Abstract
Understanding the processing of odour mixtures is a focus in olfaction research. Through a neuroethological approach, we demonstrate that different odour types, sex and habitat cues are coded together in an insect herbivore. Stronger flight attraction of codling moth males, Cydia pomonella, to blends of female sex pheromone and plant odour, compared with single compounds, was corroborated by functional imaging of the olfactory centres in the insect brain, the antennal lobes (ALs). The macroglomerular complex (MGC) in the AL, which is dedicated to pheromone perception, showed an enhanced response to blends of pheromone and plant signals, whereas the response in glomeruli surrounding the MGC was suppressed. Intracellular recordings from AL projection neurons that transmit odour information to higher brain centres, confirmed this synergistic interaction in the MGC. These findings underscore that, in nature, sex pheromone and plant odours are perceived as an ensemble. That mating and habitat cues are coded as blends in the MGC of the AL highlights the dual role of plant signals in habitat selection and in premating sexual communication. It suggests that the MGC is a common target for sexual and natural selection in moths, facilitating ecological speciation.
Keywords: chemical communication, reproductive isolation, magic trait, intracellular recordings, functional imaging, Cydia pomonella
1. Introduction
Odours typically are blends of several chemicals, in specific proportions, and the olfactory system decodes and discriminates these multi-dimensional signals rapidly and precisely. A current question is how odour blends are represented in olfactory circuits and to what extent the neural odour space reflects their ecological and evolutionary significance [1–4].
For reproduction, animals largely rely on two types of olfactory signals: sex pheromones distinguish conspecific mates, and habitat odours signal food sources for adults and offspring. Both sex and habitat odours are important mediators of premating reproductive isolation and speciation [5–7], and the neural circuitry underlying the integration of these two types of chemosensory cues is, therefore, an important target for sexual and natural selection. The interaction of sexual and natural selection is thought to be a powerful driver of speciation [8–10].
Insect herbivores are particularly suitable for studying the interaction between mating and habitat cues, especially host plant odours, owing to the importance of these signals for their ecology and evolution. Host plant shifts have probably contributed to the remarkable diversification of plant-feeding insects [11,12], and most of these rely on sex pheromones for mate finding [13,14].
Plant volatiles are recognized as sex pheromone modulators in many insect species [15,16]. Although the behavioural interaction between pheromones and host plant volatiles is well established, little is known about the neurophysiological correlates. Research on the processing of odour blends in the primary olfactory centre in the brain, the antennal lobe (AL), has focused mainly on sex pheromones or on plant volatiles, whereas the combination of these two classes of compounds has been investigated only recently [17–19].
Separate investigation of pheromones and plant volatile stimuli has led to the idea of a functional specialization of sensory processing in the AL, and that these two odour classes are represented in morphologically different regions of the AL of male moths. The macroglomerular complex (MGC) is considered to be dedicated to pheromone coding and the sexually isomorphic, ordinary glomeruli to the coding of plant volatile information [20]. Recent studies in the silk moth Bombyx mori and the noctuid moth Agrotis segetum, however, do not corroborate a strict segregation of the two subsystems, and indicate that the MGC receives lateral input from the AL [17–19].
In the codling moth Cydia pomonella (Lepidoptera, Tortricidae), a reconstruction of the glomerular structure of the AL, combined with electrophysiological recordings, suggested significant crosstalk between the pheromone and general odour subsystems [21]. Codling moth is a key pest of apple, and its sex pheromone and the behavioural role of host plant volatiles have been carefully studied [22].
We investigated the neurophysiological mechanisms regulating the interaction between female sex pheromone and behaviourally active host plant odorants, using functional imaging of the AL and intracellular recordings (IRs) of projection neurons (PNs) that transmit olfactory signals to higher brain centres. The finding that the MGC is dedicated to blends of social and environmental odours adds to our understanding of the role of chemosensory cues in premating reproductive isolation and plant–insect ecology. It also provides a new incentive for the refinement of sustainable insect control methods based on behaviour-modifying chemicals.
2. Material and methods
(a). Insects
Experiments were carried out with 2- to 3-day-old unmated codling moth C. pomonella (Lepidoptera, Tortricidae) males, which were reared for several generations on an artificial diet (Andermatt Biocontrol, Grossdietwil, Switzerland). The males were kept at 70 ± 5 per cent relative humidity, 23°C, under a 16 L : 8 D photoperiod, and they were fed with sugar water.
(b). Odour stimuli
Test odours included the main component of codling moth female sex pheromone, codlemone, (E,E)-8,10-dodecadienol (more than 99.6% chemical and isomeric purity, Shin-Etsu Chemical Co., Tokyo) and three plant volatiles, (E)-β-farnesene (93.4% pure), butyl hexanoate (97.8%, both from Bedoukian Research Inc., Danbury, CT, USA) and pear ester, (E,Z)-2,4-decadienoate (87.4%, Sigma Aldrich).
For functional imaging and IRs, solutions of test compounds in 10 µl re-distilled hexane were applied on filter paper (0.5 × 1 cm), approximately 1 h before tests. After the solvent evaporated during 1 min, one or two filter papers (compound blends) were inserted into a Pasteur pipette. Codlemone was tested at amounts of from 1 ng to 1 µg, plant compounds from 10 ng to 10 µg, in decadic steps. A continuous charcoal-filtered and moistened airstream (500 ml min−1) passed through a glass tube (10 mm i.d.) over the antenna. A stimulus controller (SFC-2/b, Syntech, Kirchzarten, Germany) injected a 0.5 s puff (500 ml min−1) through the pipettes into this glass tube. Odours were presented in randomized order. Pipettes with filter paper loaded with 10 µl of solvent were used as control.
For behavioural tests, synthetic compounds were released from a piezo sprayer [23]. Compound dilutions were delivered at 10 μl min−1 to a 20 μl glass capillary tube with a drawn-out tip. A piezo-ceramic disc vibrated the capillary at approximately 100 kHz, producing an aerosol, which evaporated a few centimetres downwind from the capillary tip at a constant rate and known chemical purity. Codlemone was tested at 0.1 pg min−1, and plant compounds at 1 and 100 pg min−1.
