Abstract
Rationale
Although reports of dextromethorphan (DXM) abuse have increased recently, few studies have examined the effects of high doses of DXM.
Objective
This study in humans evaluated the effects of supratherapeutic doses of DXM and triazolam.
Methods
Single, acute, oral doses of DXM (100, 200, 300, 400, 500, 600, 700, 800 mg/70 kg), triazolam (0.25, 0.5 mg/70kg), and placebo were administered to twelve healthy volunteers with histories of hallucinogen use, under double-blind conditions, using an ascending dose run-up design. Subjective, behavioral, and physiological effects were assessed repeatedly after drug administration for 6 hours.
Results
Triazolam produced dose-related increases in subject-rated sedation, observer-rated sedation, and behavioral impairment. DXM produced a profile of dose-related physiological and subjective effects differing from triazolam. DXM effects included increases in blood pressure, heart rate, and emesis, increases in observer-rated effects typical of classic hallucinogens (e.g. distance from reality, visual effects with eyes open and closed, joy, anxiety), and participant ratings of stimulation (e.g. jittery, nervous), somatic effects (e.g. tingling, headache), perceptual changes, end-of-session drug liking, and mystical-type experience. After 400 mg/70kg DXM, 11 of 12 participants indicated on a pharmacological class questionnaire that they thought they had received a classic hallucinogen (e.g. psilocybin). Drug effects resolved without significant adverse effects by the end of the session. In a 1-month follow up volunteers attributed increased spirituality and positive changes in attitudes, moods, and behavior to the session experiences.
Conclusions
High doses of DXM produced effects distinct from triazolam and had characteristics that were similar to the classic hallucinogen psilocybin.
Keywords: Dextromethorphan, Triazolam, Dose effects, Hallucinogen, Psychedelic, Entheogen, Drug abuse, Subjective effects, Mystical experience, Humans
INTRODUCTION
Dextromethorphan (DXM) is a widely available over-the-counter cough suppressant (e.g. Robitussin®, Coricidin®) that has been used for more than 40 years (Bem & Peck 1992). The first documented cases of abuse occurred in the mid 1960s, followed by sporadic reports of DXM abuse world-wide (Bem & Peck 1992). Over the last 10 years there has been a sharply accelerating number of reports of high dose DXM abuse confirmed by survey and epidemiological data both in the U.S. and internationally (Ziaee et al. 2005;Banken & Foster 2008;Romanelli & Smith 2009;Forrester 2011;Wilson et al. 2011). DXM abuse, sometimes referred to as “Dexing” or “Robotripping” appears to be most frequent in adolescents and young adults (Boyer 2004;Bobo et al. 2005;Falck et al. 2006;Bryner et al. 2006).
In vitro studies suggest that the primary mechanism of action of DXM is blockade of excitatory amino acid n-methyl-D-aspartate (NMDA) receptors (Church 1990;Church et al. 1994). This mechanism of action is similar to phencyclidine (PCP) (Morris et al. 2005;Newell et al. 2007) and ketamine (Sinner and Graf 2008), and is consistent with binding studies showing that DXM inhibits the binding of [3H] PCP to rat brain synaptic membranes (Murray and Leid 1984). Dextrorphan (DXO), one of the primary metabolites of DXM, also binds to the NMDA receptor, but with greater affinity than DXM (Franklin and Murray 1992;Parsons et al. 1995;Werling et al. 2007).
DXM has three major metabolites: DXO, 3-hydroxymorphinan, and 3-methoxymorphinan (Barnhart 1980). It is likely that both the parent drug DXM and its primary metabolite DXO are responsible for the drug’s psychoactive effects. However, the relative contribution(s) of each compound to DXM’s overall behavioral profile is not clear. Both DXM and DXO are self administered in PCP-trained monkeys, although DXM appears to be a less robust reinforcer than DXO (Nicholson et al. 1999;Young et al. 1981). Drug discrimination studies in rodents have shown that both DXM and DXO substitute for the stimulus effects of PCP (Nicholson et al. 1999) and that PCP substitutes for DXM in DXM-trained animals (Holtzman 1994). In monkeys, however, DXM does not always substitute for the PCP stimulus cue (Holtzman 1982;Nicholson et al. 1999). In addition, human studies suggest that the subjective effects of DXM and DXO differ, and individual differences in DXM metabolism may account for the wide variability in response to recreational doses of DXM (Zawertailo et al. 1998, 2010). Taken together, the human and non-human data suggest that both DXM and DXO are psychoactive, and that each might contribute to the behavioral effects experienced by recreational DXM users.
Several websites provide dosing information and specific instructions on the extraction of DXM from over-the-counter preparations (e.g. www.dextroverse.org) for psychoactive use. User submitted “trip reports” are also displayed on popular websites describing the effects of supratherapeutic doses of DXM. These reports often describe effects similar to classic hallucinogens (i.e. serotonergically mediated hallucinogens such as psilocybin, LSD, and mescaline) including euphoria, feelings of merging with inanimate objects, and “dream like” experiences (e.g. www.erowid.org). Although speculative, the apparently similar subjective effects produced by DXM and classic hallucinogens is consistent with the hypothesis that both serotonergic and glutamatergic neurotransmitter systems may be involved in the profile of perceptual, cognitive and mood-altering effects of a variety of hallucinogen-like compounds including dissociative anesthetics like ketamine and PCP and, more specifically, that serotonergically mediated hallucinogens may exert some of their effects via increasing cortical glutamatergic synaptic activity (Aghajanian and Marek 1999;Fantegrossi et al. 2008;Nichols 2004;Vollenweider and Kometer 2010;Fribourg et al. 2011).
Given the increase in high dose DXM abuse, it is notable that very few studies have evaluated the subjective and behavioral effects of high doses of DXM. Moreover, studies that have examined the effects of DXM in human participants have not investigated doses in the range that are frequently abused (e.g. 400–1000 mg/70 kg). Previous studies that examined lower doses of DXM (140 mg/70 kg) in alcoholics reported ethanol-like subjective effects and mild craving for alcohol (Schutz and Soyka 2000;Soyka et al. 2000). Larger doses of DXM (210–420 mg/70 kg) in recreational drug users increased ratings of liking and good effects, which are indicative of abuse liability (Zawertailo et al. 1998).
The aim of the present study was to examine the comparative clinical pharmacology and abuse liability of DXM and triazolam using a variety of physiological, subjective, and behavioral measures. Single, acute doses of DXM (100, 200, 300, 400, 500, 600, 700, 800 mg/70 kg), triazolam (0.25, 0.5 mg/70 kg), and placebo (lactose) were administered to 12 hallucinogen-experienced volunteers under double-blind conditions. The sedative-hypnotic triazolam was selected as an active comparator because of the aforementioned research showing sedative (i.e., alcohol-like) effects of lower DXM doses, and because these doses of triazolam have been well-characterized in previous abuse liability studies (e.g. Carter et al. 2006,2009;Griffiths and Johnson 2005;Mintzer and Griffiths 2005).
MATERIALS AND METHODS
Participants
This study was approved by the Institutional Review Board of Johns Hopkins University School of Medicine. Participants gave their written informed consent before beginning the study and were paid for their participation. The 12 participants (9 males) had a mean age of 27.5 years (range: 20–40 years). All were medically and psychologically healthy, and had a history of hallucinogen use. Ten participants were Caucasian (83%), one was African-American, and one was Asian-American. All volunteers reported past use of LSD (range 2–500 lifetime uses, mean: 58.2 uses, median: 10 uses) and psilocybin (range: 4–60 lifetime uses, mean: 21 uses). Seven of the twelve volunteers had used DXM previously for recreational purposes (range: 1–10 lifetime uses, mean: 4.8 uses), and three of the twelve had experience with either PCP or ketamine. Use of other hallucinogen-like drugs included Salvia divinorum (8 of 12 participants, range: 1–15 lifetime uses, mean: 4.3 uses) and MDMA (6 of 12 participants, range 1–25 lifetime uses, mean: 8.8 uses, median: 3 uses).
Individuals were excluded from participation if they had a history of substance dependence according to DSM-IV-TR criteria (excluding nicotine or caffeine), were pregnant or nursing, had a current significant medical condition or had a contraindication to receiving sedatives or anesthetics. A detailed psychiatric history was taken during the screening interview to exclude individuals with a personal or immediate family history of schizophrenia, bipolar affective disorder, delusional disorder, paranoid disorder, or schizoaffective disorder.
General Procedures
Throughout the study, general safety guidelines applicable to the study of high doses of classic hallucinogens were observed (Johnson et al. 2008). Sessions took place in an aesthetic living room-like environment. For most of the time during the sessions, participants were instructed to lie down on a couch while wearing an opaque eyeshade and headphones through which a preselected classical and world music program was played. Participants were encouraged to focus their attention on their inner experiences while not engaged in tasks.
