Abstract
Daily patterns in the foraging behaviour of birds are assumed to balance the counteracting risks of predation and starvation. Predation risks are a function of the influence of weight on flight performance and foraging behaviours that may expose individuals to predators. Although recent research sheds light on daily patterns in weight gain, little data exist on daily foraging routines in free-living birds. In order to test the predictions of various hypotheses about daily patterns of foraging, we quantified the activity of four species of passerines in winter using radio-frequency identification receivers built into supplemental feeding stations. From records of 472 368 feeder visits by tagged birds, we found that birds generally started to feed before sunrise and continued to forage at a steady to increasing rate throughout the day. Foraging in most species terminated well before sunset, suggesting their required level of energy reserves was being reached before the end of the day. These results support the risk-spreading theorem over a long-standing hypothesis predicting bimodality in foraging behaviour purportedly driven by a trade-off between the risks of starvation and predation. Given the increased energetic demands experienced by birds during colder weather, our results suggest that birds' perceptions of risk are biased towards starvation avoidance in winter.
Keywords: foraging behaviour, starvation–predation trade-off, risk-spreading theorem, resident birds, radio-frequency identification, optimal foraging
1. Introduction
Selection should act on organisms to reduce the risk of predation while foraging. The trade-off between foraging and the risk of predation should, at times, lead organisms to choose foods, foraging areas or feeding times that would be suboptimal in terms of minimizing starvation risk alone [1]. Models predicting daily foraging patterns of birds often assume a balance between the risk of starvation and the risk of predation because of the opposite effects that these two risks have in defining optimal energy reserves [2–5]. Carrying large energy reserves in the form of fat reduces the risk of starvation if food supplies are interrupted, but this strategy may be maladaptive if too much fat were deposited because increasing weight decreases flight speeds and manoeuvrability, and exposes a bird to an increased risk of predation [2,6]; but also see [7].
Foraging behaviour is clearly affected by the perceived risk of predation; recent evidence demonstrates that feeding locations are selected based on a trade-off between foraging efficiency and the need to minimize predation risk [8]. Further, birds demonstrate short-term plasticity in feeding behaviour in response to changes in the risks of starvation [9] and predation [10]. Several studies confirm that birds decrease body weight in response to a simulated increase in predation pressure [11–15], presumably because lower wing loading enhances flight performance and, thus, reduces predation risk. Even in the absence of predation risk, carrying excess fat increases metabolic costs because additional flight muscles must be maintained to support the fat reserves [16,17].
Models of optimal foraging behaviour that incorporate the risks of predation and starvation often predict a bimodal pattern in feeding throughout the day. An early morning peak in foraging activity is predicted to replenish energy reserves depleted during the previous night of fasting, thus reducing the likelihood of starvation. Following this early morning peak in activity, an extended period of relative inactivity is predicted as birds maintain low-energy reserves and avoid exposure to predators. Finally, a second peak in foraging activity is predicted late in the day as birds build energy reserves in preparation for the coming night ([4,18]; figure 1).
Figure 1.
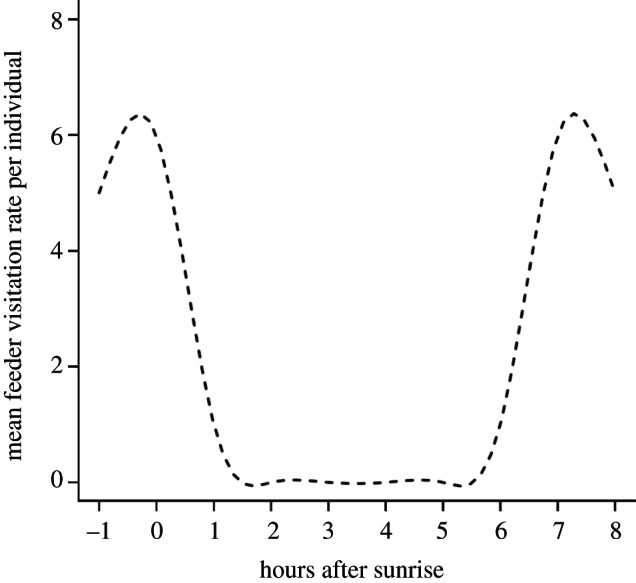
Theoretical depiction of the bimodal relationship between foraging intensity and time of day.
If these models are correct, then under the bimodal foraging hypothesis, birds should limit intensive feeding activity to times when the risk of starvation outweighs the risk of predation: following dawn and approaching dusk. This bimodal pattern would be adaptive in environments where energy can be accumulated quickly and where resources are predictable, for instance, where birds have access to supplemental food [4].
Alternatively, if predation pressures minimally influence daily foraging patterns, or if the impairment caused by carrying large fat reserves is small, then birds should focus on limiting the risk of starvation by rebuilding energy reserves at daybreak and continuing to feed throughout the day. Indeed, the risk-spreading theorem predicts constant foraging activity in the absence of complicating factors that may influence predation risk [1]. Building fat reserves in order to provide a buffer against possible interruptions to the food supply or during periods of harsh environmental conditions is adaptive in the absence of mass-dependent mortality [4,19]. Under the risk-spreading theorem, birds will continue to forage until they reach a critical threshold of stored energy. Further, birds should feed more intensively on colder days because more energy is required to maintain body temperature and more energy should be stored for the upcoming night [18].