(c). Behavioural assay
Wind tunnel experiments were conducted according to Knight et al. [24]. A fan pulled air through a charcoal filter, through a series of screens, at 0.25 m s−1 into the tunnel (1.6 × 0.6 × 0.6 m). Exhaust was expelled outside the building. Room lighting was computer-controlled to gradually decrease during a 60 min dusk period, between full light level (1330 lux) and the dark period (25 lux). Ten batches of five moths were flown consecutively to each lure, during the first 3 h of the scotophase. Male moth behaviour was recorded for up to 6 min. The following types of behaviour were recorded: wing fanning, take-off, upwind flight and contact with the screen. Proportional data were adjusted with Bartlett's correction for small sample size. An angular transformation was used to normalize proportional data prior to analysis of variance (ANOVA; Statistix v. 9, Analytical Software, Tallahassee, FL, USA). An α-level of 0.05 was used to establish significance, Tukey's method was used to compare means.
(d). Functional imaging
Individual moths were secured in a 1 ml plastic pipette, with the head protruding from the narrow end, and fixed by dental wax (Surgident, Heraeus Kulzer Inc.). The head capsule was opened between the antenna and the eyes; muscle, glands, trachea, neural sheath and the oesophagus were removed to expose the AL [25]. A calcium-sensitive dye (calcium green-2-AM dye) was dissolved in 20 per cent Pluronic F-127 in dimethyl sulfoxide (Molecular Probes, Eugene, OR, USA), and diluted in moth Ringer solution to 30 μM, and then applied to the brain, leaving the preparation in a dark and cold (5°C) environment for 3 h.
Recordings were made in vivo after incubation and washing, using an Olympus microscope (20× air objective NA 0.50; filter settings: dichroic 500 nm, emission LP 515 nm). The preparation was illuminated at 475 nm. Stimulation started at frame 12 and lasted 1 s. Images were binned twice (320 × 240 pixel) to increase signal-to-noise ratio. TILL Photonics imaging software (Gräfelfing, Germany) was used to record sequences of 40 frames (4 Hz, 200 ms exposure time), and noise was removed by a Gaussian filter. The response magnitude was calculated as the average ΔF/F for each frame, where F was estimated using a linear function fitted to the parts of the calcium fluorescence decay curve outside the potential response. The onset of the signal was set to the time of the first frame with a positive average ΔF/F. For statistical analysis, a Kruskal–Wallis test was followed by a Mann–Whitney U-test with Holm–Bonferroni correction. A three-dimensional map of the codling moth AL [21] was used to link the active area to AL glomeruli.
(e). Intracellular recordings
Insect preparation and recordings were carried out as described by Trona et al. [21]. During recordings, the brain was superfused with pH 6.9 Ringer solution delivered from a flow system. A silver ground electrode was in contact with the ringer solution. Using a micromanipulator, the AL was randomly penetrated with an electrode that was drawn from a heated glass capillary (0.5 mm i.d., Sutter Instrument Co., Novato, CA, USA) with the tip filled with 1 per cent neurobiotin (Vector Laboratories, Burlingame, CA, USA) dissolved in 0.25 mM KCl, and the remaining part was filled with 1 mM KCl.
After recordings, the AL interneuron was stained with a depolarizing current (0.5–0.7 nA, 15 min). The brain was dissected from the head capsule and stained following the protocol of Trona et al. [21]. Stained neurons were viewed in a laser scanning confocal microscope (Zeiss LSM 510, Carl Zeiss, Jena, Germany) with a 40 × 1.4 oil-immersion differential interference contrast objective. Alexa Fluor 488, fluorescein Avidin and Alexa Fluor 546-labelled structures were excited with an argon laser 488 nm (with a 505 nm long-pass filter) and a He–Ne laser (with a 560 nm long-pass filter). Stacks of X–Y confocal images (1024 × 1024 pixel) were scanned at 0.7 µm step size.
Only complete recording sessions of the entire set of test stimuli were evaluated. Responses were calculated from the number of net-spikes during 500 ms (number of spikes 500 ms before stimulus onset subtracted from the number of spikes 500 ms after stimulus onset). Net-spikes in response to control were subtracted from the net-spikes in response to odour stimuli; blend responses were considered to be synergistic/suppressive when the number of net-spikes in response to blends was significantly higher/lower than the sum of net-spikes in response to the single compounds (G-test).
3. Results
(a). Behavioural assay
Blends of the main sex pheromone component, codlemone, and host plant volatiles attracted significantly more codling moth males than single compounds (figure 1). All three plant volatiles tested: (E)-β-farnesene, butyl hexanoate and pear ester, elicited upwind orientation flights. Blending codlemone at 0.1 pg min−1 and plant volatiles at 100 pg min−1 significantly increased landings at the source, compared with codlemone alone (figure 1).
Figure 1.
