Abstract
Biogenic amines are not just ‘modulators’, they are often essential for the execution of behaviors. Here, we explored the role of biogenic amines acting on the pre-Bötzinger complex (pre-BötC), an area located in the ventrolateral medulla which is critical for the generation of different forms of breathing. Isolated in transverse slices from mice, this region continues to spontaneously generate rhythmic activities that resemble normal (eupneic) inspiratory activity in normoxia and gasping in hypoxia. We refer to these as ‘fictive eupneic’ and ‘fictive gasping’ activity. When exposed to hypoxia, the pre-BötC transitions from a network state relying on calcium-activated nonspecific cation currents (ICAN) and persistent sodium currents (INap) to one that primarily depends on the INap current. Here we show that in inspiratory neurons INap-dependent bursting, blocked by riluzole, but not ICAN-dependent bursting, required endogenously released norepinephrine acting on alpha2-noradrenergic receptors (α2-NR). At the network level, fictive eupneic activity persisted while fictive gasping ceased following the blockade of α2-NR. Blockade of α2-NR eliminated fictive gasping even in slice preparations as well as in inspiratory island preparations. Blockade of fictive gasping by α2-NR antagonists was prevented by activation of 5-hydroxytryptamine type 2A receptors (5-HT2A). Our data suggest that gasping depends on the converging aminergic activation of 5-HT2AR and α2-NR acting on riluzole-sensitive mechanisms that have been shown to be crucial for gasping.
Keywords: A1C1 neurons, gasping, norepinephrine, pacemaker, respiration
Introduction
Neuromodulators are critical for maintaining network activity and synchrony, and for determining the configuration of neuronal networks (Nadim et al., 2008; Doi & Ramirez, 2008; Grashow et al., 2009; Carrillo-Reid et al., 2009; Viemari & Tryba, 2009). The mammalian respiratory network located within the pre-Bötzinger complex (pre-BötC) is essential for breathing (Feldman & Del Negro, 2006; Tan et al., 2008), and it is ideal for studying the mechanisms underlying neuromodulation (Doi & Ramirez, 2008). When isolated in transverse slices or inspiratory island preparations, the pre-BötC continues to generate inspiratory rhythmic activity (Johnson et al., 2001; Smith et al., 1991).
Quantitative characterizations of the population activity generated in transverse slice preparations reveal that these activities resemble three distinct forms of inspiratory activities: fictive eupneic, fictive gasping and fictive sigh activity (Lieske et al., 2000; Ruangkittisakul et al., 2008). During the transition from normoxia into hypoxia, this portion of the respiratory network is reconfigured into a rhythm that has many of the characteristics of gasping (Lieske et al., 2000). During this reconfiguration, mechanisms of synaptic inhibition are significantly reduced and this is thought to contribute to (i) the loss of expiratory activity (Ramirez et al., 1997), (ii) the reconfiguration of post-inspiratory activity into inspiratory activity (Ramirez et al., 1997), (iii) the change in inspiratory activity from a bell-shaped to a decrementing activity pattern (Lieske et al., 2000) and (iv) the release of bursting properties in a subpopulation of pacemaker neurons (Tryba et al., 2003). The only pacemaker neurons that remain active throughout hypoxia are those in which bursting depends on the persistent sodium current (INap); these so-called cadmium-insensitive (CI) pacemaker neurons are also sensitive to riluzole (Peña et al., 2004). Interestingly, gasping, but not fictive eupnea is sensitive to riluzole, suggesting that these CI pacemaker neurons become the sole driver of gasping (Peña et al., 2004). Consistent with this hypothesis is the observation that gasping in the arterially-perfused brainstem and in vivo preparations is also abolished by riluzole (Paton et al., 2006; Peña & Aguileta, 2007; Peña, 2009). Based on these combined cellular and systems level findings we proposed that blockade of either the calcium-activated nonspecific cation current (ICAN) or INap alone is not sufficient to block inspiratory rhythm generation at the network level (Peña et al., 2004). Our discovery that bursting in CI pacemaker neurons depends on endogenous activation of 5-HT2AR, and that blockade of 5-HT2AR is sufficient to abolish fictive gasping, was consistent with this hypothesis (Peña et al., 2004; Tryba et al., 2006). However, our findings were not reproduced in the perfused model (Toppin et al., 2007). This discrepancy may be explained by the hypothesis that gasping depends on more than one neuromodulator and that blocking only one modulatory mechanism is not sufficient to eliminate this activity. Here, we demonstrate that bursting in CI pacemaker neurons and fictive gasping are also dependent on alpha2- noradrenergic receptor (α2-NR) activation. We propose that noradrenergic and serotonergic inputs converge on riluzole-sensitive mechanisms that are critical for the generation of gasping.
Materials and methods
Medullary transverse slice preparation and inspiratory island preparation
Brainstem transverse slice preparation from CD1 mice [home-bred; postnatal day (P)6–12] were prepared using a technique previously described in detail (Ramirez et al., 1996). The most important steps are summarized here. All surgical and experimental procedures conformed to guidelines from the French Ministry for Agriculture and Fisheries and the Institutional Animal Care and Use Committee (IACUC) at the Seattle Children’s Research Institute. The mice were quickly decapitated. The isolated brainstem was then placed in ice-cold artificial cerebrospinal fluid (aCSF) bubbled with carbogen (95% O2 and 5% CO2). The aCSF contained (in mM): NaCl, 128; KCl, 3; CaCl2, 1.5; MgCl2, 1; NaHCO3, 24; NaH2PO4, 0.5; and D-glucose, 30; pH 7.4. The brainstem, glued rostral end up onto an agar block, was mounted in a vibratome (Leica Microsystems, Waukegan, IL, USA) and serially sliced until the rostral boundary of the preBötC (Smith et al., 1991) was identified by anatomical landmarks such as disappearance of the facial nucleus and appearance of the inferior olive, the nucleus ambiguus and the hypoglossal nucleus. A single 650-μm-thick slice was then taken. Because the slices also contained regions caudal to the pre-BötC we refer to the area encompassed in the slice as the ventral respiratory group (VRG). Slices were transferred into a recording chamber, continuously superfused with oxygenated aCSF and maintained at a temperature of 30 ± 0.5 °C. To initiate and maintain fictive inspiratory rhythmic activity the potassium concentration of the perfusate was raised from 3 to 8 mM over 30 min. Only one slice was obtained per animal.
The VRG island preparation (Johnson et al., 2001) was made by taking a rhythmically active slice preparation (650 μm) and micro-dissecting with a sharp razor blade to give a wedge-shaped piece of tissue that contained the pre-BötC and the surrounding VRG.
Hypoxic conditions
Fictive gasping was induced by an hypoxic stimulus in the following manner: the incoming bath aCSF, which was saturated with carbogen during control conditions, was made hypoxic by saturating it with 95% N2 and 5% CO2 (Lieske et al., 2000; Tryba et al., 2006; Hill et al., 2011). In this study we refer to this stimulus as ‘application of hypoxic aCSF’.
