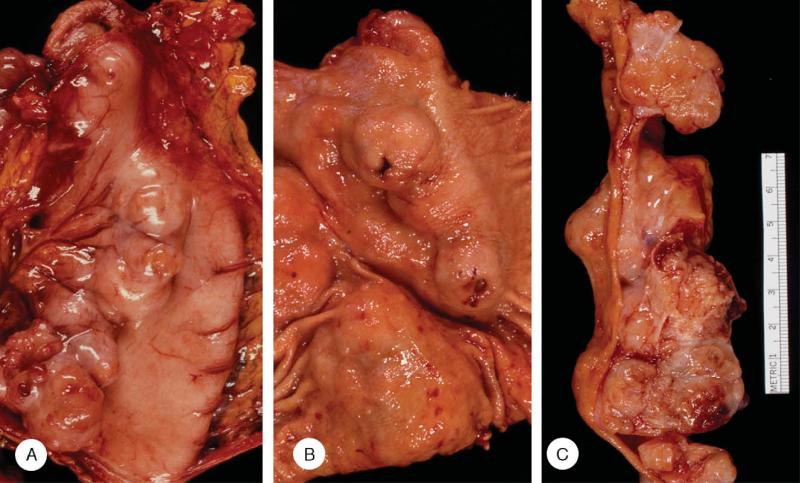
FIGURE 1.
Gross appearance of gastric stromal tumors. Tumors from the same patient at primary surgery. A, The serosal surface showed a series of juxtaposed generally hemispherical tumors high on the posterior wall and in its midportion. B, The opened stomach revealed sessile polypoid tumors high on the anterior and posterior walls, several with ulceration (arrow). C, The cut surface of the tumors showed a confluent mass of yellow and flesh-colored tumors, some with internal lobulation and areas of hemorrhage.
Most of the neoplasms were round or oval, but some were molded by adjacent tumors. Consistency ranged from soft to hard and occasionally the tumors felt gritty during cutting. The sectioned surface bulged and ranged from solid to cystic, with spaces containing hemorrhagic fluid, or grumous or friable material. The various colors included fish-flesh, pinkish tan, gray, grayish yellow, purple, and blue; the appearance was often altered by hemorrhage. Most of the tumors had delicate internal lobulation.
Scattered sessile mucosal polyps, up to 25 mm in diameter, were present in 20 cases (19%). For one patient,11 in which the stromal tumor was exophytic and did not involve the mucosa, the pathology report described soft, sessile, polypoid, edematous, and somewhat villous antral lesions that measured 2 to 3 cm.
Adenocarcinoma developed in the gastric remnant in 2 patients 39 and 40 years, respectively, after subtotal gastrectomy.
Light Microscopy
At low-power microscopy, the cells were arranged in variably shaped but generally round unencapsulated masses surrounded or separated by smooth muscle or hypocellular fibrous tissue (Fig. 2). The neoplasms distended the muscularis propria and ballooned into the submucosa or mushroomed from the serosa or both. Most of the neoplasms (86%) were hypercellular and composed almost entirely of tumor cells with minimal supporting stroma and vasculature. Linear dystrophic calcification occurred in the fibrous capsule of the occasional encapsulated tumor.
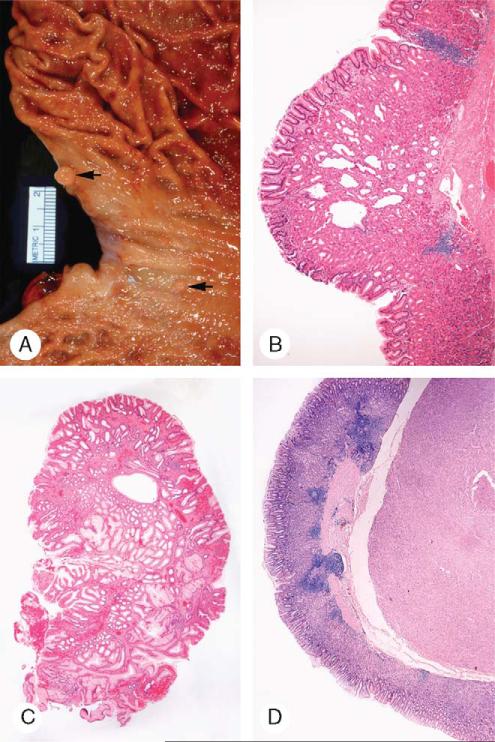
FIGURE 2.
Panoramic and low-power microscopic appearance of gastric stromal tumors. A, The partly encapsulated, patternless tumor occupied the submucosa and the muscularis propria, in which residual bands of smooth muscle were preserved. The lesion penetrated into the mucosa and externally formed a small serosal expansion. The overlying mucosa showed patchy edema, foveolar hyperplasia, and vascular ectasia (arrow). B, A coarsely lobulated tumor with residual smooth muscle bands and a nodular pattern expanded the submucosa. Superficially, it was circumscribed and still confined within the muscularis propria. A mucosal lymphoid aggregate was present (arrow).
Architecture
The cells were arranged in sheets, clusters, and fascicles. The sheets had a uniform or haphazard arrangement of the cells; fluid accumulation sometimes caused vague or distinct palisading, undulating ribboning, or pseudotrabecular arrangement. The cell clusters were usually small and tight, closely apposed with little stroma separating them, and readily apparent to indistinct. The spindle cell fascicles occasionally had an interlacing pattern.
The neoplasms had a pushing border (Figs. 2A, B) and usually abutted directly on surrounding tissue. They showed little tendency to infiltrate. Sixty-three tumors (61%) had gross or microscopic ulceration of the overlying mucosa; invasion of the lamina propria mucosa was present in 20 (19%). Necrosis was present in 8 tumors (8%). Angiolymphatic invasion was detected in 2 tumors. There was no hyperplasia of the interstitial cells of Cajal, the pacemaker cells of the gut.9,41
Cells
The medium to small-sized eosinophilic tumor cells (Fig. 3) were poorly outlined and variably shaped, polygonal, round, and spindle. On the basis of the cell shape, the tumors were classified as epithelioid (polygonal and round cells) (68%), spindle (18%), or mixed epithelioid and spindle (14%). In 1 patient, 5 separate tumors were sampled: 4 were epithelioid, and 1 was mixed epithelioid and spindle. Hypercellularity was a striking feature of most tumors (86%). With the subclassification criteria used by Miettinen et al,54 44 of 65 tumors (68%) were entirely or predominantly hypercellular and epithelioid; 8 additional tumors had a minor hypercellular epithelioid component [this pattern was rare in the Armed Forces Institute of Pathology series, in which only 78 of 1242 tumors (6%) were classified as this type]. Twelve tumors (18%) in this study group had a major hyper-cellular spindle cell component, and an additional 15 neoplasms had a minor one (this pattern was also uncommon in the AFIP series in which 139 of 1242 tumors 11% had this pattern). The 2 most common patterns in the AFIP series, the palisading and vacuolating spindle cell pattern (21%) and the sclerosing epithelioid pattern (22%), were rare in our study group: the former pattern occurred in pure form in only 1 tumor and as a minor component in 5, and the sclerosing epithelioid pattern was seen as a minor pattern in 1 tumor and in pure form in none.
