Abstract
The extinct giant moa Dinornis is one of the most remarkable known examples of reversed sexual size dimorphism (RSD), with males weighing 34–85 kg, but females weighing up to 240 kg. However, there has been little consideration of the evolutionary mechanism that produced this level of dimorphism, and most living palaeognaths also exhibit varying levels of RSD. Using male and female body mass data for extant ratites and tinamous and four extinct moa genera, and tests of phylogenetic dependence (λ) of body size evolution among these species, we investigated whether Dinornis was truly unusual with respect to RSD relative to other palaeognaths, which sex was under greater pressure to change in size over evolutionary time, and which candidate hypotheses explaining the presence and variability of RSD in the genus are most plausible. We demonstrate that the extreme level of RSD exhibited by Dinornis represents a straightforward consequence of positive allometric scaling of body size. However, Dinornis females have undergone more evolutionary change than males, and larger females from high-productivity environments are associated with greater differentiation, possibly driven by intraspecific competition and female-biased selection for increased offspring investment.
Keywords: allometry, New Zealand, palaeognath, Rensch's Rule, sexual selection, tinamou
1. Introduction
Many different forms of sexual dimorphism are displayed among birds [1], and attempts to explain complex patterns of phylogenetic variation in avian dimorphism have been the subject of considerable debate ever since Darwin [2]. Together with plumage dichromatism, the most obvious difference between males and females of many bird species is sexual body size dimorphism (SSD). Males are typically the larger sex in birds, and although female-biased SSD or ‘reversed’ sexual size dimorphism (RSD) is common in many invertebrates and poikilothermic vertebrates [3], it is only displayed in a few avian groups, notably raptors (Falconiformes and Strigiformes), wading birds (Charadriiformes) and some other mostly non-passeriform taxa [4–7].
Large numbers of hypotheses assignable to three main categories have been proposed to explain both how and why RSD evolved and is maintained in these bird groups, including ecological hypotheses (e.g. food competition between sexes); reproductive role differentiation or ‘dimorphic niche’ hypotheses; and behavioural/sexual selection hypotheses [5–8]. Following Darwin [2] and Wallace [9], these competing hypotheses can also be categorized according to whether they try to explain the evolution of male size, female size or both [10,11]. In living birds, the highest RSD levels are displayed by Accipiter hawks and jacanas; some Accipiter species have sexual dimorphism index (SDI) ((mass of heavier sex/mass of lighter sex) – 1) values greater than 1.0 (i.e. females are more than twice the size of males), whereas the maximum SDI value for jacanas is ca 0.8. This level of sexual dimorphism is almost as great as the highest ratio of male-biased size dimorphism, shown by great bustard Otis tarda (SDI = 1.48 or higher; [1,4,6,12,13]).
The most dramatic known example of avian RSD was recently recognized in the giant moa Dinornis, the largest representative of a remarkable adaptive radiation of graviportal ratites (Dinornithiformes) that occupied large-herbivore niches in New Zealand in the absence of native mammalian competitors and became extinct following the arrival of Polynesian colonists ca 700 years ago [14]. Dinornis was formerly considered to represent three size-differentiated species occurring on both the North and South Islands [14], but ancient DNA studies instead revealed that it consisted of two geographically separated allospecies, North Island Dinornis novaezealandiae and South Island Dinornis robustus [15,16]. Although Dinornis males weighed only 34–85 kg, females measured ca 2 m tall at the back and weighed up to 240 kg. Dinornis females also displayed considerable size variation, and were formerly interpreted as representing two distinct species (the smaller Dinornis novaezealandiae and the larger Dinornis giganteus [14]). The highest level of RSD in coeval Dinornis populations is observed in Late Glacial North Island populations (SDI = 1.94), whereas the smallest level is observed in high-elevation sites from northwest South Island (SDI = 0.27) [17].
Other moa species pairs that varied only in size have also been reinterpreted as intraspecific sexual dimorphs [18]. Bimodal body size measurement data considered to represent sexually dimorphic populations are available for large temporally/geographically sympatric samples of Euryapteryx curtus and Pachyornis geranoides (=P. mappini [19]), with females again provisionally identified as the larger sex [20]. RSD has now been confirmed in Euryapteryx, Pachyornis and Emeus through ancient DNA research [16].
Although the discovery of extreme RSD in Dinornis has been the subject of substantial interest, there has been little further consideration of the evolutionary mechanism that produced this level of dimorphism. The New Zealand moa radiation is phylogenetically nested within modern ratites, although its exact phylogenetic position remains debated [21–24], and the prevailing view of ratite relationships has been challenged by recent studies suggesting that tinamous are also nested within a paraphyletic ratite clade [24,25]. However, irrespective of which phylogeny is adopted, most living palaeognaths (ratites and tinamous) also exhibit varying levels of RSD, associated with prominent or exclusive paternal care of eggs and offspring, and a diverse array of mating systems ranging from monogamy to mixed polygyny/polyandry [6,26–28].
Dinornis was one of the largest birds ever to evolve [14]; by contrast, ostriches, the largest living ratites, weigh 90–130 kg [27]. An appropriate evolutionary null hypothesis must, therefore, be that extreme RSD in Dinornis may simply conform to positive allometric scaling within the palaeognath clade, rather than being an unusual deviation of this genus from the norm for their body size (for similar conclusions over the evolution of antler size in the giant deer Megaloceros see [29]).
Male body size varies more than female body size among related taxa, so that larger species are more sexually dimorphic with male-biased SSD, but less sexually dimorphic with female-biased SSD [3,30,31]. This pattern is referred to as Rensch's Rule and has led to considerable investigation into allometric trends in SSD evolution. The classic method to test for agreement with the rule is to regress male body size on female body size; if the slope of the regression is significantly greater than one, then Rensch's Rule is supported.
Allometry consistent with Rensch's Rule has been confirmed in a range of avian taxa, but significant examples are generally associated with male-biased SSD, suggesting that sexual selection acting on male size drives the evolution of this pattern of allometry [32]. Although Rensch's Rule has also been demonstrated in Charadriiformes [33], other studies have found no evidence for it in Falconiformes, Strigiformes or Tinamiformes, suggesting that it is not generally supported in birds that exclusively exhibit RSD [3,28].
The nature of allometric trends in male and female body size across palaeognaths, therefore, remains difficult to predict empirically, and a series of questions need to be addressed. Does Dinornis fit the relative pattern of SSD shown by other extant and extinct palaeognaths, or does it exhibit significantly different male and female body masses than expected for a bird of its size? Furthermore, which sex underwent the greater change in size in Dinornis, and what were the rates of body size evolution in moa and other palaeognaths, and the ecological factors associated with evolutionary differences across the group?