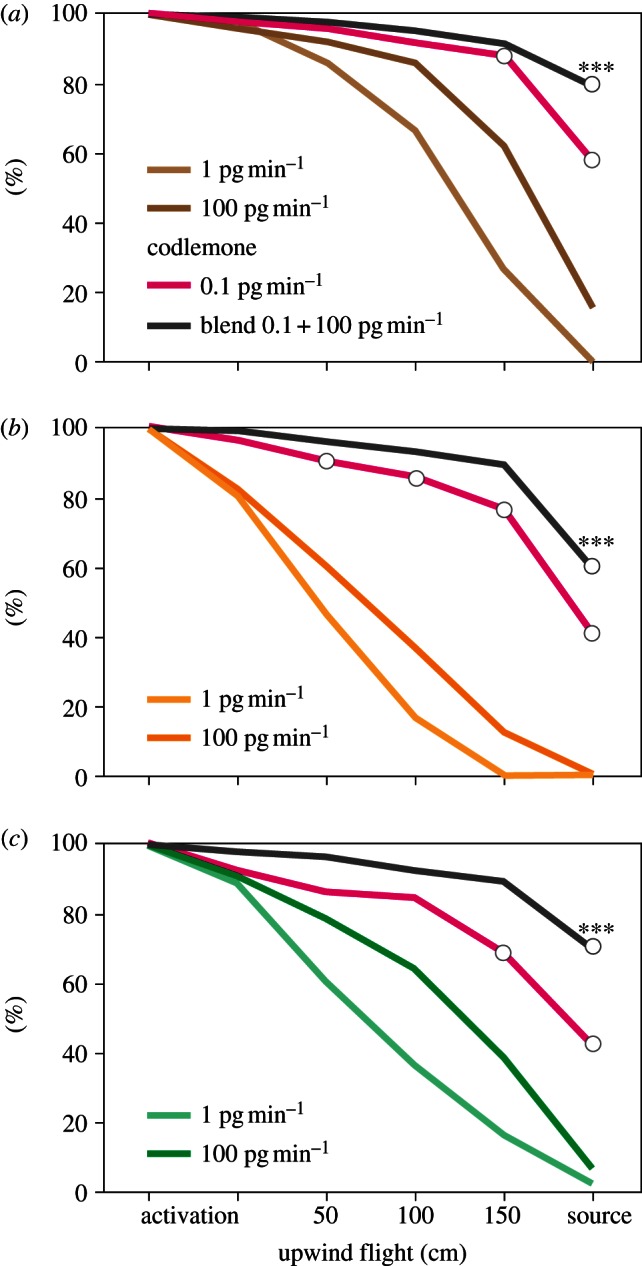
Wind tunnel attraction of codling moth C. pomonella males (n = 50) to the main pheromone compound codlemone (released at 0.1 pg min−1) and to plant volatiles (a) butyl hexanoate, (b) (E)-β-farnesene, (c) pear ester, at 1 and 100 pg min−1. Grey lines show attraction to 1 : 1000 blends of codlemone with these plant volatiles. Landings at the source are significantly increased in response to each of these two-component blends, compared with pheromone alone (***p < 0.001, two-way ANOVA; butyl hexanoate F4,45 = 45.0, β-farnesene F4,45 = 23.75, pear ester F4,45 = 24.08). Empty circles in the codlemone response curve show significant differences between codlemone and single plant volatiles alone (p < 0.0001, two-way ANOVA; butyl hexanoate F4,45 = 23.35, β-farnesene F4,45 = 53.96, pear ester F4,45 = 20.68).
(b). Functional imaging
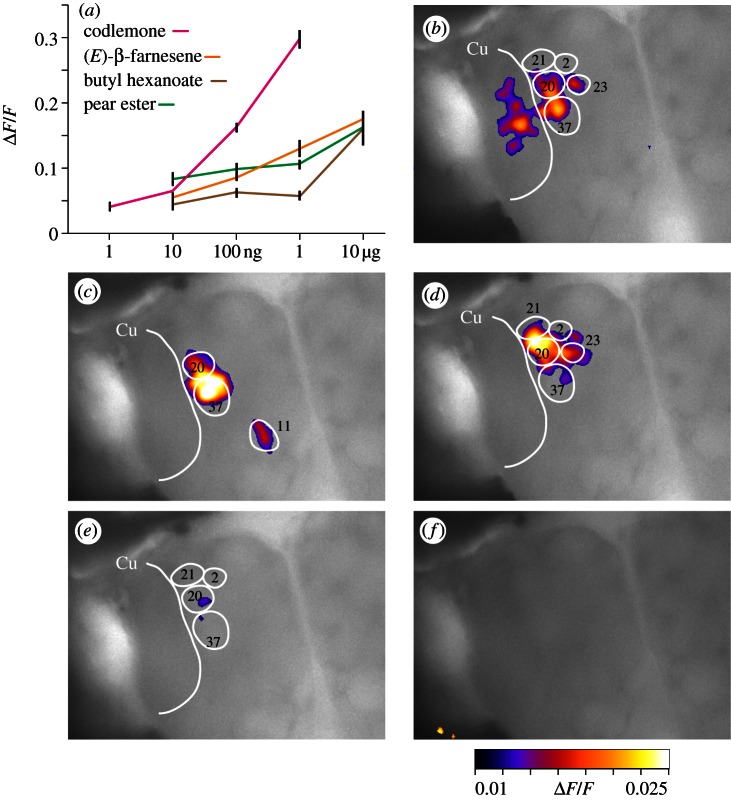
Calcium signals revealed distinct glomerular activity patterns for each odorant tested (figure 2). A threshold dose of codlemone (10 ng) elicited a significant response in the MGC, including the cumulus (Cu) and nearby satellite glomeruli (20 and 37; figure 2b). Plant volatiles alone did not elicit any response in the Cu, they instead activated satellite glomeruli and glomeruli outside the MGC (figure 2c–e). A threshold dose of pear ester (100 ng) was active in the satellite glomeruli 20 and 37, which also responded to codlemone (figure 2c) plus glomerulus 11 outside the MGC.
Figure 2.
Calcium imaging of the codling moth male AL upon stimulation with single odorants, sex pheromone (codlemone) and three plant volatiles. (a) Dose–response relationships of odour-evoked calcium signals, using an increasing dose of codlemone (n = 19), pear ester (n = 23), β-farnesene (n = 14) and butyl hexanoate (n = 19). Glomerular activation patterns in response to (b) 10 ng codlemone, (c) to 100 ng of pear ester, (d) (E)-β-farnesene and (e) butyl hexanoate, respectively, and (f) in response to the solvent (hexane). Data points show means and standard errors (s.e.), glomeruli numbers correspond to the three-dimensional atlas of the codling moth AL [21].