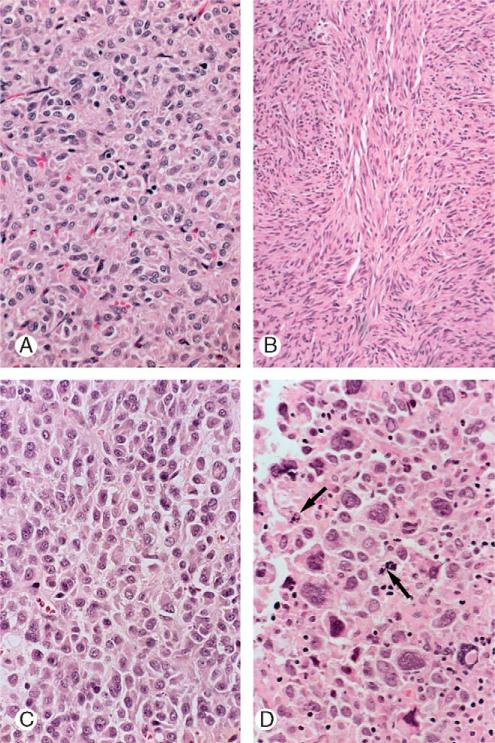
FIGURE 3.
Cytology of gastric stromal tumors. A, Patternless sheet of poorly outlined polygonal, epithelioid cells with eosinophilic cytoplasm, and polygonal, irregular, crinkled nuclei. The nucleoli are small. B, Spindle cells with elongated nuclei and eosinophilic cytoplasm arranged in interlacing fascicles. C, Dis-cohesive polygonal cells with eccentric nucleus or nuclei have a plasmacytoid appearance. The cytoplasm is homogeneous and acidophilic and features an occasional vacuole. D, Enlarged multinucleated epithelioid cells have eosinophilic cytoplasm. Mitotic figures are present (arrows).
Nuclei
The vesicular nuclei were uniform, small and medium in size, round, oval, irregular or spindle in shape, and sometimes indented or folded (Fig. 3). They were located centrally or eccentrically in the cells. The chromatin had a speckled appearance. The nucleolus was single and small. There was an occasional intranuclear vacuole. Binucleation was common and multinucleation was seen occasionally. The mitotic count ranged from 0 to 14 mitoses/50 HPFs (median, 3 mitoses/50 HPFs); 75% of cases had 5 or fewer mitoses/50 HPFs. Atypical mitotic figures were present in a few tumors. Nuclear atypicality (variation in nuclear size, shape, or chromaticity) was marked in 25% of the tumors and moderate in 40% of the lesions. Giant bizarre nuclei occurred in a few cases.
Other Findings
Intratumoral hemorrhage was common. Degenerative changes were infrequent. Edema, hyalinization, and necrosis occurred occasionally. There was no intratumoral calcification. An occasional serosal tumor had a hypocellular cavernous hemangioma-like appearance.
Immunohistochemical Profile
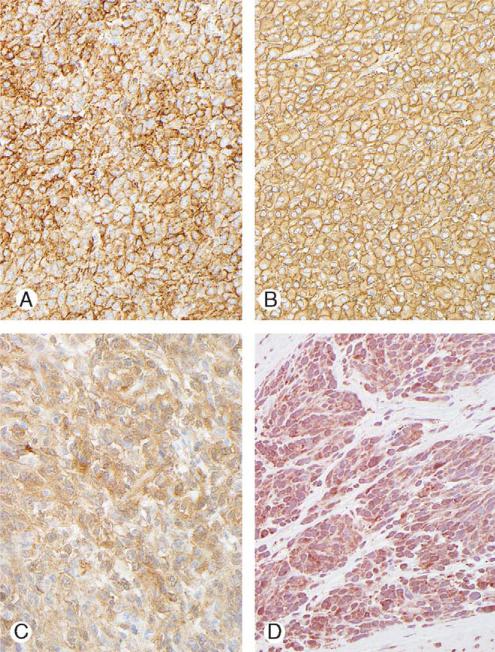
The results are summarized in Table 2 and illustrated in Figure 4. The KIT positivity (100%) was usually membranous and diffuse, and strong (77%); in the remaining cases, it was membranous. Cytoplasmic staining for CD34 was diffuse and strong, rarely involving less than 50% of the cells. PKCy showed similar staining. Staining for PDGFRA was present in 90% of the tumors tested and was cytoplasmic, weak to moderate; nuclear staining was present in 5 of 21 cases (24%). Three tumors had focal smooth muscle actin positivity. There was no staining for S100, desmin, or neurofilament. One patient had a 3 cm leiomyoma in the cardia (positive for actin and desmin, and negative for KIT and CD34).
TABLE 2.
Immunohistochemical Findings in Gastric Stromal Tumors
| Antibody | Cases Tested (No.) | Immunopositivity (%) |
|---|---|---|
| KIT | 61 | 100 |
| CD34 | 40 | 75 |
| PKCθ | 21 | 21 |
| PDGFRA | 10 | 90 |
| Smooth muscle actin | 49 | 6 |
| S100 | 31 | 0 |
| Desmin | 29 | 0 |
| Neurofilament | 29 | 0 |
FIGURE 4.
Immunostaining of gastric stromal tumors. A, Membranous and diffuse KIT positivity. B, Diffuse strong CD34 positivity. C, Strong diffuse staining for PKCy. D, Weak staining for PDGFRA.