Empirical evidence demonstrating patterns of weight gain (a proxy for foraging activity) has shown variable results. In a study of free-living blackbirds (Turdus merula), birds added the bulk of their daily weight in the first 3 h after sunrise [20]. A study of hoarding species in Sweden also demonstrated an early morning peak in weight gain, followed by consistent gains for the remainder of the day [21]. Another study showed a relatively constant rate of daily weight gain in small passerines [22], suggesting that predation risk was either constant throughout the day or not a strong driver of foraging behaviour. When great tits (Parus major) were exposed to increased perceived predation risk, birds adjusted weight gain patterns towards a late-day peak, presumably maintaining low daily fat levels in an effort to avoid predators [10]. Captive great tits, free from predation pressure, demonstrated relatively constant rates of weight gain throughout the day [23].
While these studies of weight gain are important and informative, predation risk is a function of both fat load (affecting flight performance) and feeding activity (affecting exposure to predators). Although the above mentioned studies have documented patterns of weight gain, direct measurement of daily foraging patterns of known free-living birds has been poorly detailed and insufficient to test and refine predictive models or make detailed inferences about different theoretical possibilities. Studies of foraging behaviour are impaired by the difficulties of continuously observing the activities of individual birds. As McNamara et al. [4, p. 297] noted, ‘It is not easy to compare these [model] results to the available observations because there has been little in the way of systematic investigation of foraging intensity’. Here, we quantify temporal changes in the daily foraging behaviour of individual, free-living birds in winter by tracking all feeding activity of birds at supplemental feeding stations. Using radio-frequency identification (RFID) and passive integrated transponder (PIT) technology to continuously monitor individuals, we document daily feeding behaviours in four species. Our objective was to quantify the daily feeding patterns of passerines in winter as an empirical test of existing models of optimal foraging behaviour. Specifically, we test whether feeding occurs during early- and late-day peaks in activity (bimodality) consistent with a trade-off between the risks of predation and starvation, or whether feeding is more continuous, consistent with the risk-spreading theorem.
2. Material and methods
We studied the feeding activity of black-capped chickadees (Poecile atricapillus, n = 45), tufted titmice (Baeolophus bicolor, n = 29), white-breasted nuthatches (Sitta carolinensis, n = 13) and house finches (Haemorhous mexicanus, n = 7) in two isolated woodlots near Ithaca, NY, USA (42°27′37″ N, 76°27′08″ W) over two winter seasons between December 2009 and January 2011. Accipiter hawks, primary predators of our focal species, were frequently seen in the woodlots during the study. A PIT tag (2 × 12 mm, 0.1 g) was attached to a leg band of each focal bird. Feeding stations (n = 8) were equipped with RFID data loggers [24]. Each time a PIT-tagged bird landed on a feeder, its unique identification number, date and time of visit (to the nearest second) were recorded by the RFID reader. Feeders were designed such that only one bird could visit at a time, ensuring that nearly all visits were recorded. Feeders were continuously filled with black oil sunflower seed. No other sources of supplemental food were available in the woodlots.
To model patterns of hourly feeder visitation in our data, we used generalized linear mixed models (GLMM) with a Poisson error distribution to model the total number of feeder visits within an hour as a function of time of day. Individual band number (nested within feeder location) was treated as a random factor to control for the repeated nature of the observations. We modelled hour since sunrise as a fixed effect to estimate hourly feeder visitation throughout the day. Owing to evidence of overdispersion in our data, we added an additional per-observation-level random effect (i.e. a Poisson-lognormal distribution, [25]) to more accurately estimate regression coefficients and standard errors that would otherwise be biased in the presence of overdispersion [26]. We used maximum-likelihood estimation for parameter estimation. We limited our analysis to data from December and January of each year to maintain consistency in photoperiod (approx. 9 h daylight), and pooled feeder visitation data across the two sampling years.
We created five regression models describing different patterns of feeder visitation rates in order to test predictions from the hypotheses of foraging behaviour. In each case, the response variable was the number of feeder visits per individual per hour, and sunrise was set to hour = 0. In the first model (Linear), we modelled hourly feeder use throughout the day as a linear relationship testing for a constant (or linearly increasing or decreasing) pattern in feeder visitation. Hour was modelled as a continuous fixed effect; results endorsing this model would be most consistent with the risk-spreading theorem. In the Bimodal model, we classified time of day into three bouts of activity occurring in the morning (hours 1–2), mid-day (hours 3–5) and evening (hours 6–8) periods, testing for bimodality in foraging behaviour as predicted by the bimodal foraging hypothesis. The Sunrise/Sunset model allowed for more complexity in the feeding patterns by categorizing time of day into five separate time periods (pre-sunrise = hour 1, morning = hours 0–2; mid-day = hours 3–5; late afternoon = hours 6–7; sunset = hour 8). The Discrete model allowed for arbitrary and nonlinear patterns of change in feeding activity by modelling foraging rates within each hour as being independent among the time steps. This model was the most complex as time of day was left as a categorical variable using all 10 hourly periods (including one hour before sunrise). In the final (Null) model, feeding activity did not vary with time since sunrise. These models represented a set of candidate approximating models of feeder visitation patterns; we evaluated fit using model selection methods. All approximating models were ranked using Akaike information criterion (AIC) and model weights; models with ΔAIC which differed by less than 2 were deemed to be equivalent [27].