After the slice was stabilized for 30 min under baseline conditions we exposed the slice to the hypoxic aCSF for 10 min. After 10 min of hypoxic aCSF, the inspiratory network activity was allowed to recover for 30 min to baseline conditions in aCSF equilibrated with 95% O2 and 5% CO2. This recovery period is based on a protocol used in previous studies (Blitz & Ramirez, 2002; Tryba et al., 2006). Using this protocol, fictive gasping activity generated in two consecutive 10-min applications of hypoxia were not significantly different when separated by a 30-min recovery in oxygenated aCSF (Tryba et al., 2006). In the present study, we performed the following protocol: after recovery from the first hypoxic exposure we applied yohimbine for 10 min and then exposed the slice to the second hypoxic exposure in the continued presence of yohimbine.
Fictive gasping has been characterized by a short rise time and short burst duration compared to fictive eupnea (Lieske et al., 2000). The transition from eupnea into gasping can be gradual, both in vitro (Lieske et al., 2000) and in vivo (Wang et al., 1993). During this transition, bursts with varying rise time and burst duration are typical, a characteristic which is referred to as ‘pre-gasping’ in vivo (Wang et al., 1993). In the present study we evaluated the effect of yohimbine on gasping and not on the transitory phase that characterizes pre-gasping. For this reason we evaluated the effect of yohimbine on the gasping activity measured during the last 2 min of hypoxic aCSF exposure at a time when the rise-time as well as burst duration were significantly different from fictive eupnea.
Drugs and solutions
A cocktail of antagonists for NMDA receptors [CPP-(RS), 10 μM; Tocris Cookson, Ellisville, MO, USA], non-NMDA receptors (CNQX, 20 μM; Tocris Cookson), glycine receptors (strychnine, 1 μM; SIGMA-RBI, St Louis, MO, USA) and GABAA receptors (bicuculline free base, 20 μM; SIGMA-RBI) was used to block fast synaptic transmission (Peña et al., 2004). Note that the bicuculline free base used in the present study has a very different pharmacology than the commonly used bicuculline salts (e.g. bicuculline methiodide), and the free base does not block apamin-sensitive calcium-activated potassium currents (Debarbieux et al., 1998; Seutin & Johnson, 1999). This cocktail does not block neuromodulator-evoked influences on bursting of the neurons. All drugs were initially solubilized in dimethylsulfoxide (DMSO; SIGMA-RBI). In some experiments yohimbine hydrochloride (SIGMA-RBI) rauwolscine, RX 821002 and / or DOI [(±)-2,5-dimethoxy-4-iodophenyl-2-aminopropane hydrochloride] was added to aCSF. Each drug was applied only once in a given experiment.
Electrophysiology
Extracellular recordings
In the transverse slice preparation, population activity recordings were obtained with suction electrodes positioned on the surface of the slice in the area of the nucleus ambiguous, i.e. dorsal to the pre-BötC. The signals were amplified 2000 times, filtered (low-pass 1.5 KHz, high-pass 250 Hz), rectified and integrated using an electronic filter (time constant of 30–50 ms). Integrated population activity from the VRG was always in phase with integrated inspiratory activity of the hypoglossal motor nucleus (Telgkamp & Ramirez, 1999). Therefore, it was used as a marker for inspiratory population activity (Fig. 1A). In the present study we refer to the activity generated within the transverse slice preparation and the island preparation as ‘inspiratory’ to reflect the fact that the population activity generated within the pre-BötC in slices is in phase with inspiratory activity. However, calling the activity ‘inspiratory’ does not mean that all neurons within the pre-BötC are inspiratory. Both in vivo and in vitro studies have demonstrated that the pre-BötC also contains other types of respiratory neurons including neurons rhythmically active in phase with expiration and post-inspiration (Ramirez & Lieske, 2003; Ramirez et al., 1997; Schwarzacher et al., 1995).
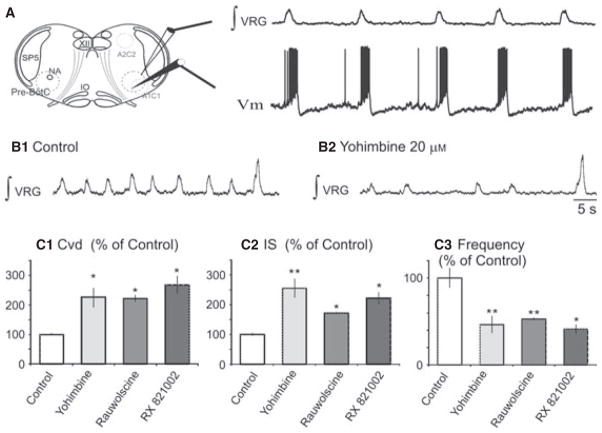
Fig. 1.
Blockade of α2NRs decreased the frequency of fictive eupneic activity and increased the irregularity. (A) Sites of integrated VRG (∫VRG) and whole-cell patch-clamp recordings (membrane potential; Vm) are illustrated in the schematic of the brainstem slice preparation including the catecholaminergic nuclei A1C1 (in the ventral part of medulla near the VRG) and A2C2 (in the dorsal medulla). (B) Fictive eupnea and fictive sigh inspiratory activities in (B1) control conditions and (B2) after application of yohimbine (20 μM). (C) Histograms summarize the differences between fictive eupneic activity in control and after application of yohimbine. We compared (C1) the Cvd and (C2) the IS to evaluate the irregularity, and (C3) the frequency; **P < 0.01, *P < 0.05. Results are expressed as mean ± SEM. VRG, ventrial respiratory group; NA, nucleus ambiguus; IO, inferior olive; Sp5, spinal trigeminal nucleus.
