Abstract
While plants are invariably attacked by numerous insects and pathogens, the consequences of multiple enemies for plant performance are poorly understood. In particular, a predictive framework is lacking for when to expect enemies to have independent versus non-independent effects on their host plant. This is problematic for weed biological control programmes where multiple enemies are frequently released with the possibility of antagonistic interactions that may reduce control. Here, we conduct an analysis of 74 unique plant–enemy–enemy combinations from 51 studies to determine the frequency of non-independent effects of natural enemies on host plant performance, and test a number of a priori predictions for determinants of independent and antagonistic effects of multiple enemies. For three-quarters of plant response measurements, enemies had independent effects on plant performance. In most of the remainder, multiple enemies led to less reduction in performance than that predicted from each enemy alone. Antagonistic effects occurred when enemies attacked the same plant part concurrently or attacked plant reproductive structures. These two predictors explained why antagonistic effects were particularly prevalent for weeds, plants in the family Asteraceae and enemies in the order Diptera. Our results suggest that a few simple rules about avoiding particular combinations of multiple enemies could improve biological control success.
Keywords: insect herbivores, plant pathogens, weed biological control, cumulative stress hypothesis, meta-analysis
1. Introduction
During their lifetime, almost all plants will be attacked by a range of natural enemies [1] that affect their survival, growth and/or reproduction [2]. Although the role of interactions among these multiple enemies in determining the total impact on their host plant may have major implications for plant populations and communities, it remains poorly understood [3]. If one enemy facilitates a second, their combined attack should reduce plant performance more than predicted from the individual attacks [4]. This synergistic response might occur if attack by the first enemy species improved the nutritional quality of the plant [5] or caused changes in plant phenology leading to increased attack by the second enemy species [6]. If one natural enemy negatively affects a second, their combined impact on plant performance is hypothesized to be less than that predicted from their individual effects [4]. This antagonistic response could be due to plant defences induced in response to the first species that negatively affect the second [7], through interference competition [8], or via changes in plant phenology that reduce attack by the second [9]. As negative interactions among phytophagous insects are the norm [10], we might expect a prevalence of antagonistic effects on plant performance. Negative interactions can also occur between arthropods and plant pathogens [4], and between plant pathogens [11].
How plants cope with multiple natural enemies is of particular interest in weed biological control. Here, multiple agents are often introduced to reduce populations of a particular weed [12], and agents also must interact with native generalists. Two broad rationales exist for introducing multiple biological control agents [13]: (i) the lottery model predicts that the introduction of more agents will more probably involve a successful agent [12], while (ii) the cumulative stress hypothesis predicts increased plant suppression with more agents, as small amounts of damage by individual species accumulate [14]. If multiple biological control agents are introduced, it would be optimal to introduce agents that will interact synergistically and counterproductive to introduce antagonistic agents. Negative interactions between agents may be a key reason for the occurrence of ‘revenge effects’ where control becomes less effective by the addition of another agent [15]. Biological control agents must also interact with native species, ideally to reduce plant performance. Predicting which types of agent interact to promote weed suppression will enable us to better select species for biological control introductions.
Two studies have previously collated evidence for non-independent effects of natural enemies on plant performance and reached opposing conclusions. A meta-analysis [16] found that on average natural enemies independently affected plant performance, but the substantial variance in responses among studies was not explained. On the other hand, a vote-counting study [17] found weak antagonistic effects on the shared host of insect herbivores and plant pathogens. While these studies were useful in establishing the overall pattern, they did not consider what traits of plants or enemies are linked to non-independent interactions. Without this knowledge, biological control programmes cannot predict the likelihood and direction of non-independent effects for any particular enemy combination. We are also not able to develop broad trait-based ecological theory of plant–enemy–enemy interactions. Our study considers numerous ecological hypotheses about how the traits, niches and taxonomic affiliations of both plants and their enemies affect the likelihood of non-independent interactions (see table 1 and electronic supplementary material). We also include more studies than previous analyses. Finally, an additional meta-analysis [10] found that the presence of one herbivorous insect significantly, albeit weakly, reduced the amount of damage caused by the second. However, here only the amount of damage to the plant was considered, neither the impact on plant performance nor the individual and combined effects of both insects on the plant as we have done.
Table 1.