After completing screening procedures, eligible individuals participated in a maximum of 11 sessions lasting 6 to 8 hours each, and a final follow-up session lasting 8 to 9 hours. Sessions were separated by at least 48 h. Participants were informed in the consent form that during the study they could receive placebo or doses of 17 psychoactive drugs from a variety of drug classes. Dextromethorphan and triazolam were among the drugs listed.
Prior to the first experimental session, the primary monitor and one assistant monitor met with each participant on two occasions before the first drug session (about 8 h total meeting time). The purpose of the meetings was to develop rapport and trust with participants and minimize the risk of adverse drug reactions (Johnson et al. 2008). During the meetings participants also practiced the experimental tasks. Participants were instructed that on experimental sessions mornings they should consume a low-fat breakfast and their usual amount of caffeine before arriving at the laboratory. They were told to refrain from using any drugs other than non-prescription pain relievers, tobacco, and caffeinated products while enrolled in the study. On each session before drug administration, participants’ urine was tested for recent use of cocaine, benzodiazepines, and opioids using an EMIT system (Syva Co., Palo Alto, CA., USA), and participants’ expired air was tested for alcohol using a breathalyzer test. Female participants were required to provide a negative result on a urine pregnancy test before the beginning of each session.
Various measures were assessed before capsule administration and repeatedly after administration, as described below. Other measures (e.g. Hallucinogen Rating Scale, States of Consciousness Questionnaire, and the Pharmacological Class Questionnaire) were completed approximately 7 hours after capsule administration when acute drug effects had resolved.
During the study, participants could receive a maximum of 11 different treatment conditions over 11 experimental sessions (placebo, 0.25 and 0.5 mg/70 kg triazolam, and 100, 200, 300, 400, 500, 600, 700 and 800 mg/70 kg DXM). The order of the three types of conditions (triazolam, DXM, and placebo) was counterbalanced across participants. Within each type of drug condition, the sequence of the doses was ascending, and dosing with triazolam or DXM was completed before proceeding to another type (e.g. both doses of triazolam were administered before proceeding to DXM dosing and vice versa). The ascending sequence was used to determine the maximum dose of DXM that could be safely tolerated by individual participants and to avoid adverse events. Higher doses were not given if the participant failed to complete both a psychomotor task (e.g., the Circular Lights task) and a computer task (e.g., the Subjective Effects Questionnaire) at any single time point during a session, or if either the participant or the investigator considered higher doses to be inadvisable.
Measures assessed repeatedly during the session
Blood pressure and heart rate
Blood pressure and heart rate were obtained before capsule administration and at 120, 240, and 360 min after administration. Blood pressure (systolic, diastolic, and mean arterial pressure using the oscillometric method with the blood pressure cuff placed on the arm) and heart rate were monitored using a Non-Invasive Patient Monitor Model 507E (Criticare Systems, Waukesha, WI, USA).
Monitor rating questionnaire
Before capsule administration and at 30, 60, 90, 120, 180, 240, 300, and 360 min after administration, a session monitor completed the Monitor Rating Questionnaire, rating 16 dimensions of the participant’s behavior or mood (dimensions are shown in Table 1). This questionnaire was similar to the monitor rating questionnaire used in previous studies of the hallucinogen psilocybin (Griffiths et al. 2006, 2011). The dimensions, expressed as peak scores in Table 1, were rated on a five-point scale from 0 to 4. The final version of this questionnaire was developed after initiation of the study and was used to evaluate the 10 participants enrolled thereafter.
Psychomotor performance measures: Balance and Circular Lights
These tasks were completed before capsule administration and at 120, 240, and 360 min after administration. The Balance task (Carter et al. 2006) involved balancing on one foot with eyes closed. The score was the number of seconds summed across both feet (60s total). The Circular Lights task is a hand eye coordination task (Mumford et al. 1995). The score was the number of correct presses (i.e., lights extinguished) in 60s.
Subjective Effects Questionnaire
This computer presented questionnaire was completed at baseline (i.e., time 0), and at 120, 240, and 360 min after capsule administration. It consisted of rating 36 items (e.g., “do you feel a drug effect”?, “Do you feel light-headed”?) on a visual analog scale (VAS). Participants were instructed to rate how they felt at the current time by using a computer mouse to click a location along each of 36, 100 mm lines anchored on opposite ends with the labels “no, not at all” and “yes, very much” respectively.
Cognitive performance measures
Various cognitive effects measures were also assessed throughout the study. These results will be reported in a separate publication.
Measures assessed at the end of the session, approximately 7 hours after capsule administration
Pharmacological Class Questionnaire
This questionnaire, modified from Rush et al. (1999), listed descriptive titles and examples of 18 classes of psychoactive drugs (see Table 3 for details). Participants were instructed to choose the drug class that most closely represented the drug effect that they experienced during the session. Then participants completed a series of visual analog scales rating how similar that day’s drug effect was to specific drugs from the previously identified drug classes. For example, participants were asked “How much did today’s drug effect feel like a CLASSIC HALLUCINOGEN (LSD, acid, psilocybin magic mushrooms, 2C-B, foxy-methoxy, DMT, ayahuasca, mescaline, peyote).” Participants were required to click a location along a 100 mm line anchored on opposite ends with the labels “no, not at all” and “yes, very much” respectively.
Mysticism scale
This 32-item paper questionnaire which was developed to assess naturally-occurring primary mystical experiences, has been extensively studied, shows cross-cultural generalizability (cf. Chapter on Mysticism in Hood et al. 2009), and has previously been shown sensitive to psilocybin (Griffiths et al. 2006, 2011; Johnson et al. 2011). A total score and three factors are measured: Interpretation, Introvertive Mysticism, and Extrovertive Mysticism. Items were rated on a 9-point scale (Griffiths et al. 2006). Participants were instructed to complete the questionnaire with reference to their experiences since they received the capsules that morning.
States of Consciousness Questionnaire
This 100-item paper questionnaire assessing possible hallucinogen experience content is rated on a six-point scale [0=none, not at all; 1=so slight, cannot decide; 2=slight; 3=moderate; 4=strong (equivalent in degree to any previous strong experience or expectation of this description); and 5=extreme (more than ever before in my life and stronger than 4)]. Forty-three items are Mystical Experience items (previously referred to as the Pahnke-Richards items), which were shown sensitive to several classic hallucinogens including psilocybin (Turek et al. 1974;Richards et al. 1977;Griffiths et al. 2006, 2011). These items assess seven domains of mystical experiences: internal unity, external unity, sense of sacredness, noetic quality, transcendence of time and space, deeply-felt positive mood, paradoxicality and ineffability. Data on each scale were expressed as a percentage of the maximum possible score. As in previous studies (Pahnke 1969;Griffiths et al. 2006, 2011), criteria for designating a volunteer as having had a “complete” mystical experience were that scores on each of the following scales had to be at least 60%: unity (either internal or external, whichever was greater), sense of sacredness, noetic quality, transcendence of time and space; positive mood; and ineffability. The remaining 57 items in the States of Consciousness Questionnaire served as distracter items.
Hallucinogen Rating Scale (HRS)
This 99-item questionnaire consists of six subscales assessing hallucinogen effects (intensity, somaesthesia, affect, perception, cognition, and volition). All six subscales of the HRS are increased by several different types of hallucinogens including intravenous N,N-dimethyltryptamine (Strassman et al. 1994), oral ayahuasca (Riba et al. 2001), oral psilocybin (Griffiths et al. 2006,2011), intramuscular ketamine (Loftwall et al. 2006),and inhaled Salvinorin A (Addy 2012). In contrast, various other drugs including d-methamphetamine (Gouzoulis-Mayfrank et al. 1999), d-amphetamine, MDMA, and mCPP (Johanson et al. 2006;Tancer and Johanson 2001, 2003) do not consistently increase all six subscales of the HRS.
End of day rating of liking the experience
One question in the HRS asked volunteers to rate “Like the experience” on a five point scale: 0=Not at all; 1=Slightly; 2= Moderately; 3=Very much; 4=Extremely. In addition to being scored as part of the HRS, this rating was also analyzed separately to provide an overall end of day assessment of liking.
Measures assessed 1 month after drug administration
Approximately one month after the final drug/placebo session, participants returned to the research facility for an 8-hour session to permit phenotyping of CYP2D6 metabolizer status and to complete the Persisting Effects Questionnaire.
Persisting Effects Questionnaire
This 143-item paper questionnaire (Griffiths et al. 2011) assessed persisting changes in attitudes, moods, behavior, and spiritual experience that volunteers attributed to their experiences during the sessions. Most items were rated on a six-point scale (0=none, not at all; 4=strong; and 5=extreme, more than ever before in your life and stronger than 4) and they addressed six categories: Attitudes about life; Attitudes about self; Mood changes; Relationships; Behavioral changes; Spirituality. Scores in each category were expressed as the percentage of the maximum possible score. Three additional questions (Griffiths et al. 2006) were: 1). How personally meaningful was the experience? (rated from 1 to 8, with 1=no more than routine, everyday experiences; 7=among the 5 most meaningful experiences of my life; and 8=the single most meaningful experience of my life). 2). How spiritually significant was the experience? (rated from 1 to 6, with 1=not at all; 5=among the 5 most spiritually significant experiences of my life; 6=the single most spiritually significant experience of my life). 3). Did the experience change your current sense of personal well-being or life satisfaction? (rated from −3 to +3, with −3=Decreased very much; 0=No change; +3=Increased very much).
