Abstract
Background
Systemic blockade of Tissue Factor (TF) attenuates acute lung injury (ALI) in animal models of sepsis but the effects of global TF deficiency are unknown.
Hypothesis
We used mice with complete knockout of mouse TF and low levels (~1%) of human TF (LTF mice) to test the hypothesis that global TF deficiency attenuates lung inflammation in direct lung injury.
Methods
LTF mice were treated with 10 μg of lipopolysaccharide (LPS) or vehicle administered by direct intratracheal (IT) injection and studied at 24 hours.
Results
Contrary to our hypothesis, LTF mice had increased lung inflammation and injury as measured by bronchoalveolar lavage cell count (3.4 × 105 WT LPS versus 3.3 × 105 LTF LPS, p=0.947) and protein (493 μg/ml WT LPS versus 1014 μg/ml LTF LPS, p=0.006), proinflammatory cytokines (TNF-α, IL-10, IL-12, p<0.035 WT LPS versus LTF LPS) and histology compared to wild type mice. LTF mice also had increased hemorrhage and free hemoglobin in the airspace accompanied by increased oxidant stress as measured by lipid peroxidation products (F2-Isoprostanes and Isofurans).
Conclusions
These findings indicate that global TF deficiency does not confer protection in a direct lung injury model. Rather, TF deficiency causes increased intra-alveolar hemorrhage following LPS leading to increased lipid peroxidation. Strategies to globally inhibit tissue factor may be deleterious in patients with ALI.
Keywords: Coagulation, free hemoglobin, acute lung injury, acute respiratory distress syndrome, alveolar hemorrhage
INTRODUCTION
Activation of the extrinsic coagulation cascade through upregulation of tissue factor (TF) dependent procoagulant activity has been implicated in the pathogenesis of both acute and chronic lung injury and may contribute to lung inflammation. In the setting of acute lung injury, TF protein is elevated both systemically 1 and locally 2 within the airspaces of the lung. Several studies have shown that systemic blockade of TF activity in the setting of sepsis attenuates organ dysfunction and acute lung injury. He et al showed that systemic blockade of TF in an intestinal ischemia-reperfusion model attenuated the severity of lung injury, leak and inflammation. 3 Welty-Wolf and colleagues found that systemic blockade of TF using either a TF blocking antibody 4 or active site inactivated factor VIIa 5,6 attenuated lung injury in an Eschericia coli model of sepsis in baboons. This group further reported that systemic blockade of TF activity attenuated lung inflammation in a model of direct lung injury using intratracheal (IT) lipopolysaccharide (LPS). 7 In summary, there is ample evidence that systemic inhibition of TF activity attenuates lung inflammation and injury induced by both direct and indirect insults. Despite this, the mechanisms by which systemic blockade of TF activity modulates coagulant and inflammatory processes in the lung environment are not clear. Studies by our group and others have shown that intra-alveolar fibrin deposition is modulated locally within the airspace by resident lung cells including the lung epithelium 2, 8–10, but effects of systemic TF inhibition on lung coagulation remain incompletely understood.
Given the compelling evidence that systemic blockade of TF ameliorates lung injury in indirect lung injury (sepsis) models but the paucity of data on the effects of global inhibition of TF in the response to acute lung inflammation caused by direct lung injury, we designed a series of experiments to test the hypothesis that global TF deficiency is protective in a model of direct lung inflammation. We used genetically manipulated mice that have global absence of mouse TF but express human TF protein at levels that are 1% of endogenous levels to prevent embryonic lethality (LTF mice). 11 Importantly, these mice have a hemostatic defect and exhibit spontaneous hemorrhage in various tissues, including the lung.12 Histologic analysis of lungs from 6 month old LTF mice showed extensive hemosiderin deposition suggestive of chronic lung hemorrhage. 12 Despite evidence of chronic lung hemorrhage in LTF mice, these mice were protected in an indirect lung injury (endotoxemia) model 13 with increased survival following systemic administration of endotoxin compared to littermate controls. How genetic deficiency of TF affects lung specific coagulation and inflammation is unknown. Here we present the results of our experiments using a model of direct lung injury, intra-tracheal lipopolysaccharide (IT LPS), in LTF mice and wild type littermate controls.
MATERIALS AND METHODS
Transgenic Mice
All experiments were approved by the Vanderbilt Institute for Animal Care and Use Committee. Transgenic LTF mice on a C57/BL6 background were used for these experiments. The mice were generated as previously described by Parry et al 11 and expressed human TF mRNA at ~1% of levels measured in normal wild type mice. Mice that were heterozygous for the murine tissue factor (mTF) gene and containing the human tissue factor (hTF) minigene were bred in order to obtain low TF mice (mTF−/−, hTF+, N=51) and wild type littermate controls (mTF+/+, hTF−, N=38).
Mouse Experimental Protocol and Tissue Collection
Mice were anesthetized with isoflurane and instilled by direct intratracheal (IT) injection with 100μl of 100 μg/ml Lipopolysaccharide (Escherichia Coli, LPS, Sigma, St. Louis, MO), 100μl of PBS (control) (Mediatech, Manassas, VA) and with 100nM recombinant murine TF (mTF) (R&D Systems, Minneapolis, MN) for selected experiments as described by Su et al. 14 After 24 hours, mice were euthanized by intraperitoneal injection of 200μl of a 23% solution of Sleepaway (Fort Dodge Animal Health, Fort Dodge, IA). All data represent 2–12 mice per group depending on the experiment. Measurements were done using all available samples were used for each measurement. Limitations in sample volumes did not allow every measurement to be made in every experimental animal. BAL was obtained by instilling 900μl 0.9% NaCl and gently aspirating the fluid. BAL was centrifuged at 1000g for 10 minutes and supernatant was collected and frozen at −80°C. The lungs were removed, flash frozen in liquid nitrogen and stored at −80°C.