Ability of experimental, plant and natural enemy attributes to explain variance in diff.RR and predict non-independent interactions. L is the statistic for the log-likelihood ratio test. Justification for inclusion of these variables can be found in the electronic supplementary material.
| variable and levels | significance in meta-analysis |
||
|---|---|---|---|
| explanatory ability |
enemies act independently at all levels? | ||
| L | p-value | ||
| response measured (density/reproductive/vegetative/survival) | 0.75 | 0.861 | yes |
| method of enemy manipulation (added/removed) | 1.22 | 0.270 | yes |
| guild or species manipulated | 1.13 | 0.288 | yes |
| experimental location (greenhouse/common garden/field) | 0.69 | 0.707 | yes |
| unit of replication (plot/plant) | 0.08 | 0.785 | yes |
| length of experiment (no. days) | 0.01 | 0.914 | n.a. |
| plant life-history (annual/biennial/perennial) | 4.69 | 0.096 | biennials: antagonistic |
| economic status (model organism/crop/weed/native) | 2.46 | 0.482 | weeds: antagonistic |
| plant functional group (forb/grass/shrub/tree/vine) | 0.49 | 0.975 | yes |
| woody or non-woody | 0.10 | 0.752 | yes |
| plant family | 18.77 | 0.547 | Asteraceae: antagonistic Ranunculaceae: synergistic |
| enemy grouping (two arthropods versus an arthropod and a plant pathogen versus two plant pathogens) | 0.23 | 0.893 | yes |
| interaction type (direct/indirect) | 3.25 | 0.072 | direct: antagonistic |
| no. of generalists present (0, 1, 2) | 0.98 | 0.612 | yes |
| both enemies in the same order | 0.03 | 0.862 | yes |
| both enemies in the same family | 0.02 | 0.882 | yes |
| both enemies in the same feeding guild | 0.006 | 0.936 | yes |
| no. of chewers present (0, 1, 2) | 1.64 | 0.441 | yes |
| no. of sap-suckers present (0, 1, 2) | 1.79 | 0.409 | yes |
| no. of miners present (0, 1, 2) | 0.26 | 0.879 | yes |
| reproductive plant parts attacked | 4.24 | 0.039 | present: antagonistic |
| leaves attacked | 0.85 | 0.356 | yes |
| roots attacked | 2.64 | 0.105 | absent: antagonistic |
| stems attacked | 0.0004 | 0.983 | yes |
| Coleoptera present | 0.009 | 0.926 | yes |
| Lepidoptera present | 0.84 | 0.358 | yes |
| Diptera present | 4.22 | 0.040 | present: antagonistic |
| Hemiptera present | 1.53 | 0.216 | yes |
| fungi present | 0.06 | 0.808 | yes |
| virus present | 0.35 | 0.554 | yes |
In this study, we ask three questions. First, we ask how prevalent are non-independent effects of two enemies on their shared host plant? Next, we perform a meta-analysis to ask whether, on average, natural enemies independently reduce plant performance? We then ask whether there are experimental factors, or attributes of plants or of the natural enemies associated with non-independence (table 1)? By identifying attributes of the species associated with non-independent impacts on host plants, we may be able to improve biological control success rates by improving selection of agents when multiple species are necessary.
2. Material and methods
(a). Data collection
We searched for papers that:
— involved two (or more) natural enemies on a focal plant species. We considered plant pathogens (fungal, viral and bacterial) and herbivorous insects and mites (‘arthropods’). While plant pathogens and arthropods are used as biological control agents, we did not restrict our search to potential or actual biological control systems; many of our species are crops and their pests or occur in natural systems;
— considered the effects of both enemies separately and together in a factorial experiment; observational studies were not included; and
— measured changes in individual plant performance—changes in vegetative parts, reproductive output or mortality. We also included measurements of plant density, although these were rare.
We initially used various search terms in Google Scholar (http://scholar.google.ca) to find data papers (including ‘herbiv*’, ‘interspecific’, ‘competition’, ‘direct interactions’, ‘indirect interactions’) but found little consistency in keywords used. After identifying a number of data papers and reviews in this manner, we found subsequent papers by exhaustively searching the citation network of each paper (i.e. papers that were cited by the focal paper or cited it). Papers were collected until 20 January 2011.
If factorial experiments tested more than two enemies, we considered them as a series of 2 × 2 interactions. There were too few studies to consider three-way interactions. We included studies that manipulated guilds of natural enemies (e.g. below-ground herbivores or defoliators) as guilds were typically dominated by one species. This increased our sample size when testing potential correlates. Studies based on guilds did not differ from those based on species (table 1).
Almost all papers measured multiple responses to determine the effects of both species on the plant. Each response was recorded. If the same response was measured multiple times, we took an overall measure if available (e.g. cumulative seed production), otherwise we used the final measurement (e.g. seed production in the final year).
(b). Prevalence of non-independent interactions
We used only studies in which experiments were analysed using a two-way ANOVA (the correct analysis for assessing independence). We categorized responses based on the model used (additive or multiplicative, see box 1). Then, we further categorized responses as independent (non-significant interaction term), synergistic (significant interaction term; greater than expected reduction in plant performance) or antagonistic (significant interaction term; less than expected reduction in plant performance).
Box 1. Effect of different statistical models for finding non-independent interactions.
Independent interactions can be identified statistically by a non-significant interaction term in a two-way ANOVA—independence is the null model. The alternatives, synergism and antagonism are identified by a significant interaction term followed by inspection of the summary data.