Phenotyping of DXM metabolism
During the 8-hour follow-up session, a very low, oral dose of DXM was administered (25 mg), and an 8-hour total urine collection was performed according to previously described procedures (Schmid et al. 1985) to identify poor or fast metabolizers of DXM (Vengurlekar et al. 2002).
Drugs
Drugs and placebo were orally administered in size 0 aqua colored opaque capsules with approximately 200 ml of water. Four identical capsules were administered in each drug session containing lactose monohydrate (Ruger Chemical Company, Linden NJ, USA) and appropriate quantities of powdered dextromethorphan (Spectrum Chemical, Gardena CA, USA) or triazolam tablets (Halcion; The Upjohn Company, Kalamazoo, MI, USA). Doses of dextromethorphan are expressed as the free base.
Data Analysis
Dextromethorphan data presentation
The maximum tolerable dose of DXM varied across the 12 volunteers; stop point doses ranged from 400–800 mg/70kg. For these stop point sessions, the missing data on the stop point sessions precluded calculating maximum or “peak” scores for subjective effects assessments. Circular lights and balance tasks were scored as “0” if a volunteer was too impaired to complete the task. “End of session” assessments were obtained for all sessions completed. The maximum dose of DXM was defined as the highest dose of DXM administered to that volunteer (i.e. the stop point dose) and the penultimate dose was the one preceding (i.e., 300–700 mg/kg).
Data were analyzed using repeated measures regression models in SAS Proc Mixed (SAS Institute Inc., Cary, NC, USA) which take into account the covariance structure of the repeated measures and handle missing data better than traditional ANOVA models (Wolfinger and Chang 1995). The implementation in PROC MIXED is preferable to GEE models for datasets with small to moderate numbers of participants. We report Type III tests of fixed effects.
For the time-course data, planned comparison t-tests were conducted between placebo and active doses at each timepoint. Analyses of peak effects and end of day measures used an exchangeable covariance structure; analyses of time-course data used an AR(1) covariance structure. For peak effects on Subjective Effect Questionnaire items and monitor ratings, the maximum value observed after drug administration for each participant was used. For the Balance and Circular Lights measures, peak effect for each participant was defined as the minimum value observed after drug administration. Because there was no predicted direction of the effects on cardiovascular measures, peak effects were examined for both maximum and minimum values observed after drug administration. Peak effect and end of session measures were analyzed first with placebo, 0.25 and 0.5 mg/70kg triazolam, and 100–300 mg/70kg DXM doses. Higher doses were not included in this analysis because some participants did not complete all the assessments at higher doses or did not receive some of the higher doses. For peak effects on Subjective Effect Questionnaire items, a second analysis used placebo, 0.25 and 0.5 mg/70kg triazolam, and the penultimate DXM dose; for all other measures, a second analysis used placebo, 0.25 and 0.5 mg/70kg triazolam, and penultimate and maximum DXM doses. For all the peak effect and end of session measures, Fisher’s LSD post hoc tests were used to compare placebo with the active doses. The mean ± standard error of the mean (SEM) is presented throughout. Statistical tests were considered significant at p≤0.05.
RESULTS
In this ascending dose-sequence design, all participants received placebo, two doses of triazolam (0.25 and 0.5 mg/70 kg) and at least four doses of DXM (100, 200, 300, and 400 mg/70 kg). The highest dose of DXM administered varied across participants (400 mg/70 kg, n=2; 500 mg/70 kg n=2; 600 mg/70 kg n=4, 700 mg/70 kg, n = 2; 800 mg/70 kg, n=2). Two of the twelve volunteers received all doses of DXM. Seven volunteers reached their stop point because of significant behavioral impairment. Two volunteers reached their stop point because the investigator judged administration of higher doses to be inadvisable. One volunteer reached a stop point because the participant would not want to receive that drug dose again.
Time-course of drug effects
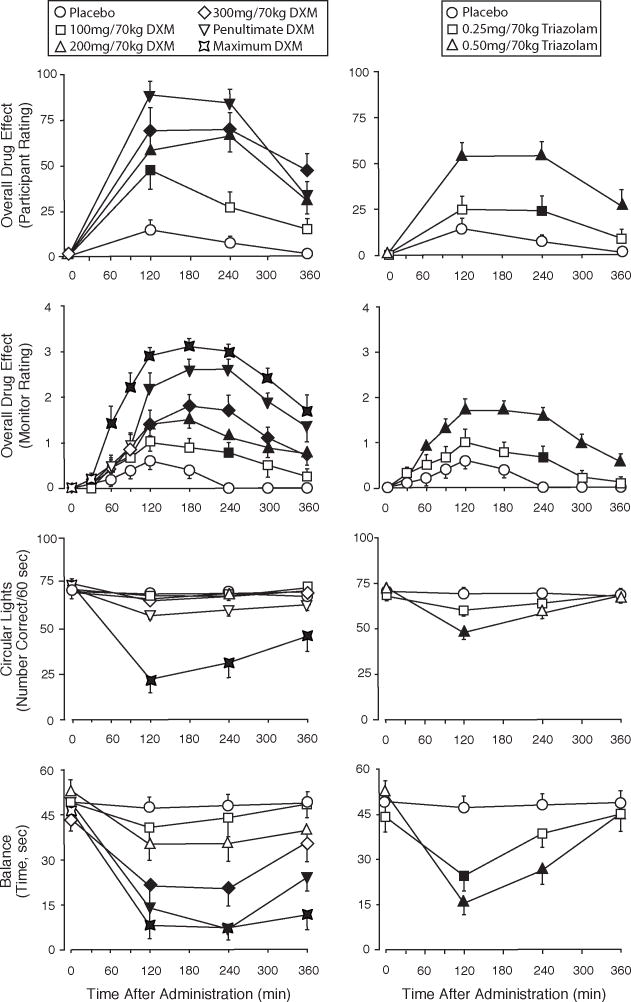
DXM and triazolam produced orderly dose- and time-related effects. Figure 1 shows illustrative time-course data for participant and monitor ratings of the magnitude of drug effect and psychomotor effects measures (Circular Lights and Balance). The time-course on cardiovascular measures (data not shown) was similar. At doses that produced significant effects, the effects were generally significant by the 2 h time-point, with maximal effects occurring at 2 to 4 h, and effects decreasing at the 6 h time-point. At the penultimate dose, DXM produced numerically higher participant ratings of drug effects than the highest dose of triazolam. Although the time to peak psychomotor effects was similar, DXM produced longer lasting deficits on Balance and Circular Lights than triazolam. The highest doses of DXM sometimes produced complete impairment of Circular Lights and Balance measures (i.e. the volunteer was unable to perform the task and thus scored a “0” for that assessment period). At the penultimate and maximum doses of DXM, deficits in Balance and Circular Lights performance were still apparent at the 6 h assessment.
Figure 1.
Time-course of effects of dextromethorphan (DXM) (left panels) and triazolam (right panels) on several measures assessed repeatedly across the session. Y-axes: participant-rated Drug Effect on a 100-point scale; monitor rating of Overall Drug Effect on a 4-point scale; Circular Lights, number correct; Balance, time in seconds. X-axes: time after drug administration in minutes. Data points show means (N=12 for all measures except for monitor ratings for which N=10), brackets show 1 SEM. Filled symbols indicate values that are significantly different from the corresponding placebo value at the same time point (p<0.05, planned comparisons). Penultimate and Maximum DXM refer to the penultimate and maximum doses of DXM administered. The maximum DXM dose is not presented for the participant rating because of missing data.
Peak effects on measures assessed repeatedly during the session
Blood pressure and heart rate
Peak maximum effects on cardiovascular measures are shown in Electronic supplementary Table 1, available online. DXM produced orderly, significant, and dose-related increases in systolic and diastolic blood pressure (20.8 mm Hg, 14.6 mm Hg, respectively at the maximum dose). In contrast, triazolam (0.25 and 0.5 mg/70 kg), showed small but significant decreases on systolic blood pressure and had no significant effects on diastolic blood pressure. Heart rate was significantly increased at the two highest DXM doses (26 beats/min at the maximum dose) and at the 0.5 mg/70 kg dose of triazolam (10.2 beats/min). Separate analyses of peak minimum cardiovascular effects generally showed smaller magnitude effects in the same directions for both drugs, with no significant effects of triazolam on systolic blood pressure.