To examine whether daily feeder use was related to temperature, we analysed data from the nearest weather station (1.56 km distant; Northeast Regional Climate Center station: 42°27′0″ N, 76°27′0″ W). We averaged hourly temperatures (°C) for each day to estimate mean daily temperature. Daily feeding rates were calculated as the total number of feeder visits for each individual. We used GLMM with a Poisson error distribution to model the total number of feeder visits within a day as a function of daily temperature. Individual band number (nested within feeder location) was treated as a random factor because the same individual birds were repeatedly visiting feeders. Average daily temperature was modelled as a linear fixed effect. For visualization purposes, we used general additive mixed modelling (GAMM) to plot the relationship between daily visitation rates and average daily temperature using package gamm4 [28] in R (v. 2.10.1; [29]).
Data used in these analyses are archived with Data Dryad (http://datadryad.org) under doi:10.5061/dryad.kn543.
3. Results
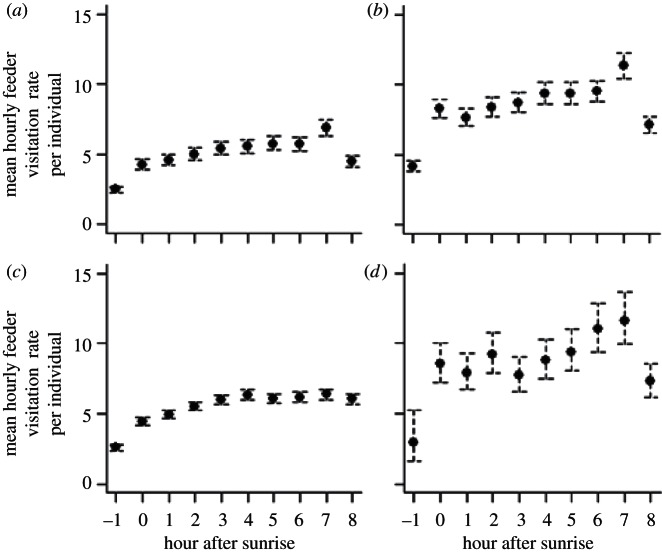
We recorded 472 368 unique feeder visits over 123 days (black-capped chickadee = 181 057; tufted titmouse = 206 595; white-breasted nuthatch = 61 384 and house finch = 23 332). For all four species, the Discrete model had the strongest support (table 1) indicating that feeder visitation varied from hour to hour but in a more complex manner than could be described by any of the simpler models in our set. Visitation rates generally increased throughout the day with no observable bimodality in feeding rates (figure 2). For all four species, feeder visitation generally began before sunrise when visitation rates were approximately half the rates following sunrise. Visitation rates generally increased from hour to hour throughout the day and peaked approximately 2 h before sunset, suggesting that birds were not feeding at their maximal possible rate for the bulk of the day. For three out of four species, we found a sharp decline in feeder visitation in the last hour before sunset. The exception to this pattern was the white-breasted nuthatch; individuals of this species typically continued visiting feeders until sunset. The only other model with support (ΔAIC < 2) was for house finches, with the Sunrise/Sunset model suggesting a discrete early morning (pre-sunrise) and sunset period of decreased activity (table 1).
Table 1.
Model selection results testing the hourly patterns of feeder visitation for black-capped chickadee, tufted titmouse, white-breasted nuthatch and house finch.
| species | model | AIC | k | ΔAIC |
|---|---|---|---|---|
| black-capped chickadee | Discrete | 50496.3 | 13 | 0 |
| Sunrise/Sunset | 50702.5 | 8 | 206.2 | |
| Linear | 51659.9 | 5 | 1163.6 | |
| Biomodal | 51773.1 | 6 | 1276.8 | |
| Null | 52721.5 | 4 | 2225.3 | |
| tufted titmouse | Discrete | 42308.0 | 13 | 0 |
| Sunrise/Sunset | 42404.0 | 8 | 96.0 | |
| Linear | 43207.6 | 5 | 899.6 | |
| Bimodal | 43323.5 | 6 | 1015.5 | |
| Null | 43796.3 | 4 | 1488.4 | |
| white-breasted nuthatch | Discrete | 16914.9 | 13 | 0 |
| Sunrise/Sunset | 16950.6 | 8 | 35.6 | |
| Bimodal | 17099.8 | 6 | 184.8 | |
| Linear | 17101.5 | 5 | 186.6 | |
| Null | 17337.8 | 4 | 422.9 | |
| house finch | Discrete | 4805.2 | 13 | 0 |
| Sunrise/Sunset | 4806.7 | 8 | 1.5 | |
| Bimodal | 4844.9 | 6 | 39.7 | |
| Linear | 4849.5 | 5 | 44.3 | |
| Null | 4860.5 | 4 | 55.3 |
Figure 2.