There are two basic null models to test whether enemies operate independently—the additive and multiplicative models. This has been appreciated in the predation literature [18], but not in the herbivory/plant pathogen literature, with the notable exceptions of [4,19]. Under the additive model, the null expectation is
where A and B are the amount of biomass removed by species A and species B, respectively, and AB is the amount removed when both species are present. However, if A removes 60 per cent of the biomass and B removes 70 per cent when each is alone, the expectation is that 130 per cent of biomass will be removed [4,18]. This conundrum is solved by using the multiplicative risk model [20] where the null expectation is
thus the expectation for the example above is that 88 per cent of biomass will be removed, a more realistic figure. The additive model is generated by conducting a two-way ANOVA without transforming the data; to generate the multiplicative model, data are simply log-transformed prior to analysis. Log-transformations have typically been used to achieve homoscedascity and normality rather than due to the underlying model.
We contacted authors to obtain raw data to determine how much null model choice matters to the identification of non-independence. We received data from 15 studies for this analysis (see the electronic supplementary material, table S1), providing us with 26 species combinations.
We re-ran analyses under both null models. Where necessary, 1 was added to the response variable. We ignored additional treatment conditions, and where three different herbivores were measured in a factorial design, we treated them as a series of two-way interactions. As such, our results cannot be compared with the results of the authors.
There were fewer cases of non-independence in this dataset than in the main study; 10.3 per cent of responses were non-independent under both models. However, in 5.1 per cent of cases, the overall conclusion of whether interactions were independent depended on model choice (assuming α = 0.05). The average change in F- and p-values was 1.38 and 0.16, respectively.
An appreciation of the different null models is needed; in approximately 5 per cent of cases model choice will fundamentally alter our interpretation. We recommend choosing the null model based on the natural history of the study system.
When studies tested natural enemy effects under different environmental conditions, we recorded only the natural enemy treatments. If separate ANOVAs were conducted on each level of the additional factor, we used the control condition or, if no control condition was indicated, we took the first presented.
Almost all studies took multiple measurements of plant performance. We determined the proportion of responses in each category (independent, synergistic and antagonistic) for each species combination and calculated the average proportion of responses in each category.
(c). Overall response and prediction of occurrence of non-independent effects: meta-analysis
(i). Data collation and data structure
Data were obtained from (in order of preference) the authors, tables in the paper or, by extraction from graphs using Plot Digitizer [21].
Some studies incorporated combinations of multiple species, often under different treatment conditions and almost all measured multiple responses. To incorporate this data structure, we ran mixed models using the R package nlme [22,23] with nested error terms: each response was nested within treatment condition, treatment was nested within species combination and species combination was nested within study.
(ii). Effect size calculation
We used log-response ratios for interactions under a multiplicative model [24]. Many ecological meta-analyses use log-response ratios; they require fewer parameters than Hedge's D allowing more studies to be incorporated [25]. In addition, Hedge's D is inherently an additive model, leading to the fundamental problem of testing for a reduction in plant performance of greater than 100 per cent (box 1, but see [16]).
For each plant performance variable (e.g. number of seeds), we calculated the difference between the reduction expected if reductions were independent and the actual reduction in plant performance.
The actual reduction in plant performance (‘log RR.AB’) was calculated as
where ‘AB’ is the mean of the response variable when both natural enemy species (A and B) were present.
To calculate the expected reduction in plant performance, we calculated the response ratio with each species alone (‘RR.A’ and ‘RR.B’)
where ‘A’ is the mean of the response variable with only species A present. A similar formula was used for species B.
We then calculated the expected reduction in plant performance (‘log RR.null’) following [24]:
We used the difference between the expected and actual values as our test statistic (‘diff.RR’). Positive values indicate a greater than expected reduction in plant performance and negative values indicate the reduction was less than expected
We could not calculate expected values under an additive null model owing to the combination of plant overcompensation and expectations of greater than 100 per cent reduction of plant performance. For a subset of individual studies (box 1), we found model choice changed the overall conclusions in approximately 5 per cent of cases, and our vote-counting results (below) showed minimal differences between the models.
(iii). Overall impact and response variables
We first tested whether the difference between the actual and expected log-response ratio was different from zero. We then tested a range of variables which we had an a priori reason to believe might be correlated with non-independent interactions (see table 1 and electronic supplementary material). Many of these variables have been shown to influence competitive interactions between herbivorous insects [10,26], so might predict the likelihood of non-independent effects on plant performance [4]. Although there has been little consideration of factors predicting how plant pathogens or pathogens and insects might interact, some of the broad ecological principles (e.g. niche overlap) demonstrated with insects might be expected to apply to these interactions.
We tested potential explanatory variables in two ways. First, we tested whether particular levels of the variable (e.g. enemies added to plants is a level of the ‘enemy manipulation’ variable) were associated with non-independent effects; if the 95% CIs of the diff.RR metric for that level included zero, there is an association with non-independent effects, either synergistic (diff.RR > 0) or antagonistic (diff.RR < 0).