Psychomotor performance
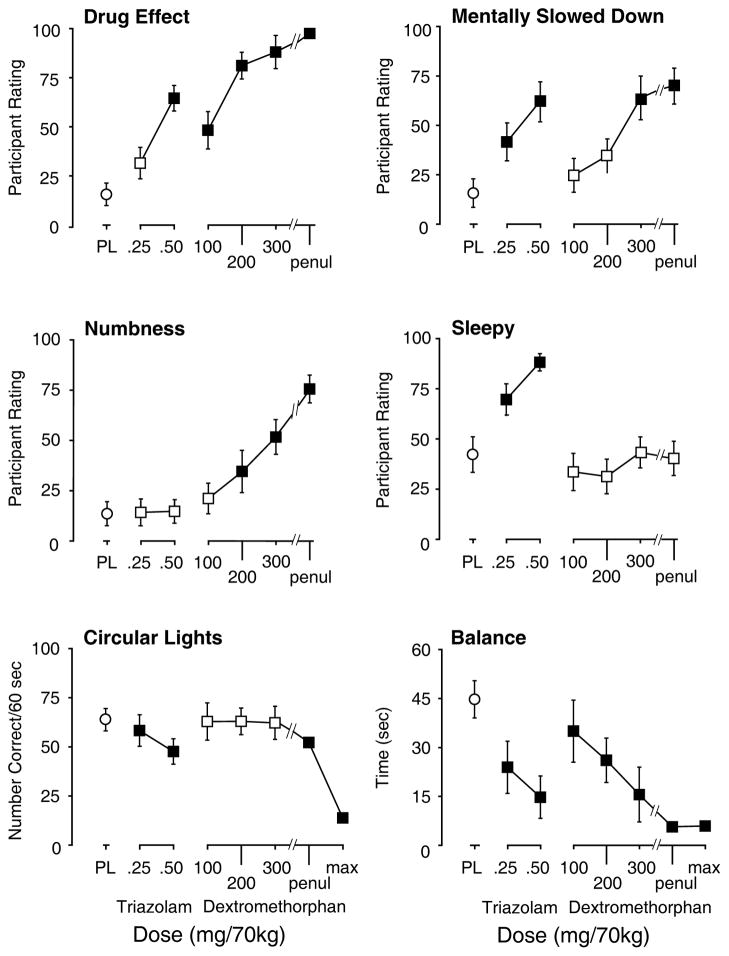
Both DXM and triazolam impaired peak performance on the Balance and Circular Lights tasks (Figure 2; Electronic supplementary Table 1). Although these effects were dose-related for both drugs the DXM dose response curve on Circular Lights was relatively flat until the maximum dose was administered. Performance on the Balance task, in contrast, was significantly and dose-dependently decreased at lower DXM doses (100–300 mg/70 kg).
Figure 2.
Dose effects of peak increases on participant ratings of four items on the Subjective Effects Questionnaire (upper and middle rows) and peak decreases on two psychomotor measures (lower row). Y-axes: participant rating of Drug Effect, Mentally Slowed Down, Numbness, and Sleepy on a 100-point scale; Circular Lights, number correct; Balance, time in seconds. X-axes: dose in mg/70kg of triazolam and dextromethorphan; PL designates placebo; ‘penul’ and ‘max’ refer, respectively, to the penultimate and maximum doses of dextromethorphan administered to each volunteer; max values are not presented for participant ratings because of missing data. Data points show means; brackets show 1 SEM; filled symbols indicate values that are significantly different from the placebo (p<0.05, planned comparisons).
Monitor ratings
Both DXM and triazolam increased peak monitor ratings of overall drug effect and motor impairment (Electronic supplementary Table 1). DXM but not triazolam produced significant and dose-related increases on monitor ratings of distance from ordinary reality, visual effects with eyes open, visual effects with eyes closed, restlessness/fidgety, joy/euphoria/peace, nausea/vomiting, psychological discomfort, unresponsive to questions, anxiety or fearfulness, and confusion/disorientation. Triazolam but not DXM produced significant increases in sedation. Neither drug significantly increased monitor ratings of stimulation/arousal, tearing/crying, or ideas of reference/paranoid thinking.
Participant ratings of subjective effects
Both drugs increased participant ratings on 13 items from the Subjective Effects Questionnaire (Table 1, Figure 2): drug effect, depressant/sedating, forgetful, mentally slowed down, fatigued/weak, limbs heavy; slurred speech, difficulty concentrating, confused/disoriented, blurred vision, unsteady, good effects, and bad effects. DXM but not triazolam increased ratings of arousing/stimulating, light-headed/dizzy, shaky/jittery, numbness/tingling, nervous/anxious, queasy/sick to stomach, hot-flushed, restless, talkative, limp/loose, headache, like drug effect, and dislike drug effect. In contrast, triazolam, but not DXM, increased ratings of sleepy, and tired/lazy, and decreased ratings of energetic and excited.
Table 1.
| Placebo | 0.25 TRZ | 0.5 TRZ | 100 DXM | 200 DXM | 300 DXM | Penultimate DXM | |
|---|---|---|---|---|---|---|---|
| Items affected by both DXM and TRZ | |||||||
| Drug Effect | 15.5 (5.7) | 31.4 (8.0) | 64.4 (6.5) | 48.1 (9.5) | 81.0 (6.8) | 87.9 (8.4) | 97.4 (1.1) |
| Depressant/Sedating | 15.2 (6.8) | 39.5 (11.0) | 72.8 (5.6) | 34.1 (8.7) | 20.9 (8.1) | 43.3 (9.1) | 42.1 (9.7) |
| Forgetful | 18.8 (6.5) | 38.3 (8.7) | 57.1 (9.4) | 28.2 (9.4) | 39.0 (8.9) | 58.4 (10.3) | 75.8 (7.2) |
| Mentally slowed down | 15.6 (7.2) | 41.5 (8.9) | 62.2 (9.7) | 24.6 (8.6) | 34.5 (8.5) | 63.8 (11.1) | 70.1 (8.7) |
| Fatigued/Weak | 7.9 (4.5) | 32.9 (9.6) | 34.2 (10.9) | 18.8 (6.3) | 29.4 (9.0) | 48.4 (11.1) | 60.0 (8.5) |
| Limbs Heavy | 6.2 (4.5) | 24.5 (9.4) | 54.8 (8.4) | 23.4 (8.5) | 31.3 (10.1) | 46.7 (9.8) | 49.0 (9.4) |
| Slurred Speech | 0.4 (0.3) | 7.8 (4.4) | 17.9 (7.0) | 10.9 (3.8) | 11.2 (5.7) | 27.6 (8.4) | 50.7 (12.2) |
| Difficulty concentrating | 20.4 (5.9) | 34.2 (9.0) | 51.3 (9.7) | 24.0 (8.9) | 37.1 (9.5) | 56.2 (10.4) | 71.7 (8.6) |
| Confused/Disoriented | 3.7 (2.9) | 12.3 (5.5) | 28.9 (7.9) | 18.0 (7.8) | 33.5 (9.9) | 48.8 (9.5) | 61.8 (10.0) |
| Blurred Vision | 0.5 (0.3) | 1.8 (1.0) | 25.3 (8.5) | 19.1 (6.9) | 29.3 (8.1) | 50.7 (10.5) | 60.1 (9.5) |
| Unsteady | 10.8 (6.3) | 18.9 (6.3) | 38.2 (9.0) | 21.1 (8.4) | 42.8 (11.3) | 65.8 (7.6) | 71.8 (7.2) |
| Good Effects | 28.1 (7.9) | 43.0 (11.9) | 65.3 (7.4) | 58.9 (9.0) | 60.7 (11.2) | 69.0 (9.1) | 74.7 (7.8) |
| Bad Effects | 12.4 (6.1) | 11.9 (7.6) | 35.8 (8.4) | 34.5 (11.3) | 40.8 (11.7) | 48.3 (8.9) | 53.3 (10.4) |
| Items affected by DXM alone | |||||||
| Arousing/Stimulating | 11.2 (7.3) | 11.8 (6.1) | 9.2 (5.3) | 37.2 (10.2) | 50.1 (11.3) | 42.3 (10.4) | 56.5 (11.5) |
| Light-Headed/Dizzy | 9.5 (3.8) | 16.6 (8.0) | 30.4 (8.9) | 28.4 (9.3) | 55.3 (11.2) | 74.9 (9.3) | 79.3 (8.0) |
| Shaky/Jittery | 4.8 (4.3) | 4.4 (2.9) | 4.3 (2.0) | 19.1 (6.4) | 31.2 (11.5) | 24.9 (7.2) | 44.3 (9.7) |
| Numbness/Tingling | 13.4 (6.0) | 14.1 (6.7) | 14.6 (5.9) | 21.0 (7.6) | 34.4 (10.5) | 51.6 (8.6) | 75.5 (7.0) |
| Nervous/Anxious | 3.9 (3.3) | 2.0 (1.8) | 9.3 (7.5) | 17.8 (6.5) | 21.5 (7.7) | 35.3 (8.1) | 19.3 (5.9) |
| Queasy/Sick to Stomach | 9.5 (4.3) | 1.3 (0.8) | 10.5 (5.8) | 21.7 (11.0) | 34.3 (8.6) | 43.1 (11.7) | 38.9 (10.7) |
| Hot/Flushed | 14.2 (7.5) | 7.3 (4.9) | 8.3 (6.2) | 20.8 (10.4) | 28.9 (8.8) | 43.3 (10.6) | 48.6 (8.6) |
| Restless | 6.8 (6.1) | 2.0 (0.9) | 13.5 (7.2) | 15.0 (5.7) | 26.4 (12.1) | 21.1 (7.1) | 44.8 (11.8) |
| Talkative | 50.3 (8.9) | 36.3 (7.5) | 34.7 (6.9) | 49.5 (6.8) | 54.3 (8.5) | 52.7 (8.6) | 71.3 (6.2) |
| Limp/Loose | 30.6 (11.4) | 45.5 (9.1) | 58.2 (8.6) | 28.6 (10.1) | 35.7 (10.3) | 56.2 (10.5) | 75.0 (6.6) |
| Headache | 6.5 (4.1) | 0.3 (0.2) | 5.8 (3.2) | 4.3 (2.0) | 20.7 (8.4) | 30.9 (9.6) | 17.7 (7.7) |
