Abstract
Life-history theory predicts that suboptimal developmental conditions may lead to faster life histories (younger age at recruitment and higher reproductive investment), but experimental testing of this prediction is still scarce in long-lived species. We report the effects of an experimental manipulation of food availability during early development and at recruitment on the onset of reproduction and reproductive performance (productivity at first breeding) in a long-lived seabird, the black-legged kittiwake Rissa tridactyla, breeding on Middleton Island, Alaska. Birds were born and raised in nests with supplemented food (‘fed’) or unsupplemented control nests (‘unfed’), and later recruited into either fed or unfed nests. Fed chicks grew faster than unfed chicks, and males grew faster than females. Birds were more likely to reproduce at younger ages when recruiting into fed nests. Faster growth during development tended to increase age at recruitment in all individuals. Social rank of individuals also affected age at recruitment: B-chicks recruited earlier than A-chicks and singletons recruited later than A- and B-chicks. Productivity increased with the age at recruitment and growth rate as chick, but much of the variability remained unexplained. We conclude that results of this study at least partially support predictions of life-history theory: younger age at first breeding for kittiwakes that experienced suboptimal natal conditions, as well as greater productivity of early recruiting kittiwakes that grew in control nests compared with those that grew in food-supplemented nests.
Keywords: recruitment, social rank, developmental stress, productivity, predictive adaptive response
1. Introduction
Whether the responses of a growing organism to environmental conditions are adaptive is a central question in life-history evolution and population ecology. Early environmental conditions, in particular developmental stress, are likely to lead to irreversible modification of phenotypes [1–3] by constraining or programming physiological or structural traits [4–9], which might affect growth, survival, reproduction and ultimately fitness [7,10–14]. Whether modification of phenotype owing to environment reflects constraint or adaptation is probably a function of the severity of the stress induced by suboptimal early conditions [9]. Critically low resources may impose severe constraints on allocation of resources to different functions, resulting in a poorly performing (‘low-quality’) individual showing delayed onset of reproduction and poor reproductive performance [6,9,15]. On the other hand, individuals experiencing suboptimal, but not critically poor, early conditions might modify the developmental processes with adaptive changes in physiology, behaviour, tissue maturation and reproductive schedule [9,16]. Since reproduction is costly and there is a trade-off between current breeding effort and subsequent attempts, it pays to reproduce early when future prospects are poor [17]. Consequently, life-history theory predicts that suboptimal early developmental conditions, operating as a proxy of future environmental and nutritional conditions, lead to early onset of reproduction and increased early reproductive investment [17,18].
In long-lived species such as seabirds, many years pass between development and reproduction, and other factors (e.g. learning, feeding conditions during the oceanic phase) are likely to further modulate the life-history strategies of an individual [19]. Although understanding reproductive dynamics is central for understanding the basic and applied ecology of long-lived seabirds, we still have limited knowledge about factors affecting age at recruitment, reproductive effort and nesting habitat selection [20,21]. In particular, whether early conditions have long-term consequences on life-history strategies remains ambiguous [22], in part, because of few longitudinal studies with the power to examine these issues (studies investigating the interaction between developmental and adult environments [9]).
We use data from a unique long-term experimental study of a long-lived seabird, the black-legged kittiwake Rissa tridactyla, to investigate how food availability during development and at recruitment influences the onset of reproduction and reproductive output at first reproduction (i.e. recruitment). Here, free-living kittiwakes were raised as chicks and later recruited into experimentally manipulated foraging conditions [23], thus allowing us to tease apart the contribution of early nutritional conditions and nutritional conditions at recruitment on reproduction.
Kittiwake chicks are vulnerable to nutritional stress during development, often suffering irreversible effects on phenotype. Deleterious effects of severe early nutritional stress on kittiwakes include low growth efficiency as chicks and compromised cognitive abilities later in life [3,24]. Chronic elevation of glucocorticoid ‘stress’ hormones, which rise in response to food shortages, causes muscle wasting, suppression of the immune function, inhibition of the gonadal axis and neuronal cell death [25], and reduces the survival of the affected chicks before fledging, or post-fledging in case they are able to fledge [26]. High fluctuations in productivity with relatively low variation in chick growth, often observed in kittiwakes [24], suggest that even relatively small changes in growth rates might have long-lasting and negative consequences, thus reflecting developmental constraints [27].