Predicted mean hourly feeder visitation rates per individual based on a generalized linear mixed model (GLMM). Feeding activity generally increased throughout the day, with a sharp decline in activity as sunset approached for three of four species. Results for (a) black-capped chickadee, (b) tufted titmouse, (c) white-breasted nuthatch and (d) house finch.
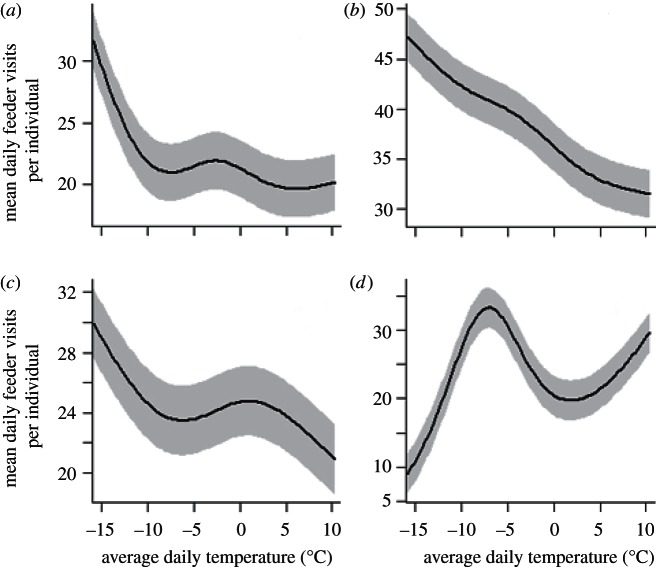
Total daily feeding activity generally increased on colder days (figure 3). The total number of daily visits increased as average temperature declined for white-breasted nuthatch (β =−0.004 ± 0.0008; p < 0.0001), black-capped chickadee (β =−0.010 ± 0.0005; p < 0.0001), tufted titmouse (β =−0.016 ± 0.00047; p < 0.0001) and house finch (β =−0.006 ± 0.0015; p < 0.0001).
Figure 3.
Predicted mean daily feeder visitation rates per individual as a function of average daily temperature (°C) based on the GAMM models. Three of four species visited feeders more often on colder days. Model results for (a) black-capped chickadee, (b) tufted titmouse, (c) white-breasted nuthatch and (d) house finch. The shaded regions represent 95% CIs.
4. Discussion
Our use of PITs and RFID-enabled bird feeders to quantify the feeding activity of free-living birds has generated a heretofore unprecedented dataset on feeding behaviour by individuals. The long-standing bimodal foraging hypothesis, proposed by models of optimal foraging behaviour for birds exposed to the risk of predation [4,5], was not supported by the activity patterns of birds in our population. Rather, we showed that birds fed relatively continuously throughout the day and terminated feeding abruptly as sunset approached. Given the extreme energetic demands faced by small passerines in temperate climates in winter, the balance between avoiding the risks of starvation and predation may be skewed towards continuous feeding in an effort to avoid starvation. Indeed, models predict that if energetic demands are sufficiently strong, feeding and weight gain may continue throughout the day [18]. In a study of five species of tits (Parus sp. and Poecile sp.) in Norway, Haftorn noted that, during December, tits foraged intensively from before sunrise until after sunset [30]. Thus, short winter days may not offer the possibility of a lull in foraging activity predicted by the bimodal foraging hypothesis.
Our results and the limited previous descriptions of foraging patterns provide support for the risk-spreading theorem over the bimodal foraging hypothesis. Observations of hummingbirds in a laboratory environment demonstrated that the birds accumulated energy reserves at a relatively constant rate throughout the day and failed to show peaks in activity in the early morning or prior to dusk [19]. Free-living white-browed babblers (Pomatostomus superciliosus) in Australia fed throughout the day with the proportion of each hour spent foraging increasing as the day progressed [31]. Similarly, black-capped chickadees in WI, USA, were recorded using feeders at an increasing rate throughout the day, with greatest visitation rates found within 2 h of sunset [32], a pattern similar to that found in the current study. The opposite pattern (decreasing rate of foraging throughout the day), however, was found in yellowhammers (Emberiza citrinella) in Sweden during some (but not all) winter months [33].
For hoarding species, the food predictability conferred by retrieving caches late in the day should lead to the greatest weight gain immediately before sunset if exposure to predation pressure increases the risk of earlier gains. Evidence from daily weight gain patterns in the hoarding willow tit (Poecile montanus), marsh tit (Poecile palustris) and European nuthatch (Sitta europaea), however, fail to show the predicted late-day peak [21]. Rather, daily weight gain patterns in this Swedish study revealed an initial peak in weight gain after sunrise followed by a relatively constant rate of gain for the remainder of the day [21]. This weight gain data matches well with the actual feeding activity demonstrated in our study. Continuous feeding, starting before sunrise, will result in an initial rapid increase in weight. The rate of gain will be lower for the remainder of the day as birds process earlier meals and defaecate (lose weight) while continuing to forage and gain weight.