| Like Drug Effect | 30.3 (8.3) | 50.2 (11.5) | 57.2 (8.4) | 61.9 (9.3) | 59.5 (10.6) | 60.1 (9.9) | 74.7 (9.0) |
| Dislike Drug Effect | 9.5 (5.3) | 9.4 (8.3) | 29.7 (8.4) | 32.3 (10.4) | 29.6 (12.2) | 44.8 (9.8) | 30.4 (8.9) |
| Items affected by TRZ alone | |||||||
| Sleepy | 42.1 (8.9) | 69.5 (7.9) | 88.1 (4.3) | 33.4 (9.3) | 31.2 (8.6) | 43.2 (7.7) | 40.2 (8.5) |
| Tired/Lazy | 37.8 (11.0) | 63.2 (9.7) | 87.3 (4.8) | 31.8 (8.4) | 29.7 (9.1) | 43.2 (9.5) | 59.3 (9.6) |
| Energetic | 61.6 (8.0) | 47.6 (5.7) | 29.0 (5.8) | 59.2 (9.3) | 57.8 (10.0) | 51.6 (8.7) | 56.6 (9.2) |
| Excited | 52.6 (9.2) | 28.8 (6.4) | 36.5 (7.4) | 45.6 (7.4) | 50.3 (9.3) | 52.6 (8.6) | 63.3 (8.9) |
Data are mean ratings on a visual analog scale (0 to 100) with 1 SEM shown in parenthesis (N=12). Bold font indicates significantly different from placebo, (Fisher’s LSD p≤.0.05); TRZ (triazolam); DXM (dextromethorphan); see text for details of statistical analyses
Six questionnaire items were not significantly affected by either drug: Alert; Comfortable; Easy-going/Mellow; Relaxed; Irritable/Grumby; Dry Mouth
Emesis
No volunteer vomited after receiving placebo, 0.25 or 0.5 mg/70 kg triazolam, or 100 or 200 mg/70kg DXM. Two of the twelve volunteers vomited after receiving 300 mg/70kg DXM. Seven of the twelve volunteers vomited after receiving a dose of 400 mg/70kg DXM or greater (6 of 12 at 400; 2 of 10 at 500; 3 or 6 at 600; 1 of 3 at 700; and 2 of 2 at 800 mg/70kg DXM). Although we cannot rule out the possibility of incomplete absorption after vomiting, vomiting typically occurred 90 minutes or longer after capsule administration, making it unlikely that significant amounts of DXM were purged before being absorbed. Vomiting at lower doses of DXM did not always predict vomiting at higher doses (e.g. 3 volunteers that vomited after 400 mg/70 kg DXM did not vomit at 500 mg/70 kg DXM).
Measures assessed at the end of the session, approximately 7 hours after capsule administration
Pharmacological Class Questionnaire
Table 2 presents the results from the Pharmacological Class Questionnaire. Most volunteers (75%, 9 of 12) correctly identified placebo, although one participant each selected stimulant, classic hallucinogen, and other. Most volunteers also correctly identified triazolam as a sedative-hypnotic, with 66.7% (8 of 12) correct at the highest dose. DXM was dose-dependently identified as a classic hallucinogen (e.g., LSD, psilocybin, DMT, mescaline etc); 91.7% (11 of 12) identified both the 400 mg/70 kg dose and their maximum dose as a classic hallucinogen. The mean (SEM) rating on the visual analog scale (1–100) of the extent of similarity to a classic hallucinogen was 92.7 (5.3) and 93.7 (5.5) at the 400 mg/70 kg and maximum dose, respectively. Table 2 also shows that, across all doses, DXM was relatively rarely identified as a dissociative anesthetic hallucinogen (e.g., like ketamine, DXM, PCP, etc).
Table 2.
Pharmacological class questionnaire: percentage of participants choosing each class1
| Drug class descriptor2 | Placebo N=12 | 0.25 TRZ N=12 |
0.5 TRZ N=12 |
100 DXM N=12 |
200 DXM N=12 |
300 DXM N=12 |
400 DXM N=12 |
500 DXM N=10 |
600 DXM N=8 |
700 DXM N=4 |
800 DXM N=2 |
Penultimate DXM N=12 |
Maximum DXM N=12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Blank/placebo | 75.0 | 41.7 | 8.3 | 25.0 | 0.0 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Alcohol | 0.0 | 0.0 | 0.0 | 0.0 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sedative-hypnotic | 0.0 | 50.0 | 66.7 | 16.7 | 16.7 | 8.3 | 0.0 | 0.0 | 12.5 | 0.0 | 0.0 | 8.3 | 8.3 |
| Muscle relaxant | 0.0 | 0.0 | 8.3 | 0.0 | 8.3 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 8.3 | 0.0 |
| Opioid | 0.0 | 0.0 | 16.7 | 0.0 | 0.0 | 0.0 | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 8.3 | 0.0 |
| Stimulant | 8.3 | 0.0 | 0.0 | 8.3 | 16.7 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Dissociative anesthetic hallucinogen | 0.0 | 0.0 | 0.0 | 8.3 | 8.3 | 25.0 | 0.0 | 20.0 | 0.0 | 0.0 | 0.0 | 8.3 | 0.0 |
| Classic hallucinogen | 8.3 | 8.3 | 0.0 | 33.3 | 41.7 | 33.3 | 91.7 | 70.0 | 87.5 | 100.0 | 100.0 | 66.7 | 91.7 |
| Other naturally occurring hallucinogen | 0.0 | 0.0 | 0.0 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Other | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 8.3 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Data are the percentage of participants selecting each class. Number of participants who received each drug condition is indicated by the numeral (N=) under each drug condition. TRZ (triazolam); DXM (dextromethorphan)
Participants were required to choose among 18 possible drugs or drug classes. For the three most relevant drug classes, the example drugs provided were: Sedative-hypnotics (Xanax, Klonapin, Ativan, Valium, Librium, Ambien, phenobarbital, pentobarbital); Classic hallucinogens (LSD, acid, psilocybin, magic mushrooms, DMT, ayahuasca, LSA, morning glory, mescaline, peyote, foxy methoxy, 2C-B); Dissociative anesthetic hallucinogens (ketamine, K, special K, dextromethorphan, DXM, cough syrup, robotripping, skittles, PCP, dippers, angel dust, embalming fluid). Ten drugs or drug classes, which were not selected by any participant, are not shown in the table (GHB and related; caffeine; ephedrine; nicotine; cannabis, MDMA, inhalant; nitrous oxide)
End of session rating of liking the experience
Triazolam did not increase end of session liking ratings, whereas DXM produced dose-related increases in liking, with significant effects at the penultimate and maximum DXM doses (Table 3).
Table 3.