Intracellular recordings
We recorded inspiratory pre-BötC neurons (one neuron per slice) using the blind patch-clamp recording method. The slice preparation was placed rostral side up and recordings were made from neurons that were no deeper than 300 μm from the surface. Inspiratory neurons were first identified in the cell-attached mode, which revealed their discharge pattern in phase with population activity. Experiments were then performed in whole-cell configuration with the neuron recorded in current-clamp (Holding current, 0 nA). We have previously demonstrated that the whole-cell configuration does not alter the firing pattern of the recorded neuron (Peña et al., 2004). In the present study we characterized the effect of yohimbine on pacemaker neurons. As we published previously (e.g. Tryba et al., 2008, 2003; Thoby-Brisson & Ramirez, 2001), neurons are considered pacemakers if they continue to exhibit voltage-dependent intrinsic bursting properties following blockade of ionotropic glutamate, GABAA and glycinergic receptors. To be considered a pacemaker these neurons must meet several criteria. Although we have already provided these criteria in detail elsewhere (Tryba et al., 2008, 2003; Thoby-Brisson & Ramirez, 2001), we briefly describe the key attributes for identifying pacemakers. Pacemaker neurons often exhibit ectopic bursting when embedded in the inspiratory network, as exemplified in Ramirez et al. (2004). After isolating a neuron from chemical synaptic input by bath-applying CNQX, CPP, strychnine and bicuculline, a neuron is considered a potential pacemaker if it: (i) continues to burst in the absence of VRG population bursts; and (ii) exhibits voltage-dependent bursting properties. Specifically, a brief depolarizing current injection needs to evoke a burst or a hyperpolarizing current needs to terminate an ongoing burst. It is expected that either of these reset the ongoing pacemaker bursting rhythm. Depolarizing current injection increases, and injected hyperpolarizing current decreases, the bursting frequency. For examples of voltage dependency in inspiratory pacemaker neurons, the reader is referred to figures and experiments already published in Tryba et al. (2008, 2003, 2006); Viemari & Ramirez (2006); Thoby-Brisson & Ramirez (2001). After synaptic isolation with CNQX, CPP, bicuculline and strychnine, we routinely use cadmium (Cd2+), a broad-spectrum calcium channel blocker, to discriminate between different types of pacemaker neurons. Two basic types of inspiratory bursting pacemaker neurons can be discriminated based on their sensitivity to cadmium (Thoby-Brisson & Ramirez, 2001; Peña et al., 2004): Neurons in which bursting is abolished by 200 μM Cd2+ are referred to as cadmium-sensitive (CS) pacemaker neurons (Fig. 2); these neurons have been shown to be typically flufenamic acid (FFA)-sensitive (Peña et al., 2004). Neurons that continue to burst in 200 μM Cd2+ are referred to as CI pacemaker neurons (Fig. 3; also Peña et al., 2004). These pacemaker neurons are riluzole-sensitive (Peña et al., 2004). We use 200 μM Cd2+ because this concentration ensures the blockade of all voltage-activated calcium currents in VRG neurons, as previously demonstrated (Elsen & Ramirez, 1998). We also assume that following bath application of 200 μM Cd2+ all calcium-dependent chemical synaptic transmission should be fully blocked. Although it would be an interesting issue to investigate whether CI and CS pacemaker neurons are present in different rostrocaudal locations, this very specific question is beyond the scope of the present study. Here and in all our prior studies, we aimed at producing slices consistently within the same rostrocaudal plane. During the course of this and all of our prior studies over the past decade, we consistently recorded CI and CS pacemakers. However, the exact rostrocaudal boundaries of the pre-BötC have not been determined in our study for mice. However, despite this uncertainty, it seems plausible to assume that at least some of the recorded pacemaker neurons were located within the preBötC.
Fig. 2.
Yohimbine did not affect the bursting properties of CS inspiratory pacemaker neurons. (A) Recording of an inspiratory pacemaker neuron that produced bursts when embedded in the network. (B) This neuron continued to burst in the presence of the cocktail. (C) Application of yohimbine (20 μM) did not change the bursting properties of the CS pacemaker neuron. (D) Application of 200 μM cadmium abolished burst properties of this particular type of pacemaker neuron. Loss of bursting properties was confirmed by current injection. (E) The histogram represents the time course of the burst frequency of synaptically isolated CS pacemaker neurons during a 10-min bath application of yohimbine. Each bin represents the burst frequency for a time window of 1 min. The percentage of control frequency was calculated based on the mean frequency 1 min before bath application of yohimbine (grey bin). Results are expressed as mean ± SEM.
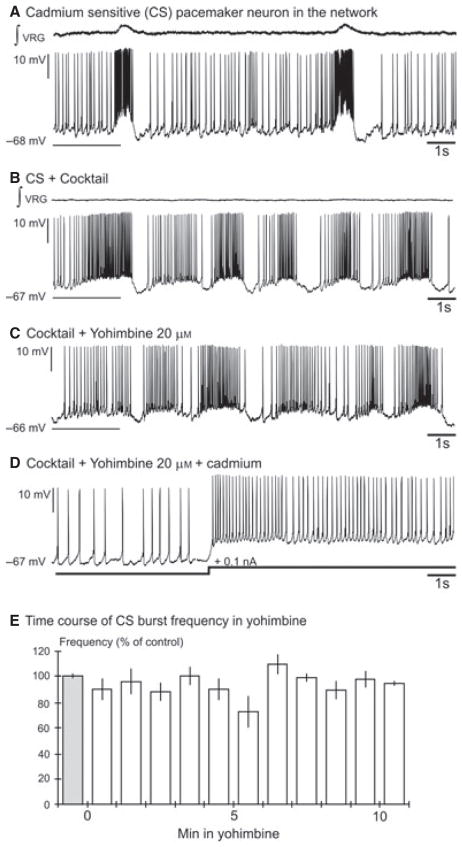
Fig. 3.
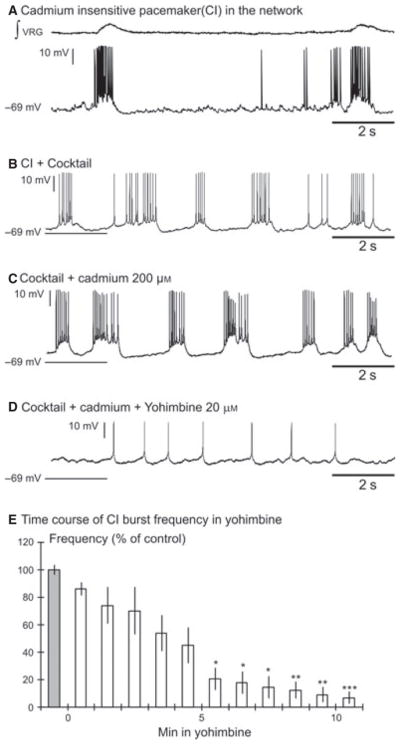
Yohimbine abolished the burst properties of CI inspiratory pacemaker neurons. (A) Recording of an inspiratory pacemaker neuron in the network. (B) This neuron continued to burst after blockade of the fast synaptic transmission by the cocktail and (C) after application of cadmium. (D) Yohimbine (20 μM) abolished the burst properties of the CI pacemaker neuron. (E) The histogram represents the time course of the burst frequency of synaptically isolated CI pacemaker neurons (n = 8) during a 10-min bath application of yohimbine. Each bin represents the burst frequency for a time window of 1 min. The percentage of control frequency was calculated based on the mean frequency 1 min before bath application of yohimbine (grey bin); ***P < 0.001, **P < 0.01, *P < 0.05, Friedman test. Results are expressed as mean ± SEM.
The patch electrodes were pulled from filamented borosilicate glass tubes (G150F-4; Warner Instruments, Hamden, CT, USA) and filled with a solution containing (in mM) K-gluconic acid, 140; CaCl2·6H2O, 1; EGTA, 10; MgCl2·6H2O, 2; Na2ATP, 4; and HEPES, 10. The composition of this intracellular solution and the lack of adverse effects on pacemaker activity have already been addressed (Peña et al., 2004; Del Negro et al., 2005).
Data analysis
All recordings were stored on a personal computer using AxoTape (Version 2.0; Axon Instruments, Union City, CA, USA) and analyzed offline using customized analysis software written with IGOR Pro (Wavemetrics, Lake Oswego, OR, USA). Bursts were automatically detected by the IGOR program as described in detail in our previous study (Viemari & Ramirez, 2006). This computerized method was only reliable when used in conjunction with qualitatively good recordings that were characterized by a good signal-to-noise ratio. In the case of intracellular recordings this automated program could detect bursts, but the amplitude and burst duration values were not useful because of the presence of action potentials. For this reason we used cursor values to assess changes in the membrane potential (Vm) of automatically detected, intracellularly recorded bursts. Unless noted otherwise, measured parameter values are reported in the text as mean ± SEM. Statistical significance is considered at the 95% confidence level, and Friedman, Kruskal–Wallis, Mann–Whitney rank-sum and Wilcoxon tests were used for analysis as appropriate.