Indeed, our results generally support the risk-spreading theorem with the exception of the apparent early termination in foraging activity near the end of the day when birds could continue to build reserves before nightfall. We find the peak in feeding activity 2 h before sunset to be particularly interesting; this pattern demonstrates that birds were not feeding at a maximal rate throughout the day as would be expected if starvation risk alone was dictating feeding behaviour. This late-day peak in foraging activity implies that birds were restricting foraging behaviour earlier in the day, possibly in response to a low but non-trivial predation risk. Birds, therefore, may be spreading foraging risk throughout the day until engaging in a late bout of increased foraging activity in order to minimize the risk of overnight starvation. Following this bout of increased foraging behaviour, they rapidly decrease their activity as dusk approaches. The early termination of feeding under lighting conditions that allow for continued feeding may imply either satiation or an increase in predation risk later in the day. The potential for increased predation risk could be related to the relatively high weight achieved by birds after a day of foraging or to an increase in predator activity at the end of the day. Crepuscular and nocturnal predators such as owls often begin foraging before sunset, and this potential addition to the predator community could increase the risk of predation such that late-day foraging is no longer offset by the fitness gains of continued feeding. Previous research on wild zebra finches (Taeniopygia guttata) during the breeding season [34] and dark-eyed juncos (Junco hyemalis) during winter [35] also demonstrated an early termination to feeding, with birds relinquishing more than an hour of potential feeding time in the evening. Predation pressure is probably not constant over the course of a day [36], and a consistent late-day peak in predator activity (e.g. if owls are primary predators of our focal species) could explain the current results. Indeed, different foraging strategies may arise because birds are exposed to various foraging constraints, predation risks, or abilities to compensate for various risks [20]. A recent study of weight regulation in great tits even demonstrated a complex interaction between predation pressure and temperature, with greater predation pressure associated with lower average weight in cold winters and greater weight in warmer winters [20]. Efforts at modelling optimal foraging routines continue to evolve [37], and further studies of fattening patterns [10] and foraging behaviour (current study) in free-living populations will inform future models.
Although we did not find evidence in support of the bimodal foraging hypothesis, some empirical evidence does suggest that bimodal activity patterns do exist under certain conditions. Captive rock finches (Lagonostica sanguinodorsalis) demonstrated a bimodal pattern in daily feeding activity regardless of predation risk, with weight increasing rapidly in the last 3 h of the day [38]. Captive zebra finches also demonstrated bimodality in feeding patterns in the absence of predation pressure [39]. Bimodality was observed in a study of sparrows (Passer domesticus) where the abundance of birds at feeders during the morning peak was 1.6 times that recorded during the mid-day lull, and the afternoon peak abundance was 1.9 times that of the mid-day lull [40]. Similarly, observations of wild flocks of white-crowned sparrows (Zonotrichia leucophrys) in winter indicated that diurnal feeding patterns followed a bimodal pattern with peaks immediately after sunrise and before sunset [41]. Previous studies attempting to quantify daily foraging routines, however, have often recorded the numbers of individuals feeding in unmarked populations. Quantifying differences in the foraging activity of known individuals at different times of the day over extended periods of time (current study) has not been feasible previously.
One drawback of our approach is that we were limited to examining the use of supplemental food. Birds were certainly feeding on natural and cached food supplies, but this foraging activity was missed given the limitations of our tracking technology [42]. By focusing on a population with access to supplemental food, however, we were able to quantify feeding behaviours while controlling for scarcity. Foraging models suggest that if the food supply is predictable, then birds can wait until the end of the day to feed, thereby minimizing weight (and hence, predation risk) while still maintaining the ability to quickly acquire necessary resources before nightfall [3,43]. Indeed, the nature of stochasticity in the food supply turns out to be crucial for modifying model predictions [4]. Despite the fact that birds in our population had unrestricted access to predictable, high-energy food, we failed to find evidence of either bimodality or a late-day peak. The relatively low rates of feeding at supplemental stations at daybreak could be a result of early morning cache retrieval [44]. If caches are exploited and depleted as the day progresses, then birds may be expected to visit feeders more later in the day in order to continue feeding or to replenish caches. Caching cannot entirely explain our observed patterns, however, because one of the species studied (house finch) does not cache.
We found that temperature did strongly influence daily feeding patterns. Previous studies have also found that birds are more likely to visit supplemental feeding stations during periods of inclement weather [45,46] and may use daily temperature or snowfall as proximate cues to assess how much fat to accumulate [47,48]. The tendency of birds to visit the supplemental feeders with greater frequency on colder days is consistent with the idea that birds feed until they reach a satiation threshold.
In summary, our study demonstrates that daily feeding activity in free-living birds generally supports predictions of the risk-spreading theorem (relatively constant activity) over the bimodal foraging pattern predicted, if birds face a significant risk of predation. As such, the risk of starvation is the over-riding factor determining foraging activity in our population. Physiological constraints acting on food ingestion and fat accumulation may also be a contributing factor to the observed patterns [18,49]. For instance, digestive constraints may prevent concentrated bursts of foraging activity even if food availability is unlimited as at supplemental feeding stations. Further, birds may behave differently if food availability were not reliable. Additional research is required to examine how birds respond to stochasticity in the food supply; the sensitivity of birds to stochasticity could be experimentally tested by periodically limiting access to the feeders and quantifying changes in feeding behaviours pre- and post-manipulation. Manipulating perceived predation risks via introducing model predators [50,51] or playbacks of predator vocalizations would also be informative for further elucidating the influence of perceived predation risk on foraging activities. Examining the influence of social status on foraging behaviours may also reveal intraspecific differences related to social hierarchies [23]. Additional studies of free-living populations are required to further explore the relative importance of predation and starvation risks in influencing foraging behaviour.