Tumor Risk Classification
Based on the tumor size and mitotic activity, the risk of recurrence or metastasis was determined to be very low in 1 case, low in 13 cases, intermediate in 12 cases, and high in 11 cases. There was no correlation of the assessed risk with subsequent development of metastasis, tumor recurrence, or tumor-specific death. In the patient who had a very low-risk tumor, new gastric stromal tumors, and metastasis developed. Serial tumor development was associated with 13 low-risk tumors (62%), 6 of 12 intermediate-risk tumors (50%), and 4 of 11 high-risk tumors (36%). Among the 6 patients who died of the tumor, the lesions were low-risk in 3, intermediate-risk in 1, and high-risk in 2.
Metastasis
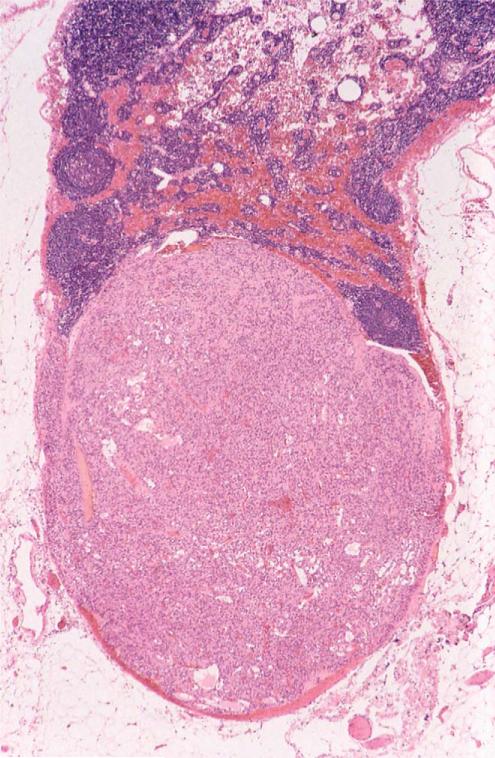
Histologically confirmed stromal tumor metastasis was found at primary surgery in 28 patients (27%): metastasis was to lesser curvature lymph nodes (n = 15), liver (n = 14), and peritoneum (n = 8). Subsequently, metastasis developed in an additional 21 patients. The sites of metastasis at primary and subsequent abdominal operations were lymph nodes (30 cases), liver 26 (cases), and peritoneum and omentum (14 cases). Fifty-seven lymph nodes contained tumor metastasis (Fig. 5) at primary or secondary gastric surgery. Thirteen patients had metastasis to 1 or more lymph nodes only, which were usually located on the lesser gastric curvature. One hundred and sixty-two lymph nodes from 55 other patients were free of metastasis. Extra-abdominal meta-stasis occurred in 5 patients.
FIGURE 5.
Lymph node with gastric stromal tumor metastasis.
Follow-up
Follow-up ranged from 1 to 60 years (mean, 22.3 y; median, 21 y). Only 6 patients had less than 5 years of follow-up. Seventy-seven patients (74%) were alive at the last follow-up, 1 to 60 years after first detection of a gastric tumor. Twenty-five patients (24%) had metastatic or residual gastric stromal tumor. Fifty-nine patients had pulmonary chondromas, and 6 had primary or metastatic paraganglioma. In 17 of 80 patients (21%) who initially underwent subtotal gastric resection, 1 or more new stromal tumors developed in the gastric remnant requiring 1 or more additional operations. The mean interval between primary and secondary surgery was 10.3 years (median, 8 y; range, 0.5 to 46 y). The median number of new tumor episodes was 2 (maximum, 6).
Twenty-six patients (25%) were dead: 14 (13%) died of metastatic or residual gastric stromal tumor at a mean age of 45 years (range, 30 to 69 y; the 3 youngest deceased patients were aged 30, 31, and 32 y). One patient died of complications of abdominal radiotherapy for the gastric stromal tumor. The median survival time after the first gastric surgery was 26.5 years (range, 16 to 60 y). The estimated stromal tumor-specific survival rates (95% confidence interval, number still at risk were as follows: at 10 y (100%), 100% to 100%, 81); at 20 years, 92.9% (86.3% to 99.9%, 51); at 30 years, 77.6% (66.0% to 91.4%, 28); and at 40 years, 73.3% (60.2% to 89.4%, 13). Other causes of death in the group were paraganglioma (5 patients), gastric adenocarcinoma (1 patient and probably a second), colonic adenocarcinoma (1 patient), and unrelated conditions (4 patients).
Twenty-four patients were alive with metastatic gastric stromal tumor: metastasis was to the liver (10 patients), to liver and peritoneum (9 patients), and peritoneum (5 patients). One patient had residual gastric tumor. Twenty-five patients were alive without known gastric stromal tumor. Sixteen patients were treated with imatinib mesylate; there was no tumor response in 10 (62%) and a minor, partial, or questionable response in the remainder.
Gastric Mucosal Lesions
Gastric mucosa was available for study in 52 cases. Three types of lesion were encountered: lymphoid aggregates (34 cases), benign polyps (19 cases), and adenocarcinoma (2 cases and probably a third).
The mucosal lymphoid aggregates (Figs. 2B, 6B, D) were single and scattered deep in an otherwise normal mucosa impinging on and sometimes extending into the muscularis mucosae and submucosa. Germinal centers were common. The aggregates were not associated with Helicobacter pylori organisms, active chronic gastritis, or lymphocytic gastritis.
FIGURE 6.
Gastric mucosal lesions in cases of gastric stromal tumor. A, Two small sessile polyps are in the gastric cardia (arrows). B, Cystic fundic gland polyp featured proliferated fundic glands, several of which are dilated. C, Proliferated foveolar cells created a hyperplastic mucosal polyp. D, Lymphoid aggregates extended into and through the thickened muscularis mucosa covering a circumscribed stromal tumor.
The polyps (Figs. 6A–C) measured 1 to 25 mm and were multiple and sessile and located throughout the mucosa but mostly in the proximal stomach. They included hyperplastic (most frequent), fundic gland, and indeterminate types. None had dysplastic features.
The 2 histologically proven carcinomas occurred in women, aged 50 and 67 years. The tumors were discovered 39 and 57 years after subtotal gastrectomy.
Esophageal and Duodenal Lesions
Sixteen patients (15%) had 1 or more asymptomatic esophageal leiomyomas, ranging in diameter from 4 mm to 2 cm. One of them also had a 1.5 cm esophageal stromal tumor. One patient had an esophageal carcinoid tumor.