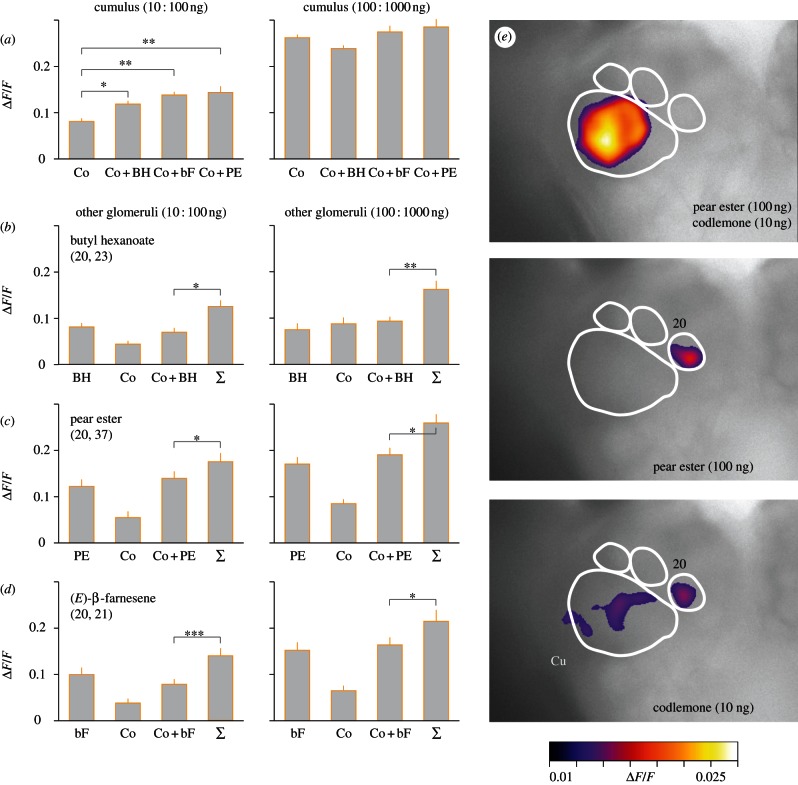
Blends of 10 ng codlemone plus 100 ng of each plant volatile compound produced a strong synergistic interaction in the Cu (figure 3a,e). This synergistic effect was not seen at a 10-fold higher dose (figure 3a). Although several of the glomeruli surrounding the Cu responded to plant volatiles and codlemone (figures 2b–e and 3e), there was no synergistic interaction in these glomeruli: outside the Cu, the activity elicited by blends was significantly lower than the sum of the activity elicited by the single compounds (figure 3b–d).
Figure 3.
Calcium imaging of the codling moth male AL following stimulation with two-component blends of sex pheromone (codlemone) and plant volatiles, butyl hexanoate, pear ester and β-farnesene. Odour-evoked activity was measured in the Cu and other responding glomeruli. Response in the Cu (a), showing a synergistic blend interaction for 10 : 100 ng blends (*p < 0.05, **p < 0.01, Kruskal–Wallis test followed by Mann–Whitney U-test with Holm–Bonferroni correction, n = 30 males). At a higher dose, blends (100 : 1000 ng) were not significantly different from codlemone (p = 0.36, Kruskal–Wallis test, n = 30 males). Response of glomeruli outside the Cu (b–d) to plant compounds, codlemone, their blends and the summed responses to single compounds (∑): butyl hexanoate, satellite glomerulus 20 and glomerulus 23 (*p < 0.05 and **p < 0.01, n = 26; b); pear ester, satellite glomeruli 20, 37 (*p < 0.05, n = 30; c); β-farnesene, satellite glomeruli 20, 21 (***p < 0.001 and *p < 0.05, one-sided t-test, n = 31; d). Bars show the standard error of the mean (s.e.m.). Representative recording of codlemone, pear ester and their blend (e). Glomeruli numbers correspond to the atlas of codling moth AL [21].
(c). Intracellular recordings
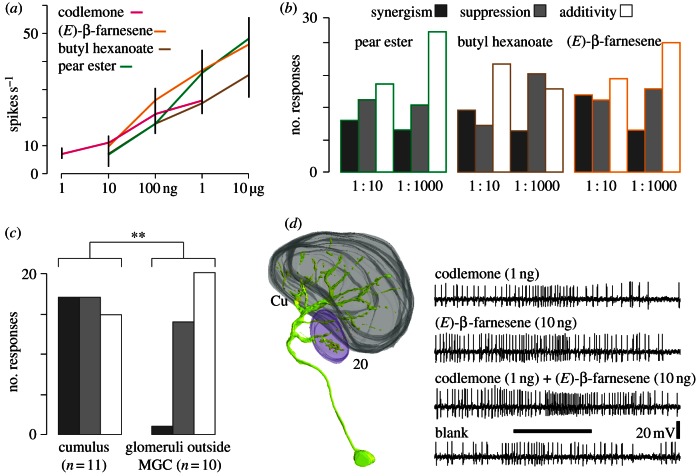
Figures 4 and 5 show the blend response of AL output neurons. Based on a dose–response test with single compounds (figure 4a), codlemone and individual plant volatiles were combined in a ratio of 1 : 10 and 1 : 1000. The number of synergistic, suppressive and additive responses of AL neurons to blends of codlemone and plant volatiles, in the Cu and surrounding glomeruli is shown in figure 4b,c.
Figure 4.
Responses of AL neurons to single compounds and binary blends. Intracellular recordings of AL neurons with increasing doses of codlemone (n = 12), butyl hexanoate (n = 10), pear ester (n = 11) and β-farnesene (n = 12) (a). Histograms of synergistic, suppressive and additive responses of 69 physiologically characterized interneurons to blends of codlemone and plant volatiles (b). Number of synergistic, suppressive and additive responses of neurons innervating Cu and glomeruli outside the MGC (**p < 0.005, χ2-test) (c). Three-dimensional reconstruction of a multi-glomerular PN innervating the Cu and the satellite glomerulus 20, showing a synergistic response to a blend of codlemone and (E)-β-farnesene. The horizontal bar shows the stimulus period (500 ms) (d).