BAL Cell Count and Differential
Manual cell counts using a hemacytometer and cytospins/differentials were completed with fresh BAL fluid. Cytospins were stained with Hema 3 staining kit (Thermo Fisher Scientific).
Lung mTF Western
Protein was extracted from frozen lung tissue using a radioimmunoprecipitation assay (RIPA) buffer containing 50mM Tris, 100mM NaCl, 1mM EDTA, 0.5% sodium deoxycholate, 1% Triton-x-100, and 0.1% SDS with Protease Inhibitor Cocktail (Roche, Indianapolis, IN) and kept on ice. Lung tissue was homogenized in 1ml of RIPA buffer and spun at 13,000 rpm at 4°C for 5 minutes. Supernatant was removed and placed in NuPAGE LDS sample buffer (Invitrogen, Carlsbad, CA) with 100mM DTT, vortexed, boiled for 5 minutes and stored at −80°C. Protein was separated by SDS-PAGE, using 10% polyacrylamide gels (BioRad, Hercules, CA), electro blotted onto a PVDF membrane (Millipore, Burlington, MA) and blocked with Odyssey Blocking Buffer (LI-COR, Lincoln, NB), washed with PBS 0.1% Tween, and incubated with anti-TF primary antibody (R&D Systems, Minneapolis, MN) against mTF at 1:1000 overnight at 4°C. Blots were washed and incubated with secondary antibody (LI-COR) at 1:15000 for 1 hour at room temperature and visualized with the Odyssey infrared imaging system (LI-COR). Densitometric measurements were collected. Actin was used as a protein loading control.
Lung hTF PCR
RNA was isolated from approximately 30mg of lung tissue with the Pure Link RNA Mini Kit (Invitrogen) with Trizol. cDNA was synthesized using the SuperScript VILO kit (Invitrogen). Real Time-PCR assay was perfomed using Taqman Universal Master Mix and commercially available primers/probes for human TF (Applied Biosystems) on a Step-One PCR instrument (Applied Biosystems).
mTF Immunostaining
Paraffin embedded lung sections were de-paraffinized prior to antigen retrieval (Retrievit-6, Innogenex, San Ramon, CA) for 20 minutes in a rice cooker. Slides were rinsed with DI water and incubated for 20 minutes in 0.3% H2O2 in cold methanol then rinsed and blocked (Powerblock, Biogenex, Fremont, CA and 0.4% Triton) for 15 minutes in a humid environment. Slides were rinsed and incubated overnight at 4°C with 1:200 goat polyclonal anti-TF Ab (R&D Systems, Minneapolis, MN), rinsed, and incubated with a biotinylated rabbit anti-goat secondary antibody (Innogenex, San Ramon, CA) for 20 minutes at room temperature. Slides were developed with NovoRed (Vector, Burlingame, CA) for 30 seconds and counterstained with Methyl Green for 10 seconds.
Lung Wet-to-Dry Weight Ratio
The left lung was removed and placed on a piece of pre-weighed aluminum foil. The foil + lung was weighed and placed in an 80°C oven for 2 days. The foil + lung was weighed again and the ratio was calculated ([lung+foil before drying]/[lung+foil after drying]).
BAL Clot Time Measurements
Clot time was measured using a mechanical clot detection system (STart4 Coagulometer; Diagnostica Stago, Asnieres, France). Briefly, 25 μl of BAL was warmed for 15 min at 37°C. Samples were then incubated with 25 μl of pooled citrated mouse plasma (Bioreclamation, East Meadow, NY). Clot time was determined as recalcification time following the addition of 25 μl of 50 mM calcium chloride. Measurements were done in duplicate.
BAL Protein and Cytokine Measurements
Protein was measured in BAL using the BCA protein assay according to manufacturers instructions (Pierce, Rockford, IL). Cytokines were measured by electrochemiluminescence in a mouse 7-plex proinflammatory cytokine assay according to manufacturers instructions (Meso Scale Discovery, Gaithersburg, MD).
Lung Histology and Scoring
Formalin fixed and paraffin embedded lung sections were stained with H+E. 10 images were captured at 40x power across 2 lobes of each lung. Each images was scored on a 5 point scale modified from Frank, et al 15 for septal thickening, edema, inflammation, and hemorrhage in a blinded fashion. The scores for each parameter were averaged for the 10 images and the averages were added to generate a total lung injury score.
Free Hemoglobin Measurements
Free hemoglobin was measured in undiluted cell free pulmonary edema fluid collected as previously described 2 from critically ill mechanically ventilated patients with acute lung injury (ALI) or control patients with severe hydrostatic pulmonary edema (HYDRO) using the low hemoglobin analyzer from HemoCue (Cypress, CA) according to manufacturer’s instructions. Human studies were approved by the Vanderbilt Institutional Review Board.