2. Material and methods
(a). Body mass data
We collected body mass data for all extant ratite species and moa species for which both male and female measurements are available, and also for two tinamou genera (table 1). SSD was calculated as log(male) minus log(female) body mass [38]. Direct body mass measurements were used for extant ratites, whereas moa body masses were calculated using an algorithm based on available skeletal measurement data. Femoral circumference, an accurate predictor of body mass in large birds, has recently been used to estimate body masses for some moa taxa [19,39]; however, published femoral circumference data are unavailable for moa samples containing identified sexual dimorphs [16,17,20], so a regression equation developed for birds in general (and irrespective of sex) based on femoral length [40], representing another widely used predictor of avian body mass also used in recent moa studies [14,17,39], was used instead. Although the use of a broad-taxon regression to calculate moa body masses could conceivably affect accuracy of estimation if moa deviated consistently in size from taxon-wide trends, different moa species show considerable variation in robust or gracile leg morphology [14,41], suggesting that our estimates are unlikely to be consistently biased, and the high R2 value for this equation (0.99 [40]) further indicates that our mass estimates are likely to be accurate and so useful for investigating patterns of palaeognath evolution. Femoral length data were not compared directly because published data are largely unavailable for extant ratites. Median body masses for North and South Island Dinornis, E. curtus and P. geranoides were calculated from available femoral measurement data [17,20]; body mass data were also calculated for Dinornis populations displaying maximum and minimum recorded RSD levels [17]. Body mass data calculated for male and female specimens of Emeus crassus identified using molecular sexing [16] were also incorporated to increase the number of included moa taxa, although it is important to note that these specimens originate from different collection localities, meaning that comparable male and female body mass estimates may be confounded by geographical and temporal size variation recorded in many moa lineages and populations [14,20]. Palaeognath body masses, and an indication of the degree and direction of SSD across the group, are shown in figure 1.
Table 1.
Palaeognath body masses and SSD, calculated as log(male mass) minus log(female mass). (Moa highlighted in bold. Mean body masses measured from living birds used for extant palaeognaths, and body mass estimates derived from Quaternary skeletal remains used for moa species. Dinornis body mass estimates vary widely from different Pleistocene and Holocene environments, and so median, minimum, and maximum estimates are used for each species.)
| taxon | measure | male mass (g) | female mass (g) | SSD | source |
|---|---|---|---|---|---|
| Apteryx australis | mean | 2335.8 | 2690.9 | −0.061 | [27,34–36] |
| Apteryx haastii | mean | 2278.2 | 2760.3 | −0.083 | [35,37] |
| Apteryx mantelli | mean | 2069.3 | 2484.1 | −0.079 | [27] |
| Apteryx owenii | mean | 1133.8 | 1350.6 | −0.076 | [27,34–36] |
| Casuarius casuarius | mean | 31 650 | 45 750 | −0.160 | [34,37] |
| Dinornis novaezealandiae | median | 56 600 | 11 1976 | −0.297 | [14,17] |
| min | 37 800 | 10 9000 | −0.460 | ||
| max | 75 000 | 126 000 | −0.225 | ||
| Dinornis robustus | median | 80 333 | 151 723 | −0.276 | [14,17] |
| min | 70 000 | 89 000 | −0.104 | ||
| max | 84 000 | 151 723 | −0.257 | ||
| Dromaius novaehollandiae | mean | 31 500 | 36 900 | −0.069 | [27,34,37] |
| Emeus crassus | mean | 42 800 | 68 300 | −0.203 | [16] |
| Eudromia elegans | mean | 582 | 738 | −0.103 | [28] |
| Euryapteryx curtus | mean | 17 200 | 28 100 | −0.213 | [20] |
| Pachyornis mappini | mean | 31 800 | 51 400 | −0.209 | [20] |
| Rhea americana | mean | 28 350 | 21 546 | 0.119 | [27,34] |
| Struthio camelus | mean | 115 000 | 100 000 | 0.061 | [27] |
| Tinamus guttatus | mean | 638.3 | 737.5 | −0.063 | [27] |
| Tinamus major | mean | 982.1 | 1107.1 | −0.052 | [27,34] |
| Tinamus solitarius | mean | 1284.0 | 1476.4 | −0.061 | [27] |
| Tinamus tao | mean | 1565.0 | 1648.1 | −0.022 | [27,34] |
Figure 1.
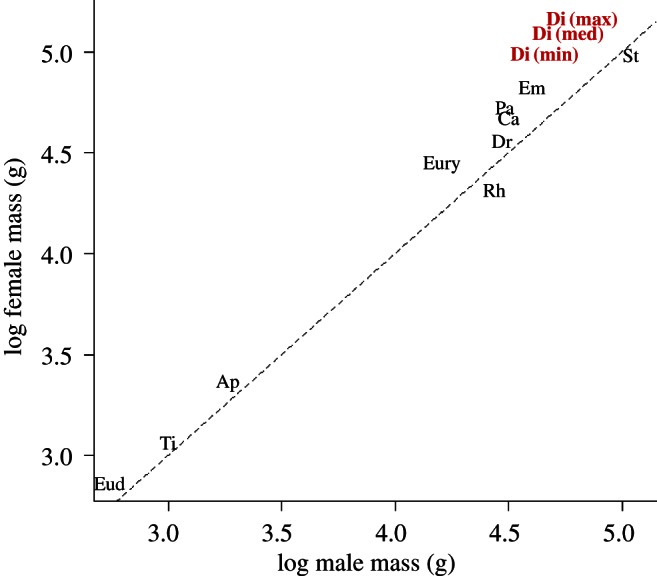
Male and female body sizes and SSD in ratites. Minimum, median and maximum body size estimates shown for Dinornis in bold. Dashed line represents slope of 1.0. Ap, Apteryx; Ca, Casuarius; Em, Emeus; Eud, Eudromia; Eury, Euryapteryx; Di, Dinornis; Dr, Dromaius; Pa, Pachyornis; Rh, Rhea; St, Struthio; Ti, Tinamus. (Online version in colour.)