In seabirds, whether early suboptimal conditions lead to faster life histories (i.e. accelerated reproduction, high early reproductive investment) or, because of developmental constraints, lead instead to delayed reproduction and poor reproductive performance is an unresolved issue [19]. Age at first breeding partially reflects a trade-off between lifetime reproduction and survival, since breeding too early in life can substantially increase post-breeding mortality [28–30], but delaying breeding for too long will reduce lifetime reproductive success [19]. Thus, there are two competing hypotheses about how seabirds may respond to early adversity. A poor start could result in (i) earlier onset of reproduction owing to the adoption of faster life histories or (iii) delayed reproduction owing to the potential higher breeding costs for birds that had a poorer start [31]. Birds with a poorer start may also be constrained by their inferiority as competitors [21,32], reducing their access to mates and vacant sites, and thus imposing a ‘slower’ life history. In addition, kittiwakes adopting faster life-history strategies are expected to increase their early reproductive investment [17].
Environmental conditions experienced during the early stages of life may determine changes in phenotype that make the organism better equipped specifically for the nutritional and environmental conditions it will encounter later in life (the predictive adaptive response, PAR [9,33]). Whether or not these predictive developmental pathways are beneficial depends on how closely the conditions experienced during development predict those later in life, although evidence for PARs where benefits are achieved in future environments is still scarce, and the occurrence of PARs has rarely been either experimentally or theoretically tested [9,34]. In the case of a PAR, the prediction is that reproductive performance of an individual is higher when environmental conditions during its development match those during its reproduction [9].
The main goal of this study was to use our unique dataset to investigate whether early developmental conditions impose constraints on reproductive schedule, performance and nesting habitat selection, or induce adaptive shifts in life-history strategies of kittiwakes. Our specific objectives were (i) to test whether food supply during development, social rank (i.e. hatching order) and sex affect chick growth; (ii) to test whether there is an acceleration or delay in recruitment for kittiwakes growing in suboptimal conditions; (iii) to test whether nest selection at recruitment (between food-supplemented or control nests) is influenced by the food availability that recruiting birds experienced when they were chicks; (iv) to test the effects of early conditions and breeding conditions on reproductive performance, as well as the occurrence of a PAR, i.e. higher reproductive performance of kittiwakes when early conditions and conditions at recruitment are matched.
2. Material and methods
(a). Methods
On Middleton Island (59°26′ N, 146°20′ W), Gulf of Alaska, black-legged kittiwakes nest on an abandoned US Air Force radar tower [23]. The colony on Middleton Island was failing in the early 1990s (from 166 000 birds in 1981 to fewer than 25 000 in 1999), partly because of successional changes in breeding habitat following an earthquake. A large-scale supplemental feeding experiment was initiated in 1996 to test whether the food was limiting productivity [23].
The black-legged kittiwake is a medium-sized colonial gull breeding on vertical cliffs of the coasts and islands in the Northern Hemisphere. Previous work showed that a substantial fraction of adults in Atlantic colonies starts breeding between 3 and 6 years old [27,35], while less information is available for Pacific colonies. Breeders show high site fidelity and lay clutches of one to three eggs [27]. Chicks remain in the nest until they are nearly adult size. Peak mass of chicks in the nest is more than 90 per cent of adult weight [36], and adult body weight is highly repeatable [37]. Food availability for breeding kittiwakes varies markedly from year to year [38,39].
The following are most salient features of the supplemental feeding experimental set-up [23]. The radar tower is a 12-walled polygon where artificial nest sites have been constructed on the upper walls, permitting observations and capture of breeders and their chicks from inside the building through sliding one-way windows. Since 1996, a feeding experiment divides birds into two treatments: supplementally fed (hereafter ‘fed’ group) and control group (i.e. ‘unfed’ group). Panels (12-wall segments) of unfed nests alternate with panels of fed nests so that environmental conditions are replicated. Treatments were assigned to the same panels over years. Capelin (Mallotus villosus) was used as the supplemental food for birds in fed panels. Kittiwakes (parents and chicks) were fed three times a day from inside the tower; fish were continuously given through a plastic tube passing through the wall at each nest site until birds were sated. For all recruiters included in our dataset, supplemental food was provided in fed nests for the duration of breeding season (from arrival until fledging chicks).
Nests were checked twice daily during the entire breeding season to determine content. Chicks were banded for individual identification and measured every 5 days to the nearest 0.1 g from the day of hatching until fledging or day 40 post-hatching. First-, second- and third-hatched chicks were recorded as A-, B- and C-chicks, respectively; if only a single chick hatched it was recorded as singleton.