Lung Isoprostane and Isofuran Measurements
F2-IsoPs and IsoFs were quantified in mouse lung homogenates and human BAL by stable isotope dilution gas chromatography/negative ion chemical ionization mass spectrometry (GC/NICI/MS) as described previously. 16, 17
Statistical Analysis
Non-normally distributed data were natural log transformed prior to statistical analysis and are displayed as boxplots or dot plots. Normally distributed data are displayed as bar graphs showing mean and SEM. Comparison of 3 or more groups was done using one way ANOVA with post hoc Tukey test. Comparison of 2 groups was done using a Students t test for normally distributed or transformed data. Percent body weight loss was non-normally distributed but could not be log transformed because the majority of the values were negative numbers. For this analysis, a Kruskall-Wallis test was performed followed by pairwise Mann-Whitney U tests for comparisons between groups. All analyses were done with SPSS software version 19 for Macintosh. For all analyses, p<0.05 was considered statistically significant.
RESULTS
TF levels and coagulation in LTF mice
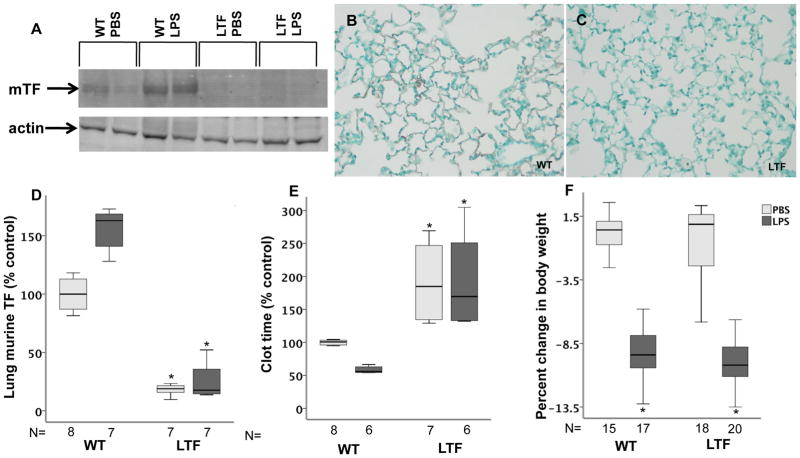
Murine TF, measured by Western blot, was present in low levels in lung homogenates in wild type mice and modestly increased following treatment with IT LPS (p=0.116 WT PBS versus WT LPS) (Figure 1A and D). LTF mice had no detectable mTF in either treatment group. Immunostaining showed mTF protein lining the airspace in WT mice (Figure 1B) but no detectable lung staining for TF in LTF mice (Figure 1C). Because of very low levels of expression, human TF protein could not be detected by Western blotting and expression was measured in low TF mice by PCR. hTF mRNA was detectable in LTF mice but was not detected in WT mice and LPS treatment had no effect on hTF expression (data not shown). In WT mice, clot time decreased with LPS treatment compared to PBS treatment (Figure 1E) consistent with induction of TF-dependent procoagulant activity in the alveolar compartment. In LTF mice, BAL clot time was prolonged compared to WT mice (Figure 1E) in both treatment groups consistent with the deficiency of TF and was not significantly shortened in response to LPS treatment. Both WT and LTF mice lost a significant amount of body weight 24 hours after treatment with IT LPS (Figure 1F).
Figure 1.
TF expression. Wild type mice have low but detectable levels of murine TF expression at base line that increases following IT LPS treatment while murine TF levels are undetectable in LTF mice. Panel A shows a representative western blot for murine TF and Panel D shows densitometric quantification from 4 independent experiments. *p=<0.001 versus both wild type groups. Panel B and C show immunostaining for murine TF (red/brown) in WT (B) and LTF (C) mice 24 hours after IT LPS. While wild type mice have a shortened BAL clot time in response to IT LPS (Panel E), LTF mice have a prolonged clot time that is not shortened by IT LPS. *p<0.001 versus WT. Panel F shows weight change 24 hours after treatment with LPS. *p<0.001 versus PBS treatment.
LTF mice are not protected from lung inflammation induced by IT LPS
Both WT and LTF mice had increased BAL total cell counts 24 hours after IT LPS (Figure 2A). There was more variability in the inflammatory response in LTF mice compared to WT with some LTF mice exhibiting an exaggerated inflammatory response, one third of the LTF mice had BAL cell counts that were higher than the highest counts in wild type mice. In WT mice treated with LPS, neutrophils predominated (88% neutrophils, 12% macrophages) while LTF mice had a higher percentage of macrophages (45% neutrophils, p=0.513 versus WT LPS, 55% macrophages, p=0.513 versus WT LPS) (Figure 2B). In addition, LPS treated low TF mice had significantly increased BAL levels of IL-10, IL-12 and TNF-α and a non-significant increase in IFN-γ, IL-1β, IL-6 and IL-8 (Table 1). There was also a modest increase in IL-6 and IL-8 in PBS treated LTF mice compared to WT.
Figure 2.
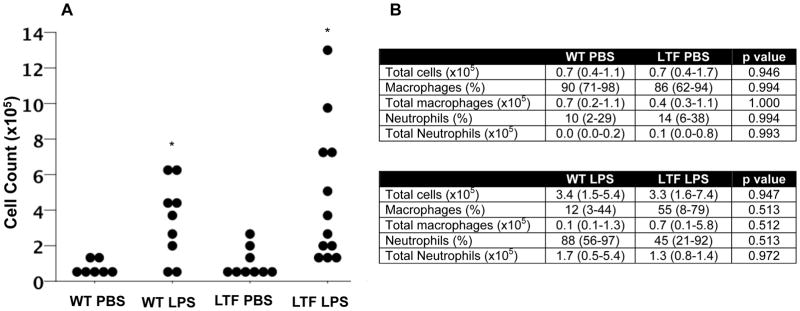
Intratracheal LPS induces lung inflammation. Dot plot of BAL cell counts 24 hours after IT LPS administration. BAL total cell count was increased in both wild type and low TF mice compared to saline treated animals (Panel A). *p<0.005 versus PBS treatment by ANOVA of log transformed data with post hoc Tukey test. Panel B shows median (IQR) total BAL macrophage and neutrophil counts and percent counts.