(b). Phylogenies
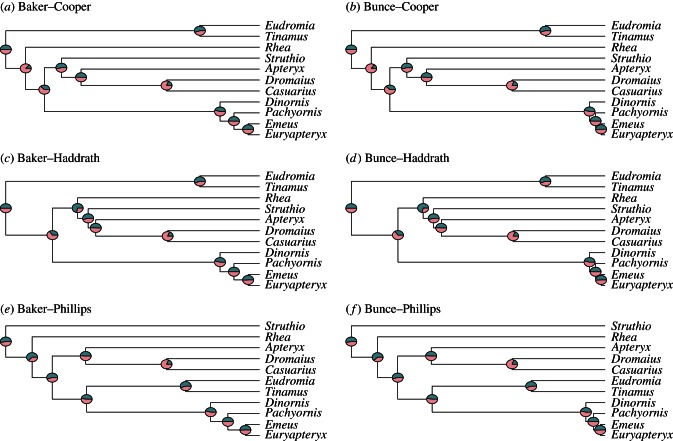
Several different phylogenetic studies, representing alternative hypotheses about the evolutionary relationships of extant ratites, tinamous and moa, have been published over the past decade. These studies have investigated both higher-order relationships of ratite and tinamou genera and the placement of moa, and also finer-scale evolutionary relationships between different moa genera and species. To ensure that our analyses were not biased towards any particular evolutionary hypothesis and to investigate any differences in RSD evolution associated with different hypotheses, we used topologies and branch lengths from three studies on palaeognaths that incorporated some moa taxa (hereafter referred to as the Cooper [21], Haddrath [22] and Phillips [24] phylogenies) and also two ancient DNA studies on moa that were fully resolved at the species level, which differ mainly in the timing of morphological radiation of different moa clades (hereafter referred to as the Baker [42] and Bunce [43] phylogenies). Using these hypotheses, we constructed six composite phylogenies, representing alternative hypotheses for examining the evolution of ratite RSD (figure 2). Other recent hypotheses of ratite and tinamou relationships could not be used because they did not provide branch length data [23], or because there was no congruence between novel tree topologies lacking moa and the phylogenetic placement of moa in other studies, making it impossible to add moa into the higher-order palaeognath phylogeny [25].
Figure 2.
(a–f) Hypotheses of phylogenetic relationships between extinct and extant palaeognath genera, and showing ratios of male : female phylogenetically independent body mass contrasts among ratites exhibiting female-biased SSD. Pies plotted at nodes of candidate phylogenies indicate value of male contrast (dark) relative to that of female contrast (pale); pies that are 50% dark and 50% pale indicate equal contrast values for both sexes. When testing Rensch's Rule in taxa with female-biased SSD we expect male contrasts to be larger than female contrasts (i.e. pies should have larger dark than pale segments), whereas in taxa with male-biased SSD the reverse should be true. (Online version in colour.)
Following previous studies [44,45], we used genus-level rather than species-level trees to avoid potential confounding problems. Many ratite genera are species-poor (comprising only 1–2 species [27]), and separate male and female body mass estimates are similarly only available for 1–2 species within different moa genera, such that available data are effectively at the genus level for these taxa. However, five extant kiwi (Apteryx) species are now recognized [46]. The number of Apteryx species in the dataset, combined with the small body mass of this genus, has the potential to disproportionately influence phylogenetically controlled analyses at the species level. Furthermore, fossil species and extant species may be defined according to different taxonomic criteria, and revisions to the taxonomy of extinct versus extant species are often not contemporaneous or consistent with one another. Indeed, whereas species-level taxonomy for most extant ratites and genus-level taxonomy for moa are both well understood, moa species-level taxonomy is notoriously unstable and has been in a state of continuing revision in recent years [14–16,19,42,43]. Tinamous are genus- and species-rich, and so we selected two representative genera (Tinamus and Eudromia) for which male and female body mass data are available and which are close to the root of the tinamou clade [47]. We obtained a divergence date for these genera from ref. [48]. We calculated body masses and SSD for each genus using the means of all congeners sampled. Inclusion of taxa with missing data values can influence estimates of scaling parameters [49], and so our trees did not include genera for which no separate male and female body mass values were available.
(c). Phylogenetic analyses
Our primary research question concerned the distinctiveness of Dinornis with respect to the apparent extremity of its RSD: are Dinornis species particularly sexually dimorphic given their body size and phylogenetic position? If a trait exhibits phylogenetic signal, then the phylogeny should explain at least some proportion of the distribution of character values among taxa. We investigated this prediction using the test statistic lambda (λ) [50], a tree-transformation parameter that gradually eliminates phylogenetic structure by multiplying off-diagonal elements of the variance/covariance matrix describing tree topology and branch lengths by values between 0 and 1 (λ = 1 indicates complete phylogenetic dependence, whereas λ = 0 indicates no phylogenetic dependence [49]). We did not calculate a related measure of evolutionary rate, kappa (κ) [49,50], because κ will always tend towards one in phylogenies that contain few nodes or that do not include all species, as is the case in our study.
The trait value for the root of a phylogeny can also be estimated from its topology, branch lengths and species trait values. The root value is equivalent to that obtained from the ‘squared-change parsimony’ algorithm and is also the maximum-likelihood estimate under Brownian motion. The independent contrasts algebra can also be used to compute a standard error or confidence interval. We derived the root trait value and its standard error from phylogenetic regression analysis in which the independent variable is set to a constant (1) and the dependent variable is the trait of interest. Upon removal from the tree, species with unusual trait values for their phylogenetic position will affect λ and root trait estimates more than taxa with trait values more typical of the clade [51]. We therefore tested for distinctiveness of Dinornis by first estimating λ and different root body size measures across the entire phylogeny, and then removing each genus from the phylogeny one at a time and re-estimating λ and root body size. We also conducted a final iteration in which we removed both genera of tinamous simultaneously. We performed this procedure for three root body size measures (male body mass, female body mass and SSD), repeating it for all six composite phylogenies and for all three Dinornis body mass estimates.
We also conducted a simple test of Rensch's Rule using each of our six candidate phylogenies. We calculated six sets of phylogenetically independent contrasts, one for each phylogeny, where each set comprised both male and female body mass contrasts (cf. [28]), to calculate differences in body mass values between pairs of sister taxa and scale them by their expected variances (the so-called ‘contrasts’; [52]). Contrasts therefore represent the result of evolutionary divergence that occurs after the origin of each pair, and are independent of phylogenetic associations. We used reduced major axis regression to determine the slope of the relationship between male (independent) and female (dependent) contrasts for each phylogenetic hypothesis. If Rensch's Rule is supported in ratites, then negative allometry among taxa with RSD is expected (i.e. males of sister taxa should differ more than females).