Fifteen patients (14%) had duodenal lesions: small stromal tumors (4 patients), mucosal lymphoid aggregates (4 patients, abundant in 1), interstitial cell of Cajal hyperplasia (1 patient),59 direct extension of gastric stromal tumor into the duodenum (1 patient), and other or indeterminate lesions (5 patients).
DISCUSSION
Carney triad is 1 of the several syndromes that feature gastric stromal tumors as a major component (Table 3). Features of the triad neoplasm include occurrence at a young age, female predilection, tumor multifocality, slow growth, frequent metastasis (often to lymph nodes), lack of response to imatinib therapy, and sometimes a fatal outcome. Features of the usual sporadic gastric stromal tumor (GIST) include occurrence in older patients (>50 y), equal sex distribution, single tumor occurrence, very infrequent metastasis and not to lymph nodes, generally favorable response to imatinib therapy, and usually a benign natural history with infrequent mortality.54
TABLE 3.
Syndromes Featuring Gastric Stromal Tumors (GISTs) in Chronologic Order of Description
| Syndrome | Components | Autosomal Dominant Inheritance | Mutation |
|---|---|---|---|
| Carney triad15 | Gastric stromal tumor, pulmonary chondroma, extra-adrenal paraganglioma, esophageal leiomyoma, and adrenal adenoma | No | Unknown |
| Unnamed43,44 | Esophageal, small intestinal and gastric GISTs, skin pigmentation, urticaria pigmentosa, and mast cell tumors | Yes | Germline KIT exon 11 |
| Familial GISTs57 | Gastric GISTs | Yes | Germline KIT exon 13 |
| Unnamed33 | Small intestinal and gastric GISTs, myenteric plexus abnormalities | Yes | Unknown |
| Carney-Stratakis syndrome16,49 | Paraganglioma and gastric GISTs | Yes | Germline SDHB, SDHC, SDHD |
| Unnamed19 | Gastric GISTs, large hands | Yes | Germline PDGFRA 2675G > T |
| Neurofibromatosis7,25,79 | Small intestinal and gastric GISTs | Positive family history; presumably yes | KIT and PDGFRA |
| Unnamed17 | Gastric stromal tumors, gastric and small intestinal fibromas, and lipomas | No family history; presumably yes | Germline PDGFRA (V561D) |
Further comparison of the clinical, pathologic, behavioral, and molecular genetic features of the triad and the sporadic tumors showed that they were virtually all different (Table 4), although the tumors likely share a common ICC lineage; the similarities were limited to cell shape (spindle and epithelioid) and KIT and CD34 immunopositivity. The differences between the neoplasms were so many and of such a nature that it was difficult to avoid concluding that they were in fact different neoplasms. Because of the differences, we believe that it is now inappropriate and misleading to label the triad tumor simply as GIST. A different title is needed for it because of its special properties.
TABLE 4.
Comparison of Selected Findings in Sporadic Gastrointestinal Stromal Tumors (GISTs) and Carney Triad Gastric Stromal Tumors
| Feature | Sporadic GIST | Carney Triad Stromal Tumor |
|---|---|---|
| Cause | Somatic KIT or PDGFRA mutation35,36 | Unknown; no KIT, PDGFRA, or SDH mutations46 |
| Sex ratio | Approximately equal54 | Female predilection (88%)15 |
| Age, Y | > 50 (75%), median, 5861 | Range, 6-53; mean, 215 |
| No. tumors | Single61 | Multiple15 |
| Location | Body > antrum54 | Antrum > body15 |
| Cell type | Spindle > mixed > epithelioid54 | Epithelioid > mixed > spindle (present study) |
| Mitotic activity | Low (< 1 mitosis/10 high-power fields) | Variable 0-14 mitoses/50 high-power fields (present study) |
| Pleomorphism | Rare or variable54,61 | Marked to moderate (present study) |
| Gastric mucosal polyps | No | Yes (18%) (present study) |
| Immunophenotype | KIT+, PDGFRA+55 | KIT+, PDGFRA+ (present study) |
| Serial stromal tumors | No61 | Yes (present study) |
| Comparative genomic hybridization | 14q > 22q > 1p loss31 | 1p > other loss46 |
| Metastatic rate | < 1%54,61 | 49/104 (47%) (present study) |
| Metastatic sites | Liver, peritoneum, bone, lung | Lymph node, liver, peritoneum, bone, lung, and brain |
| Response to imatinib mesylate therapy | Yes | None or inconclusive |
| Mortality | 17% dead; 0.8% alive with metastatic tumor54 | I6% dead*; 25% alive with residual or metastatic tumor15 |
| Associated conditions | None54 | Pulmonary chondroma, paraganglioma, esophageal leiomyoma, adrenocortical adenoma15 |
Owing to gastric stromal tumor.
In the 1940s and 1950s, most spindle cell tumors of the gut were interpreted as leiomyomas or leiomyosarcomas.1 In 1962, Martin et al45 described intramural gastric tumors that had groups and cords of large round and oval cells with clear cytoplasm lacking myofibrils. Shortly afterward, Stout70 studied 69 tumors with these features and concluded that the terms leiomyoma and leiomyosarcoma were incorrect for them; in their stead, he offered leiomyoblastoma and malignant leiomyoblastoma.
In 1969, Enzinger et al27 introduced the descriptor “epithelioid” for the tumors in recognition of the round and oval shape of their cells. Later, Appleman and Helwig8 adopted this terminology in a major article entitled “Epithelioid leiomyoma and leiomyosarcoma (leiomyoblastoma).” This was the nomenclature in use when the triad was first reported in 1977.18
In 1983, Mazur and Clark48 examined the ultra-structure of 28 gastric tumors classified as leiomyomas or leiomyosarcomas and found myofilaments in only 2, leading them to conclude that the majority of the neoplasms had not arisen in smooth muscle. In 1987, Saul et al63 introduced the title GIST as an umbrella term for spindle and epithelioid cell tumors distributed throughout the entire gut.
The use of the term GIST for stromal lesions throughout the gut (and elsewhere) has resulted in the inevitable use of the tautologic terms gastric GIST and small intestinal GIST.42 Indeed, the title of this article refers to “gastric gastrointestinal stromal tumors.” GIST is a useful umbrella term for all the stromal tumors of the gut. However, it seems inappropriate to apply it to retroperitoneal and omental,51 and other extraintestinal stromal tumors. Furthermore, it is preferable to refer to gut stromal tumors by their anatomic location because the tumors have location-dependent characteristics, behavior, and associations.