Table 1.
Median (IQR) BAL cytokines in WT and LTF mice treated with PBS or LPS. P values are for comparison of WT vs LTF within each treatment group.
| Cytokine (pg/ml) | WT PBS N=7 | LTF PBS N=6 | p value | WT LPS N=5 | LTF LPS N=6 | p value |
|---|---|---|---|---|---|---|
| IFN-γ | 0.0(0.0–0.1) | 0.2 (0.1–0.4) | 0.405 | 3.2(0.7–17.5) | 96.6 (1.2–222.3) | 0.230 |
| IL-1β | 1.3(0.6–2.4) | 1.4 (0.4–11.0) | 0.986 | 43.1 (23.7–55.4) | 75.5 (14.1–167.4) | 0.937 |
| IL-10 | 0.3 (0.0–2.1) | 0.1 (0.0–1.9) | 0.961 | 15.0 (8.7–19.1) | 62.8 (45.3–96.3) | 0.034 |
| IL-12 | 0.0 (0.0–1.1) | 0.1 (0.0–1.9) | 0.924 | 23.0 (17.0–36.6) | 148.2 (0.0–354.9) | 0.008 |
| IL-6 | 0.5 (0.0–2.1) | 27.9 (1.0–83.0) | 0.021 | 897.8 (548.5–1124.4) | 2255.1 (845.2–5113.1) | 0.591 |
| IL-8 | 7.7 (3.4–15.1) | 32.9 (13.0–63.4) | 0.012 | 649.3 (522.6–1034.8) | 1704.3 (797.9–2470.9) | 0.464 |
| TNF-α | 0.8 (0.3–1.1) | 1.2 (0.5–1.9) | 0.917 | 170.7 (108.3–544.5) | 985.9 (452.4–2958.7) | 0.027 |
LTF mice have increased pulmonary edema in response to IT LPS
Pulmonary capillary leak was assessed by lung wet-to-dry weight ratios (Figure 3A) and total BAL protein (Figure 3B). Both WT and LTF mice had modest but non-significant increases in wet-to-dry weight ratio following treatment with IT LPS without a significant difference between genotypes. BAL protein, however, was increased in response to IT LPS in both groups and LTF mice had BAL protein levels that were 2-fold higher than WT mice.
Figure 3.
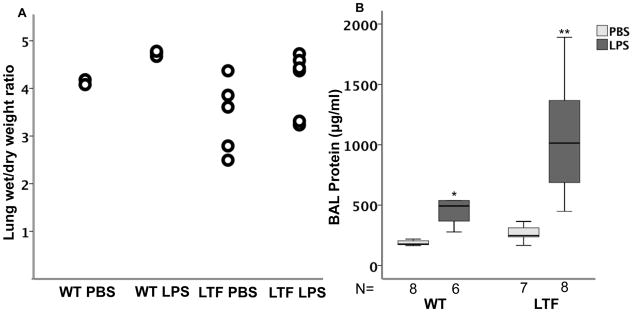
IT LPS increases lung edema. IT LPS causes a modest but non-significant increase in lung wet to dry weight ratio (Panel A) but there are no differences between WT and LTF. In contrast, BAL total protein is significantly increased by IT LPS (Panel B). *p<0.007 versus WT PBS and LTF LPS groups, **p<0.007 versus all other groups.
WT and LTF LPS treated mice have increased histologic lung injury
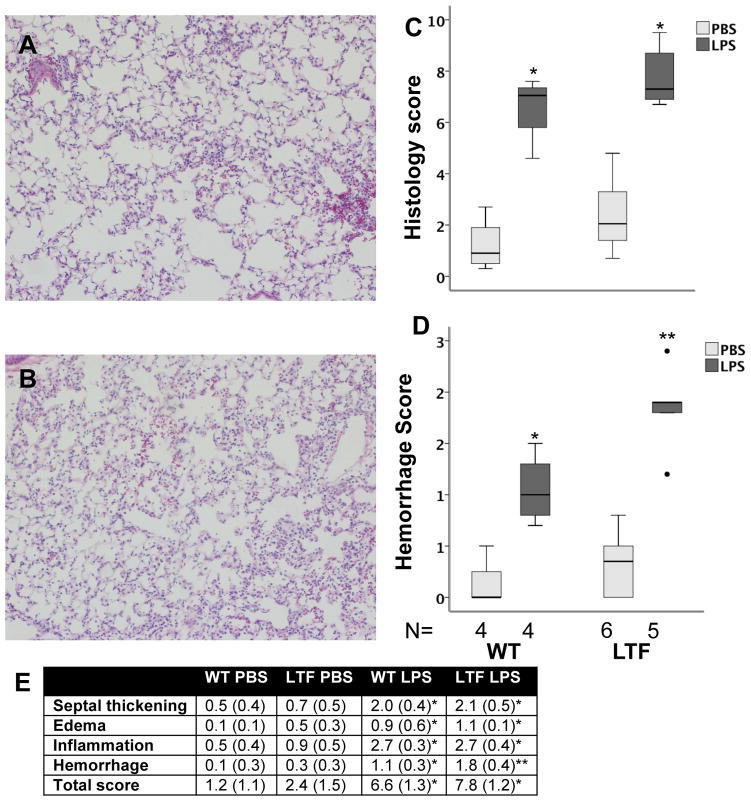
The degree of lung injury was assessed histologically using a 5 point scoring system in a blinded fashion. Both WT (Figure 4A) and LTF (Figure 4B) mice had increased histologic evidence of lung injury 24 hours after IT LPS treatment (Figure 4C and E). LTF mice had significantly more alveolar hemorrhage (Figure 4D and E, Figure 5A) compared to WT mice.