(b). Data and statistical analysis
The dataset includes information for 113 kittiwakes that were chicks in the experimental nests from 1996 to 2006, and recruited (laid eggs for the first time) into the experimental nests from 2001 up to 2011. No new recruits were observed in 2012. For each individual, we recorded sex S, social rank R (A-chick, B-chick, C-chick, singleton), year of birth Yb, year at recruitment, feeding treatment (fed/unfed) at the nestling stage TN, feeding treatment (fed/unfed) at breeding TB, reproductive success at first breeding Rs, chicks fledged per nest (productivity) at first breeding relative to the mean productivity in that year for fed or unfed nests Fr and age at recruitment α (see the electronic supplementary material, figure S1). Henceforth, we refer to reproductive success at first breeding and (relative) productivity at first breeding as reproductive success and (relative) productivity. Relative productivity Fr controls for inter-annual and experimental treatment effects on reproductive performance [23]. Indeed, the mean number of chicks fledged per nest in unfed and fed treatments was consistently different across years (paired t-test, p < 0.01), with 0.365 more chicks fledging per nest on average in fed compared with unfed nests. In all years except 2002, mean productivity was greater in fed nests. It is probable that an equal productivity occurs in fed and unfed nests when natural food availability is particularly high (which occurs rarely). The most parsimonious explanation for greater productivity of unfed nests in 2002 is sampling error.
Fledging mass is a common proxy for quality (in particular for post-fledging survival) in many seabird species [19], including kittiwakes [27]. We used peak body mass Pm (i.e. maximum recorded mass for a chick before fledging) and daily growth during the linear phase Gm (between day 5 and day 20, from approx. 75 to 300 g) to characterize growth during the nestling phase.
Unless otherwise noted, in the models described below, we included interactions between explanatory variables in the starting model only when biologically interpretable. For both Gm and Pm, we used as (categorical) explanatory variables R, S, Yb and TN. We first fitted the complete model (i.e. with full interaction between variables since all interactions were potentially biologically interpretable) and then selected the best model using AIC [40,41]. For both Gm and Pm as response variables, we excluded from the analysis two chicks of rank C. For Gm, we excluded from the analysis one chick with growth during the linear phase less than 5 g d−1; while for Pm, we excluded from the analysis one chick with peak weight more than 550 g. In both cases, the subjects were clear outliers (figure 1a). We excluded one chick which was not measured at 5 days post-hatch and which precluded the calculation of Gm.
Figure 1.
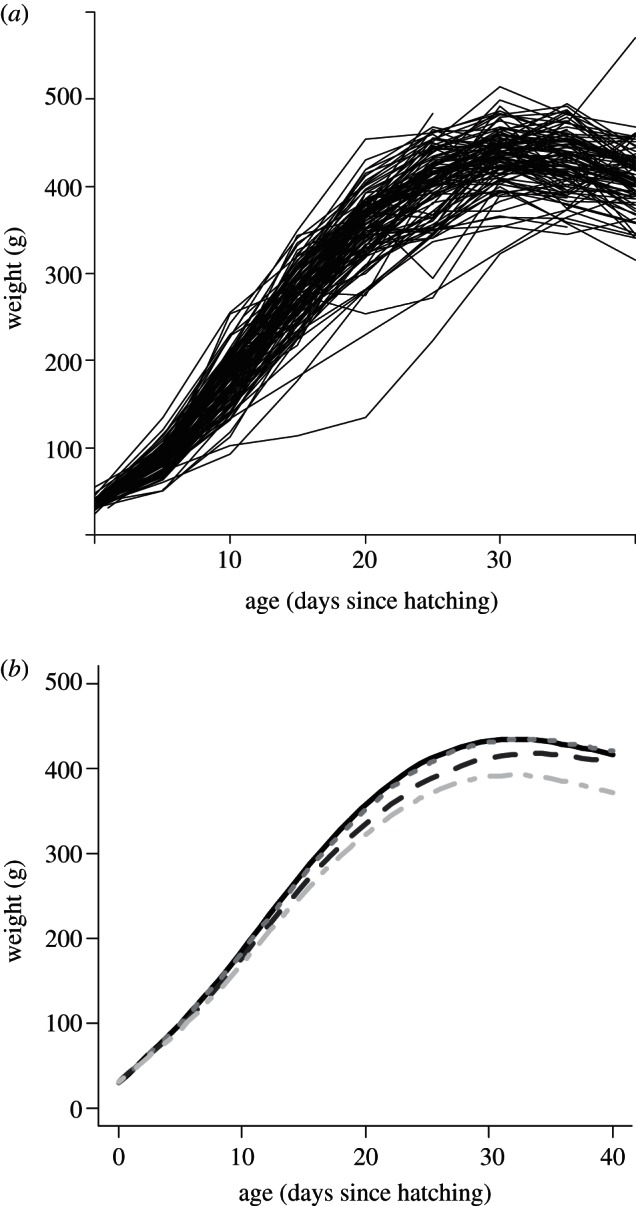
(a) Growth trajectories of the 113 kittiwake chicks that developed in supplementally fed or control (unfed) nests and later recruited into the breeding colony; (b) locally weighted linear regression of body mass on age of fed (solid line), unfed (dashed line), male (dotted line) and female (dash-dotted line) chicks. Rate of increase in body mass during the linear phase was higher for chicks growing in fed nests and for males.