Second, we tested whether different levels of the variables (e.g. enemies added versus removed of the ‘enemy manipulation’ variable) could explained variance in diff.RR by comparing models with the explanatory variable to the null model using log-likelihood ratios (L). If the log-likelihood test yields a significant p-value, the model containing the variable is a better fit to the data than the null model. Note that while a particular level of a variable may be significantly associated with non-independent effects (i.e. the mean and 95% CIs do not cross zero), the inclusion of the variable may not explain much variance if other levels of the variable have overlapping effects (i.e. the 95% CIs overlap).
Following identification of significant variables, we looked for situations (using Fisher's exact test) where there was a non-random association between the two significant variables, for example, were reproductive plant parts more commonly attacked when the plant was an Aster? When a significant association was found, we examined whether the significance of one variable was retained after prior inclusion of the other variable in the regression model.
3. Results
(a). Literature search
In total, we found 51 papers meeting our criteria, giving us 74 unique plant–enemy–enemy combinations (listed in electronic supplementary material, table S1). Our dataset contained 45 unique plant–arthropod–arthropod combinations, 22 plant–pathogen–arthropod combinations and seven plant–pathogen–pathogen combinations. Not all studies could be incorporated into both analyses.
(b). Prevalence of non-independent interactions
On average, 77 per cent of responses in each species combination had independent effects on plant performance under a multiplicative model, and 74 per cent had independent effects under an additive model. A total of 14–22% of responses (under a multiplicative and additive model, respectively) were antagonistic, i.e. multiple enemies had less effect on plant performance in combination than predicted. In the remainder, responses were synergistic.
(c). Overall response and prediction of non-independent effects: meta-analysis
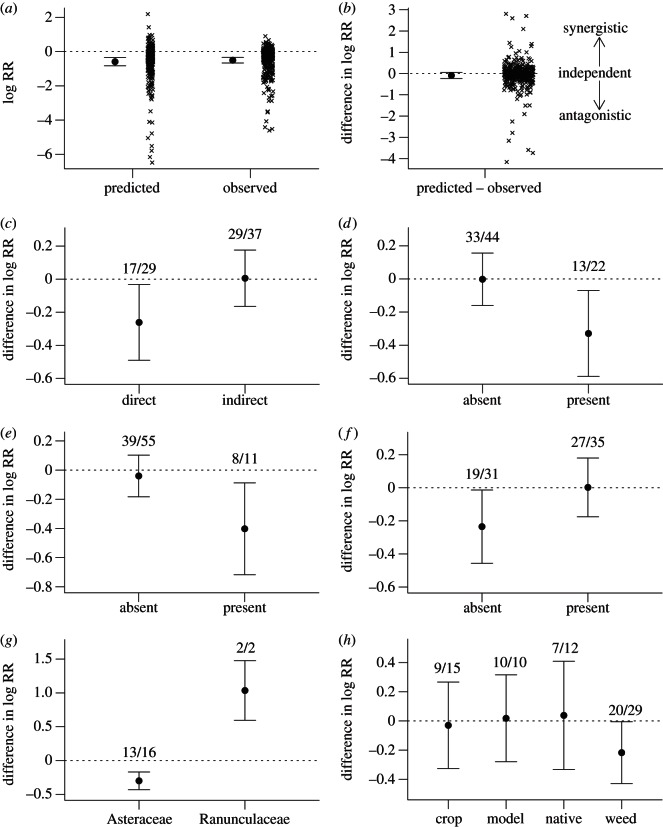
The meta-analysis confirmed the vote-counting results. When two species attacked a plant, plant performance did not differ from that predicted by their effects alone under the multiplicative model (F1,235 = 1.55; p = 0.215; figure 1a,b). The variance around the mean however is substantial, and understanding this variance is key to understanding the factors correlated with either antagonistic or synergistic effects.
Figure 1.
(a) Mean log-response ratio (log RR ± 95% CIs) for the observed reduction in plant performance when two species are present on a plant overlaps that predicted for the two species independently under the multiplicative model. Crosses represent individual measurements from the papers used. (b) Mean difference between predicted and observed log-response ratio (log RR ± 95% CIs) is not significantly different than zero, but tend to be more negative (antagonistic) than positive (synergistic). Crosses represent individual measurements. (c–f) Predictors associated with non-independent impacts on plant performance within the meta-analysis. The numbers above the error bars are the number of studies/number of species combinations. (c) Direct versus indirect interactions; (d) attack to reproductive plant parts (i.e. seeds, fruits and flowers); (e) attack by Diptera; (f) attack to roots; (g) attack to Asteraceae and Ranunulaceae; and (h) attack to plants of differing economic status. Note variation in y-axis scale.
We tested whether experimental factors or attributes of either the plants or natural enemies correlated with non-independent reductions in plant performance. Of the 32 variables examined, only seven were associated with significant non-independent effects of multiple enemies, almost all of which were antagonistic (table 1). Here, we highlight significant results—full results are presented in the electronic supplementary material.