Our methods avert potential difficulties for investigating SSD in clades containing extinct taxa, caused by models for predicting SSD requiring information about the relationship between body size and reproductive success for each sex [53]. This information is unavailable for nearly all extinct taxa. Our analyses do not require information on sexual behaviour or reproductive output of extinct species, and do not rely on a priori inferences regarding mechanisms driving male and female body sizes in ratites, but instead examine whether the pattern and rate of change itself can reveal the relative plausibility of different evolutionary hypotheses.
3. Results
(a). Phylogenetic distinctiveness of body size and sexual body size dimorphism
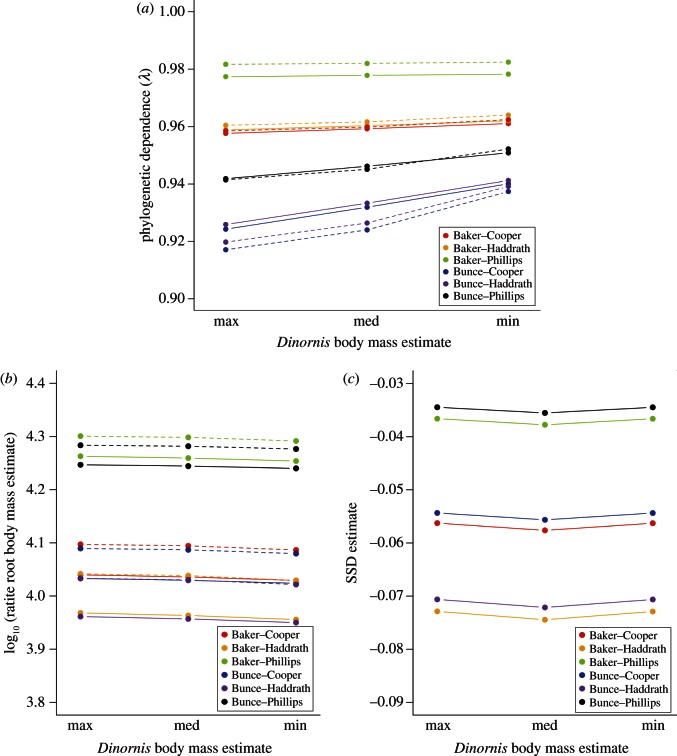
When we retained all genera in analyses and compared estimates of λ across all phylogenies and Dinornis body mass estimates, all values of λ were very close to one (range = 0.917–0.982). For analyses of both male and female genus-level body masses, there was some variation in λ depending upon the combination of phylogenies used (figure 3a). The lowest λ estimates were obtained using Bunce's moa phylogeny combined with Cooper's or Haddrath's palaeognath phylogeny (0.917–0.941), whereas the highest estimates were obtained using Baker's moa phylogeny combined with Phillips' palaeognath phylogeny (0.977–0.982). Using Bunce's moa phylogeny shows increasing phylogenetic dependence with decreasing Dinornis body mass estimate (i.e. smaller estimates of Dinornis body mass appear to be closer to that predicted by phylogeny alone); however, λ does not change with Dinornis body mass using Baker's moa phylogeny. Within one sex and phylogenetic hypothesis, λ also varied according to which Dinornis body mass estimate was used, although the nature of the variation depended upon which phylogeny was used. However, no values of λ for any analyses were significantly different from one (0.05 < p < 0.10 in all cases). For SSD, no phylogenies produced any shifts in λ away from one (all values > 0.9999).
Figure 3.
Estimates of (a) λ for male and female ratite body masses, (b) root body mass for male and female ratites, and (c) root SSD of body size, across six phylogenies, three estimates of Dinornis body mass, and including all genera. Male trends shown as solid lines and female trends shown as dashed lines in first two plots.
Root estimates of ‘ancestral’ palaeognath body mass are 8.91–18.20 kg for males and 10.47–19.95 kg for females, depending on phylogeny and Dinornis body size estimate. These values are intermediate among the body masses of taxa included in the analyses, as expected when the estimate is derived directly from taxa in the phylogeny. Both species-level phylogenetic hypotheses for moa (Baker/Bunce) predicted fairly similar root estimates of ratite body mass; where the palaeognath phylogeny was held constant, differences in root mass estimate using these phylogenies ranged from 119 to 665 g. Altering the higher-order palaeognath phylogeny unsurprisingly produced more disparate estimates; when moa phylogeny was held constant, differences in root mass estimate ranged from 1.64 to 9.02 kg. The highest root body mass estimates were predicted for hypotheses incorporating Phillips' phylogeny, in which small-bodied tinamous are nested within the larger-bodied ratites (figure 3b). Root estimates of palaeognath body mass were always smaller for males than for females (figure 3b). This difference in root estimates between the sexes also meant that the root estimate of SSD was female-biased, with the least female-biased estimates produced by Phillips' phylogeny and the most female-biased estimates produced by Haddrath's phylogeny (figure 3c).
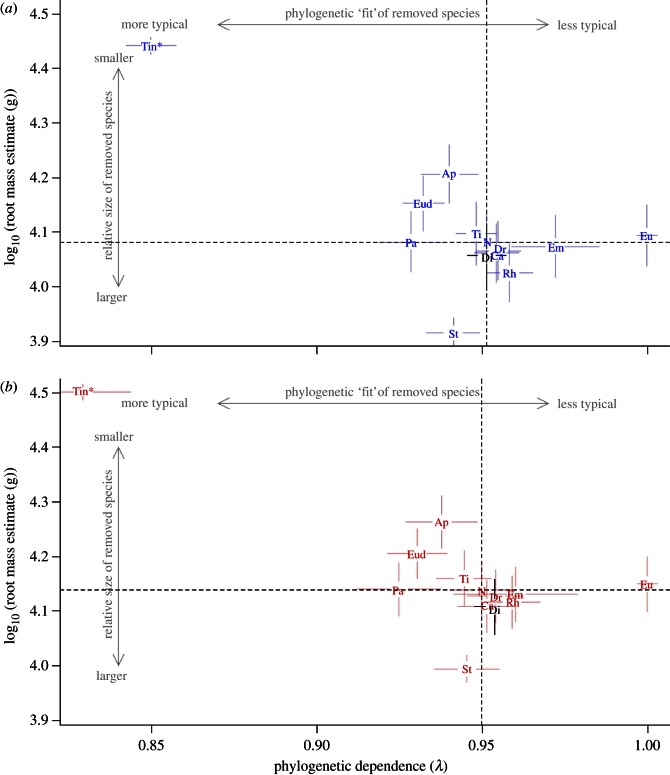
When we removed each genus from the phylogenies in turn and recalculated λ and root body mass estimates, palaeognath taxa showed different patterns defined by body mass and phylogenetic dependence (figure 4a,b). A group of small- and large-bodied genera including Apteryx, Eudromia, Tinamus, Pachyornis and Struthio have body masses approximately in line with what is expected solely on the basis of phylogeny, and removal of these taxa caused reductions in λ. Removal of both tinamou genera simultaneously caused a large reduction in λ of about 0.1 (figure 4a,b). A second group of typically larger-bodied genera including Casuarius, Dinornis, Dromaius, Emeus, Euryapteryx and Rhea all have phylogenetically more atypical body masses, and removal of these taxa caused increases in λ. In this group, Dinornis females were more atypical than males and Emeus females were less atypical than males. Casuarius, Dromaius and Rhea females and males had only a small effect on λ; in Euryapteryx, both sexes caused a large increase in λ of 0.05.