Figure 4.
Lung histology 24 hours after IT LPS. IT LPS induced lung inflammation and injury in both WT (Panel A) and LTF (Panel B) mice. LPS treatment increased lung injury in both genotypes (Panel C) [*p<0.002 versus PBS treatment]. Lung hemorrhage was more severe in LPS treated LTF mice compared to WT (Panel D) [*p<0.027 versus all other groups,** p<0.019 versus all other groups]. Panel E shows the individual components of the score [* p<0.05 vs PBS treatment in both genotypes, ** p<0.05 vs PBS treatment in both genotypes and WT LPS treatment].
Figure 5.
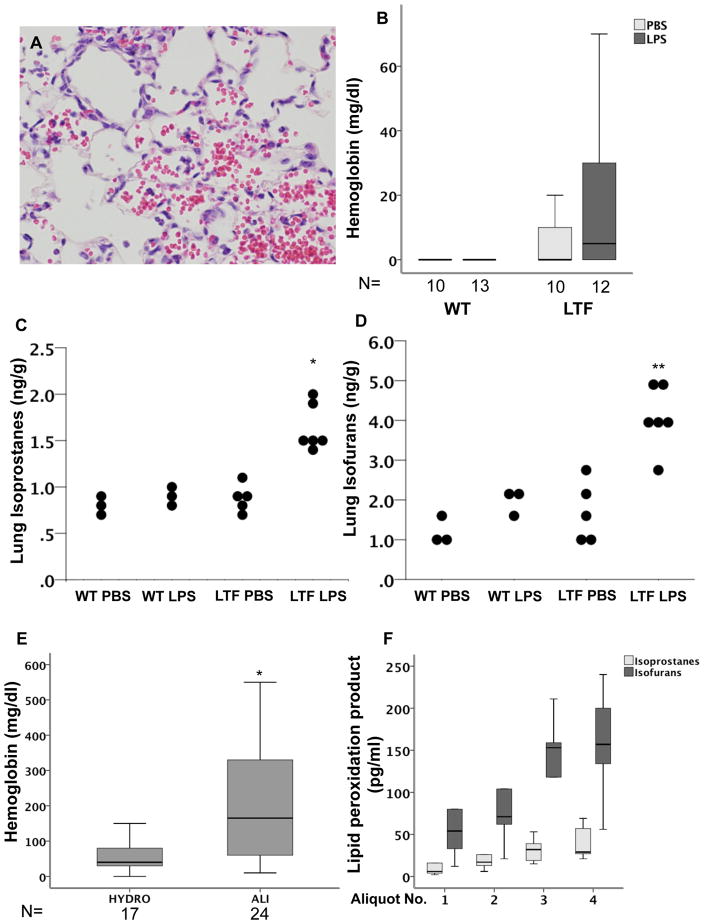
Free hemoglobin and lipid peroxidation. LTF mice have increased alveolar hemorrhage (Panel A) and increased free hemoglobin in BAL fluid (Panel B) both basally and in response to IT LPS. LTF mice also have an increase in total lung isporostanes (Panel C) and isofurans (Panel D) in response to IT LPS (*p<0.001 versus all other groups and **p<0.021 versus all other groups). Patients with ALI also have increased levels of free hemoglobin in the lung compared to patients with hydrostatic edema (Panel E) (*p=0.007). In patients with a diagnosis of alveolar hemorrhage, BAL isoprostanes and isofurans increase with increasing aliqout number (Panel F).
LTF mice have higher levels of free hemoglobin in the lung and elevated isoprostanes and isofurans compared to WT mice
Because Low TF mice were not protected from IT LPS induced lung inflammation and had increased bleeding into the airspaces, we hypothesized that release of free hemoglobin into the airspaces might be contributing to lung injury and inflammation in the low TF mice. Low TF mice treated with IT LPS had higher levels of free hemoglobin in the cell free BAL than WT mice (Figure 5B). To test whether the free hemoglobin is contributing to lung injury through increased oxidant stress we measured total lung isoprostanes (Figure 5C) and isofurans (Figure 5D) in lung homogenates. LPS treated LTF mice had higher levels of both isoprostanes and isofurans in the lung compared to LPS treated WT mice. To test whether these findings are applicable to human disease, we measured free hemoglobin in undiluted pulmonary edema fluid from patients with acute lung injury (ALI) compared to control patients with severe hydrostatic pulmonary edema and found that free hemoglobin levels were significantly elevated in patients with ALI (Figure 5E). Finally, to test whether free hemoglobin in human lungs is associated with markers of lipid peroxidation we measured BAL ispoprostanes and isofurans in sequential BAL aliquots from 5 patients with diffuse alveolar hemorrhage (Figure 5F). In patients with alveolar hemorrhage, sequential aliquots of BAL fluid are progressively bloodier. As the aliquot number increased, BAL isoprostane and isofuran levels increased.