The independence of multiple enemy effects depended on whether both natural enemies attack the same plant part concurrently (direct interactions) or differed in either plant part or time (indirect interactions, mediated through the host plant). Direct interactions were antagonistic, whereas indirect interactions were independent (figure 1c). Multiple enemy effects also depended on which part of the plant was attacked. Attack to seeds, fruits and flowers was significantly associated with antagonistic interactions (figure 1d), as was attack of both enemies to above-ground parts of the plant (i.e. neither attacked the roots; figure 1f).
Several taxonomic and life-history characteristics of the enemies and the host plant were also associated with non-independent effects. Antagonistic effects occurred when at least one of the enemies was a Diptera (figure 1e), and when the host plant was in the Asteraceae (figure 1g). Ranunculaceae hosts were significantly associated with synergistic effects (figure 1g), however, few conclusions can be drawn as this finding is based on only two studies. While biennial plants were associated with antagonistic effects, this result is driven by a study of seed-head predators on knapweeds [27]. Finally, plants categorized as weeds were associated with antagonistic effects (figure 1h).
There are two important caveats to the above results. First, we examined whether plant–enemy–enemy communities sharing a certain attribute (e.g. biennial life-history) had on average non-independent effects on plant performance, rather than asking if variance in multiple enemy effects on plant performance could be explained by differences in attributes between species combinations (e.g. annual versus biennial versus perennial). If the latter question is asked, only location of attack (whether reproductive parts are attacked, table 1) and the presence of Dipteran enemies (table 1) significantly explained the variance in results. In addition, interaction type (i.e. direct versus indirect interactions) and plant life-history were marginally significant (0.05 < p < 0.10; table 1).
Second, so far we have considered the explanatory variables separately, however, the variables can be correlated with each other (table 2). These covariance effects can be teased apart using multiple regression. For example, weeds were more commonly in the family Asteraceae and were significantly associated with direct interactions (table 2). However, as weeds were rarely attacked by root herbivores, these two characteristics were inversely associated (table 2). When added to a model already containing direct interactions, weeds were no longer significantly associated with antagonism (interaction type: F1,43 = 3.43, p = 0.07; weed: F1.43 = 1.01, p = 0.32), meaning that the significant association between antagonism and weeds is entirely driven by the covariation of weeds and interaction type. Similarly, both Asteraceae and Diptera were significantly associated with attack to reproductive structures (table 2). As the second variable in a model with reproductive structures, neither Asteraceae (reproductive parts: F1,44 = 4.56, p = 0.038; Asteraceae: F1,19 = 2.47, p = 0.13) nor Diptera (reproductive parts: F1,44 = 4.85, p = 0.033; Diptera: F1,20 = 2.82, p = 0.11) retained their significant association with antagonism. Finally, root attack is no longer significant when incorporated into a model following interaction type (interaction type: F1,43 = 3.39, p = 0.073; root attack: F1,43 = 0.79, p = 0.38) as root attack was almost always coupled with above-ground attack. By contrast, the significance of both interaction type and attack to reproductive structures was unaffected by inclusion of the second variable in models, indicating they drive the association with antagonistic interactions.
Table 2.
Fisher's exact tests for association between explanatory variables. Only explanatory variables with levels significantly associated with non-independent effects on plant performance are included in these analyses.
| direct interactions | reproductive structures attacked | roots attacked | Asteraceae | Diptera | |
|---|---|---|---|---|---|
| weeds | p = 0.0004 | p = 0.20 | p < 0.0001a | p = 0.041 | p = 1 |
| direct interactions | — | p = 0.12 | p < 0.001a | p = 0.58 | p = 0.52 |
| reproductive structures attacked | — | — | p = 0.44 | p = 0.035 | p = 0.004 |
| roots attacked | — | — | — | p = 1 | p = 0.32 |
| Asteraceae | — | — | — | — | p = 0.0027 |
aInverse association.
In summary, the primary predictors of antagonistic effects were direct interactions between enemies and attack to plant reproductive parts. Although other features of enemies and hosts are correlated with non-independent effects, these are largely realized through covariance with these two primary predictors. There are no robust predictors of synergism.
4. Discussion
Almost all plants must contend with attack from multiple natural enemies. Our analysis shows that, on average, multiple enemies reduce plant performance independently. This confirms the result of an earlier meta-analysis [16], but contrasts with conclusions of non-independent, antagonistic effects of multiple enemies on plant performance [17] and damage [10] in two other literature reviews.
Several reasons could explain why our results did not support the prediction that on average multiple enemies would have antagonistic effects on plant performance, i.e. that the overall impact would be less than predicted from the individual enemy impacts. First, strong effects of herbivores on each other's fitness (competition or facilitation) may not always translate into non-independent effects on plant performance. Much stronger negative effects of multiple insect herbivores on each other's fitness than on the amount of damage done have been documented and attributed to greater effects of plant quality than quantity in mediating herbivore interactions [10]. Second, the prevalence of independent effects of multiple natural enemies may reflect the often small influence of enemies on their host plants and the ability of plants to compensate for this damage. Third, unlike many earlier analyses (box 1), our meta-analysis used a multiplicative risk model in the context of a two-way ANOVA to assess independence, the most biologically meaningful assessment for answering these types of question. Finally, the prevalence of antagonistic effects of multiple enemies on reductions in plant performance may depend on the characteristics of the natural enemies and the plants. Indeed, non-independent interactions were common (approx. 25% of responses, predominantly antagonistic), and depended on two attributes of natural enemies.