The mean rise time and duration of the VRG population bursts obtained in each preparation were assessed for the two last minutes of a 10-min hypoxic exposure. The VRG population burst rise time was measured between 20 and 80% of the burst onset, as described in more detail in Lieske et al. (2000). Burst duration was measured as the width at half-maximum amplitude. To analyze the irregularity of the VRG population, we calculated an irregularity score (IS) by applying a formula for consecutive cycle length values: Sn = 100 × ABS − (Pn − Pn−1) / Pn−1, where Sn is the score of nth cycle, Pn is its period, Pn−1 is the period of preceding burst and ABS is the absolute value. To estimate the cycle duration variability we also calculated the coefficient of variability of cycle duration, defined as the ratio between the SD and the mean cycle duration as measured during 30–60 successive cycles (Viemari et al., 2004).
Results
Fictive eupneic activity required activation of α2-NR
The transverse medullary slice preparation contains medullary noradrenergic groups (Fig. 1A; also Zanella et al., 2006) which continue to exert an endogenous modulatory effect on the inspiratory rhythm. Medullary slice preparations also generate fictive sighs (Fig. 1B), but the effect of yohimbine on fictive sighs was not investigated in the present study. The α2-NR antagonist yohimbine (20 μM) blocked the endogenous activation of α2-NR and significantly depressed the mean inspiratory rhythm frequency, by 58 ± 6% (from 0.22 ± 0.03 to 0.1 ± 0.02 Hz; Mann–Whitney test, P = 0.008, n = 9; Fig. 1, B and C3). To assess the effects of this blockade on the regularity of the rhythm we calculated the coefficient of variability (mean / SD: Cvd; Viemari et al., 2004) and the IS (Telgkamp et al., 2002). Yohimbine (20 μM, Fig. 1, C1 and C2) increased the Cvd by 126 ± 32% and IS of the cycle period by 166 ± 32% (0.26 ± 0.04 vs. 0.58 ± 0.09 for Cvd, Mann–Whitney test, P = 0.017; and 28 ± 4 vs. 66 ± 7 for IS, Mann–Whitney test, P = 0.008, n = 9; Fig. 1, C1 and C2). To determine whether the action of yohimbine was on α2-NR, we used other α2-NR antagonists rauwolscine and RX 821002. The effects of yohimbine were mimicked by rauwolscine (20 μM) and RX 821002 (20 μM; Fig. 1C). Rauwolscine (n = 4) decreased significantly the rate of inspiratory rhythm, by 56 ± 3% (from 0.18 ± 0.01 to 0.08 ± 0.01 Hz, P = 0.010). The Cvd and IS of the cycle period increased respectively by 120 ± 11 and 75 ± 4% (0.25 ± 0.01 vs. 0.55 ± 0.02 for Cvd, Mann–Whitney test, P = 0.024; and 32 ± 1 vs. 56 ± 2 for IS, Mann–Whitney test, P = 0.029; Fig. 1C). Application of RX 821002 (Fig. 1C; n = 3) had the same effect than that caused by yohimbine and raulwolscine, i.e. it decreased the rate of inspiratory rhythm by 59 ± 5% (Mann–Whitney test, P = 0.024) and increased significantly the Cvd and IS, by 167 ± 30% (Mann–Whitney test, P = 0.029) and 122 ± 20% (Mann–Whitney test, P = 0.046), respectively.
Blockade of α2-NR affected CI but not CS bursting properties
Here we recorded from 14 pacemaker neurons that continued to generate bursting activity in a cocktail of antagonists for NMDA receptors, non-NMDA receptors, glycine receptors and GABAA receptors. This cocktail blocks synaptic transmission involving ionotropic glutamate receptors and receptor-coupled anion channels. Following application of the cocktail we bath-applied yohimbine (20 μM). In all six recorded CS pacemaker neurons yohimbine did not affect burst duration (1.12 ± 0.22 vs. 1.54 ± 0.37 s, Wilcoxon test, P = 0.42), burst amplitude (17.62 ± 6.10 vs. 20.00 ± 6.45 mV, Wilcoxon test, P = 0.42), burst area (9.15 ± 4.16 vs. 12.13 ± 7.04 a.u., Wilcoxon test, P = 0.84) or burst frequency (0.47 ± 0.07 vs. 0.45 ± 0.11 Hz, Wilcoxon test, P = 1). Yohimbine completely abolished burst generation in seven of eight CI pacemaker neurons neurons after 10 min but in one neuron yohimbine completely abolished burst generation after 15 min. (Fig. 3D and E; n = 8, Friedman test, Dunn’s post-test). Yohimbine did not affect the membrane potential of either CS (−64.2 ± 3.2 vs. −64.03 ± 3.6 mV) or CI pacemaker neurons (−58.8 ± 5.4 vs. −59.2 ± 4.7 mV). Our data indicate that bursting in CI pacemaker neurons depends on α2-NR activation (shown here) as well as 5-HT2AR activation as previously demonstrated by Tryba et al. (2006). We tested the hypothesis that gasping activity also depends on α2-NR activity.
Fictive gasping required activation of α2-NR
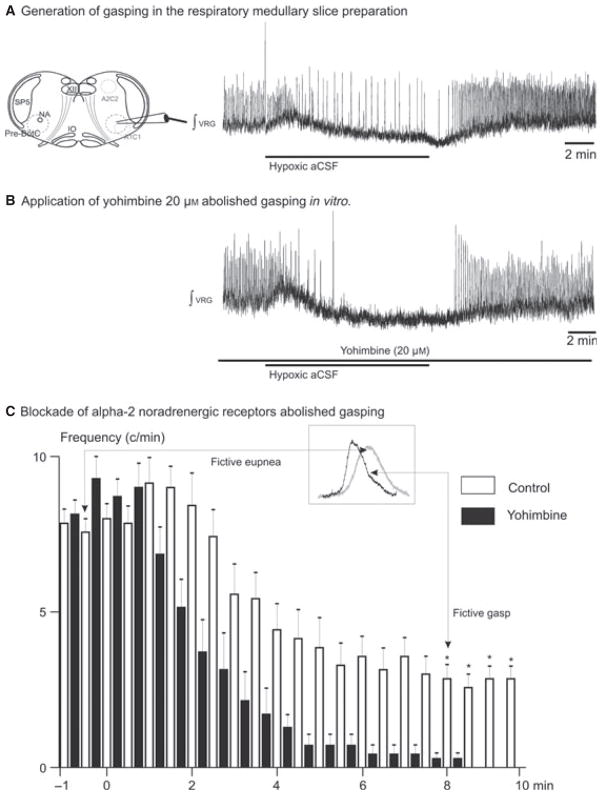
In hypoxia the inspiratory network reconfigures into a network state that we refer to as fictive gasping (Lieske et al., 2000). This pattern is characterized by a change in shape of the integrated VRG population burst. We calculated both the rise time and the VRG burst duration during the last 2 min of the 10-min hypoxic exposure. Compared to control, the rise time and the VRG burst duration were significantly shorter in hypoxic conditions (0.41 ± 0.02 vs. 0.31 ± 0.02 s, Mann–Whitney test, P = 0.029 for duration and 0.2 ± 0.02 vs. 0.12 ± 0.03 s, Mann–Whitney test, P = 0.029). Here, we evaluated the effects of blocking α2-NR on the VRG population in hypoxia. The inspiratory rhythm was recorded in seven slices during 10-min exposures to a hypoxic aCSF. All seven preparations exhibited fictive gasping under these conditions (Fig. 4A). After 10 min of hypoxia the inspiratory network activity was allowed to recover (30 min) to baseline conditions in aCSF equilibrated with carbogen. After allowing the inspiratory network to recover, we applied yohimbine (20 μM) and exposed the slice to a second application of the hypoxic aCSF. Following blockade of α2-NR the frequency of the VRG burst population decreased dramatically and ceased during the last 2 min of the second hypoxic exposure (Fig. 4C). Fictive gasping that we characterized during this time window was abolished (Fig 4B; Kruskal–Wallis test, Dunn’s post-test, P < 0.05). Despite the continuous application of yohimbine, inspiratory VRG burst population returned during the period of recovery in all preparations.