Volunteer ratings on questionnaires completed approximately 7 h after drug administration1
| Placebo | 0.25 TRZ | 0.5 TRZ | 100 DXM | 200 DXM | 300 DXM | Penultimate DXM | Maximum DXM | |
|---|---|---|---|---|---|---|---|---|
| Like the Experience? | ||||||||
| Rating (0 to 4) | 1.25 (0.28) | 1.67 (0.28) | 1.25 (0.22) | 1.33 (0.33) | 1.67 (0.48) | 1.75 (0.28) | 2.58 (0.38) | 2.83 (0.37) |
| Hallucinogen Rating Scale (HRS) 2 | ||||||||
| Intensity | 0.31 (0.16) | 0.98 (0.28) | 1.42 (0.14) | 1.40 (0.25) | 1.77 (0.25) | 2.15 (0.26) | 2.44 (0.17) | 3.08 (0.14) |
| Somaesthesia | 0.26 (0.07) | 0.21 (0.07) | 0.28 (0.04) | 0.60 (0.12) | 1.10 (0.23) | 1.22 (0.17) | 1.54 (0.20) | 1.87 (0.23) |
| Affect | 0.53 (0.08) | 0.41 (0.08) | 0.34 (0.05) | 0.69 (0.14) | 0.85 (0.17) | 0.95 (0.13) | 1.35 (0.23) | 1.91 (0.25) |
| Perception | 0.20 (0.08) | 0.22 (0.08) | 0.21 (0.07) | 0.53 (0.17) | 0.55 (0.15) | 1.03 (0.17) | 1.57 (0.23) | 1.94 (0.35) |
| Cognition | 0.27 (0.08) | 0.24 (0.11) | 0.32 (0.08) | 0.43 (0.13) | 0.75 (0.17) | 1.11 (0.16) | 1.73 (0.25) | 2.11 (0.33) |
| Volition | 1.01 (0.10) | 1.14 (0.20) | 1.43 (0.22) | 1.08 (0.13) | 1.21 (0.11) | 1.44 (0.10) | 1.55 (0.10) | 2.01 (0.18) |
| Mysticism Scale 3 | ||||||||
| Interpretation | 32.3 (6.5) | 25.2 (4.7) | 22.2 (4.8) | 33.9 (5.8) | 47.4 (9.6) | 63.0 (7.7) | 78.5 (7.4) | 84.8 (6.7) |
| Introvertive | 22.1 (4.9) | 24.3 (3.6) | 23.4 (5.0) | 39.6 (6.5) | 40.6 (8.5) | 66.7 (8.2) | 77.8 (8.0) | 95.3 (3.9) |
| Extrovertive | 16.4 (4.0) | 15.0 (3.7) | 13.3 (3.4) | 20.5 (4.7) | 31.3 (6.6) | 34.2 (5.9) | 47.1 (6.3) | 50.7 (6.3) |
| Total (max score = 288) | 70.8 (14.1) | 64.5 (11.1) | 58.8 (12.8) | 94.0 (15.8) | 119.3 (24.2) | 163.8 (19.8) | 203.4 (19.5) | 230.8 (15.7) |
| States of Consciousness Questionnaire 4 | ||||||||
| Internal Unity | 1.0 (1.0) | 2.4 (2.1) | 0 (0) | 3.7 (2.1) | 7.0 (4.6) | 25.7 (8.9) | 43.3 (7.8) | 63.6 (9.8) |
| External Unity | 4.7 (3.0) | 5.5 (3.5) | 0.9 (0.7) | 5.0 (3.2) | 6.7 (4.6) | 17.7 (7.0) | 33.9 (7.4) | 48.8 (10.4) |
| Sense of Sacredness | 3.7 (2.4) | 3.6 (2.1) | 0.3 (0.3) | 7.1 (3.4) | 11.7 (6.7) | 29.7 (10.3) | 48.4 (10.0) | 61.8 (11.1) |
| Intuitive knowledge | 1 (0.7) | 0.5 (0.5) | 0.5 (0.5) | 3.5 (2.5) | 13.0 (5.9) | 20.0 (6.1) | 50.9 (9.0) | 66.4 (9.6) |
| Transcendence time/space | 1.3 (1.0) | 3.9 (1.2) | 6.1 (1.4) | 14.5 (6.3) | 8.5 (3.4) | 33.3 (8.0) | 48.9 (8.1) | 68.9 (8.9) |
| Deeply-felt positive mood | 10.6 (4.0) | 13.8 (3.1) | 8.8 (1.7) | 14.9 (4.7) | 18.6 (8.2) | 35.1 (9.0) | 52.0 (8.4) | 59.0 (8.9) |
| Ineffability | 4.4 (2.4) | 7.6 (4.1) | 0.7 (0.7) | 12.8 (4.0) | 14.4 (4.5) | 30.4 (8.4) | 53.1 (10.1) | 69.5 (7.1) |
| Total | 4.4 (1.9) | 6.1 (2.0) | 3.3 (0.6) | 10.4 (3.7) | 12.1 (5.1) | 30.1 (8.0) | 49.1 (8.1) | 64.6 (8.8) |
Data are means with 1 SEM shown in parenthesis (N=12 for Mysticism Scale, Hallucinogen Rating Scale, and Like the Experience rating; N=11 for States of Consciousness Questionnaire). Bold font indicates significant differences from placebo, (Fisher’s LSD p≤.0.05); TRZ (triazolam); DXM (dextromethorphan); see text for details of statistical analyses.
Maximum scores were 4.25 for Intensity and 4.0 for the other subscales.
Maximum possible scores were 108, 108, 72, and 288 for Interpretation, Introvertive, Extrovertive, and Total, respectively.
Data are expressed as a percentage of maximum possible score.
Hallucinogen Rating Scale (HRS)
DXM showed significant and generally dose-related increases on all 6 subscales of the HRS (Table 3). These effects included perceptual changes (e.g., visual and auditory hallucinations, illusions, and synesthesias), mood changes (e.g., feelings of transcendence, grief, joy, and/or anxiety), and cognitive changes (e.g., sense of meaning and/or ideas of reference). In contrast, the highest dose of triazolam (0.5 mg/70 kg) significantly affected only 2 of the 6 scales on the HRS (Intensity of experience; impaired Volition), both of which might be expected to be increased by a sedative-hypnotic drug.
Measures of mystical experience
Triazolam did not increase measures of mystical experience, whereas DXM produced dose-related increases on both the Mysticism scale and the States of Consciousness Questionnaire. At the maximum dose of DXM, seven of eleven volunteers (64%) met a priori criteria on the States of Consciousness Questionnaire for having a “complete” mystical type experience, and two of eleven (18%) met these criteria at the penultimate DXM dose. No volunteer met these criteria after placebo, the two doses of triazolam, or 100–300 mg/70 kg DXM.
Measures assessed 1 month after drug administration
Persisting Effects Questionnaire
Approximately one month after the final study session, participants rated current persisting effects that they attributed to the experiences during any of the sessions. Positive changes in attitudes about life and self, positive mood changes, positive social effects, positive behavior changes, and increased spirituality showed intermediate rates of endorsement which varied between 46% to 61% of maximum possible scores (Electronic supplementary Table 2, available online). Negative or decreased ratings on these same dimensions were relatively low.
On the question probing how personally meaningful the session experiences were, 33% (4 of 12) of the volunteers rated the session experiences as “among the 5 most meaningful experiences of my life” and another 25% (3 of 12) rated the experiences as “among the 10 most meaningful experiences of my life.” On the question about the spiritual significance, 58% of the volunteers (7 of 12) rated the experiences as “among the 5 most spiritually significant experiences of my life”. All 12 volunteers reported that study participation increased their current sense of personal well-being and life satisfaction. One volunteer (8%) reported that his sense of personal well being and life satisfaction increased “slightly” after study participation, seven volunteers (58%), reported it increased “moderately”, and four (33%) reported that it increased “very much.” Mean ratings for these three questions are shown in Electronic supplementary Table 2, available online.
Although the follow up questionnaire did not allow differentiation between DXM vs triazolam experiences, in unstructured interviews at follow up, volunteers attributed the positive persisting effects to sessions on which they thought they had received a classic hallucinogen (i.e. DXM sessions).
Urine Phenotype Results
Of the 11 volunteers assessed for CYP2D6 status, one was a poor metabolizer. That individual received a maximum dose of 600 mg/70 kg DXM but there were no obvious differences in that volunteer’s data.
DISCUSSION
This double blind, ascending dose-response study demonstrates that DXM produces orderly dose-and time-related subjective and psychomotor effects. On several subjective effects measures, the effects of DXM were similar to triazolam, with both producing dose-related increases in subjective domains reflecting a sedative drug effect (e.g. increases in depressant/sedating, mentally slowed down, fatigued/weak, confused/disoriented, and unsteady). Consistent with this, both drugs impaired psychomotor measures (Circular Lights and Balance). Interestingly, although triazolam produced a dose-related decrease in Circular Lights scores, the DXM dose response curve was relatively flat until the maximum dose was administered. The maximal dose of DXM generally produced greater effects than the highest dose of triazolam on most of these measures
Despite these similarities, DXM and triazolam were clearly distinguishable on other volunteer rated subjective measures. Triazolam but not DXM, increased subjective ratings of sleepy and tired/lazy, decreased ratings of energetic and excited, and increased monitor ratings of sedation. Triazolam but not DXM was identified as a sedative-hypnotic by most volunteers. DXM but not triazolam increased blood pressure and heart rate, and produced feelings of stimulation (e.g., increased ratings of arousing/stimulating, shaky/jittery, nervous/anxious, restless, and talkative) as well as other somatic effects (e.g. light-headed/dizzy, numbness/tingling, queasy/sick to stomach, and headache). On monitor ratings, DXM increased ratings of distance from ordinary reality, visual effects with eyes open and with eyes closed, restless/fidgety, joy/euphoria/peace, nausea/vomiting, and psychological discomfort, and, at the maximum dose only, DXM increased monitor ratings of unresponsive to questions, anxiety or fearfulness, and confusion/disorientation. At the end of sessions participants reported liking DXM but not triazolam, and DXM increased scores on the Mysticism Scale and States of Consciousness Questionnaire. On the HRS, DXM but not triazolam increased the subscales of somaesthesia, affect, perception, and cognition. Finally, on the Pharmacological Class Questionnaire, participants identified the high doses of DXM as a classic hallucinogen (e.g., like LSD, psilocybin, DMT, mescaline etc). Thus, DXM produced effects that were clearly different from the effects of triazolam.