Figure 4.
Estimates of λ plotted against root estimate of palaeognath male body mass (a) and female body mass (b), using median estimates of Dinornis body mass and averaged across all six phylogenetic hypotheses, showing how excluding each genus from analyses changes the estimates. Dashed lines intersect at the values of λ and estimated root body mass when all genera are included in analyses. Lines radiating from plotted genera are standard error bars. If a genus is small-bodied compared with the root estimate when all taxa are included, it will appear in one of the upper quadrants, because excluding it increases the root estimate; similarly, large-bodied genera appear in one of the lower quadrants. Genera with phylogenetically atypical body masses appear to the right of the vertical dashed line. ‘Tin’ denotes change in λ and root estimate when both tinamou genera are excluded; abbreviations otherwise as for figure 1. (Online version in colour.)
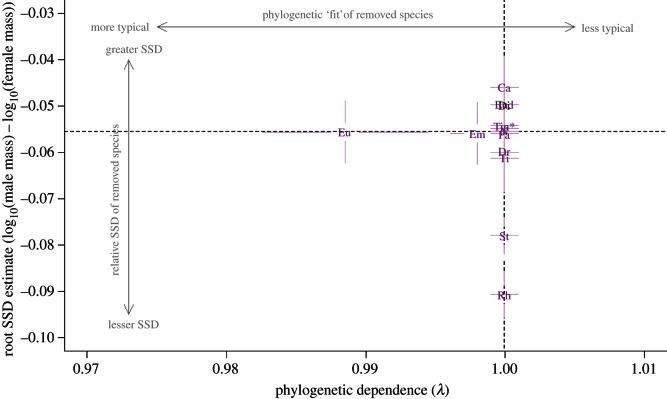
This procedure was also repeated for SSD (figure 5). Four genera (Dromaius, Rhea, Struthio and Tinamus) exhibited SSD lower than that estimated when all genera were included, and three other genera (Casuarius, Dinornis and Eudromia) exhibited SSD higher than this estimate. However, although removal of Eudromia and Emeus caused very slight λ shifts, in no case did λ depart significantly from one upon removal of a genus from a phylogeny. Root estimates of SSD varied slightly when most genera were omitted, but standard errors around these estimates overlapped extensively for most genera; only removal of taxa with male-biased SSD (Struthio and Rhea) caused large changes in SSD root estimate (figure 5).
Figure 5.
Estimates of λ plotted against root estimate of SSD averaged across all six phylogenetic hypotheses, showing how excluding each genus from analyses changes the estimates. Dashed lines intersect at the values of λ and estimated root body size when all genera are included in analyses. Lines radiating from plotted genera are standard error bars. Genera exhibiting strongly female-biased SSD appear above the black dashed line, whereas those with less female-biased or male-biased SSD appear below the line. Abbreviations as for figure 1. (Online version in colour.)
(b). Rensch's Rule
Our phylogenetically controlled tests did not support Rensch's Rule in palaeognaths. In all tests, the 95% confidence limits around the slope always included one, and the 95% confidence limits around the intercept always included zero (table 2). This isometric pattern contrasts with the allometric relationships expected under Rensch's Rule. Rensch's Rule also predicts that male body mass contrasts should be larger than female body mass contrasts where female-biased SSD occurs, whereas the opposite should be true where male-biased SSD occurs. This was not the case for palaeognaths, in which male and female body mass contrasts were often similar and showed no specific bias towards either sex (figure 2). This similarity of contrasts between the sexes was most pronounced when we used Phillips' phylogeny.
Table 2.
Slopes (β), intercepts (a), and upper/lower confidence limits for regression of female on male phylogenetic contrasts of body mass. (Three estimates of Dinornis body mass used in all analyses. n = 10 contrasts.)
| Dinornis size estimate | phylogenetic hypothesis | β | lower 95% | upper 95% | a | lower 95% | upper 95% |
|---|---|---|---|---|---|---|---|
| Median | Baker–Cooper | 1.002 | 0.898 | 1.118 | 0.0050 | −0.0042 | 0.014 |
| Baker–Haddrath | 1.004 | 0.898 | 1.122 | 0.0056 | −0.0039 | 0.015 | |
| Baker–Phillips | 1.025 | 0.932 | 1.128 | 0.0064 | −0.0030 | 0.016 | |
| Bunce–Cooper | 1.016 | 0.920 | 1.121 | 0.0045 | −0.0062 | 0.015 | |
| Bunce–Haddrath | 1.018 | 0.921 | 1.125 | 0.0052 | −0.0058 | 0.016 | |
| Bunce–Phillips | 1.030 | 0.941 | 1.128 | 0.0062 | −0.0047 | 0.017 | |
| Minimum | Baker–Cooper | 0.989 | 0.891 | 1.099 | 0.0054 | −0.0031 | 0.014 |
| Baker–Haddrath | 0.992 | 0.892 | 1.031 | 0.0059 | −0.0028 | 0.015 | |
| Baker–Phillips | 1.016 | 0.926 | 1.114 | 0.0066 | −0.0022 | 0.015 | |
| Bunce–Cooper | 1.001 | 0.913 | 1.097 | 0.0048 | −0.0047 | 0.014 | |
| Bunce–Haddrath | 1.002 | 0.913 | 1.101 | 0.0055 | −0.0044 | 0.015 | |
| Bunce–Phillips | 1.018 | 0.935 | 1.108 | 0.0063 | −0.0035 | 0.016 | |
| Maximum | Baker–Cooper | 0.999 | 0.904 | 1.105 | 0.0053 | −0.0032 | 0.014 |
| Baker–Haddrath | 1.002 | 0.904 | 1.109 | 0.0059 | −0.0029 | 0.015 | |
| Baker–Phillips | 1.022 | 0.936 | 1.116 | 0.0067 | −0.0020 | 0.015 | |
| Bunce–Cooper | 1.012 | 0.928 | 1.103 | 0.0050 | −0.0045 | 0.015 | |
| Bunce–Haddrath | 1.014 | 0.928 | 1.106 | 0.0057 | −0.0041 | 0.016 | |
| Bunce–Phillips | 1.026 | 0.948 | 1.110 | 0.0066 | −0.0031 | 0.016 |
4. Discussion
Our analyses of composite phylogenies find no support for the existence of Rensch's Rule in palaeognaths, and provide important insights into patterns of male and female body size evolution in moa and other species. Using a series of different source phylogenies to examine the evolution of sexual dimorphism, in the absence of independent critical assessment of the relative robustness of each phylogeny, inevitably leads to some variation in inference about evolutionary patterns in this character state across the group. However, despite this variation in source phylogenies and evolutionary outputs, there is a strong consensus across our results that all observed levels of SSD in all palaeognaths are extremely close to what would be expected, given their body size and phylogenetic position alone. The evolution of SSD in palaeognaths is, therefore, completely correlated with phylogeny, with no genus out of the ordinary with respect to relative size differences between males and females.