Fig. 4.
Blocking α2-NRs eliminated gasping in the transverse slice preparation in vitro. (A) Gasping was evoked after application of hypoxic aCSF. (B) The blockade of α2-NRs prevented generation of gasping in the transverse slice preparation. (C) Histogram showing fictive inspiratory activity during application of hypoxic aCSF (control, open bins). After application of yohimbine the VRG burst frequency decreased and ceased during the last 2 min of application of the hypoxic aCSF (filled bins, n = 6). The asterisks indicate significant differences between control burst frequency and following yohimbine application. The onset of the 10-min application of the hypoxic aCSF was at the origin. VRG, ventral respiratory group; NA, nucleus ambiguus; IO, inferior olive; Sp5, spinal trigeminal nucleus. *P < 0.05, Kruskal-Wallis test, Dunn’s post-test. Results are expressed as mean ± SEM.
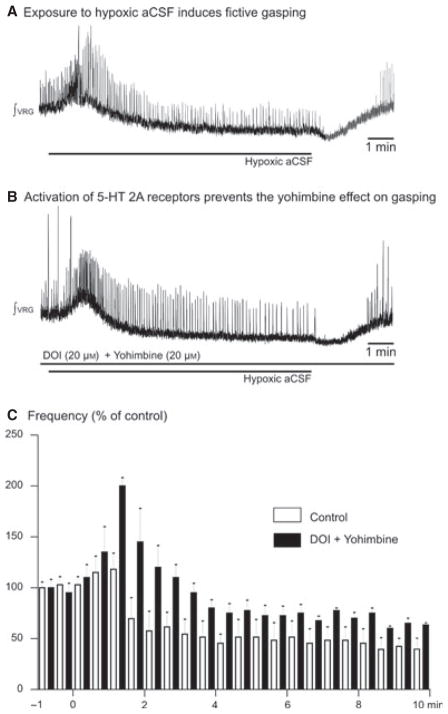
To further investigate the role of neuromodulators we repeated the same set of experiments after exposure to DOI 20 μM (a 5-HT2AR agonist; n = 5, Fig. 5A and B) before application of yohimbine. 5-HT2AR antagonists have also been shown to abolish gasping (Tryba et al., 2006). Application of DOI excited the inspiratory network (Peña & Ramirez, 2002). Subsequent application of yohimbine did not significantly depress the rate of the VRG burst population (Fig. 5C; Friedman test) and fictive gasping was not abolished during the last 2 min of hypoxic exposure, which differs from the situation in which we antagonized α2-NR alone.
Fig. 5.
Activation of 5-HT2A receptors prevented the abolition of gasping in vitro. (A) Gasping was evoked after application of hypoxic aCSF. (B) Application of DOI (20 μM) prevented the effect of blocking α2-NRs in the transverse slice preparation. (C) Histogram showing fictive inspiratory activity during application of hypoxic aCSF (control, open bins). After application of DOI and yohimbine the VRG burst frequency was maintained during the last minutes of application of hypoxic aCSF (filled bins, n = 5). The onset of the 10-min application of hypoxic aCSF was at the origin.
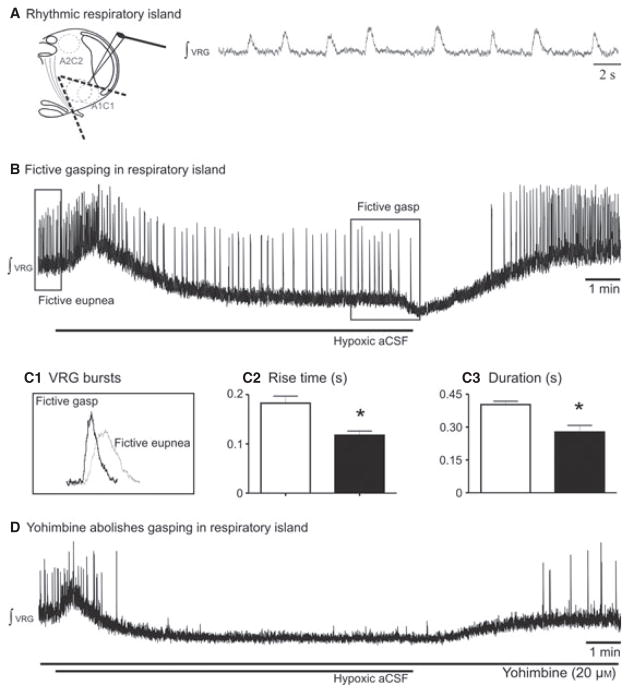
Fictive gasping was generated in inspiratory islands
It is already known that in the inspiratory island, a preparation that contains the pre-BötC (Fig. 6A; also Johnson et al., 2001), generates inspiratory activity in vitro, but whether this network was sufficient to elaborate gasping was not investigated. In a set of experiments we exposed inspiratory islands to hypoxia. In all experiments fictive gasping was induced (Fig. 6B, n = 5) during hypoxia. Fictive gasping generated in the inspiratory island has characteristics similar to those generated in slice preparations. Specifically, the rise time and the burst duration of fictive gasping were significantly shorter than those of fictive eupnea (0.40 ± 0.03 vs. 0.28 ± 0.06 s, Mann–Whitney test, P = 0.01 for duration; and 0.18 ± 0.03 vs. 0.12 ± 0.02 s, Mann– Whitney test, P = 0.024; Fig. 6C). However, the values of the rise time and the burst duration of fictive eupnea or fictive gasping in the inspiratory island were not significantly different from those obtained in slices.
Fig. 6.
Blocking α2-NRs eliminated gasping in the inspiratory island preparation in vitro. (A) Schematic of the inspiratory island that generated rhythmic inspiratory activity. (B) Gasping was evoked after application of hypoxic aCSF. (C1) Characteristics of gasping in the inspiratory island. VRG burst during application of hypoxic aCSF in black and in control in grey. (C2 and 3) Histograms show fictive eupnea (open bars) and fictive gasps (solid bars). C2 shows the shorter rise time of fictive gasps and (C3) the shorter burst duration. (D) Blockade of alpha-2 noradrenergic receptors prevents generation of gasping in the inspiratory island preparation. *P < 0.05, Mann–Whitney test. Results are expressed as mean ± SEM.