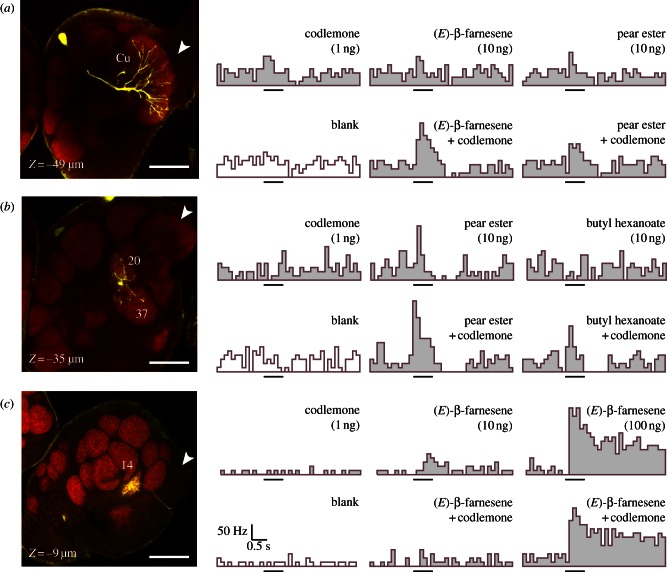
Figure 5.
Single confocal sections and spike frequency histograms (spikes s−1) of physiologically and morphologically characterized PNs in the codling moth male AL. (a) Synergistic responses of a PN innervating the Cu to blends of codlemone with pear ester and β-farnesene. (b) Synergistic responses of a multi-glomerular PN, innervating the satellite glomeruli 20 and 37, to blends of codlemone with pear ester and butyl hexanoate. (c) Suppressive responses of a PN innervating the glomerulus 14, to a blend of codlemone and (E)-β-farnesene at different blend ratios. Confocal sections: entrance of the antennal nerve (arrowheads), depth from anterior side of the AL (Z), scale bars (50 µm), glomeruli numbers correspond to the three-dimension AL atlas [21]. Histograms: stimulus period (bars, 500 ms).
Analysis of 69 successful recordings demonstrates that odour blend interaction was not merely additive (p < 0.05, G-test). Of the neurons showing a synergistic blend response, 52 per cent responded only to blends, and not to single compounds. Suppressive responses comprised both a decreased excitatory phase (53%) and complete response suppression (47%; figure 4b).
Twenty-nine neurons were successfully stained: 11 PNs arborizing in the Cu, five PNs in satellite glomeruli surrounding the Cu, 10 PNs in glomeruli outside the MGC and, in addition, three local interneurons (LNs). The Cu was innervated by uni-glomerular PNs (figure 5a), and by one multi-glomerular PN that also arborized in the satellite glomerulus 20 (figure 4d). Spike frequency histograms for selected PNs in response to compound blends are shown in figure 5. A statistical comparison of the blend effects in stained PNs revealed a significant difference: synergism occurred almost exclusively in the Cu, whereas blend stimulation of glomeruli outside the MGC mostly had a additive or suppressive effect (figures 4c and 5c).
4. Discussion
(a). Neural ensemble coding of sex pheromone and host plant odour in the macroglomerular complex of the male moth antennal lobe
Understanding how stimulation with a blend of odorants generates a unique perception in the brain is a current research question. What adds to the complexity of olfactory coding is the integration of separate, independent signals—sex and habitat odours—which are together required to generate appropriate behavioural responses during mate finding.
We combined functional imaging and IRs to study odour blend processing in the codling moth C. pomonella, and show that the behavioural synergism between sex pheromone and host plant odorants is mirrored neurophysiologically. The MGC in the AL integrates signals from conspecific insects with habitat odours, and synergistic interactions between these two classes of odours occur both at the input and output level. This demonstrates that processing of sex pheromone and plant volatiles, which insects encounter as an ensemble in nature, does not use functionally separate pathways [17,18].
Blend enhancement and suppression in the AL may stem from odour interference in antennal sensory neurons [19,26] and ultimately at the olfactory receptor level [27]. However, in codling moth, pheromone–plant volatile blends enhance the Cu response while they simultaneously suppress surrounding glomeruli in a ‘centre-surround’ manner. Such complex coding may instead rely on lateral excitatory or inhibitory interconnections between glomeruli through LNs [2,28]. Functional studies of LNs will be essential to understand olfactory processing in the AL.
IRs of PNs, which connect the AL to higher brain centres, further corroborate that the MGC processes blends of plant volatiles and sex pheromone. Synergistic, blend-specific responses have been shown in the silk moth B. mori [17] and in codling moth, where PNs innervate the Cu and satellite glomeruli of the MGC [21].
An antagonistic interaction modality was shown in the black cutworm Agrotis ipsilon. A floral volatile, which inhibits male attraction to pheromone, suppresses the pheromone response in the AL [18] and in PNs innervating the MGC [19]. This suggests that odours with different ecological roles may differently affect pheromone coding. A wiring diagram of input and output signals in the codling moth AL, based on a more complete panel of ecologically relevant odorants, from host and non-host plants or associated mutualistic micro-organisms [29,30], will reveal whether glomerulus morphology and position in the AL correlates with the behavioural role of the respective key stimuli [31].
(b). Behavioural and ecological physiology of pheromone–plant odour blend perception
Mate recognition in insects, and especially in habitat-specific plant-feeding species, involves two main elements: sexual communication and recognition of larval and adult food plants, which frequently serve as rendezvous sites. Both mate and host finding largely rely on olfactory signals [14,32] that play a fundamental role in speciation [6,33].
In the codling moth, host plant odour is part of the mate-finding signal. The plant volatiles chosen for this study are distinctive for the main hosts, pear and apple, respectively. They mediate female attraction for oviposition [29,34–37], and they synergize male attraction to female sex pheromone. The MGC, in the olfactory centre of the moth brain, is the focal point for processing blends of pheromone and these plant signals.
Speciation is thought be facilitated by multiple-effect or ‘magic’ traits, which are subject to divergent selection and which contribute to non-random mating [9,10]. The MGC interconnects mate and host choice, and would accordingly be considered as a multiple-effect trait. Host choice seemingly is under divergent selection in codling moth, which forms distinct host races on apple, pear, walnut, plum and apricot. These differ in spring emergence and diapause initiation, in close association with host flowering and fruit maturation [38,39], and the genetically distinct walnut strain is adapted to toxic walnut metabolites [40–42]. Females of several strains preferentially oviposit on their respective host fruit [29,38].