The extreme level of RSD observed in Dinornis can, therefore, be interpreted as predominantly an allometric scaling effect associated with the overall increase in body size shown by this moa lineage, presumably in response to independent selective pressures and niche availability in New Zealand terrestrial environments during the Cenozoic, rather than a specific adaptation in itself. Body size evolution in Dinornis was also associated with other substantial biological changes, including marked acceleration in juvenile growth rate relative to other moa taxa [41]. In comparison with other moa, on the basis of both relative magnitude and phylogenetic dependence of body size, Dinornis is most similar to Emeus; the most unusual moa genus instead appears to be Euryapteryx, whose body size, while similar to predicted ancestral body sizes, is appreciably small for its phylogenetic position (figure 4).
However, there is also variation in relative phylogenetic dependence of body size between different Dinornis sexes and allopatric populations. Although observed levels of intersexual variation in phylogenetic dependence are relatively small in comparison with some other palaeognaths (figure 4), we show that Dinornis females are phylogenetically more atypical in body size than males. In contrast to male Dinornis, female Dinornis body masses, therefore, evolved at least slightly independently of phylogeny, indicating that there has been some adaptation of female body size to ecological conditions. Results from the most recent and densely sampled moa species-level phylogeny [43] further suggest that larger Dinornis body mass estimates are phylogenetically more atypical. These findings together indicate that Dinornis females have undergone more evolutionary change than males, and that smaller Dinornis females (the ‘novaezealandiae’ morph) are closer to the expected female body mass value based on phylogeny, whereas larger Dinornis females (the ‘giganteus’ morph) are associated with greater levels of adaptation. It is, therefore, possible that whereas one factor may be responsible for the origin of RSD in moa, another factor could have maintained or modified it [8].
Darwin [2] believed that sexual selection on males was the main driver of sexual dimorphism, whereas Wallace [9] considered that natural selection produced different female phenotypes associated with effective nest defence in different habitats. Selection on males for aerodynamic ability (for display or attack) is suggested to have driven RSD evolution in raptors and shorebirds, but this is unlikely in flightless palaeognaths [28]. Conversely, female-based intrasexual competition and dominance in intraspecific interactions (e.g. selection for fecundity, offspring investment and/or defence of breeding territory) have been invoked to explain RSD in mammals and birds [1,7,28]. These hypotheses are consistent with past selective pressures on New Zealand's K-selected avifauna, which evolved in the absence of mammalian predators; many New Zealand birds are not only flightless and large-bodied, but also have low reproductive rates, prolonged immaturity and high investment in individual young [14,54]. Studies of moa cortical growth marks demonstrate that these birds exaggerated the K-selected life-history strategy and took up to a decade to reach skeletal maturity [41]. Intraspecific competition was, therefore, probably a major factor in moa evolution, with selection for increased investment to produce competitively fit offspring probably more intense among females than males. Observed variation in female Dinornis body masses may also represent variation in expression of this probable adaptive response, as size variation is associated with available nutritional content of vegetation [14,17]: females were largest in low-altitude regions characterized by high-productivity open forest-shrubland mosaics, and smallest in lower-productivity subalpine zones and montane forests where resource limitation may have constrained body size.
Examples of apparent sexual selection in nature are frequently the source of scientific and popular interest, but can remain critically untested within appropriate evolutionary frameworks. Our study not only helps to clarify patterns of body size change in palaeognaths and the relative extent to which phylogeny versus selective processes have driven body size evolution in this group, but also demonstrates that the recently discovered extreme RSD in Dinornis was in fact largely driven by positive allometric scaling related to its huge body size. Although the giant moa was in many other respects extremely ecologically and evolutionarily unusual, this level of sexual dimorphism is no different from what we would expect based on wider evolutionary patterns across ratites as a whole.
Acknowledgements
We thank Ed Braun, Rob Freckleton and Tamás Székely for discussion and ideas. This research was supported by a NERC Postdoctoral Research Fellowship (V.O.), and a NERC Postdoctoral Fellowship and a Royal Society University Research Fellowship (S.T.T.).