Effects of mTF replacement in LTF mice
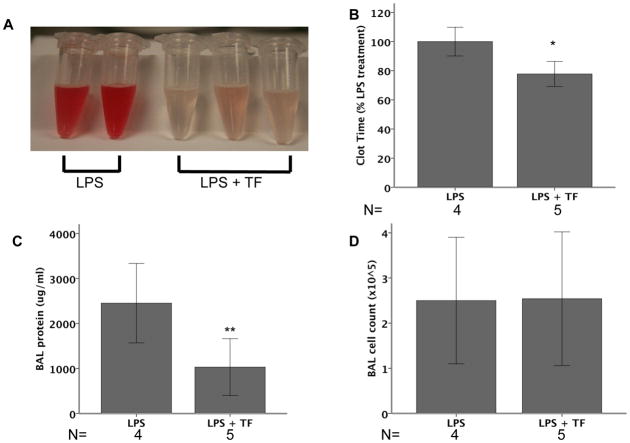
LTF mice were treated with LPS or LPS plus recombinant murine tissue factor (mTF) and analyzed at 24 hours. LTF mice treated with mTF had visibly decreased BAL hemorrhage (Figure 6A) and a shortened clot time (Figure 6B) suggesting that replacement of TF into the airspace partially restored TF procoagulant activity. mTF treatment attenuated lung permeability induced by IT LPS as measured by BAL protein (Figure 6C) but did not affect inflammatory cell influx (Figure 6D).
Figure 6.
Effects of TF replacement in LTF mice. LTF mice were treated with 10μg LPS IT (N=4) or 10μg LPS + 100 nM recombinant mTF IT (N=5) and harvested at 24 hours. With the administration of mTF, LTF mice had visibly reduced BAL hemorrhage (Panel A) and significantly shortened BAL clot time (Panel B) compared to LPS treated mice that did not receive recombinant TF. BAL total protein was higher in mice given mTF (Panel C) but BAL cell counts did not differ (Panel D). (*p=0.011, **p=0.031).
DISCUSSION
Contrary to our hypothesis, global deficiency of TF is not protective in a model of direct lung inflammation. LTF mice have influx of inflammatory cells into the lung in response to IT LPS with BAL cell counts that are comparable to WT mice. In addition, LTF mice have increased BAL cytokines, histologic severity of lung injury and alveolar capillary barrier permeability compared to WT mice. These findings are in distinct contrast to prior reports that systemic inhibition of TF was protective in either a direct or indirect model of acute lung injury 4–7. All of these studies suggest that inhibition of coagulation is protective in models of acute lung injury. While the increases in lung injury in LTF mice in our studies are modest, the fact that our findings contradict prior work in this field is particularly interesting.
TF mediated coagulation is upregulated in the lung in the setting of inflammation and injury 2, 18–20 but whether TF in the lung is protective or injurious is not completely understood. Other studies have shown that TF inhibition and deficiency protects against lung injury. One important difference between our study and previous reports is that we used a model of global TF deficiency while the previous studies have administered pharmacologic TF inhibitors systemically, which may or may not have modulated coagulation in the lung. We have previously shown that coagulation is modulated locally by the lung epithelium. 2, 8, 9, 21 Because coagulation is regulated locally in the airspace independent of the circulation, modulation of coagulation using inhibitors delivered systemically as in the previous studies might have very different effects compared to global TF deficiency. In the current study, global TF deficiency did not protect against lung inflammation in response to a direct insult. Interestingly, the same LTF mice treated with systemic LPS have decreased systemic inflammation and improved survival compared to control mice treated with LPS.22 These findings suggest that mice with global TF deficiency respond quite differently to local versus systemic inflammatory insults.
One observation that we made in our initial experiments was that the BAL from LTF mice appeared blood tinged while the BAL from WT mice was clear. LTF mice are known to have chronic hemorrhage into the lungs 12 and might be expected to have increased hemorrhage in response to injury. In addition, LTF mice also had significantly increased BAL protein and modestly increased lung wet to dry weight ratios which may have contributed to increased bleeding in the lungs. The discrepancy between BAL protein and wet to dry weight ratios may be explained by the relatively mild epithelial injury in LTF mice that allows for preserved alveolar fluid clearance in the setting of increased permeability. To understand whether blood in the lung augmented lung inflammation, we studied the potential injurious effects of bleeding into the lungs in LTF mice. In addition to intact RBCs in the airspace as seen on histology, LTF mice had red-tinged BAL fluid after the removal of cellular components, suggestive of free hemoglobin. To quantify this observation we measured free hemoglobin in cell free BAL in both WT and LTF mice and found that only 13% of WT mice had any detectable free hemoglobin in the BAL after LPS treatment while 41% of LTF mice had measureable levels (Figure 5B). Since free hemoglobin is a potent oxidant and could potentially contribute to lung inflammation and injury, we measured lung isoprostanes and isofurans as a marker of oxidant-mediated lipid peroxidation and found that both were elevated in the lungs of LTF mice compared to WT (Figure 5C, D). Thus, one potential mechanism whereby LTF mice have increased lung inflammation and injury is through the harmful effects of free hemoglobin. Free hemoglobin is a potent inducer of oxidant stress. Free hemoglobin oxidizes lipids and can cause extensive cell damage. 23 Hemoglobin can potentiate vascular injury induced by oxidized LDL 24 or activated neutrophils. 25 Although cell free hemoglobin has been implicated in the pathogenesis of transfusion related acute lung injury, 26, 27 little is know about the direct effects of free hemoglobin in the airspace. Our data suggest that free hemoglobin in the airspace may be injurious by inducing lipid peroxidation since LTF mice have both elevated levels of free hemoglobin and an increase in markers of lipid peroxidation.
