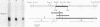Abstract
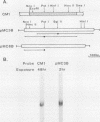
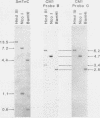
A chicken calmodulin pseudogene with no introns was previously shown to hybridize under stringent conditions with an mRNA species present in skeletal and cardiac muscles, yet it would not hybridize to calmodulin mRNA (J. P. Stein, R. P. Munjaal, L. Lagace', E. C. Lai, B. W. O'Malley, and A. R. Means, Proc. Natl. Acad. Sci. USA 80:6485-6489, 1983). Using the pseudogene as a probe, we isolated a full-length cDNA corresponding to this mRNA from a chicken breast muscle library and showed by sequence analysis that it encodes slow-muscle troponin C and not the pseudogene product. Hybridization between the calmodulin pseudogene and slow-muscle troponin C cDNA is due to a short region of high homology in those nucleotides that encode helices B and C of troponin C and calmodulin. Genomic Southern analysis showed the calmodulin pseudogene and the gene for slow-muscle troponin C to exist as distinct single copies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis G. F., Hieter P. A., McBride O. W., Swan D., Leder P. Processed genes: a dispersed human immunoglobulin gene bearing evidence of RNA-type processing. Nature. 1982 Mar 25;296(5855):321–325. doi: 10.1038/296321a0. [DOI] [PubMed] [Google Scholar]
- Keller L. R., Emerson C. P., Jr Synthesis of adult myosin light chains by embryonic muscle cultures. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1020–1024. doi: 10.1073/pnas.77.2.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagacé L., Chandra T., Woo S. L., Means A. R. Identification of multiple species of calmodulin messenger RNA using a full length complementary DNA. J Biol Chem. 1983 Feb 10;258(3):1684–1688. [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Bruns G. A., Morton C. C., Orkin S. H. The human phosphoglycerate kinase multigene family. HLA-associated sequences and an X-linked locus containing a processed pseudogene and its functional counterpart. J Biol Chem. 1985 Jun 10;260(11):6982–6992. [PubMed] [Google Scholar]
- Nojima H., Sokabe H. Structure of rat calmodulin processed genes with implications for a mRNA-mediated process of insertion. J Mol Biol. 1986 Aug 5;190(3):391–400. doi: 10.1016/0022-2836(86)90010-0. [DOI] [PubMed] [Google Scholar]
- Putkey J. A., Draetta G. F., Slaughter G. R., Klee C. B., Cohen P., Stull J. T., Means A. R. Genetically engineered calmodulins differentially activate target enzymes. J Biol Chem. 1986 Jul 25;261(21):9896–9903. [PubMed] [Google Scholar]
- Putkey J. A., Slaughter G. R., Means A. R. Bacterial expression and characterization of proteins derived from the chicken calmodulin cDNA and a calmodulin processed gene. J Biol Chem. 1985 Apr 25;260(8):4704–4712. [PubMed] [Google Scholar]
- Putkey J. A., Ts'ui K. F., Tanaka T., Lagacé L., Stein J. P., Lai E. C., Means A. R. Chicken calmodulin genes. A species comparison of cDNA sequences and isolation of a genomic clone. J Biol Chem. 1983 Oct 10;258(19):11864–11870. [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Daubas P., Akimenko M. A., Cohen A., Garner I., Guenet J. L., Buckingham M. A single locus in the mouse encodes both myosin light chains 1 and 3, a second locus corresponds to a related pseudogene. Cell. 1984 Nov;39(1):129–140. doi: 10.1016/0092-8674(84)90198-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. Regulation of muscle differentiation: isolation and purification of chick actin messenger ribonucleic acid and quantitation with complementary deoxyribonucleic acid probes. Biochemistry. 1980 May 27;19(11):2506–2514. doi: 10.1021/bi00552a032. [DOI] [PubMed] [Google Scholar]
- Stein J. P., Munjaal R. P., Lagace L., Lai E. C., O'Malley B. W., Means A. R. Tissue-specific expression of a chicken calmodulin pseudogene lacking intervening sequences. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6485–6489. doi: 10.1073/pnas.80.21.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cowan N. J. Diverse mechanisms in the generation of human beta-tubulin pseudogenes. Science. 1982 Aug 6;217(4559):549–549. doi: 10.1126/science.6178164. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cripe T. P., Gwo-Shu Lee M., Cowan N. J. Evidence that a human beta-tubulin pseudogene is derived from its corresponding mRNA. Nature. 1982 May 6;297(5861):83–84. doi: 10.1038/297083a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M. The amino acid sequence of troponin C from chicken skeletal muscle. FEBS Lett. 1976 Nov;70(1):254–256. doi: 10.1016/0014-5793(76)80769-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M. Troponin C from rabbit slow skeletal and cardiac muscle is the product of a single gene. Eur J Biochem. 1980 Jan;103(1):179–188. doi: 10.1111/j.1432-1033.1980.tb04302.x. [DOI] [PubMed] [Google Scholar]