As has already been indicated (Table 4), the features of the gastric tumors in the triad differed significantly from those of sporadic GISTs. Metastasis, a cardinal feature of malignancy, was found at primary surgery in a quarter of the triad patients. This behavior may have been at least partly attributable to tumor size (mean diameter, 6.3 cm). Surprisingly, the neoplasms were generally circumscribed and showed little local or lymphovascular invasion. This natural history, culminating in fatal metastasis for a substantial number of patients, indicated that the stromal tumors under study were malignant mesenchymal neoplasms. As the accepted general term for such tumors is sarcoma, we believe that the designation gastric stromal sarcoma is more accurate and the appropriate title for the gastric neoplasm in the triad.
The behavior of the metastases was unpredictable: some exhibited little or no progression (patients had hepatic and disseminated peritoneal metastases for up to 30 y without symptoms); others progressed rapidly and proved fatal. As an illustration of indolent behavior, a recent follow-up note on a patient (now aged 37 y) who had 6 abdominal operations for gastric tumor recurrence and liver and peritoneal metastases, starting at age 13, stated that “she looked and was feeling extremely well” and that “previously noted abnormal areas in her abdomen (by computed tomography examination) had not enlarged and cystic lesions near her liver had reduced in number,” and that “there was no evidence of tumor progression.” By comparison, the neoplasm had a progressive course in some patients, leading to the death of several in their early 30s. Presumably, some of the surviving patients with metastases will eventually die of the tumor.
Other differences between the triad and sporadic stromal gastric tumors deserve comment: the young age of the patients with the gastric stromal sarcoma (this term is used henceforth in this article to refer to the triad gastric tumors), tumor multifocality, lymph node meta-stasis, lack of response to imatinib therapy, the presence of mucosal lesions, and the molecular characteristics. The median age at detection of the gastric stromal sarcomas was 18 years; the youngest patient was aged 6 years. Two patients presented with bilateral leg lymphedema, one as a “child” and the other at age 12, suggesting that the gastric sarcomas had already spread at an early age. The sarcoma probably arose in many instances in the first decade of life and might even have been congenital; a lung lesion (likely a chondroma) was detected in 1 patient at 18 months of age.
In connection with multifocality, Miettinen et al54 made no mention of multiple and serial GIST development in a study of 1765 gastric GISTs. Rubin61 indicated that sporadic gastric GIST usually occurred as a single neoplasm. However, sporadic multifocal gastric GISTs have been reported in a small number of patients. Agaimy and Wünch2 described 43 minute sclerosing stromal tumors in 35 patients; thus, 1 or more of the group had more than 1 tumor; interestingly, all these neoplasms occurred in the proximal stomach, none in the antrum, as is usually the case in the triad. Haller et al32 studied 4 patients who each had a clinically apparent gastric GIST and 1 to 3 similar incidental tumors.
More recently, Agaimy et al4 reported 27 GISTs in 11 patients (mean age, 75 y). The tumors were located in the gastric body and fundus and generally small (mean diameter, 9 mm). Microscopically, they were uniformly spindle celled and the majority had KIT mutations. Thus, it seems that sporadic gastric GIST is rarely multifocal and in these circumstances spindle celled. Elderly patients with multiple tumors are unlikely to show other components of the triad or a GIST-related syndrome. GIST may rarely be multicentric with primary tumors in 2 of these locations: stomach, small intestine, omentum, and peritoneum.30,51
Mesenchymal neoplasms rarely metastasize to lymph nodes.29 In a study of a large number of patients with sarcoma, nodal metastasis was found in 46 of 1772 patients (2.6%), usually from angiosarcoma, embryonal rhabdomyosarcoma, or epithelioid sarcoma. We found lymph node metastasis in approximately one-third of patients with gastric stromal sarcoma. Rubin61 stated that gastric GIST metastasized to lymph nodes in <1% of cases. Miettinen and Lasota52 indicated that nodal dissection was not necessary for GISTs because the tumor did not metastasize to lymph nodes, and later a detailed review54 of 1765 gastric GISTs made no mention of lymph node metastasis. More recently, Miettinen et al53 studied 44 cases of gastric GISTs in children and young adults and did not encounter lymph node metastasis.
However, lymph node metastasis has been reported in a few children and young adults with multifocal gastric tumors3,60,64 and in 2 adults, 1 with multifocal tumor.72 The findings in these reports and in our study indicate that lesser curvature and other regional nodes should be examined in all patients with multiple gastric stromal tumors. Further clinical examination and investigation and follow-up of these patients may reveal other components of the triad; the mean interval between the detection of the first and second component of the syndrome was 8.4 years; the 3 longest intervals between development of the components were 21, 24, 25, and 26 years.15 Even if a second component tumor of the triad never develops in the patients with lymph node metastasis, it is still possible that they have the disorder incompletely expressed. Gastric stromal sarcoma lymph node involvement however is not limited to the triad. We found lymph node metastasis in 3 of 5 patients with the familial paraganglioma-gastric stromal sarcoma syndrome (Carney-Stratakis syndrome).16,49 Thus, gastric GIST lymph node metastasis may indicate of a syndrome-related neoplasm.
The agent imatinib mesylate (formerly known as STI571) (Gleevec in the United States, Novartis Corp; Glivec in Europe, Novartis International A6, Basel, Switzerland) has proved to have a dramatically positive effect on sporadic gastric GIST and its metastases.24,39 It was ineffective in 1 reported triad patient26 and ineffective or equivocally effective in 16 patients in our study suggesting that the 2 tumors develop through different mechanisms.
A series of gastric mucosal lesions—lymphoid aggregates, polyps, and adenocarcinomas—was an unexpected finding in a study of intramural gastric tumors. The frequent, isolated mucosal lymphoid aggregates, sometimes with germinal centers, are unexplained. These aggregates are very rarely seen in normal mucosa56; most authors state that they do not occur. The aggregates are a well-known clue to Helicobacter pylori infection, but Helicobacter organisms were not seen in our specimens. Nor were the findings those of lymphocytic gastritis.76 The aggregates are obviously unrelated to the prominent peripheral lymphoid cuff and intratumoral lymphocytes characteristic of gastric schwannoma.22
The presence of gastric mucosal thickenings without further amplification was mentioned in the description of the original group of Mayo Clinic patients with the triad.14 In this study, we found cystic fundic or hyperplastic polyps or both in one-fifth of the patients. One patient with the triad reportedly had juvenile gastric polyposis.34 Cystic fundic gland polyps have been described in several syndromes, including the Peutz-Jegher syndrome (familial adenomatous polyposis), Cowden disease, and Gardner syndrome.69 The triad should be added to this list.