Given that DXM is an NMDA antagonist, it is interesting that several of the effects of DXM showed similarities to those observed in previous research with the classic serotonergically mediated hallucinogen psilocybin (Griffiths et al. 2011). Comparing across studies, both DXM and psilocybin produced dose-related increases on all six subscales of the Hallucinogen Rating Scale (HRS) and on two questionnaires assessing mystical-type experience. Furthermore, monitors rated DXM as producing dose-related increases on distance from ordinary reality and visual effects with eyes open and closed. An important caveat is that few studies have investigated the specificity of these measures to identify hallucinogens vs. other drugs. The most compelling observation suggesting the similarity of DXM to classic hallucinogens is from the Pharmacological Class Questionnaire on which these hallucinogen-experienced volunteers rated the similarity of the compound they received to 18 different classes of psychoactive drugs. At both 400 mg/70 kg and the maximum DXM dose, 11 of the 12 volunteers (91.7%) chose “classic hallucinogens” (e.g. LSD, psilocybin, DMT, ayahuasca, mescaline) as the drug they thought they most likely received during the experimental session. The single volunteer who did not choose “classic hallucinogens” after 400 mg/70 kg DXM, selected that class after the next dose of DXM (500 mg/70 kg). Thus, between 400–500 mg/70 kg DXM, all volunteers reported that they had received a classic hallucinogen. Furthermore, mean ratings on a visual analog scale (1–100) of the extent to which the drug received that day was similar to a classic hallucinogen were 93 and 94 at the 400 mg/70 kg and maximum dose of DXM respectively.
Despite these similarities between DXM and psilocybin, there are notable differences. Most prominently, high doses of DXM produce severe behavioral impairment and vomiting, which generally do not occur after high doses of psilocybin. Furthermore, psilocybin but not DXM produced increases in monitor ratings of stimulation/arousal, tearing crying, and ideas of reference/paranoid thinking (Griffiths et al. 2011). The conclusion of similarity between DXM and classic hallucinogens is also significantly limited by the study design that did not use a classic hallucinogen as the active comparator and the reliance of comparison of data across different studies. It will be important for future studies to directly compare DXM with psilocybin in the same participants.
The finding that an NMDA antagonist produces subjective effects that are similar to serotonergically mediated hallucinogens is consistent with cellular and preclinical behavioral studies suggesting serotonergic-glutamatergic interactions in the mechanism of action of classic hallucinogens (Fantegrossi et al. 2008;Nichols 2004;Vollenweider and Kometer 2010). On the basis of electrophysiological studies, Aghajanian and Marek (1999) postulated that changes in glutamatergic transmission in the cerebral cortex may be responsible for the cognitive and perceptual effects of hallucinogens. Drug discrimination studies also suggest functional interactions between serotonergic and glutamatergic neurotransmitter systems in the action of hallucinogens (Winter et al. 2000, 2005). Reviewing recent data, Vollenweider and Kometer (2010) proposed that the classic hallucinogens increase glutamate levels in the prefrontal cortex via 5-HT2A receptors localized on pyramidal cells in the cortex.
An important caveat to interpreting the hallucinogen-like effects of DXM is the unknown extent to which volunteers’ expectancies about and histories of prior hallucinogen use influenced the study results. All participants had some prior experience with hallucinogens and indicated that they were familiar with previous research performed in our laboratory with psilocybin. Thus, it is plausible that the volunteers erroneously assumed that they would receive psilocybin in the study. Expectancy is believed to be an important determinant of the effects of classic hallucinogens (Metzner et al. 1965). This might also explain why most volunteers misidentified DXM as a classic hallucinogen rather than a dissociative hallucinogen. It is also intriguing that individuals with substantial prior hallucinogen experience reported that the laboratory experience was unusually meaningful and spiritually significant. In addition to expectancy, it seems likely that the psychological preparation, social support (before, during and after sessions), and comfortable and safe setting might have contributed to the participants’ positive ratings of their session experiences. Notably, despite these expectancies and setting conditions, triazolam did not produce such hallucinogen-like effects but instead produced subjective sedation and impaired motor performance similar to that observed in previous studies in non-drug using volunteers without expectancies (Carter et al. 2009). Future research should evaluate the relative importance of set and setting conditions as determinants of the effects of various drugs
The present study shows that with appropriate volunteer screening, preparation, and monitoring during sessions, high doses of DXM can be administered safely, and potentially adverse effects including sensory distortions and transient anxiety can be managed. Only one participant reached a stop point by indicating that he/she did not want to receive that drug dose again. This participant reported post-session headache that he/she attributed to DXM. Although most volunteers experienced a variety of seemingly unpleasant effects including nausea and vomiting at some point during experimental sessions, these effects were tolerable. This is likely because volunteers were informed of the wide range of potentially disturbing effects. Additionally, the primary monitor remained accessible via cell phone to each volunteer for the entire time that the participant was enrolled in the study to provide additional support and manage possible delayed effects, but no volunteer called and/or reported distress after the sessions. No lasting distress or persisting perceptual phenomena were reported at the one-month follow up.
In conclusion, the present study demonstrated that, when administered to volunteers under carefully controlled and supportive conditions, the NMDA antagonist DXM was readily distinguished from triazolam and produced effects on several measures that were similar to those produced by the classic hallucinogen psilocybin. This finding is consistent with a growing body of literature suggesting the importance of serotonergic-glutamatergic interactions in the mechanism of action of the classic hallucinogens. Studies directly comparing the effects of DXM with psilocybin in the same volunteers and examining the effects of NMDA and serotonin blockers could provide important insights into the extent of shared underlying mechanisms of action.
Supplementary Material
Acknowledgments
This research was supported by NIDA grant DA003889. We would like to thank Mary Cosimano, M.S.W., Lilian Salinas, and Jenna Cohen, for serving as assistant session monitors. We thank John Yingling for technical assistance, Barine Duman Majewska, J.D. for database programming, and Linda Felch for statistical assistance.
Footnotes
Disclosures:
The study was conducted in compliance with United States laws. Lawrence Carter is a current employee of Jazz Pharmaceuticals and owns stock options in the company.