Elevated free hemoglobin in the airspace is not unique to this mouse model of acute lung injury. To determine the clinical significance of our findings, we measured free hemoglobin or isoprostanes/isofurans in the airspaces of two patient cohorts. Patients with acute lung injury had very high levels of free hemoglobin in the airspace compared to critically ill mechanically ventilated controls with cardiogenic pulmonary edema. Patients with diffuse alveolar hemorrhage had progressively increasing BAL isoprostanes and isofurans as their sequential BAL aliquots become more bloody. These findings suggest that liberation of free hemoglobin into the airspace and intraalveolar lipid peroxidation may be important mechanisms of clinical acute lung injury.
To confirm that the effects of LPS in LTF mice were a result of TF deficiency, we repeated our experiments in LTF mice and reconstituted lung TF by co-injection of a recombinant murine TF protein with LPS. In these experiments, TF replacement attenuated lung hemorrhage and permeability induced by LPS and augmented lung coagulation. However, mTF replacement had no effects on lung inflammation. These data suggest that free hemoglobin mediates the increased lung permeability and lipid peroxidation in LTF mice and can be reversed by TF replacement while TF replacement does not mediate changes in inflammation. Others have shown that circulating free hemoglobin mediates vascular permeability. In a murine model of sickle cell disease with extensive intravascular hemolysis, free hemoglobin induces endothelial permeability in the lungs. 28 Our study is the first to suggest that free hemoglobin in the airspace may be a potent mediator of lung permeability. Although we have not measured circulating free hemoglobin in our studies the fact that replacement of TF in the airspace attenuates lung hemorrhage as well as permeability suggest that it is the intra-alveolar hemoglobin that is mediating these effects. Admittedly our study did not specifically test the independent effects of free hemoglobin so it may be that free hemoglobin is simply a marker of increased permeability. In addition to it’s role in modulating free hemoglobin, the effects of TF deficiency on lung inflammation may be mediated through a different mechanism such as loss of PAR-2 signaling in LTF mice or lack of adequate intra-alveolar TF replacement. Understanding the mechanism of LTF effects on lung inflammation will be the focus of future studies.
The study has some limitations. First, by using mice with a global TF deficiency we were unable to identify which cell type or types are most critically involved in the lung response to IT LPS. However, our results do show that global TF deficiency does not protect against direct lung injury, and in fact enhances several indices of acute lung injury including BAL cytokines as well as BAL protein and histologic injury. Interestingly, LTF mice may have increased susceptibility to even mild insults. In our LTF mice treated with PBS we observed increased BAL IL-6 and IL-8, higher levels of BAL protein, and mild increases in histology score and BAL cell counts although not all of these findings were statistically significant. This response to a very mild insult, IT PBS, highlights the critical nature of TF in limiting lung injury and inflammation. Second, our studies were not designed to test whether the effects of TF deficiency are mediated through the procoagulant function of TF or whether the effects are modulated through noncoagulant functions of TF such as signaling effects through protease activated receptors. Along these lines, it is unknown whether human TF can signal through mouse PAR-2. While it is possible that the human protein cannot signal through murine PAR-2 we would not expect this to affect our results since we are studying LTF mice which would be expected to have reduced PAR-2 signaling as a result of TF deficiency. Our results do show that global TF deficiency leads to increased bleeding into the lung and a concomitant increase in lung injury. Finally, although we have shown that free hemoglobin and markers of lipid peroxidation are elevated in the lungs of LTF mice there may be additional mechanisms that explain the increased lung inflammation seen in these animals.
In summary, mice with a global deficiency of tissue factor are not protected from the effects of IT LPS as they have modestly increased lung inflammation, injury and permeability compared to LPS treated WT mice. Our data suggest that intra-alveolar bleeding in LTF mice in response to IT LPS may contribute to lung injury through the oxidant effects of free hemoglobin. Furthermore, we demonstrate that many of the indices of injury can be attenuated with IT TF replacement. These findings are relevant to human acute lung injury and highlight the complexity of coagulation pathways in the acutely injured lung. Two clinical trials related to acute lung inflammation have been completed using recombinant tissue factor pathway inhibitor (TFPI, Tifacogin®), one in severe sepsis 29 and one in severe community acquired pneumonia. 30 Neither of these trials showed a clinical benefit of TFPI. This study included patients with both direct and indirect causes of ALI and TF blockade may have different effects depending on the cause of ALI. Furthermore, the specific roles of TF in the lung and vascular compartment may be different as our TF replacement studies might suggest. Future studies will focus on compartmentalized manipulation of the TF pathway in both direct and indirect ALI.
Acknowledgments
Funding
K08 HL 090785 to JAB, T32 HL087738 to SCS, HL 105479 to WEL, NIH GM42056 to LJR, K24 HL103836 and American Heart Association Established Investigator Award to LBW.