Acknowledgements
This research was carried out in strict accordance with the guidelines for the Use of Wild Birds in Research of the Ornithological Council and approved by the Cornell University Institutional Animal Care and Use Committee (protocol no. 2008-0058). Ringing and tagging was licensed by the USGS Bird Banding Laboratory (permit no. 23245 to D.N.B.).
E. Bridge designed the RFID circuit boards that made this research possible. J. DeCoste, S. Dean, S. MacLean, A. Potter and M. Savoca provided assistance in the field. The research benefited from discussion with J. Dickinson and A. Dhondt and with students in the Dickinson and Dhondt laboratory groups. We thank the Cornell Laboratory of Ornithology and the thousands of participants in Project FeederWatch for financial support.
References
- 1.Houston AI, McNamara JM, Hutchinson JMC. 1993. General results concerning the trade-off between gaining energy and avoiding predation. Phil. Trans. R. Soc. Lond. B 341, 375–397 10.1098/rstb.1993.0123 (doi:10.1098/rstb.1993.0123) [DOI] [Google Scholar]
- 2.Lima SL. 1986. Predation risk and unpredictable feeding conditions: determinants of body mass in birds. Ecology 67, 377–385 10.2307/1938580 (doi:10.2307/1938580) [DOI] [Google Scholar]
- 3.Houston AI, McNamara JM. 1993. A theoretical investigation of the fat reserves and mortality levels of small birds in winter. Ornis Scand. 24, 205–219 10.2307/3676736 (doi:10.2307/3676736) [DOI] [Google Scholar]
- 4.McNamara JM, Houston AI, Lima SL. 1994. Foraging routines of small birds in winter: a theoretical investigation. J. Avian Biol. 25, 287–302 10.2307/3677276 (doi:10.2307/3677276) [DOI] [Google Scholar]
- 5.Pravosudov V, Lucas J. 2001. Daily patterns of energy storage in food-caching birds under variable daily predation risk: a dynamic state variable model. Behav. Ecol. Sociobiol. 50, 239–250 10.1007/s002650100361 (doi:10.1007/s002650100361) [DOI] [Google Scholar]
- 6.Kullberg C, Fransson T, Jakobsson S. 1996. Impaired predator evasion in fat blackcaps (Sylvia atricapilla). Proc. R. Soc. Lond. B 263, 1671–1675 10.1098/rspb.1996.0244 (doi:10.1098/rspb.1996.0244) [DOI] [Google Scholar]
- 7.Kullberg C. 1998. Does diurnal variation in body mass affect take-off ability in wintering willow tits? Anim. Behav. 56, 227–233 10.1006/anbe.1998.0765 (doi:10.1006/anbe.1998.0765) [DOI] [PubMed] [Google Scholar]
- 8.Quinn JL, Cole EF, Bates J, Payne RW, Cresswell W. 2012. Personality predicts individual responsiveness to the risks of starvation and predation. Proc. R. Soc. B 279, 1919–1926 10.1098/rspb.2011.2227 (doi:10.1098/rspb.2011.2227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bednekoff P, Krebs J. 1995. Great tit fat reserves: effects of changing and unpredictable feeding day length. Funct. Ecol. 9, 457–462 10.2307/2390009 (doi:10.2307/2390009) [DOI] [Google Scholar]
- 10.MacLeod R, Gosler AG, Cresswell W. 2005. Diurnal mass gain strategies and perceived predation risk in the great tit Parus major. J. Anim. Ecol. 74, 956–964 10.1111/j.1365-2656.2005.00993.x (doi:10.1111/j.1365-2656.2005.00993.x) [DOI] [Google Scholar]
- 11.Gosler A, Greenwood J, Perrins C. 1995. Predation risk and the cost of being fat. Nature 377, 621–623 10.1038/377621a0 (doi:10.1038/377621a0) [DOI] [Google Scholar]
- 12.Carrascal LM, Polo V. 1999. Coal tits, Parus ater, lose weight in response to chases by predators. Anim. Behav. 58, 281–285 10.1006/anbe.1999.1142 (doi:10.1006/anbe.1999.1142) [DOI] [PubMed] [Google Scholar]
- 13.Gentle LK, Gosler AG. 2001. Fat reserves and perceived predation risk in the great tit, Parus major. Proc. R. Soc. Lond. B 268, 487–491 10.1098/rspb.2000.1405 (doi:10.1098/rspb.2000.1405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmer C, Boos M, Poulin N, Gosler A, Petit O, Robin J-P. 2011. Evidence of the trade-off between starvation and predation risks in ducks. PLoS ONE 6, e22352. 10.1371/journal.pone.0022352 (doi:10.1371/journal.pone.0022352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tvardíková K, Fuchs R. 2010. Do birds behave according to dynamic risk assessment theory? A feeder experiment. Behav. Ecol. Sociobiol. 65, 727–733 10.1007/s00265-010-1075-0 (doi:10.1007/s00265-010-1075-0) [DOI] [Google Scholar]