Contributor Information
Chad J. Reissig, Email: chadreissig@gmail.com, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA, Telephone: 716 228-5243
Lawrence P. Carter, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Little Rock, AR 72205
Matthew W. Johnson, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA
Miriam Z. Mintzer, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA
Margaret A. Klinedinst, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA
Roland R. Griffiths, Email: rgriff@jhmi.edu, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA. Department of Neuroscience, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA
References
- Addy PH. Acute and post-acute behavioral and psychological effects of Salvinorin A in humans. Psychopharmacology (Berl) 2012;220:195–204. doi: 10.1007/s00213-011-2470-6. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Banken JA, Foster H. Dextromethorphan. Ann N Y Acad Sci. 2008;1139:402–411. doi: 10.1196/annals.1432.003. [DOI] [PubMed] [Google Scholar]
- Barnhart JW. The urinary excretion of dextromethorphan and three metabolites in dogs and humans. Toxicol Appl Pharmacol. 1980;55:43–48. doi: 10.1016/0041-008x(80)90218-5. [DOI] [PubMed] [Google Scholar]
- Bem JL, Peck R. Dextromethorphan. An overview of safety issues. Drug Saf. 1992;7:190–199. doi: 10.2165/00002018-199207030-00004. [DOI] [PubMed] [Google Scholar]
- Bobo WV, Miller SC, Martin BD. The abuse liability of dextromethorphan among adolescents: A review. J Child and Adolesc Substance Abuse. 2005;14 (4):55–75. [Google Scholar]
- Boyer EW. Dextromethorphan abuse. Pediatr Emerg Care. 2004;20:858–863. doi: 10.1097/01.pec.0000148039.14588.d0. [DOI] [PubMed] [Google Scholar]
- Bryner JK, Wang UK, Hui JW, Bedodo M, MacDougall C, Anderson IB. Dextromethorphan abuse in adolescence: an increasing trend: 1999–2004. Arch Pediatr Adolesc Med. 2006;160:1217–1222. doi: 10.1001/archpedi.160.12.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, Mintzer MZ. Cognitive, psychomotor, and subjective effects of sodium oxybate and triazolam in healthy volunteers. Psychopharmacology (Berl) 2009;206:141–154. doi: 10.1007/s00213-009-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology. 2006;31:2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- Church J. Dextromethorphan, dysphoria and NMDA receptors. Neuromodulatory effects of dextromethorphan: role of NMDA receptors in responses. Trends Pharmacol Sci. 1990;11:146–147. doi: 10.1016/0165-6147(90)90063-e. [DOI] [PubMed] [Google Scholar]
- Church J, Sawyer D, McLarnon JG. Interactions of dextromethorphan with the N-methyl-D-aspartate receptor-channel complex: single channel recordings. Brain Res. 1994;666:189–194. doi: 10.1016/0006-8993(94)90771-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75:17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, et al. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester MB. Dextromethorphan abuse in Texas, 2000–2009. J Addict Dis. 2011;30:243–247. doi: 10.1080/10550887.2011.581986. [DOI] [PubMed] [Google Scholar]
- Franklin PH, Murray TF. High affinity [3H]dextrorphan binding in rat brain is localized to a noncompetitive antagonist site of the activated N-methyl-D-aspartate receptor-cation channel. Mol Pharmacol. 1992;41:134–146. [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, Hermle L, Spitzer M, Sass H. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology (Berl) 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl) 2011;218:649–665. doi: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66(Suppl 9):31–41. [PubMed] [Google Scholar]
- Holtzman SG. Phencyclidine-like discriminative stimulus properties of opioids in the squirrel monkey. Psychopharmacology (Berl) 1982;77:295–300. doi: 10.1007/BF00432758. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus effects of dextromethorphan in the rat. Psychopharmacology (Berl) 1994;116:249–254. doi: 10.1007/BF02245325. [DOI] [PubMed] [Google Scholar]
- Hood RW, Jr, Hill PC, Spilka B. The psychology of religion: an empirical approach. 4. Guilford; New York: 2009. [Google Scholar]
- Johanson CE, Kilbey M, Gatchalian K, Tancer M. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans trained to discriminate among d-amphetamine, meta-chlorophenylpiperazine and placebo. Drug Alcohol Depend. 2006;81:27–36. doi: 10.1016/j.drugalcdep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22:603–620. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Sewell RA, Griffiths RR. Psilocybin dose-dependently causes delayed, transient headaches in healthy volunteers. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2011.10.029. (published online 2011, in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Griffiths RR, Mintzer MZ. Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Exp Clin Psychopharmacol. 2006;14:439–449. doi: 10.1037/1064-1297.14.4.439. [DOI] [PubMed] [Google Scholar]
- Metzner R, Litwin G, Weil G. The relation of expectation and mood to psilocybin reactions: a questionnaire study. Psychedelic Review. 1965;5:3–39. [Google Scholar]
- Mintzer MZ, Griffiths RR. An abuse liability comparison of flunitrazepam and triazolam in sedative drug abusers. Behav Pharmacol. 2005;16:579–584. doi: 10.1097/01.fbp.0000172736.11994.3c. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Rush CR, Griffiths RR. Abecarnil and alprazolam in humans: behavioral, subjective and reinforcing effects. J Pharmacol Exp Ther. 1995;272:570–580. [PubMed] [Google Scholar]
- Murray TF, Leid ME. Interaction of dextrorotatory opioids with phencyclidine recognition sites in rat brain membranes. Life Sci. 1984;34:1899–1911. doi: 10.1016/0024-3205(84)90121-8. [DOI] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K, Huang XF. Short and long term changes in NMDA receptor binding in mouse brain following chronic phencyclidine treatment. J Neural Transm. 2007;114:995–1001. doi: 10.1007/s00702-007-0668-x. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Hayes BA, Balster RL. Evaluation of the reinforcing properties and phencyclidine-like discriminative stimulus effects of dextromethorphan and dextrorphan in rats and rhesus monkeys. Psychopharmacology (Berl) 1999;146:49–59. doi: 10.1007/s002130051087. [DOI] [PubMed] [Google Scholar]
- Pahnke WN. Psychedelic drugs and mystical experience. Int Psychiatry Clin. 1969;5:149–162. [PubMed] [Google Scholar]
- Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartmann S, Danysz W. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology. 1995;34:1239–1258. doi: 10.1016/0028-3908(95)00092-k. [DOI] [PubMed] [Google Scholar]
- Riba J, Anderer P, Jane F, Saletu B, Barbanoj MJ. Effects of the South American psychoactive beverage ayahuasca on regional brain electrical activity in humans: a functional neuroimaging study using low-resolution electromagnetic tomography. Neuropsychobiology. 2004;50:89–101. doi: 10.1159/000077946. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodriguez-Fornells A, Strassman RJ, Barbanoj MJ. Psychometric assessment of the Hallucinogen Rating Scale. Drug Alcohol Depend. 2001;62:215–223. doi: 10.1016/s0376-8716(00)00175-7. [DOI] [PubMed] [Google Scholar]
- Richards WA, Rhead JC, DiLeo FB, Yensen R, Kurland AA. The peak experience variable in DPT-assisted psychotherapy with cancer patients. J Psychedelic Drugs. 1977;9:1–10. [Google Scholar]
- Romanelli F, Smith KM. Dextromethorphan abuse: clinical effects and management. J Am Pharm Assoc (2003) 2009;49:e20–e25. doi: 10.1331/JAPhA.2009.08091. [DOI] [PubMed] [Google Scholar]
- Rush CR, Frey JM, Griffiths RR. Zaleplon and triazolam in humans: acute behavioral effects and abuse potential. Psychopharmacology (Berl) 1999;145:39–51. doi: 10.1007/s002130051030. [DOI] [PubMed] [Google Scholar]
- Schmid B, Bircher J, Preisig R, Kupfer A. Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther. 1985;38:618–624. doi: 10.1038/clpt.1985.235. [DOI] [PubMed] [Google Scholar]
- Schutz CG, Soyka M. Dextromethorphan challenge in alcohol-dependent patients and controls. Arch Gen Psychiatry. 2000;57:291–292. doi: 10.1001/archpsyc.57.3.291. [DOI] [PubMed] [Google Scholar]
- Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008:313–333. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- Soyka M, Bondy B, Eisenburg B, Schutz CG. NMDA receptor challenge with dextromethorphan - subjective response, neuroendocrinological findings and possible clinical implications. J Neural Transm. 2000;107:701–714. doi: 10.1007/s007020070071. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72:33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Tancer ME, Johanson CE. The subjective effects of MDMA and mCPP in moderate MDMA users. Drug Alcohol Depend. 2001;65:97–101. doi: 10.1016/s0376-8716(01)00146-6. [DOI] [PubMed] [Google Scholar]
- Vengurlekar SS, Heitkamp J, McCush F, Velagaleti PR, Brisson JH, Bramer SL. A sensitive LC-MS/MS assay for the determination of dextromethorphan and metabolites in human urine--application for drug interaction studies assessing potential CYP3A and CYP2D6 inhibition. J Pharm Biomed Anal. 2002;30:113–124. doi: 10.1016/s0731-7085(02)00134-6. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11:642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Werling LL, Keller A, Frank JG, Nuwayhid SJ. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Exp Neurol. 2007;207:248–257. doi: 10.1016/j.expneurol.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Wilson MD, Ferguson RW, Mazer ME, Litovitz TL. Monitoring trends in dextromethorphan abuse using the National Poison Data System: 2000–2010. Clin Toxicol (Phila) 2011;49:409–415. doi: 10.3109/15563650.2011.585429. [DOI] [PubMed] [Google Scholar]
- Winter JC, Doat M, Rabin RA. Potentiation of DOM-induced stimulus control by non-competitive NMDA antagonists: a link between the glutamatergic and serotonergic hypotheses of schizophrenia. Life Sci. 2000;68:337–344. doi: 10.1016/s0024-3205(00)00934-6. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rice KC, Rabin RA. Serotonergic/glutamatergic interactions: potentiation of phencyclidine-induced stimulus control by citalopram. Pharmacol Biochem Behav. 2005;81:694–700. doi: 10.1016/j.pbb.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfinger R, Chang M. Comparing the SAS GLM and MIXED procedures for repeated measurements analysis. SUGI Proceedings.1995. [Google Scholar]
- Young AM, Herling S, Winger GD, Woods JH. Comparison of discriminative and reinforcing effects of ketamine and related compounds in the rhesus monkey. NIDA Res Monogr. 1981;34:173–179. [PubMed] [Google Scholar]
- Zawertailo LA, Kaplan HL, Busto UE, Tyndale RF, Sellers EM. Psychotropic effects of dextromethorphan are altered by the CYP2D6 polymorphism: a pilot study. J Clin Psychopharmacol. 1998;18:332–337. doi: 10.1097/00004714-199808000-00014. [DOI] [PubMed] [Google Scholar]
- Zawertailo LA, Tyndale RF, Busto U, Sellers EM. Effect of metabolic blockade on the psychoactive effects of dextromethorphan. Hum Psychopharmacol. 2010;25:71–79. doi: 10.1002/hup.1086. [DOI] [PubMed] [Google Scholar]
- Ziaee V, Akbari HE, Hoshmand A, Amini H, Kebriaeizadeh A, Saman K. Side effects of dextromethorphan abuse, a case series. Addict Behav. 2005;30:1607–1613. doi: 10.1016/j.addbeh.2005.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.