A comparison of the female sex pheromones of closely related Cydia species further corroborates the role of plant volatiles in reproductive isolation. Only few species share the same pheromone, but these all feed on host plants belonging to different families. For example, pea moth C. nigricana (Leguminosae) and pear moth C. pyrivora (Pyrus), the sibling species of codling moth, use codlemone acetate (E,E)-8,10-dodecadienyl acetate, which is a strong pheromone antagonist in codling moth males [43].
Pheromone and host odour communication is also highly integrated in other insects, for example in Drosophila [44] and in bark beetles, where non-host volatiles, as opposed to host volatiles, have an antagonistic effect on host and mate finding [45]. In the two pheromone races of the European corn borer Ostrinia nubilalis, male preference for females of the same race leads to premating isolation [46,47], which is reinforced by preferential attraction to volatiles of their respective host plants, mugwort and maize [48,49].
Ecological speciation, following host plant shifts, has probably contributed to the remarkable diversity of phytophagous insects [11,33]. Our study provides physiological data which suggest that mate recognition systems evolve in concert with chemosensory adaptation to new hosts and ecological niches, and that sexual selection cannot be separated from natural selection in male insect herbivores.
(c). Practical implication
Our knowledge of codling moth chemical ecology has led to the successful development of species-specific and safe population control by pheromone-mediated mating disruption. In spite of orchard applications on 200 000 ha [50], the behavioural mechanisms underlying the disruption of mating are still under debate [51,52], and a better understanding of them will give leads for improvement. Our study demonstrates that it will be useful to consider the physiological and behavioural effect of plant volatiles on mating disruption, because, in nature, pheromone and plant volatiles are perceived together.
Acknowledgements
We thank Valerio Mazzoni for advice on statistical analysis, and Duane Larson, USDA, ARS, Wapato, WA for assistance with the flight tunnel bioassays. This research is part of the Linnaeus programme Insect Chemical Ecology, Ethology and Evolution (IC-E3) and financially supported by Formas, SLU and IASMA.
References
- 1.Chittka L, Brockmann A. 2005. Perception space: the final frontier. PLoS Biol. 3, 564–568 10.1371/journal.pbio.0030137 (doi:10.1371/journal.pbio.0030137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masse NY, Turner GC, Jefferis G.SXE 2009. Olfactory information processing in Drosophila. Curr. Biol. 19, R700–R713 10.1016/j.cub.2009.06.026 (doi:10.1016/j.cub.2009.06.026) [DOI] [PubMed] [Google Scholar]
- 3.Su C-Y, Menuz K, Carlson JR. 2009. Olfactory perception: receptors, cells, and circuits. Cell 139, 45–59 10.1016/j.cell.2009.09.015 (doi:10.1016/j.cell.2009.09.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy VN. 2011. Olfactory maps in the brain. Annu. Rev. Neurosci. 34, 233–258 10.1146/annurev-neuro-061010-113738 (doi:10.1146/annurev-neuro-061010-113738) [DOI] [PubMed] [Google Scholar]
- 5.White CS, Lambert DM, Foster SP. 1994. Chemical signals and the recognition concept. In Speciation and the recognition concept: theory and application (eds Lambert DM, Spencer H.), pp. 301–326 Baltimore, MA: John Hopkins University Press [Google Scholar]
- 6.Smadja C, Butlin RK. 2009. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102, 77–97 10.1038/hdy.2008.55 (doi:10.1038/hdy.2008.55) [DOI] [PubMed] [Google Scholar]
- 7.Mendelson TC, Shaw KL. 2012. The (mis)concept of species recognition. Trends Ecol. Evol. 27, 421–427 10.1016/j.tree.2012.04.001 (doi:10.1016/j.tree.2012.04.001) [DOI] [PubMed] [Google Scholar]
- 8.Maan ME, Seehausen O. 2011. Ecology, sexual selection and speciation. Ecol. Lett. 14, 591–602 10.1111/j.1461-0248.2011.01606.x (doi:10.1111/j.1461-0248.2011.01606.x) [DOI] [PubMed] [Google Scholar]
- 9.Servedio MR, Van Doorn GS, Kopp M, Frame AM, Nosil P. 2011. Magic traits in speciation: ‘magic’ but not rare? Trends Ecol. Evol. 26, 389–397 10.1016/j.tree.2011.04.005 (doi:10.1016/j.tree.2011.04.005) [DOI] [PubMed] [Google Scholar]
- 10.Merrill RM, Wallbank RWR, Bull V, Salazar PCA, Mallet J, Stevens M, Jiggins CD. 2012. Disruptive ecological selection on a mating cue. Proc. R. Soc. B 279, 4907–4913 10.1098/rspb.2012.1968 (doi:10.1098/rspb.2012.1968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fordyce JA. 2010. Host shifts and evolutionary radiations of butterflies. Proc. R. Soc. B 277, 3735–3743 10.1098/rspb.2010.0211 (doi:10.1098/rspb.2010.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janz N. 2011. Ehrlich and Raven revisited: mechanisms underlying codiversification of plants and enemies. Annu. Rev. Ecol. Evol. Syst. 42, 71–89 10.1146/annurev-ecolsys-102710-145024 (doi:10.1146/annurev-ecolsys-102710-145024) [DOI] [Google Scholar]