The significance of the occurrence of 2 cases of gastric adenocarcinoma among the 64 patients who underwent subtotal gastrectomy is unknown. Schafer et al66 concluded that there was no increased risk of developing gastric cancer after subtotal gastrectomy. In contrast, Tersmette et al73 concluded that there was up to a four-fold to five-fold increase in risk after the operation.
Finally, molecular genetic studies of tumors from 37 patients included in this study20,26,71 did not show the coding sequence abnormalities of the KIT and PDGFRA genes in triad stromal sarcomas that occur in sporadic gastric GISTs. Additionally, there were no coding sequence abnormalities in the SDHB, SDHC, and SDHD genes that are involved in the pathogenesis of familial paragangliomas. Comparative genomic hybridization has shown frequent deletion of the 1 cen-q21 chromosomal region, a locus that involves the SDHC gene.46 Other chromosomal abnormalities found included loss of the 1p region.
In 1977, when first presented, the triad seemed a bizarre clinical and pathologic puzzle, destined to become a medical curiosity because of its rarity and failure to conform to the pattern of the known multicentric tumor syndromes. But the odd features of the syndrome bespoke a molecular explanation that is being assiduously sought today.
ACKNOWLEDGMENTS
The authors thank the many colleagues who provided material and follow-up information for the study. The authors also thank the patients and their families for generous cooperation.
REFERENCES
- 1.Ackerman LV. Surgical Pathology. Chapt 9, Gastrointestinal Tract. Mosby; St Louis: 1959. pp. 332–334. [Google Scholar]
- 2.Agaimy A, Wünch PH. Lymph node metastasis in gastrointestinal stromal tumours (GIST) occurs preferentially in young patients <40 years: an overview based on our case material and the literature. Langenbecks Arch Surg. 2009;394:375–381. doi: 10.1007/s00423-008-0449-5. [DOI] [PubMed] [Google Scholar]
- 3.Agaimy A, Wünsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113–120. doi: 10.1097/01.pas.0000213307.05811.f0. [DOI] [PubMed] [Google Scholar]
- 4.Agaimy A, Dirnhofer S, Wünsch PH, et al. Multiple sporadic gastrointestinal stromal tumors (GISTs) of the proximal stomach are caused by different somatic KIT mutations suggesting a field effect. Am J Surg Pathol. 2008;32:1553–1559. doi: 10.1097/PAS.0b013e31817587ea. [DOI] [PubMed] [Google Scholar]
- 5.Alberto VO, Kelleher D, Denholm RD, et al. A calcified lung tumor and microcytic anemia in a young woman: partial expression of the Carney triad. Surgeon. 2008;6:249–251. doi: 10.1016/s1479-666x(08)80036-4. [DOI] [PubMed] [Google Scholar]
- 6.Allievi A, Araya V, Calvar C, et al. Incomplete Carney's triad and arterial hypertension in a young woman. Medicina (B Aires) 2006;66:43–45. [PubMed] [Google Scholar]
- 7.Andersson J, Shito H, Meis-Kindblom JM, et al. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol. 2005;29:1170–1176. doi: 10.1097/01.pas.0000159775.77912.15. [DOI] [PubMed] [Google Scholar]
- 8.Appleman HD, Helwig EB. Gastric epithelioid leiomyoma and leiomyosarcoma (leiomyoblastoma). Cancer. 1976;38:708–728. doi: 10.1002/1097-0142(197608)38:2<708::aid-cncr2820380215>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Barajas-López C, Berezin I, Daniel EE, et al. Pacemaker activity recorded in interstitial cells of Cajal in the gastrointestinal tract. Am J Physiol. 1989;257:C830–C835. doi: 10.1152/ajpcell.1989.257.4.C830. [DOI] [PubMed] [Google Scholar]
- 10.Bladen JC, Moosajee MB, Duncan Bassett JH. A tense case—Carney's triad. J R Soc Med. 2004;97:540–541. doi: 10.1258/jrsm.97.11.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brassier D. Jeune femme de 27 ans admise pour masse abdominale. J Chir. 1998;135:133–136. [Google Scholar]
- 12.Byrne CM, Daneshjoo R, Ma-Wyatt J, et al. Gastrointestinal haemorrhage as a presentation of Carney's triad. ANZ J Surg. 2007;77:88–90. doi: 10.1111/j.1445-2197.2006.03892.x. [DOI] [PubMed] [Google Scholar]
- 13.Cameron S, Ramadori G, Füzesi L, et al. Successful liver transplantation in two cases of metastatic gastrointestinal stromal tumors. Transplantation. 2005;80:283–284. doi: 10.1097/01.tp.0000164141.34293.6b. [DOI] [PubMed] [Google Scholar]
- 14.Carney JA. The triad of gastric epithelioid leiomyosarcoma, functioning extra-adrenal paraganglioma, and pulmonary chondroma. Cancer. 1979;43:374–382. doi: 10.1002/1097-0142(197901)43:1<374::aid-cncr2820430152>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543–552. doi: 10.4065/74.6.543. [DOI] [PubMed] [Google Scholar]
- 16.Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132–139. doi: 10.1002/ajmg.10235. [DOI] [PubMed] [Google Scholar]
- 17.Carney JA, Stratakis CA. Stromal, fibrous and fatty gastrointestinal tumors in a patient with PDGFRA mutation. Am J Surg Pathol. 2008;32:1412–1420. doi: 10.1097/PAS.0b013e31816250ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carney JA, Sheps SG, Go VLW, et al. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296:1517–1518. doi: 10.1056/NEJM197706302962609. [DOI] [PubMed] [Google Scholar]
- 19.Chompret A, Kannengiesser C, Barrios M, et al. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology. 2004;126:318–321. doi: 10.1053/j.gastro.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 20.Colwell AS, D'Cunha J, Maddaus J. Carney's triad paragangliomas. Cardiovasc Surg. 2001;121:1011–1102. doi: 10.1067/mtc.2001.112820. [DOI] [PubMed] [Google Scholar]
- 21.Convey RP, Grainger AJ, Bhatnagar NK, et al. Lung abscess complicating chondromas in Carney's syndrome. Eur Respir J. 1998;11:1409–1411. doi: 10.1183/09031936.98.11061409. [DOI] [PubMed] [Google Scholar]
- 22.Daimaru Y, Kido H, Hashimoto H, et al. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunocytochemical study. Hum Pathol. 1998;19:257–264. doi: 10.1016/s0046-8177(88)80518-5. [DOI] [PubMed] [Google Scholar]