Yohimbine (20 μM) application abolished fictive gasping (Fig. 6D, n = 5) similar to that observed in the slice preparation. These experiments suggest that A1C1 neurons (catecholaminergic nuclei A1C1 in the ventral part of medulla near the VRG) preserved in the inspiratory islands are sufficient to sustain gasping in vitro.
Discussion
Activation of α2-NR sustained fictive gasping
Here we showed that a subpopulation of inspiratory pacemaker neurons, the CI pacemaker neurons, is endogenously activated by α2-NRs. This has important implications for the hypoxic response of the respiratory network. During hypoxia CI pacemaker neurons remain active while CS pacemaker neurons and many non-pacemakers are inhibited, rendering the remaining network sensitive to the blockade of CI pacemaker neurons (Peña et al., 2004). Consistent with the hypothesis that gasping is dependent on CI pacemaker activity, fictive gasping is also eliminated following blockade of endogenous 5-HT2AR activation (Tryba et al., 2006). However, a subsequent study suggested that blockade of 5-HT receptor activation alone produced no significant effect on gasping in the arterially-perfused brainstem preparation (Toppin et al., 2007; St-John & Leiter, 2008). In Pet-1 knockout mice, in which serotonin levels are reduced, gasping was also still generated (St-John et al., 2009). However, in this study it was not clear whether the respiratory network was fully reconfigured. The in vivo preparation was exposed to just 40 s of ischemia and, while the inspiratory burst had a shorter rise time, the duration of time of inspiration, one of the key criteria for gasping, was not different from eupnea (St-John et al., 2009). In another study, it was demonstrated in the Pet-1 knockout mouse that the onset of gasping and the restoration of eupnea are delayed once hypoxic apnea develops (Erickson & Sposato, 2009). Thus, while it is unclear to what extent gasping depends on serotonergic activation, none of these studies addressed whether gasping may also involve noradrenergic drive. Our findings indicate that noradrenaline is probably an additional neuromodulator important for gasping, as fictive gasping was eliminated following blockade of endogenous α2-NR activation. We demonstrated that α2-NR activation modulates riluzole-sensitive bursting mechanisms in a similar way to the activation of 5-HT2AR (Peña & Ramirez, 2002). We also demonstrated that α2-NR activation does not affect ICAN-dependent bursting in CS pacemaker neurons as has previously been shown for 5-HT2AR (Pena & Ramirez, 2002; Tryba et al., 2006). The noradrenergic modulatory effect on inspiratory network activity appears to be in close proximity to the pre-BötC as yohimbine also blocked gasping-like activity in inspiratory islands. However, even in this preparation we cannot exclude the possibility that presynaptic terminals from other brain regions were still intact and were releasing neuromodulators. However, what we can conclude is that this island preparation will probably include A1C1 catecholaminergic neurons (Zanella et al., 2006) because this region probably overlaps with the preBötC.
Activation of α2-NRs was required to generate regular fictive eupneic activity
Endogenous 5-HT2AR and α2-NR activation are also involved in the generation of normal inspiratory activity. Under baseline conditions, significant depression and increased irregularity of the inspiratory rhythm occurs following blockade of 5-HT2AR (Peña & Ramirez, 2002) and, as shown here, also following blockade of α2-NR. In both cases, the irregularity could potentially be attributed to the specific loss of riluzole-sensitive mechanisms under normoxic conditions (Peña & Ramirez, 2002). It is important to note that the blockade of the aminergic receptors abolished specifically bursting and not the generation of action potentials. Taken together our studies (Peña & Ramirez, 2002; Viemari & Ramirez, 2006) support the hypothesis that noradrenergic and serotonergic endogenous drive are necessary to sustain the riluzole-sensitive bursting mechanism which is important for the control of inspiratory frequency and regularity, and also for gasping. In control conditions, however, inspiratory rhythm generation did not entirely rely on this riluzole-sensitive mechanism. This is not surprising given that other rhythm-generating and modulatory mechanisms also contribute to inspiratory rhythm generation. These other mechanisms include ICAN (Peña et al., 2004), various excitatory synaptic mechanisms (Peña et al., 2004; Lieske & Ramirez, 2006a, b; Pace et al., 2007; Del Negro & Hayes, 2008; Rubin et al., 2009) and inhibitory mechanisms (Ramirez et al., 1997; Shao & Feldman, 1997; Busselberg et al., 2001) as well as various additional modulatory mechanisms (Doi & Ramirez, 2008, 2010). We did not investigate the intracellular mechanisms that underlie this modulatory effect. However, it is well established that α2-NRs produce an intracellular inhibition of adenylate cyclase via G proteinαi. α2-NR receptors can also be linked to increased phosphoinositide turnover via G proteinβγ stimulation of Phospholipase C and subsequent activation of protein kinase C (PKC) (Boehm et al., 1996; Gesek, 1996; Dorn et al., 1997; Talaia et al., 2006; Doi & Ramirez, 2008). In the inspiratory network, blockade of endogenous activation of 5-HT2AR reduced fast and persistent sodium current (Peña & Ramirez, 2002). Peña & Ramirez (2002) also showed that activation of PKC masked the effect of the 5-HT2AR blockade. Here, we demonstrated that activating 5-HT2AR with DOI, presumably acting via PKC, prevented the abolition of gasping in the presence of yohimbine.
Common pathways may mediate the effects of 5-HT2AR and α2-NR
Thus, our study suggests that 5-HT2AR and α2-NR activation may possibly share the same PKC pathway. The effects on riluzole-sensitive mechanisms, CI pacemaker neurons and the effects on gasping at the network level that depend on the same mechanisms also suggest that these two neuromodulators could potentially act on the persistent sodium current via the PKC pathway. However, the exact intracellular mechanisms that govern the observed convergence on the same type of bursting mechanism will require further investigation. At the systems level, the present study is consistent with a prior in vivo study in which we demonstrated that the effects on breathing caused by NE and 5-HT were not additive (Doi & Ramirez, 2010). Together, these findings support the perspective that these two neuromodulators act on common downstream targets.
Data presented here suggest that the noradrenergic release which is important for inspiratory rhythm generation and gasping arises from A1C1. This is interesting as neurons in this region are particularly active during hypoxia (Teppema et al., 1997). Although an important role for medullary noradrenergic neurons in cardiorespiratory regulation has been well established (Champagnat et al., 1979; Guyenet, 1991; Haxhiu et al., 2001), it is much less clear whether these neurons also play a crucial role in inspiratory rhythmogenesis. A large percentage of tyrosine hydroxylase (TH)-expressing neurons within the A1 noradrenergic group are activated during hypercapnia and hypoxia (Teppema et al., 1997) whereas the rostral end of C1 shows a reduced level of TH–Fos colocalisation. The A1 and A2 noradrenergic cell groups are also activated after paradoxical sleep (PS) or rapid eye movement (REM) sleep deprivation, indicating that those noradrenergic neurons might also contribute to PS inhibition (Leger et al., 2009). During REM sleep there is a significant decrease in both noradrenergic and serotonergic neuronal activity (Luppi et al., 2006), which could potentially increase the probability of a resuscitation failure. While these considerations cannot prove any link between aminergic disturbances and a failure to resuscitate, it is conceivable that an imbalance in these important modulators could have detrimental effects that are worsened in a state-dependent manner. Doi & Ramirez (2010) investigated state-dependent neuromodulation but state dependency is difficult to replicate in in vitro slice preparations and was therefore not investigated in the present study. Nevertheless, our data support an important role for medullary noradrenergic inputs that converge partly with serotonergic inputs. However, it must be emphasized that medullary noradrenergic input is not the only noradrenergic drive important for breathing, and neurons from the pontine noradrenergic nuclei are probably as important (Viemari, 2008; Doi & Ramirez, 2008, 2010).