References
- 1.Székely T, Lislevand T, Figuerola J. 2007. Sexual size dimorphism in birds. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn DJ, Blanckenhorn WU, Székely T.), pp. 27–37 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Darwin C. 1871. The descent of man and selection in relation to sex. London, UK: Murray [Google Scholar]
- 3.Fairbairn DJ. 1997. Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Ann. Rev. Ecol. Syst. 28, 659–687 10.1146/annurev.ecolsys.28.1.659 (doi:10.1146/annurev.ecolsys.28.1.659) [DOI] [Google Scholar]
- 4.Amadon D. 1977. Further comments on sexual size dimorphism in birds. Wilson Bull. 89, 619–620 [Google Scholar]
- 5.Mueller HC, Meyer K. 1985. The evolution of reversed sexual dimorphism in size: a comparative analysis of the Falconiformes of the Western Palearctic. Curr. Ornithol. 2, 65–101 10.1007/978-1-4613-2385-3_2 (doi:10.1007/978-1-4613-2385-3_2) [DOI] [Google Scholar]
- 6.Jehl JRJ, Murray BGJ. 1986. The evolution of normal and reverse sexual dimorphism in shorebirds and other birds. Curr. Ornithol. 3, 1–86 10.1007/978-1-4615-6784-4_1 (doi:10.1007/978-1-4615-6784-4_1) [DOI] [Google Scholar]
- 7.Mueller HC. 1986. The evolution of reversed sexual dimorphism in owls: an empirical analysis of possible selective factors. Wilson Bull. 98, 387–406 [Google Scholar]
- 8.Hedrick AV, Temeles EJ. 1989. The evolution of sexual dimorphism in animals: hypotheses and tests. Trends Ecol. Evol. 4, 136–138 10.1016/0169-5347(89)90212-7 (doi:10.1016/0169-5347(89)90212-7) [DOI] [PubMed] [Google Scholar]
- 9.Wallace AR. 1889. Darwinism: an exposition of the theory of natural selection with some of its applications. London, UK: MacMillan [Google Scholar]
- 10.Massemin S, Korpimäki E, Wiehn J. 2000. Reversed sexual size dimorphism in raptors: evaluation of the hypotheses in kestrels breeding in a temporally changing environment. Oecologia 124, 26–32 10.1007/s004420050021 (doi:10.1007/s004420050021) [DOI] [PubMed] [Google Scholar]
- 11.Swaddle JP, Karubian J, Pruett-Jones S. 2000. A novel evolutionary pattern of reversed sexual dimorphism in fairy wrens: implications for sexual selection. Behav. Ecol. 11, 345–349 10.1093/beheco/11.3.345 (doi:10.1093/beheco/11.3.345) [DOI] [Google Scholar]
- 12.Johnsgard PA. 1991. Bustards, hemipodes, and sandgrouse: birds of dry places. Oxford, UK: Oxford University Press [Google Scholar]
- 13.Alonso JC, Magaña M, Alonso JA, Palacín C, Martín CA, Martín B. 2009. The most extreme sexual size dimorphism among birds: allometry, selection, and early juvenile development in the great bustard (Otis tarda). Auk 126, 657–665 10.1525/auk.2009.08233 (doi:10.1525/auk.2009.08233) [DOI] [Google Scholar]
- 14.Worthy TH, Holdaway RN. 2002. The lost world of the moa. Bloomington, IN: Indiana University Press [Google Scholar]
- 15.Bunce M, Worthy TH, Ford T, Hoppitt W, Willerslev E, Drummond A, Cooper A. 2003. Extreme reversed sexual size dimorphism in the extinct New Zealand moa Dinornis. Nature 425, 172–175 10.1038/nature01871 (doi:10.1038/nature01871) [DOI] [PubMed] [Google Scholar]
- 16.Huynen L, Millar CD, Scofield RP, Lambert DM. 2003. Nuclear DNA sequences detect species limits in ancient moa. Nature 425, 175–178 10.1038/nature01838 (doi:10.1038/nature01838) [DOI] [PubMed] [Google Scholar]
- 17.Worthy TH, Bunce M, Cooper A, Scofield P. 2005. Dinornis: an insular oddity, a taxonomic conundrum reviewed. Monogr. Soc. Hist. Nat. Balears 12, 377–390 [Google Scholar]
- 18.Cracraft J. 1976. The species of moa (Aves: Dinornithidae). Smithson. Contrib. Paleobiol. 27, 189–205 [Google Scholar]
- 19.Worthy TH, Scofield RP. 2012. Twenty-first century advances in knowledge of the biology of moa (Aves: Dinornithiformes): a new morphological analysis and moa diagnoses revised. NZ J. Zool. 39, 87–153 10.1080/03014223.2012.665060 (doi:10.1080/03014223.2012.665060) [DOI] [Google Scholar]
- 20.Worthy TH. 1987. Sexual dimorphism and temporal variation in the North Island moa species Euryapteryx curtus (Owen) and Pachyornis mappini Archer. Rec. Natl. Mus. NZ 3, 59–70 [Google Scholar]
- 21.Cooper A, Lalueza-Fox C, Anderson S, Rambaut A, Austin JM, Ward R. 2001. Complete mitochondrial genome sequences of two extinct moas clarify ratite evolution. Nature 409, 704–706 10.1038/35055536 (doi:10.1038/35055536) [DOI] [PubMed] [Google Scholar]
- 22.Haddrath O, Baker AJ. 2001. Complete mitochondrial DNA genome sequences of extinct birds: ratite phylogenetics and the vicariance biogeography hypothesis. Proc. R. Soc. Lond. B 268, 929–945 10.1098/rspb.2001.1587 (doi:10.1098/rspb.2001.1587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourdon E, de Ricqlès A, Cubo J. 2009. A new Transantarctic relationship: morphological evidence for a Rheidae-Dromaiidae-Casuariidae clade (Aves, Palaeognathae, Ratitae). Zool. J. Linn. Soc. 156, 641–663 10.1111/j.1096-3642.2008.00509.x (doi:10.1111/j.1096-3642.2008.00509.x) [DOI] [Google Scholar]
- 24.Phillips MJ, Gibb GC, Crimp EA, Penny D. 2010. Tinamous and moa flock together: mitochondrial genome sequence analysis reveals independent losses of flight among ratites. Syst. Biol. 59, 90–107 10.1093/sysbio/syp079 (doi:10.1093/sysbio/syp079) [DOI] [PubMed] [Google Scholar]
- 25.Harshman J, et al. 2008. Phylogenomic evidence for multiple losses of flight in ratite birds. Proc. Natl Acad. Sci. USA 105, 13 462–13 467 10.1073/pnas.0803242105 (doi:10.1073/pnas.0803242105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handford P, Mares MA. 1985. The mating systems of ratites and tinamous: an evolutionary perspective. Biol. J. Linn. Soc. 25, 77–104 10.1111/j.1095-8312.1985.tb00387.x (doi:10.1111/j.1095-8312.1985.tb00387.x) [DOI] [Google Scholar]