- 13.Wyatt TD. 2003. Pheromones and animal behaviour: communication by smell and taste. Cambridge, UK: Cambridge University Press [Google Scholar]
- 14.Johansson BG, Jones TM. 2007. The role of chemical communication in mate choice. Biol. Rev. 82, 265–289 10.1111/j.1469-185X.2007.00009.x (doi:10.1111/j.1469-185X.2007.00009.x) [DOI] [PubMed] [Google Scholar]
- 15.Landolt PJ, Phillips TW. 1997. Host plant influence on sex pheromone behaviour of phytophagous insects. Annu. Rev. Entomol. 42, 371–391 10.1146/annurev.ento.42.1.371 (doi:10.1146/annurev.ento.42.1.371) [DOI] [PubMed] [Google Scholar]
- 16.Reddy GVP, Guerrero A. 2004. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 9, 253–261 10.1016/j.tplants.2004.03.009 (doi:10.1016/j.tplants.2004.03.009) [DOI] [PubMed] [Google Scholar]
- 17.Namiki S, Iwabuchi S, Kanzaki R. 2008. Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori. J. Comp. Physiol. A 194, 501–515 10.1007/s00359-008-0325-3 (doi:10.1007/s00359-008-0325-3) [DOI] [PubMed] [Google Scholar]
- 18.Chaffiol A, Kropf J, Barrozo R, Gadenne C, Rospar JP, Anton S. 2012. Plant odour stimuli reshape pheromonal representation in neurons of the antennal lobe macroglomerular complex of a male moth. J. Exp. Biol. 215, 1670–1680 10.1242/jeb.066662 (doi:10.1242/jeb.066662) [DOI] [PubMed] [Google Scholar]
- 19.Deisig N, et al. 2012. Differential interactions of sex pheromone and plant odour in the olfactory pathway of a male moth. PLoS ONE 7, e33159. 10.1371/journal.pone.0033159 (doi:10.1371/journal.pone.0033159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galizia CG, Rössler W. 2010. Parallel olfactory system in insects: anatomy and function. Annu. Rev. Entomol. 55, 399–420 10.1146/annurev-ento-112408-085442 (doi:10.1146/annurev-ento-112408-085442) [DOI] [PubMed] [Google Scholar]
- 21.Trona F, Anfora G, Bengtsson M, Witzgall P, Ignell R. 2010. Coding and interaction of sex pheromone and plant volatile signals in the antennal lobe of the codling moth Cydia pomonella. J. Exp. Biol. 213, 4291–4303 10.1242/jeb.047365 (doi:10.1242/jeb.047365) [DOI] [PubMed] [Google Scholar]
- 22.Witzgall P, Stelinski L, Gut L, Thomson D. 2008. Codling moth management and chemical ecology. Annu. Rev. Entomol. 53, 503–522 10.1146/annurev.ento.53.103106.093323 (doi:10.1146/annurev.ento.53.103106.093323) [DOI] [PubMed] [Google Scholar]
- 23.El-Sayed A, Gödde J, Arn H. 1999. Sprayer for quantitative application of odor stimuli. Environ. Entomol. 28, 947–953 [Google Scholar]
- 24.Knight AL, Light DM, Trimble RM. 2011. Identifying (E)-4,8-dimethyl-1,3,7-nonatriene plus acetic acid as a new lure for male and female codling moth (Lepidoptera: Tortricidae). Environ. Entomol. 40, 420–430 10.1603/EN10283 (doi:10.1603/EN10283) [DOI] [Google Scholar]
- 25.Carlsson MA, Galizia CG, Hansson BS. 2002. Spatial representation of odors in the antennal lobe of the moth Spodoptera littoralis (Lepidoptera: Noctuidae). Chem. Senses 27, 231–244 10.1093/chemse/27.3.231 (doi:10.1093/chemse/27.3.231) [DOI] [PubMed] [Google Scholar]
- 26.Ochieng SA, Park KC, Baker TC. 2002. Host plant volatiles synergize responses of sex pheromone-specific olfactory receptor neurons in male Helicoverpa zea. J. Comp. Physiol. A 188, 325–333 10.1007/s00359-002-0308-8 (doi:10.1007/s00359-002-0308-8) [DOI] [PubMed] [Google Scholar]
- 27.Pregitzer P, Schubert M, Breer H, Hansson BS, Sachse S, Krieger J. 2012. Plant odorants interfere with detection of sex pheromone signals by male Heliothis virescens. Front. Cell. Neurosci. 6, 42. 10.3389/fncel.2012.00042 (doi:10.3389/fncel.2012.00042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tootoonian S, Laurent G. 2010. Electric times in olfaction. Neuron 67, 903–905 10.1016/j.neuron.2010.09.011 (doi:10.1016/j.neuron.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 29.Witzgall P, Ansebo L, Yang Z, Angeli G, Sauphanor B, Bengtsson M. 2005. Plant volatiles affect oviposition by codling moths. Chemoecology 15, 77–83 10.1007/s00049-005-0295-7 (doi:10.1007/s00049-005-0295-7) [DOI] [Google Scholar]
- 30.Witzgall P, et al. 2012. “This is not an apple”—yeast mutualism in codling moth. J. Chem. Ecol. 38, 949–957 10.1007/s10886-012-0158-y (doi:10.1007/s10886-012-0158-y) [DOI] [PubMed] [Google Scholar]
- 31.Ramdya P, Benton R. 2010. Evolving olfactory systems on the fly. Trends Genet. 26, 307–316 10.1016/j.tig.2010.04.004 (doi:10.1016/j.tig.2010.04.004) [DOI] [PubMed] [Google Scholar]
- 32.Bruce TJA, Pickett JA. 2011. Perception of plant volatile blends by herbivorous insects—finding the right mix. Phytochemistry 72, 1605–1611 10.1016/j.phytochem.2011.04.011 (doi:10.1016/j.phytochem.2011.04.011) [DOI] [PubMed] [Google Scholar]
- 33.Smadja CM, Canback B, Vitalis R, Gautier M, Ferrari J, Zhou JJ, Butlin RK. 2012. Large-scale candidate gene scan reveals the role of chemoreceptor genes in host plant specialization and speciation in the pea aphid. Evolution 66, 2723–2738 10.1111/j.1558-5646.2012.01612.x (doi:10.1111/j.1558-5646.2012.01612.x) [DOI] [PubMed] [Google Scholar]