Potential clinical implications
The finding that the inspiratory network depends critically on converging endogenous aminergic drive during hypoxia but not during normoxic conditions has potentially interesting clinical implications. Gasping is a vital mechanism for survival in response to several life-threatening conditions (Fewell, 2005; Thach, 2008), and gasping is a critical component in the arousal response that leads to spontaneous recovery from hypoxic apnea (Adolph, 1969; Jacobi & Thach, 1989). A disturbance in the generation of gasping and the arousal response has been implicated in Sudden Infant Death Syndrom (SIDS; Poets et al., 1999; Sridhar et al., 2003). Moreover, SIDS has also been associated with disturbances in serotonergic and noradrenergic systems (Obonai et al., 1998; Ozawa et al., 2003; Weese-Mayer et al., 2004; Viemari, 2008; Gaultier & Gallego, 2008; de Pontual et al., 2003; Kinney, 2005; Weese-Mayer et al., 2008; Duncan et al., 2010). Although the direct link between a disturbance in aminergic modulation and SIDS remains unclear the convergence of serotonin and norepinephrine onto the same neurons that are critical for gasping suggests that a disturbance in noradrenergic mechanisms could have equally detrimental consequences as a disturbance in serotonin. At the same time, this convergence may constitute an important safety mechanism protecting the network against the disturbance of a single modulator. The dependency on more than one factor is indeed one of the core hypotheses of the triple risk model according to Filiano & Kinney (1994). Thus, a disturbance in one neuromodulator may increase the risk for SIDS but other risk factors (e.g. Allen et al., 2011; Rubens et al., 2008) must be present to ultimately cause SIDS.
Acknowledgments
This study was supported by NIH grants (RO1 HL / NS60120 and PPG HL090554-01: project 3).
Abbreviations
- 5-HT2AR
5-hydroxytryptamine type 2A receptors
- A1C1
catecholaminergic nuclei A1C1
- aCSF
artificial cerebrospinal fluid
- CI
cadmium-insensitive
- CS
cadmium-sensitive
- Cvd
coefficient of variability (mean / SD)
- FFA
flufenamic acid
- ICAN
calcium-activated nonspecific cation current
- INap
persistent sodium current
- IS
irregularity score
- P
postnatal day
- PKC
protein kinase C
- pre- BötC
pre-Bötzinger complex
- VRG
ventral respiratory group
- α2-NR
alpha2-noradrenergic receptor
References
- Adolph EF. Regulations during survival without oxygen in infant mammals. Respir Physiol. 1969;7:356–368. doi: 10.1016/0034-5687(69)90019-x. [DOI] [PubMed] [Google Scholar]
- Allen T, Juric-Sekhar G, Campbell S, Mussar KE, Seidel K, Tan J, Zyphur M, Villagracia L, Stephanian D, Koch H, Ramirez JM, Rubens DD. Inner ear insult suppresses the respiratory response to carbon dioxide. Neuroscience. 2011;175:262–272. doi: 10.1016/j.neuroscience.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol. 2002;87:2964– 2971. doi: 10.1152/jn.2002.87.6.2964. [DOI] [PubMed] [Google Scholar]
- Boehm S, Huck S, Freissmuth M. Involvement of a phorbol ester-insensitive protein kinase C in the alpha2-adrenergic inhibition of voltage-gated calcium current in chick sympathetic neurons. J Neurosci. 1996;16:4596–4603. doi: 10.1523/JNEUROSCI.16-15-04596.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busselberg D, Bischoff AM, Paton JF, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflugers Arch. 2001;441:444–449. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- Carrillo-Reid L, Tecuapetla F, Ibanez-Sandoval O, Hernandez-Cruz A, Galarraga E, Bargas J. Activation of the cholinergic system endows compositional properties to striatal cell assemblies. J Neurophysiol. 2009;101:737–749. doi: 10.1152/jn.90975.2008. [DOI] [PubMed] [Google Scholar]
- Champagnat J, Denavit-Saubie M, Henry JL, Leviel V. Catecholaminergic depressant effects on bulbar respiratory mechanisms. Brain Res. 1979;160:57–68. doi: 10.1016/0006-8993(79)90600-0. [DOI] [PubMed] [Google Scholar]
- Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of I-AHP in reticularis neurons. J Neurophysiol. 1998;79:2911–2918. doi: 10.1152/jn.1998.79.6.2911. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Hayes JA. A ‘group pacemaker’ mechanism for respiratory rhythm generation. J Physiol (Lond) 2008;586:2245–2246. doi: 10.1113/jphysiol.2008.153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW, II, Oswald KJ, McCluskey TS, Kuhel DG, Liggett SB. Alpha 2A-adrenergic receptor stimulated calcium release is transduced by Gi-associated G(beta gamma)-mediated activation of phospholipase C. Biochemistry. 1997;36:6415–6423. doi: 10.1021/bi970080s. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsen FP, Ramirez JM. Calcium currents of rhythmic neurons recorded in the isolated respiratory network of neonatal mice. J Neurosci. 1998;18:10652–10662. doi: 10.1523/JNEUROSCI.18-24-10652.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Sposato BC. Autoresuscitation responses to hypoxia-induced apnea are delayed in newborn 5-HT-deficient Pet-1 homozygous mice. J Appl Physiol. 2009;106:1785–1792. doi: 10.1152/japplphysiol.90729.2008. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE. Protective responses of the newborn to hypoxia. Respir Physiol Neurobiol. 2005;149:243–255. doi: 10.1016/j.resp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- Gaultier C, Gallego J. Neural control of breathing: insights from genetic mouse models. J Appl Physiol. 2008;104:1522–1530. doi: 10.1152/japplphysiol.01266.2007. [DOI] [PubMed] [Google Scholar]
- Gesek FA. Alpha 2-adrenergic receptors activate phospholipase C in renal epithelial cells. Mol Pharmacol. 1996;50:407–414. [PubMed] [Google Scholar]
- Grashow R, Brookings T, Marder E. Reliable neuromodulation from circuits with variable underlying structure. Proc Natl Acad Sci USA. 2009;106:11742–11746. doi: 10.1073/pnas.0905614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. Central noradrenergic neurons: the autonomic connection. Prog Brain Res. 1991;88:365–380. doi: 10.1016/s0079-6123(08)63823-6. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Tolentino-Silva F, Pete G, Kc P, Mack SO. Monoaminergic neurons, chemosensation and arousal. Respir Physiol. 2001;129:191–209. doi: 10.1016/s0034-5687(01)00290-0. [DOI] [PubMed] [Google Scholar]
- Hill AA, Garcia AJ, III, Zanella S, Upadhyaya R, Ramirez JM. Graded reductions in oxygenation evoke graded reconfiguration of the isolated respiratory network. J Neurophysiol. 2011;105:625–639. doi: 10.1152/jn.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi MS, Thach BT. Effect of maturation on spontaneous recovery from hypoxic apnea by gasping. J Appl Physiol. 1989;66:2384–2390. doi: 10.1152/jappl.1989.66.5.2384. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Koshiya N, Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Botzinger complex “island”. J Neurophysiol. 2001;85:1772–1776. doi: 10.1152/jn.2001.85.4.1772. [DOI] [PubMed] [Google Scholar]
- Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol. 2005;8:507–524. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]