- 23.Delemarre L, Aronson D, van Rijn R, et al. Respiratory symptoms in a boy revealing Carney triad. Pediatr Blood Cancer. 2008;50:399–401. doi: 10.1002/pbc.21071. [DOI] [PubMed] [Google Scholar]
- 24.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 25.DeRaedt T, Cools J, Debiec-Rychter M, et al. Intestinal neurofibromatosis is a subtype of familial GISTs and results from a dominant activating mutation in PDGFRA. Gastroenterology. 2006;131:1907–1912. doi: 10.1053/j.gastro.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Diment J, Tamborini E, Casali P, et al. Carney triad; case report and molecular analysis of gastric tumor. Hum Pathol. 2005;36:112–116. doi: 10.1016/j.humpath.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Enzinger FM, Lattes R, Torloni H. Histological Typing of Soft Tissue Tumors. International Histological Classification of Tumours No 3. World Health Organization; Geneva: 1969. [Google Scholar]
- 28.Fletcher CDM, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 29.Fong Y, Coit DG, Woodruff JM, et al. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72–77. doi: 10.1097/00000658-199301000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gasparotto D, Rossi S, Bearzi I, et al. Multiple primary sporadic gastrointestinal stromal tumors in the adult: an underestimated entity. Clin Cancer Res. 2008;14:5715–5721. doi: 10.1158/1078-0432.CCR-08-0622. [DOI] [PubMed] [Google Scholar]
- 31.Gunawan B, von Heydebreck A, Sander B, et al. An oncogenic tree model in gastrointestinal stromal tumours (GISTs) identifies different pathways of cytogenetic evolution with prognostic implications. J Pathol. 2007;211:463–470. doi: 10.1002/path.2128. [DOI] [PubMed] [Google Scholar]
- 32.Haller F, Schulten H-J, Armbrust T, et al. Multicentric sporadic gastrointestinal stromal tumors (GISTs) of the stomach with distinct clonal origin: differential diagnosis to familial and syndromal GIST variants and peritoneal metastasis. Am J Surg Pathol. 2007;31:933–937. doi: 10.1097/01.pas.0000213440.78407.27. [DOI] [PubMed] [Google Scholar]
- 33.Handra-Luca A, Fléjou J-F, Molas G, et al. Familial multiple gastrointestinal stromal tumours with associated abnormalities of the myenteric plexus layer and skenoid fibers. Histopathology. 2001;39:359–363. doi: 10.1046/j.1365-2559.2001.01214.x. [DOI] [PubMed] [Google Scholar]
- 34.Hatara M, Aragon G, Borum M. Incomplete Carney triad, metastatic GIST and JPS in a young woman: a case report and review of the literature. Am J Gastroenterol. 2008;130:811, S319. [Google Scholar]
- 35.Heinrich MC, Corless ML, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 36.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 37.Horenstein MG, Hitchcock TA, Tucker JA. Dual CD117 expression in gastrointestinal stromal tumor (GIST) and paraganglioma of Carney triad: a case report. Int J Surg Pathol. 2005;13:87–92. doi: 10.1177/106689690501300113. [DOI] [PubMed] [Google Scholar]
- 38.Ishimoto M, Suzuki H, Yanagimoto M, et al. Carney's triad. J Clin Anesth (Jpn) 1998;22:1155–1156. [Google Scholar]
- 39.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 40.Kawamura M, Takahashi Y, Sakata Y. Carney's triad with gastric leiomyosarcoma and multiple pulmonary hamartomas; a case report. J Jpn Assoc Chest Surg (Jpn) 1994;8:190–195. [Google Scholar]
- 41.Kindblom L-G, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT). Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 42.Liegl B, Hornick JL, Lazar AJF. Contemporary pathology of gastrointestinal stromal tumors. Hematol Oncol Clin N Am. 2009;23:49–68. doi: 10.1016/j.hoc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Maeyama H, Hidaka E, Ota H, et al. Familial gastrointestinal stromal tumor with hyperpigmentation: association with a germline mutation of the c-kit gene. Gastroenterology. 2001;120:210–215. doi: 10.1053/gast.2001.20880. [DOI] [PubMed] [Google Scholar]
- 44.Marshall JB, Diaz-Arias AA, Bochna GS, et al. Achalasia due to diffuse leiomyomatosis and inherited as an autosomal dominant disorder. Report of a family study. Gastroenterology. 1990;98:1358–1365. doi: 10.1016/0016-5085(90)90357-7. [DOI] [PubMed] [Google Scholar]
- 45.Martin JF, Bazin P, Feroldi J, et al. Tumeurs myoides intra-murales de l'estomac. Ann d'Anat Pathol. 1960;5:484–497. [PubMed] [Google Scholar]
- 46.Matyakhina L, Bei TA, McWhinney SR, et al. Genetics of Carney triad: recurrent losses in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab. 2007;92:2938–2943. doi: 10.1210/jc.2007-0797. [DOI] [PubMed] [Google Scholar]
- 47.Mazas-Artasona L, Romeo M, Felices R, et al. Gastro-oesophageal leiomyoblastomas and multiple pulmonary chondromas: an incomplete variant of Carney's triad. Brit J Radiol. 1988;61:1181–1184. doi: 10.1259/0007-1285-61-732-1181. [DOI] [PubMed] [Google Scholar]
- 48.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 49.McWhinney SR, Passini B, Stratakis CA, International Carney triad and Carney-Stratakis syndrome consortium Familial gastrointestinal stromal tumors and germ-line mutations [Letter]. N Engl J Med. 2007;357:1054–1056. doi: 10.1056/NEJMc071191. [DOI] [PubMed] [Google Scholar]
- 50.Mendes W, Ayoub A, Chapchap P, et al. Association of gastrointestinal stromal tumor (leiomyosarcoma), pulmonary chondroma, and nonfunctional retroperitoneal paraganglioma. Med Pediatr Oncol. 1998;31:537–540. doi: 10.1002/(sici)1096-911x(199812)31:6<537::aid-mpo18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 51.Miettinen M, Monihan JM, Sarloma-Rikala M, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery. Clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Miettinen M, Lasota J. Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchow's Archiv. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 53.Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults. A clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005;29:1373–1381. doi: 10.1097/01.pas.0000172190.79552.8b. [DOI] [PubMed] [Google Scholar]