- 16.Brodin A. 2001. Mass-dependent predation and metabolic expenditure in wintering birds: is there a trade-off between different forms of predation? Anim. Behav. 62, 993–999 10.1006/anbe.2001.1844 (doi:10.1006/anbe.2001.1844) [DOI] [Google Scholar]
- 17.Carrascal LM, Polo V. 2006. Effects of wing area reduction on winter body mass and foraging behaviour in coal tits: field and aviary experiments. Anim. Behav. 72, 663–672 10.1016/j.anbehav.2005.11.027 (doi:10.1016/j.anbehav.2005.11.027) [DOI] [Google Scholar]
- 18.Bednekoff PA, Houston AI. 1994. Avian daily foraging patterns: effects of digestive constraints and variability. Evol. Ecol. 8, 36–52 10.1007/BF01237664 (doi:10.1007/BF01237664) [DOI] [Google Scholar]
- 19.Wolf LL, Hainsworth FR. 1977. Temporal patterning of feeding by hummingbirds. Anim. Behav. 25, 976–989 10.1016/0003-3472(77)90049-5 (doi:10.1016/0003-3472(77)90049-5) [DOI] [Google Scholar]
- 20.Cresswell W. 1998. Diurnal and seasonal mass variation in blackbirds Turdus merula: consequences for mass-dependent predation risk. J. Anim. Ecol. 67, 78–90 10.1046/j.1365-2656.1998.00174.x (doi:10.1046/j.1365-2656.1998.00174.x) [DOI] [Google Scholar]
- 21.Lilliendahl K. 2002. Daily patterns of body mass gain in four species of small wintering birds. J. Avian Biol. 33, 212–218 10.1034/j.1600-048X.2002.330302.x (doi:10.1034/j.1600-048X.2002.330302.x) [DOI] [Google Scholar]
- 22.Koivula K, Orell M, Lahti K. 2002. Plastic daily fattening routines in willow tits. J. Anim. Ecol. 71, 816–823 10.1046/j.1365-2656.2002.00646.x (doi:10.1046/j.1365-2656.2002.00646.x) [DOI] [Google Scholar]
- 23.Lange H, Leimar O. 2004. Social stability and daily body mass gain in great tits. Behav. Ecol. 15, 549–554 10.1093/beheco/arh044 (doi:10.1093/beheco/arh044) [DOI] [Google Scholar]
- 24.Bridge ES, Bonter DN. 2011. A low-cost radio frequency identification device for ornithological research. J. Field Ornithol. 82, 52–59 10.1111/j.1557-9263.2010.00307.x (doi:10.1111/j.1557-9263.2010.00307.x) [DOI] [Google Scholar]
- 25.Hinde J. 1982. Compound regression models. In GLIM 82: Proc. Int. Conf. on Generalized Linear Models, London, UK, 1982 (ed. Gilchrist R.), pp. 109–121 Berlin, Germany: Springer [Google Scholar]
- 26.Breslow N. 1990. Tests of hypotheses in overdispersed Poisson regression and other quasi-likelihood models. J. Am. Stat. Assoc. 85, 565–571 10.1080/01621459.1990.10476236 (doi:10.1080/01621459.1990.10476236) [DOI] [Google Scholar]
- 27.Burnham KP, Anderson DR. 2002. Model selection and inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 28.Wood SN. 2006. Generalized additive models: an introduction with R. London, UK: Chapman & Hall [Google Scholar]
- 29.R Core Development Team 2009. R: a language environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 30.Haftorn S. 1989. Seasonal and diurnal body weight variations in titmice, based on analyses of individual birds. Wilson Bull. 101, 217–235 [Google Scholar]
- 31.Taylor SG, Paul WL. 2006. Minimal diurnal change in foraging time in an Australian passerine, the white-browed babbler Pomatostomus superciliosus. J. Avian Biol. 37, 527–531 10.1111/j.0908-8857.2006.03530.x (doi:10.1111/j.0908-8857.2006.03530.x) [DOI] [Google Scholar]
- 32.Brittingham MC, Temple SA. 1992. Use of winter bird feeders by black-capped chickadees. J. Wildl. Manage. 56, 103–110 10.2307/3808797 (doi:10.2307/3808797) [DOI] [Google Scholar]
- 33.van der Veen IT. 2000. Daily routines and predator encounters in yellowhammers Emberiza citrinella in the field during winter. Ibis 142, 413–420 10.1111/j.1474-919X.2000.tb04437.x (doi:10.1111/j.1474-919X.2000.tb04437.x) [DOI] [Google Scholar]
- 34.Mariette MM, Pariser EC, Gilby AJ, Magrath MJL, Pryke SR, Griffith SC. 2011. Using an electronic monitoring system to link offspring provisioning and foraging behavior of a wild passerine. Auk 128, 26–35 10.1525/auk.2011.10117 (doi:10.1525/auk.2011.10117) [DOI] [Google Scholar]