Attack by directly interacting natural enemies (i.e. occurring on the same plant part at the same time) reduced plant performance less than predicted, whereas those that interacted indirectly (i.e. through changes in plant quality) had independent effects on plant performance. This agrees with traditional competition theory that predicts the strongest competitive interactions when species use resources concurrently [10], i.e. occur in the same niche. For example, rust mites and powdery mildew both attack leaf epidermal cells, so damage by rust mites to the leaf epidermal cells reduces the size of powdery mildew colonies [4]. Although herbivores can affect each other even when spatio-temporally separated [10,26], these indirect interactions did not translate into non-independent reductions in plant performance in our analysis. Interactions between above- and below-ground herbivores are variable indicating a high degree of context-dependency [28], and our analysis only found evidence for antagonistic effects on plant performance when both enemies were above-ground (figure 1f).
Antagonistic interactions were significantly associated with attack to reproductive parts. This could not be attributed to increased levels of interference competition [8,27] as attack to reproductive structures was not statistically associated with direct interactions. Optimal defence theory [29] predicts that defence compounds should be concentrated in more valuable plant parts, such as reproductive structures. If defence compounds generated in response to damage are concentrated in valuable reproductive parts, then such induced defences will result in antagonistic effects of enemies when at least one enemy attacks reproductive parts, as we found. Alternatively, enemy-induced desynchronization of flight times and flowering/fruiting phenology could be responsible for this antagonistic effect [30] although changes in phenology can also synchronize the plant and enemy [6].
We have shown, through multiple regression, that these two simple predictors—whether the interaction was direct and whether reproductive structures were attacked—explained the other correlates of antagonistic effects of multiple enemies. For example, antagonistic effects were also found when species combinations included a plant in the family Asteraceae or a Dipteran enemy, because both were associated with attacks to reproductive plants parts. Similarly, we found that antagonistic effects found for plants categorized as weeds could be explained by the association of weeds with direct interactions (i.e. same plant part at the same time). Attack to roots was associated with indirect interactions (spatial or temporal separation).
(a). Implications for weed biological control
Many biological control programmes release multiple enemies; up to 25 have been released against a single target [12]. Such a strategy is often justified by the hypothesis that each additional enemy increases the cumulative stress to the plant [14]. Our results may appear to support this general strategy in that the independent effects of most multiple enemies reduced plant performance overall. However, we caution that in most cases successful control (i.e. plant population decline) is attributable to one agent [12]. Furthermore, increasing the number of enemies increases the chance that some interactions will be non-independent, simply due to sampling effects [15]. For example, our vote-counting results suggest that if three enemy species are introduced (average number of agents introduced is 3.4 [12]), the chance of including an antagonistic interaction in each of the species pairs is 65 per cent—calculated as 0.863, where 0.86 is the approximate probability found in the vote-counting analysis of a random pair having a non-antagonistic interaction (i.e. independent or synergistic) under the multiplicative model.
The likelihood of non-independent effects can be predicted to some degree by considering the location of attack and whether the enemies directly interact. Direct interactions were associated with antagonistic interactions, supporting earlier calls to exclude direct competitors from biological control programmes [31]. For example, by simply excluding direct competitors the likelihood of avoiding antagonistic interactions increases to 68 per cent with three agents. Attack to reproductive structures was also associated with antagonistic interactions. It has been suggested that pre-dispersal seed predators might be poor biological control agents as weeds are rarely seed limited [32]. Here, we show that they also interact with other agents in an antagonistic manner; again if three species were to be introduced, by avoiding species that attack reproductive structure, the probability of preventing antagonistic interactions increases to 88 per cent.
We caution that our results are relevant only for predicting plant performance; it is less well understood how natural enemy attack to an individual plant scales up to plant population declines [2] and/or reductions in rates of spread. This reflects the larger problem of a lack of data on the long-term impacts of natural enemies on plant populations. Additionally, experiments are needed to assess if independent reductions in plant performance result in independent reductions in plant population density.
5. Conclusions
Natural enemies sharing a host plant tend to reduce plant performance independently. Approximately, one quarter of responses are non-independent; generally antagonistic. Non-independent responses can be predicted to some degree based on attributes of the enemies involved. Our results suggest that increasing the number of natural enemies in biological control programmes is a double-edged sword—although on average the net performance of plants may decrease, so does the likelihood of plant performance increasing owing to the inclusion of a species pair with strong antagonistic interactions. The latter effect could be partially reduced by two simple rules: (i) avoid introducing species that attack the same part of the plant at the same time, and (ii) assess the impact on plant performance of species attacking reproductive structures in conjunction with already existing agents.