- Leger L, Goutagny R, Sapin E, Salvert D, Fort P, Luppi PH. Noradrenergic neurons expressing Fos during waking and paradoxical sleep deprivation in the rat. J Chem Neuroanat. 2009;37:149–157. doi: 10.1016/j.jchemneu.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. I Effects of alterations in synapse strength. J Neurophysiol. 2006a;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. II Intrinsic modulation by metabotropic glutamate receptors. J Neurophysiol. 2006b;95:1334–1344. doi: 10.1152/jn.00506.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100:271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Nadim F, Brezina V, Destexhe A, Linster C. State dependence of network output: modeling and experiments. J Neurosci. 2008;28:11806–11813. doi: 10.1523/JNEUROSCI.3796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonai T, Yasuhara M, Nakamura T, Takashima S. Catecholamine neurons alteration in the brainstem of sudden infant death syndrome victims. Pediatrics. 1998;101:285–288. doi: 10.1542/peds.101.2.285. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Takashima S, Tada H. Alpha2-adrenergic receptor subtype alterations in the brainstem in the sudden infant death syndrome. Early Hum Dev. 2003;75(Suppl):S129–S138. doi: 10.1016/j.earlhumdev.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBotzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Peña F. Neuronal network properties underlying the generation of gasping. Clin Exp Pharmacol Physiol. 2009;36:1218–1228. doi: 10.1111/j.1440-1681.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- Peña F, Aguileta MA. Effects of riluzole and flufenamic acid on eupnea and gasping of neonatal mice in vivo. Neurosci Lett. 2007;415:288–293. doi: 10.1016/j.neulet.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res. 1999;45:350–354. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- de Pontual L, Nepote V, ttie-Bitach T, Al HH, Trang H, Elghouzzi V, Levacher B, Benihoud K, Auge J, Faure C, Laudier B, Vekemans M, Munnich A, Perricaudet M, Guillemot F, Gaultier C, Lyonnet S, Simonneau M, Amiel J. Noradrenergic neuronal development is impaired by mutation of the proneural HASH-1 gene in congenital central hypoventilation syndrome (Ondine’s curse) Hum Mol Genet. 2003;12:3173–3180. doi: 10.1093/hmg/ddg339. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Lieske SP. Commentary on the definition of eupnea and gasping. Respir Physiol Neurobiol. 2003;139:113–119. doi: 10.1016/s1569-9048(03)00195-2. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJA, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol. 1996;491:799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ, Richter DW. Respiratory rhythm generation in mammals: synaptic and membrane properties. Respir Physiol. 1997;110:71–85. doi: 10.1016/s0034-5687(97)00074-1. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Peña F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, Poon BY, Funk GD, Ballanyi K. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci. 2008;28:2447–2458. doi: 10.1523/JNEUROSCI.1926-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens DD, Vohr BR, Tucker R, O’Neil CA, Chung W. Newborn oto-acoustic emission hearing screening tests: preliminary evidence for a marker of susceptibility to SIDS. Early Hum Dev. 2008;84:225–229. doi: 10.1016/j.earlhumdev.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci USA. 2009;106:2939–2944. doi: 10.1073/pnas.0808776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol. 1995;73:1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW. Recent advances in the pharmacology of quaternary salts of bicuculline. Trends Pharmacol Sci. 1999;20:268–270. doi: 10.1016/s0165-6147(99)01334-6. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex – a brain-stem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol. 2003;36:113–122. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of alpha1-adrenergic receptors and serotonin 5-HT2 receptors. J Appl Physiol. 2008;104:665–673. doi: 10.1152/japplphysiol.00599.2007. [DOI] [PubMed] [Google Scholar]
- St-John WM, Li A, Leiter JC. Genesis of gasping is independent of levels of serotonin in the Pet-1knockoutmouse. J Appl Physiol. 2009;107:679–685. doi: 10.1152/japplphysiol.91461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaia C, Queiroz G, Pinheiro H, Moura D, Gonçalves J. Involvement of G-protein betagamma subunits on the influence of inhibitory alpha2-autoreceptors on the angiotensin AT1-receptor modulation of noradrenaline release in the rat vas deferens. Neurochem Int. 2006;49:698–707. doi: 10.1016/j.neuint.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Tan WB, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgkamp P, Ramirez JM. Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. J Neurophysiol. 1999;82:2163–2170. doi: 10.1152/jn.1999.82.5.2163. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol. 2002;88:206–213. doi: 10.1152/jn.2002.88.1.206. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Thach BT. Some aspects of clinical relevance in the maturation of respiratory control in infants. J Appl Physiol. 2008;104:1828–1834. doi: 10.1152/japplphysiol.01288.2007. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol. 2001;86:104–112. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- Toppin VAL, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol. 2007;103:220–227. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Ramirez JM. Stabilization of bursting in respiratory pacemaker neurons. J Neurosci. 2003;23:3538–3546. doi: 10.1523/JNEUROSCI.23-08-03538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol. 2008;99:2114–2125. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC. Noradrenergic modulation of the respiratory neural network. Respir Physiol Neurobiol. 2008;164:123–130. doi: 10.1016/j.resp.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Tryba AK. Bioaminergic neuromodulation of respiratory rhythm in vitro. Respir Physiol Neurobiol. 2009;168:69–75. doi: 10.1016/j.resp.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Bevengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. J Neurosci. 2004;24:928–937. doi: 10.1523/JNEUROSCI.3065-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Fung ML, St-John WM. Pontile regulation of ventilatory activity in the adult rat. J Appl Physiol. 1993;74:2801–2811. doi: 10.1152/jappl.1993.74.6.2801. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Curran ME, Silvestri JM, Marazita ML. Sudden infant death syndrome: case–control frequency differences at genes pertinent to early autonomic nervous system embryologic development. Pediatr Res. 2004;56:391–395. doi: 10.1203/01.PDR.0000136285.91048.4A. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Ramirez JM. Autonomic dysregulation in young girls with Rett syndrome during nighttime in-home recordings. Pediatr Pulmonol. 2008;43:1045–1060. doi: 10.1002/ppul.20866. [DOI] [PubMed] [Google Scholar]
- Zanella S, Roux JC, Viemari JC, Hilaire G. Possible modulation of the mouse respiratory rhythm generator by A1 / C1 neurones. Respir Physiol Neurobiol. 2006;153:126–138. doi: 10.1016/j.resp.2005.09.009. [DOI] [PubMed] [Google Scholar]