- 54.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach. A clinicopathologic, immunohistochemical and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 55.Miselli F, Millefanti C, Conca E, et al. PDGFRA immunostaining can help in the diagnosis of gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:738–743. doi: 10.1097/PAS.0b013e31815c47e8. [DOI] [PubMed] [Google Scholar]
- 56.Neutra MR, Padykula HA. The gastrointestinal tract. In: Weiss L, editor. Modern Concepts in Gastrointestinal Histology. Elsevier; New York, Amsterdam, Oxford: 1983. pp. 664–666. [Google Scholar]
- 57.Nishida T, Hirota S, Taniguchi M, et al. Familial gastrointestinal stromal tumors with germline mutation of the KIT gene. Nat Genet. 1998;19:323–324. doi: 10.1038/1209. [DOI] [PubMed] [Google Scholar]
- 58.Online Mendelian Inheritance in Man, OMIM (TM) Center for Medical Genetics, John Hopkins University; National Center for Biomedical Information, National Library of Medicine; Baltimore, MD: Bethesda, MD: 1999. Updated 11-8-99. [Google Scholar]
- 59.Perez-Atayde AR, Shamberger RC, Kozakewich HWP. Neuroectodermal differentiation of the gastrointestinal tumors in the Carney triad. An ultrastructural and immunohistochemical study. Am J Surg Pathol. 1993;17:706–714. doi: 10.1097/00000478-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Prakash S, Sarrna L, Socci N, et al. Gastrointestinal stromal tumors in children and young adults. A clinicopathologic, molecular, and genomic study of 15 cases and a review of the literature. J Pediatr Hematol Oncol. 2005;27:179–187. doi: 10.1097/01.mph.0000157790.81329.47. [DOI] [PubMed] [Google Scholar]
- 61.Rubin BP. Gastrointestinal stromal tumours: an update. Histopathology. 2006;48:83–96. doi: 10.1111/j.1365-2559.2005.02291.x. [DOI] [PubMed] [Google Scholar]
- 62.Sans N, Durand G, Giron J, et al. Triade de Carney. Mise au point: à propos d'un cas. J Radiol. 2000;81:39–42. [PubMed] [Google Scholar]
- 63.Saul SH, Rast ML, Brooks JJ. The immunohistochemistry of gastrointestinal stromal tumors. Evidence supporting origin from smooth muscle. Am J Surg Pathol. 1987;11:464–473. doi: 10.1097/00000478-198706000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Sauseng W, Benesch M, Lackner H, et al. Clinical, radiological, and pathological findings in children with gastrointestinal stromal tumors of the stomach. Pediatr Hematol Oncol. 2007;24:209–219. doi: 10.1080/08880010601104687. [DOI] [PubMed] [Google Scholar]
- 65.Sawhney SA, Chapman AD, Carney JA, et al. Incomplete Carney triad—a review of two cases. QJM. 2009;102:647–653. doi: 10.1093/qjmed/hcp078. [DOI] [PubMed] [Google Scholar]
- 66.Schafer LW, Larson DE, Melton LJ, et al. The risk of gastric carcinoma after surgical treatment for benign ulcer disease. A population-based study in Olmsted County, Minnesota. N Engl J Med. 1983;309:1210–1213. doi: 10.1056/NEJM198311173092003. [DOI] [PubMed] [Google Scholar]
- 67.Scopsi L, Collini P, Muscolino G. A new observation of the Carney triad with long follow-up period and additional tumors. Cancer Detect Prev. 1999;23:435–443. doi: 10.1046/j.1525-1500.1999.99047.x. [DOI] [PubMed] [Google Scholar]
- 68.Sigmund G, Buitrago-Telléz CH, Torhorst J, et al. Radiologie des gastrointestinalen Stromatumors (GIST). Gleichzeitig ein Beitrag zum Carney-syndrom. Fortschr Röntgenstr. 2000;172:287–294. doi: 10.1055/s-2000-121. [DOI] [PubMed] [Google Scholar]
- 69.Stolte M, Sticht T, Eidt S, et al. Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy. 1994;26:659–665. doi: 10.1055/s-2007-1009061. [DOI] [PubMed] [Google Scholar]
- 70.Stout AP. Bizarre smooth muscle tumors of the stomach. Cancer. 1962;15:400–409. doi: 10.1002/1097-0142(196203/04)15:2<400::aid-cncr2820150224>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 71.Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Int Med. 2009;266:43–52. doi: 10.1111/j.1365-2796.2009.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tashiro T, Hasegawa T, Omatsu M, et al. Gastrointestinal stromal tumour of the stomach showing lymph node metastases [Letter]. Histopathology. 2005;47:438–439. doi: 10.1111/j.1365-2559.2005.02133.x. [DOI] [PubMed] [Google Scholar]
- 73.Tersmette AC, Giardiello FM, Tygat GN, et al. Carcinogenesis after remote peptic ulcer surgery: the long-term prognosis after partial gastrectomy. Scand J Gastroenterol Suppl. 1995;212:96–99. doi: 10.3109/00365529509090306. [DOI] [PubMed] [Google Scholar]
- 74.Valverde K, Henderson M, Smith CR, et al. Typical and atypical Carney's triad presenting with malignant hypertension and papilledema. J Pediatr Hematol/Oncol. 2001;23:519–524. doi: 10.1097/00043426-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Wintermark P, Boubaker A, Gebhard S, et al. Adrenal mass in Carney triad. J Endo Genet. 2001;2:229–240. [Google Scholar]
- 76.Vio A, Sanna S, Morgagni P, et al. Considerazioni su una singolare associazione tumorale: la triade di Carney. Chir Oggi. 2001;18:25–33. [Google Scholar]
- 77.Wu T-T, Hamilton SR. Lymphocytic gastritis: association with etiology and topology. Am J Surg Pathol. 1999;23:153–158. doi: 10.1097/00000478-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Yanada M, Shimada J, Daishiro K, et al. A case of Carney's triad. J Jpn Assoc Chest Surg (Jpn) 2003;17:39–43. [Google Scholar]
- 79.Yutaka T, Sakurai S, Sakuma Y, et al. Gastrointestinal stromal tumors in neurofibromatosis type 1 (von Recklinghausen's disease). Am J Surg Pathol. 2005;29:755–763. doi: 10.1097/01.pas.0000163359.32734.f9. [DOI] [PubMed] [Google Scholar]