- 27.Davies SJJF. 2002. Ratites and tinamous. Oxford, UK: Oxford University Press [Google Scholar]
- 28.Tubaro PL, Bertelli S. 2003. Female-biased sexual size dimorphism in tinamous: a comparative test fails to support Rensch's Rule. Biol. J. Linn. Soc. 80, 519–527 10.1046/j.1095-8312.2003.00252.x (doi:10.1046/j.1095-8312.2003.00252.x) [DOI] [Google Scholar]
- 29.Gould SJ. 1973. Positive allometry of antlers in the ‘Irish elk’, Megaloceros giganteus. Nature 244, 375–376 10.1038/244375a0 (doi:10.1038/244375a0) [DOI] [Google Scholar]
- 30.Fairbairn DJ, Preziosi RF. 1994. Sexual selection and the evolution of allometry for sexual size dimorphism in the waterstrider, Aquarius remigis. Am. Nat. 144, 101–118 10.1086/285663 (doi:10.1086/285663) [DOI] [Google Scholar]
- 31.Abouheif E, Fairbairn DJ. 1997. A comparative analysis of allometry for sexual dimorphism: assessing Rensch's Rule. Am. Nat. 149, 540–562 10.1086/286004 (doi:10.1086/286004) [DOI] [Google Scholar]
- 32.Dale J, Dunn PO, Figuerola J, Lislevand T, Székely T, Whittingham LA. 2007. Sexual selection explains Rensch's Rule of allometry for sexual size dimorphism. Proc. R. Soc. B 274, 2971–2979 10.1098/rspb.2007.1043 (doi:10.1098/rspb.2007.1043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Székely T, Freckleton RP, Reynolds JD. 2004. Sexual selection explains Rensch's Rule of size dimorphism in shorebirds. Proc. Natl Acad. Sci. USA 101, 12 224–12 227 10.1073/pnas.0404503101 (doi:10.1073/pnas.0404503101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunning JB. 1993. CRC handbook of avian body masses. Boca Raton, FL: CRC Press [Google Scholar]
- 35.Heather B, Robertson A. 1997. The field guide to the birds of New Zealand. Oxford, UK: Oxford University Press [Google Scholar]
- 36.del Hoyo J, Elliot A, Sargatal J. 1992. Handbook of the birds of the world. Volume 1: ratites to ducks. Barcelona, Spain: Lynx Edicions [Google Scholar]
- 37.Marchant S, Higgins PJ. 1990. Handbook of Australian, New Zealand and Antarctic birds. Volume 1. Ratites to ducks. Melbourne, Australia: Oxford University Press [Google Scholar]
- 38.Smith RJ. 1999. Statistics of sexual size dimorphism. J. Hum. Evol. 36, 423–459 10.1006/jhev.1998.0281 (doi:10.1006/jhev.1998.0281) [DOI] [PubMed] [Google Scholar]
- 39.Dickison MR. 2007. The allometry of giant flightless birds. PhD thesis, Duke University, Durham, NC [Google Scholar]
- 40.Prange HD, Anderson JF, Rahn H. 1979. Scaling of skeletal mass to body mass in birds and mammals. Am. Nat. 113, 103–122 10.1086/283367 (doi:10.1086/283367) [DOI] [Google Scholar]
- 41.Turvey ST, Green OR, Holdaway RN. 2005. Cortical growth marks reveal extended juvenile development in New Zealand moa. Nature 435, 940–943 10.1038/nature03635 (doi:10.1038/nature03635) [DOI] [PubMed] [Google Scholar]
- 42.Baker AJ, Huynen LJ, Haddrath O, Millar CD, Lambert DM. 2005. Reconstructing the tempo and mode of evolution in an extinct clade of birds with ancient DNA: the giant moas of New Zealand. Proc. Natl Acad. Sci. USA 102, 8257–8262 10.1073/pnas.0409435102 (doi:10.1073/pnas.0409435102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunce M, et al. 2009. The evolutionary history of the extinct ratite moa and New Zealand Neogene palaeogeography. Proc. Natl Acad. Sci. USA 107, 16 201–16 206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alroy J. 1996. Constant extinction, constrained diversification, and uncoordinated stasis in North American mammals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 127, 285–311 10.1016/S0031-0182(96)00100-9 (doi:10.1016/S0031-0182(96)00100-9) [DOI] [Google Scholar]
- 45.Foote M. 2001. Inferring temporal patterns of preservation, origination, and extinction from taxonomic survivorship analysis. Paleobiology 27, 602–630 (doi:10.1666/0094-8373(2001)027<0602:ITPOPO>2.0.CO;2) [DOI] [Google Scholar]
- 46.Tennyson AJD, Palma RL, Robertson HA, Worthy TH, Gill BJ. 2003. A new species of kiwi (Aves, Apterygiformes) from Okarito, New Zealand. Rec. Auckland Mus. 40, 55–64 [Google Scholar]
- 47.Bertelli S, Giannini NP, Goloboff PA. 2002. A phylogeny of the tinamous (Aves: Palaeognathiformes) based on integumentary characters. Syst. Biol. 51, 959–979 10.1080/10635150290102492 (doi:10.1080/10635150290102492) [DOI] [PubMed] [Google Scholar]
- 48.Chojnowski JL, Kimball RT, Braun EL. 2008. Introns outperform exons in analyses of basal avian phylogeny using clathrin heavy chain genes. Gene 410, 89–96 10.1016/j.gene.2007.11.016 (doi:10.1016/j.gene.2007.11.016) [DOI] [PubMed] [Google Scholar]
- 49.Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 10.1086/343873 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 50.Pagel M. 1997. Inferring evolutionary processes from phylogenies. Zool. Scr. 26, 331–348 10.1111/j.1463-6409.1997.tb00423.x (doi:10.1111/j.1463-6409.1997.tb00423.x) [DOI] [Google Scholar]
- 51.Birkhead TR, Immler S, Pellatt EJ, Freckleton R. 2006. Unusual sperm morphology in the Eurasian bullfinch (Pyrrhula pyrrhula). Auk 123, 383–392 10.1642/0004-8038(2006)123[383:USMITE]2.0.CO;2 (doi:10.1642/0004-8038(2006)123[383:USMITE]2.0.CO;2) [DOI] [Google Scholar]
- 52.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15 10.1086/284325 (doi:10.1086/284325) [DOI] [Google Scholar]
- 53.Arak A. 1988. Sexual dimorphism in body size: a model and a test. Evolution 42, 820–825 10.2307/2408874 (doi:10.2307/2408874) [DOI] [PubMed] [Google Scholar]
- 54.Bourdon E, Castanet J, de Ricqlès A, Scofield P, Tennyson A, Lamrous H, Cubo J. 2009. Bone growth marks reveal protracted growth in New Zealand kiwi (Aves, Apterygidae). Biol. Lett. 5, 639–642 10.1098/rsbl.2009.0310 (doi:10.1098/rsbl.2009.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]