The authors would like to thank Bill Zackert for his excellent technical assistance.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors have no conflicts of interest to disclosures
References
- 1.Gando S, Kameue T, Matsuda N, Hayakawa M, Morimoto Y, Ishitani T, et al. Imbalances between the levels of tissue factor and tissue factor pathway inhibitor in ARDS patients. Thromb Res. 2003;109(2–3):119–24. doi: 10.1016/s0049-3848(03)00151-8. [DOI] [PubMed] [Google Scholar]
- 2.Bastarache JA, Wang L, Geiser T, Wang Z, Albertine KH, Matthay MA, et al. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax. 2007;62(7):608–16. doi: 10.1136/thx.2006.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He X, Han B, Mura M, Li L, Cypel M, Soderman A, et al. Anti-human tissue factor antibody ameliorated intestinal ischemia reperfusion-induced acute lung injury in human tissue factor knock-in mice. PLoS One. 2008;3(1):e1527. doi: 10.1371/journal.pone.0001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welty-Wolf KE, Carraway MS, Ortel TL, Ghio AJ, Idell S, Egan J, et al. Blockade of Tissue Factor-Factor X binding attenuates sepsis-induced respiratory and renal failure. Am J Physiol Lung Cell Mol Physiol. 2005 doi: 10.1152/ajplung.00155.2005. [DOI] [PubMed] [Google Scholar]
- 5.Welty-Wolf KE, Carraway MS, Miller DL, Ortel TL, Ezban M, Ghio AJ, et al. Coagulation blockade prevents sepsis-induced respiratory and renal failure in baboons. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1988–96. doi: 10.1164/ajrccm.164.10.2105027. [DOI] [PubMed] [Google Scholar]
- 6.Carraway MS, Welty-Wolf KE, Miller DL, Ortel TL, Idell S, Ghio AJ, et al. Blockade of tissue factor: treatment for organ injury in established sepsis. Am J Respir Crit Care Med. 2003;167(9):1200–9. doi: 10.1164/rccm.200204-287OC. [DOI] [PubMed] [Google Scholar]
- 7.Miller DL, Welty-Wolf K, Carraway MS, Ezban M, Ghio A, Suliman H, et al. Extrinsic coagulation blockade attenuates lung injury and proinflammatory cytokine release after intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 2002;26(6):650–8. doi: 10.1165/ajrcmb.26.6.4688. [DOI] [PubMed] [Google Scholar]
- 8.Bastarache JA, Wang L, Wang Z, Albertine KH, Matthay MA, Ware LB. Intra-alveolar tissue factor pathway inhibitor is not sufficient to block tissue factor procoagulant activity. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L874–81. doi: 10.1152/ajplung.00372.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Bastarache JA, Wickersham N, Fang X, Matthay MA, Ware LB. Novel role of the human alveolar epithelium in regulating intra-alveolar coagulation. Am J Respir Cell Mol Biol. 2007;36(4):497–503. doi: 10.1165/rcmb.2005-0425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware LB, Bastarache JA, Wang L, Matthay MA. Modulation of Intra-alveolar Fibrin Deposition by the Alveolar Epithelium In Acute Lung Injury: Clinical and Experimental Evidence. Proc Am Thorac Soc. 2008;5(3):358–9. [Google Scholar]
- 11.Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest. 1998;101(3):560–9. doi: 10.1172/JCI814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen B, Holscher T, Sato Y, Pawlinski R, Mackman N. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105(7):2777–82. doi: 10.1182/blood-2004-09-3724. [DOI] [PubMed] [Google Scholar]
- 13.Pawlinski R, Pedersen B, Schabbauer G, Tencati M, Holscher T, Boisvert W, et al. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103(4):1342–7. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol. 2005;175(4):2598–605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- 15.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med. 2002;165(2):242–9. doi: 10.1164/ajrccm.165.2.2108087. [DOI] [PubMed] [Google Scholar]
- 16.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ., 2nd Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(26):16713–8. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts LJ. Analysis of F2-isoprostanes by gas chromatography mass spectrometry negative ion chemical ionization. In: Hensley K, Floyd RA, editors. Methods in Biological Oxidative Stress. Humana Press; 2003. pp. 33–39. [Google Scholar]
- 18.Drake TA, Cheng J, Chang A, Taylor FB., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. The American journal of pathology. 1993;142(5):1458–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Tang H, Ivanciu L, Popescu N, Peer G, Hack E, Lupu C, et al. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. The American journal of pathology. 2007;171(3):1066–77. doi: 10.2353/ajpath.2007.070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Till JW, Levi M, Bresser P, Schultz MJ, Gouma DJ, Boermeester MA. Early procoagulant shift in the bronchoalveolar compartment of patients with secondary peritonitis. The Journal of infectious diseases. 2006;194(9):1331–9. doi: 10.1086/508290. [DOI] [PubMed] [Google Scholar]
- 21.Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L514–21. doi: 10.1152/ajplung.00442.2002. [DOI] [PubMed] [Google Scholar]
- 22.Pawlinski R, Mackman N. Tissue factor, coagulation proteases, and protease-activated receptors in endotoxemia and sepsis. Crit Care Med. 2004;32(5 Suppl):S293–7. doi: 10.1097/01.ccm.0000128445.95144.b8. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157(3):175–88. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Nagy E, Eaton JW, Jeney V, Soares MP, Varga Z, Galajda Z, et al. Red cells, hemoglobin, heme, iron, and atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(7):1347–53. doi: 10.1161/ATVBAHA.110.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64(5):648–55. [PubMed] [Google Scholar]
- 26.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature medicine. 2002;8(12):1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Gladwin MT. Bad blood: the risks of red cell storage. Nature medicine. 2010;16(4):381–2. doi: 10.1038/nm0410-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh S, Fang T, Ofori-Acquah SF. Spatiotemporal Dysfunction of the vascular permeability barrier in transgenic mice with sickle cell disease. Anemia. 2012;2012:6. doi: 10.1155/2012/582018. Article ID 582018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290(2):238–47. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 30.Wunderink RG, Laterre PF, Francois B, Perrotin D, Artigas A, Vidal LO, et al. Recombinant tissue factor pathway inhibitor in severe community-acquired pneumonia: a randomized trial. American journal of respiratory and critical care medicine. 2011;183(11):1561–8. doi: 10.1164/rccm.201007-1167OC. [DOI] [PubMed] [Google Scholar]