Acknowledgements
We thank all those who kindly provided data for the comparison of different models (box) and/or provided summary data for the meta-analysis: Nick Barber; Esther Gerber; Kristen Hladun; Tamaru Hunt-Joshi; Scott Johnson; Rick Karban; David Knochel; Peter McEvoy; Charles Mitchell; Betty Owor, James Legg and International Institute of Tropical Agriculture; Katja Poveda; Ken Puliafico; Jennifer Rudgers; Lincoln Smith; Pete Turner. We also thank all those who looked unsuccessfully for data files. John Richardson, Charles Krebs and Peter McEvoy gave helpful comments on a draft of this manuscript. Funding was provided by a UBC 4yF to AEAS and NSERC grants to J.H.M. and D.S.S. All authors contributed to study design. A.E.A.S. collated and analysed the data with assistance from D.S.S. A.E.A.S. wrote the manuscript with assistance from both D.S.S. and J.H.M.
References
- 1.Thompson JN. 1998. Coping with multiple enemies: 10 years of attack on Lomatium dissectum plants. Ecology 79, 2550–2554 10.1098/rspb.2006.3587 (doi:10.1098/rspb.2006.3587) [DOI] [Google Scholar]
- 2.Maron JL, Crone E. 2006. Herbivory: effects on plant abundance, distribution and population growth. Proc. R. Soc. B 273, 2575–2584 10.1098/rspb.2006.3587 (doi:10.1098/rspb.2006.3587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strauss SY, Irwin RE. 2004. Ecological and evolutionary consequences of multispecies plant–animal interactions. Ann. Rev. Ecol. Evol. Syst. 35, 435–466 10.1146/annurev.ecolsys.35.112202.130215 (doi:10.1146/annurev.ecolsys.35.112202.130215) [DOI] [Google Scholar]
- 4.Fournier V, Rosenheim JA, Brodeur J, Diez JM, Johnson MW. 2006. Multiple plant exploiters on a shared host: testing for non-additive effects on plant performance. Ecol. Appl. 16, 2382–2398 10.1890/1051-0761(2006)016[2382:MPEOAS]2.0.CO;2 (doi:10.1890/1051-0761(2006)016[2382:MPEOAS]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 5.Johnson SN, Hawes C, Karley AJ. 2009. Reappraising the role of plant nutrients as mediators of interactions between root- and foliar-feeding insects. Funct. Ecol. 23, 699–706 10.1111/j.1365-2435.2009.01550.x (doi:10.1111/j.1365-2435.2009.01550.x) [DOI] [Google Scholar]
- 6.Masters GJ, Jones TH, Rogers M. 2001. Host-plant mediated effects of root herbivory on insect seed predators and their parasitiods. Oecologia 127, 246–250 10.1007/s004420000569 (doi:10.1007/s004420000569) [DOI] [PubMed] [Google Scholar]
- 7.Bezemer TM, Wagenaar R, Van Dam NM, Wäckers FL. 2003. Interactions between above- and belowground insect herbivores as mediated by the plant defense system. Oikos 101, 555–562 10.1034/j.1600-0706.2003.12424.x (doi:10.1034/j.1600-0706.2003.12424.x) [DOI] [Google Scholar]
- 8.Juenger T, Bergelson J. 1998. Pairwise versus diffuse natural selection and the multiple herbivores of scarlet gilia, Ipomopsis aggregata. Evolution 52, 1583–1592 10.2307/2411332 (doi:10.2307/2411332) [DOI] [PubMed] [Google Scholar]
- 9.Milbrath LR, Nechols JR. 2004. Indirect effect of early-season infestations of Trichosirocalus horridus on Rhinocyllus conicus (Coleoptera: Curculionidae). Biol. Control 30, 95–109 10.1016/j.biocontrol.2003.09.017 (doi:10.1016/j.biocontrol.2003.09.017) [DOI] [Google Scholar]
- 10.Kaplan I, Denno RF. 2007. Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecol. Lett. 10, 977–994 10.1111/j.1461-0248.2007.01093.x (doi:10.1111/j.1461-0248.2007.01093.x) [DOI] [PubMed] [Google Scholar]
- 11.Power AG. 1996. Competition between plant viruses in a complex plant-pathogen system. Ecology 77, 1004–1010 10.2307/2265571 (doi:10.2307/2265571) [DOI] [Google Scholar]
- 12.Denoth M, Frid L, Myers JH. 2002. Multiple agents in biological control: improving the odds? Biol. Control 24, 20–30 10.1016/S1049-9644(02)00002-6 (doi:10.1016/S1049-9644(02)00002-6) [DOI] [Google Scholar]
- 13.Swope SM, Parker IM. 2010. Trait-mediated interactions and lifetime fitness of the invasive plant Centaurea solstitialis. Ecology 91, 2284–2293 10.1890/09-0855.1 (doi:10.1890/09-0855.1) [DOI] [PubMed] [Google Scholar]