- 34.Bengtsson M, Bäckman A-C, Liblikas I, Ramirez MI, Borg-Karlson A-K, Ansebo L, Anderson P, Löfqvist J, Witzgall P. 2001. Plant odor analysis of apple: antennal response of codling moth females to apple volatiles during phenological development. J. Agric. Food Chem. 49, 3736–3741 10.1021/jf0100548 (doi:10.1021/jf0100548) [DOI] [PubMed] [Google Scholar]
- 35.Light DM, et al. 2001. A pear-derived kairomone with pheromonal potency that attracts male and female codling moth, Cydia pomonella (L.). Naturwissenschaften 88, 333–338 10.1007/s001140100243 (doi:10.1007/s001140100243) [DOI] [PubMed] [Google Scholar]
- 36.Coracini M, Bengtsson M, Liblikas I, Witzgall P. 2004. Attraction of codling moth males to apple volatiles. Entomol. Exp. Appl. 110, 1–10 10.1111/j.0013-8703.2004.00124.x (doi:10.1111/j.0013-8703.2004.00124.x) [DOI] [Google Scholar]
- 37.Hern A, Dorn S. 2004. A female-specific attractant for the codling moth, Cydia pomonella, from apple fruit volatiles. Naturwissenschaften 91, 77–80 10.1007/s00114-003-0484-6 (doi:10.1007/s00114-003-0484-6) [DOI] [PubMed] [Google Scholar]
- 38.Phillips PA, Barnes MM. 1975. Host race formation among sympatric apple, walnut, and plum populations of the codling moth, Laspeyresia pomonella. Ann. Entomol. Soc. Am. 68, 1053–1060 [Google Scholar]
- 39.Pashley DP, Bush GL. 1979. The use of allozymes in studying insect movement with special reference to the codling moth, Laspeyresia pomonella L. (Olethreutidae). In Movement of highly mobile insects: concepts and methodology in research (eds Rabb RL, Kennedy GG.), pp. 333–341 Raleigh, NC: North Carolina State University Press [Google Scholar]
- 40.Thaler R, Brandstätter A, Meraner A, Chabicovski M, Parson W, Zelger R, Dalla Via J, Dallinger R. 2008. Molecular phylogeny and population structure of the codling moth (Cydia pomonella) in central Europe. II. AFLP analysis reflects human-aided local adaptation of a global pest species. Mol. Phylogenet. Evol. 48, 838–849 10.1016/j.ympev.2008.05.027 (doi:10.1016/j.ympev.2008.05.027) [DOI] [PubMed] [Google Scholar]
- 41.Cisneros FH, Barnes MM. 1974. Contribution to the biological and ecological characterization of apple and walnut host races of codling moth, Laspeyresia pomonella L.: moth longevity and oviposition capacity. Environ. Entomol. 3, 402–406 [Google Scholar]
- 42.Piskorski R, Dorn S. 2011. How the oligophage codling moth Cydia pomonella survives on walnut despite its secondary metabolite juglone. J. Insect Physiol. 57, 744–750 10.1016/j.jinsphys.2011.02.014 (doi:10.1016/j.jinsphys.2011.02.014) [DOI] [PubMed] [Google Scholar]
- 43.Witzgall P, et al. 1996. Sex pheromones and attractants in the Eucosmini and Grapholitini (Lepidoptera, Tortricidae). Chemoecology 7, 13–23 10.1007/BF01240633 (doi:10.1007/BF01240633) [DOI] [Google Scholar]
- 44.Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GSXE, Benton R. 2011. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478, 236–240 10.1038/nature10428 (doi:10.1038/nature10428) [DOI] [PubMed] [Google Scholar]
- 45.Zhang QH, Schlyter F. 2004. Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric. Forest Entomol. 6, 1–19 10.1111/j.1461-9555.2004.00202.x (doi:10.1111/j.1461-9555.2004.00202.x) [DOI] [Google Scholar]
- 46.Karpati Z, Olsson S, Hansson BS, Dekker T. 2010. Inheritance of central neuroanatomy and physiology related to pheromone preference in the male European corn borer. BMC Evol. Biol. 10, 286. 10.1186/1471-2148-10-286 (doi:10.1186/1471-2148-10-286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lassance JM, Groot AT, Lienard MA, Antony B, Borgwardt C, Andersson F, Hedenstrom E, Heckel DG, Lofstedt C. 2010. Allelic variation in a fatty-acyl reductase gene causes divergence in moth sex pheromones. Nature 466, 486–489 10.1038/nature09058 (doi:10.1038/nature09058) [DOI] [PubMed] [Google Scholar]
- 48.Malausa T, Bethenod MT, Bontemps A, Bourguet D, Cornuet JM, Ponsard S. 2005. Assortative mating in sympatric host races of the European corn borer. Science 308, 258–260 10.1126/science.1107577 (doi:10.1126/science.1107577) [DOI] [PubMed] [Google Scholar]
- 49.Leppik E, Frerot B. 2012. Volatile organic compounds and host-plant specialization in European corn borer E and Z pheromone races. Chemoecology 22, 119–129 10.1007/s00049-012-0104-z (doi:10.1007/s00049-012-0104-z) [DOI] [Google Scholar]
- 50.Witzgall P, Kirsch P, Cork A. 2010. Sex pheromones and their impact on pest management. J. Chem. Ecol. 36, 80–100 10.1007/s10886-009-9737-y (doi:10.1007/s10886-009-9737-y) [DOI] [PubMed] [Google Scholar]
- 51.Cardé RT, Minks AK. 1995. Control of moth pests by mating disruption: successes and constraints. Annu. Rev. Entomol. 40, 559–585 10.1146/annurev.ento.40.1.559 (doi:10.1146/annurev.ento.40.1.559) [DOI] [Google Scholar]
- 52.Miller JR, McGhee PS, Siegert PY, Adams CG, Huang J, Grieshop MJ, Gut LJ. 2010. General principles of attraction and competitive attraction as revealed by large-cage studies of moths responding to sex pheromone. Proc. Natl Acad. Sci. USA 107, 22–27 10.1073/pnas.0908453107 (doi:10.1073/pnas.0908453107) [DOI] [PMC free article] [PubMed] [Google Scholar]