- 35.Lima SL. 1988. Initiation and termination of daily feeding in dark-eyed juncos: influences of predation risk and energy reserves. Oikos 53, 3–11 10.2307/3565656 (doi:10.2307/3565656) [DOI] [Google Scholar]
- 36.McNamara JM, Barta Z, Houston AI, Race P. 2005. A theoretical investigation of the effect of predators on foraging behaviour and energy reserves. Proc. R. Soc. B 272, 929–934 10.1098/rspb.2004.3037 (doi:10.1098/rspb.2004.3037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polo V, Bautista LM. 2006. Daily routines of body mass gain in birds. 1. An exponential model. Anim. Behav. 72, 503–516 10.1016/j.anbehav.2005.09.024 (doi:10.1016/j.anbehav.2005.09.024) [DOI] [Google Scholar]
- 38.Brandt MJ, Cresswell W. 2009. Diurnal foraging routines in a tropical bird, the rock finch Lagonosticta sanguinodorsalis: how important is predation risk? J. Avian Biol. 40, 90–94 10.1111/j.1600-048X.2008.04389.x (doi:10.1111/j.1600-048X.2008.04389.x) [DOI] [Google Scholar]
- 39.Dall S, Witter M. 1998. Feeding interruptions, diurnal mass changes and daily routines of behaviour in the zebra finch. Anim. Behav. 55, 715–725 10.1006/anbe.1997.0749 (doi:10.1006/anbe.1997.0749) [DOI] [PubMed] [Google Scholar]
- 40.Beer JR. 1961. Winter feeding patterns in the house sparrow. Auk 78, 63–71 10.2307/4082235 (doi:10.2307/4082235) [DOI] [Google Scholar]
- 41.Morton ML. 1967. Diurnal feeding patterns in white-crowned sparrows, Zonotrichia leucophrys gambelii. Condor 69, 491–512 10.2307/1366149 (doi:10.2307/1366149) [DOI] [Google Scholar]
- 42.Bonter DN, Bridge ES. 2011. Applications of radio frequency identification (RFID) in ornithological research: a review. J. Field Ornithol. 82, 1–10 10.1111/j.1557-9263.2010.00302.x (doi:10.1111/j.1557-9263.2010.00302.x) [DOI] [Google Scholar]
- 43.Lima SL. 1985. Maximizing feeding efficiency and minimizing time exposed to predators: a trade-off in the black-capped chickadee. Oecologia 66, 60–67 10.1007/BF00378552 (doi:10.1007/BF00378552) [DOI] [PubMed] [Google Scholar]
- 44.Pravosudov VV, Grubb TC. 1997. Management of fat reserves and food caches in tufted titmice (Parus bicolor) in relation to unpredictable food supply. Behav. Ecol. 8, 332–339 10.1093/beheco/8.3.332 (doi:10.1093/beheco/8.3.332) [DOI] [Google Scholar]
- 45.Chamberlain DE, Vickery JA, Glue DE, Robinson RA, Conway GJ, Woodburn RJW, Cannon AR. 2005. Annual and seasonal trends in the use of garden feeders by birds in winter. Ibis 147, 563–575 10.1111/j.1474-919x.2005.00430.x (doi:10.1111/j.1474-919x.2005.00430.x) [DOI] [Google Scholar]
- 46.Zuckerberg B, Bonter DN, Hochachka WM, Koenig WD, DeGaetano AT, Dickinson JL. 2011. Climatic constraints on wintering bird distributions are modified by urbanization and weather. J. Anim. Ecol. 80, 403–413 10.1111/j.1365-2656.2010.01780.x (doi:10.1111/j.1365-2656.2010.01780.x) [DOI] [PubMed] [Google Scholar]
- 47.Rogers CM, Smith JN. 1993. Life-history theory in the nonbreeding period: trade-offs in avian fat reserves? Ecology 74, 419–426 10.2307/1939303 (doi:10.2307/1939303) [DOI] [Google Scholar]
- 48.Gosler A. 2002. Strategy and constraint in the winter fattening response to temperature in the great tit Parus major. J. Anim. Ecol. 71, 771–779 10.1046/j.1365-2656.2002.00642.x (doi:10.1046/j.1365-2656.2002.00642.x) [DOI] [Google Scholar]
- 49.Heath JP, Gilchrist HG, Ydenberg RC. 2010. Interactions between rate processes with different timescales explain counterintuitive foraging patterns of arctic wintering eiders. Proc. R. Soc. B 277, 3179–3186 10.1098/rspb.2010.0812 (doi:10.1098/rspb.2010.0812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lima SL, Bednekoff PA. 2011. On the perception of targeting by predators during attacks on socially feeding birds. Anim. Behav. 82, 535–542 10.1016/j.anbehav.2011.06.007 (doi:10.1016/j.anbehav.2011.06.007) [DOI] [Google Scholar]
- 51.Tilgar V, Moks K, Saag P. 2010. Predator-induced stress changes parental feeding behavior in pied flycatchers. Behav. Ecol. 22, 23–28 10.1093/beheco/arq164 (doi:10.1093/beheco/arq164) [DOI] [Google Scholar]