- 14.Harris P. 1981. Stress as a strategy in the biological control of weeds. In Beltsville Symp. in Agricultural Research. 5. Biological control in crop production (ed. Papavizas G.), May 1980, Beltsville, MA, pp. 333–340 Totowa, NY: Allanheld, Osmun [Google Scholar]
- 15.McEvoy PB, Coombs EM. 1999. Biological control of plant invaders: regional patterns, field experiments, and structured population models. Ecol. Appl. 9, 387–401 10.1890/1051-0761(1999)009[0387:BCOPIR]2.0.CO;2 (doi:10.1890/1051-0761(1999)009[0387:BCOPIR]2.0.CO;2) [DOI] [Google Scholar]
- 16.Morris WF, et al. 2007. Direct and interactive effect of enemies and mutualists on plant performance: a meta-analysis. Ecology 88, 1021–1029 10.1890/06-0442 (doi:10.1890/06-0442) [DOI] [PubMed] [Google Scholar]
- 17.Hatcher PE. 1995. Three-way interactions between plant pathogenic fungi, herbivorous insects and their host plants. Biol. Rev. 70, 639–694 10.1111/j.1469-185X.1995.tb01655.x (doi:10.1111/j.1469-185X.1995.tb01655.x) [DOI] [Google Scholar]
- 18.Sih A, Englund G, Wooster D. 1998. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 13, 350–355 10.1016/S0169-5347(98)01437-2 (doi:10.1016/S0169-5347(98)01437-2) [DOI] [PubMed] [Google Scholar]
- 19.Willis AJ, Ash JE, Groves RH. 1993. Combined effects of two arthropod herbivores and water stress on growth of Hypericum species. Oecologia 96, 517–525 10.1007/BF00320509 (doi:10.1007/BF00320509) [DOI] [PubMed] [Google Scholar]
- 20.Soluk DA, Collins NC. 1988. Synergistic interactions between fish and stoneflies: facilitation and interference among stream predators. Oikos 52, 94–100 10.2307/3565987 (doi:10.2307/3565987) [DOI] [Google Scholar]
- 21.Huwaldt JA. 2010. Plot digitizer. See http://plotdigitizer.sourceforge.net/.
- 22.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 23.Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team 2011. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–101. See http://cran.r-project.org/web/packages/nlme/index.html [Google Scholar]
- 24.Darling ES, Côté IM. 2008. Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286 10.1111/j.1461-0248.2008.01243.x (doi:10.1111/j.1461-0248.2008.01243.x) [DOI] [PubMed] [Google Scholar]
- 25.Hedges LV, Gurevitch J, Curtis PS. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2) [DOI] [Google Scholar]
- 26.Denno RF, McClure MS, Ott JR. 1995. Interspecific interactions in phytophagous insects: competition revisited and resurrected. Annu. Rev. Entomol. 40, 297–331 10.1146/annurev.en.40.010195.001501 (doi:10.1146/annurev.en.40.010195.001501) [DOI] [Google Scholar]
- 27.Smith L, Mayer M. 2005. Field cage assessment of interference among insects attacking seed heads of spotted and diffuse knapweed. Biocontrol Sci. Technol. 15, 427–442 10.1080/09583150400016902 (doi:10.1080/09583150400016902) [DOI] [Google Scholar]
- 28.Johnson SN, Clark KE, Hartley SE, Jones TH, McKenzie SW, Koricheva J. 2012. Aboveground-belowground herbivore interactions: a meta-analysis. Ecology 93, 2208–2215 10.1890/11-2272.1 (doi:10.1890/11-2272.1) [DOI] [PubMed] [Google Scholar]
- 29.Zangerl AR, Rutledge CE. 1996. The probability of attack and patterns of constitutive and induced defense: a test of optimal defense theory. Am. Nat. 147, 599–608 10.1086/285868 (doi:10.1086/285868) [DOI] [Google Scholar]
- 30.Poveda K, Steffan-Dewenter I, Scheu S, Tscharntke T. 2003. Effects of below- and above-ground herbivores on plant growth, flower visitation and seed set. Oecologia 135, 601–605 [DOI] [PubMed] [Google Scholar]
- 31.Story JM, Boggs KW, Good WR, Harris P, Nowierski RM. 1991. Metzneria paucipunctella Zeller (Lepidoptera: Gelechiidae), a moth introduced against spotted knapweed: its feeding strategy and impact on two introduced Urophora spp. (Diptera: Tephritidae). Can. Entomol. 123, 1001–1007 10.4039/Ent1231001-5 (doi:10.4039/Ent1231001-5) [DOI] [Google Scholar]
- 32.Myers JH, Risley C. 2000. Why reduced seed production is not necessarily translated into successful biological weed control. In Proc. X Int. Symp. on Biological Control of Weeds (ed. Spencer NR.), 4–14 July 1999, Bozeman, MT, pp. 569–581 Bozeman, MT: Montana State University [Google Scholar]