We used a Cox proportional hazard model [42] to analyse age at first breeding. Hazard, the probability of an event that has not happened previously occurring, is appropriate for events that can happen once. In our case, the hazard hi(a) corresponds to the chance of kittiwake i recruiting for the first time at age a
| 2.1 |
where ho(a) is the baseline hazard for age a, 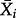 is a vector of covariates for kittiwake i and
is a vector of covariates for kittiwake i and 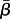 is a vector of coefficients to be estimated. We used as categorical variables S, R, TN, TB and as covariates Gm and Pm. We excluded from the analysis two chicks of rank C. We selected the best model using AIC.
is a vector of coefficients to be estimated. We used as categorical variables S, R, TN, TB and as covariates Gm and Pm. We excluded from the analysis two chicks of rank C. We selected the best model using AIC.
Cohort effects on local juvenile survival are well documented in seabird species [3,43], but we did not model cohort effects since that would introduce an obvious bias given the limited time frame for recruitment of late cohorts.
We used a χ2-test of independence to test whether chicks from fed/unfed nests were more likely to recruit into fed/unfed nests than expected by chance alone.
We used ordinary least-square regression models to model the relationship between Fr and S, R, TN, TB with covariates Gm, Pm and age at recruitment α, including pair interactions between covariates and factors. We excluded two chicks from the analysis of rank C.
Lack of independence among individuals (i.e. members of a reproductive pair) may create pseudo-replication for reproductive traits. Among the kittiwakes included in the dataset there were eight pairs. Therefore, we fitted the model for relative productivity Fr (i) using males only (i.e. the most abundant sex, electronic supplementary material, figure S1), (ii) randomly excluding one pair member. In both cases, results very similar to those provided by the model obtained with the complete dataset.
We visually checked for violation of linearity and homoscedasticity of data and residuals in the linear models, and we confirmed assumptions of the Cox proportional hazard model [42].
We did not discriminate among models with ΔAIC < 2 [41]. In the case of multiple models with ΔAIC < 2, we proceeded with model averaging without shrinking model parameters [41]. We report the relative importance of the explanatory variables (including their interactions), computed as a sum of the Akaike weights over all of the models in which the parameter of interest appears.
For the χ2-test of independence for preference of treatment at recruitment, we evaluated significance at the 0.05 level. All analyses were run with R v. 2.12.1 [44].
3. Results
(a). Nestling growth
Increase in body mass accelerated slightly in the first days after hatching, followed by a period in which the daily increase in weight was approximately constant (figure 1a). For most individuals, there was a loss of about 10 per cent of body mass before fledging. The best model for body mass growth rates during the linear phase included sex and feeding treatment as explanatory variables (figure 1b and table 1) but no interaction between the explanatory variables (table 1). Across all individuals, daily growth was (mean±s.d.) 18.0±2.11 g d−1. Males grew faster than females (males = 18.2±2.08 g; females = 16.9±1.89 g), and chicks in fed nests grew faster than chicks in unfed nests (fed = 18.7±2.1 g; unfed = 17.3±1.90 g). After accounting for sex and feeding treatment, social rank did not explain variability in daily growth during the linear phase.
Table 1.
Estimates (±s.e.) of coefficients included in the best model with AICc (AIC corrected for finite sample size) selection for daily growth (Gm, ordinary least-square regression, ols) and peak mass (Pm, ols) of chicks. Colon (:) denotes interaction between variables. We also report statistical significance of models and parameters and coefficients of determination 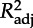 . TN is the experimental treatment at the chick stage; Gm, daily growth rate (g d−1) during the chick stage; α, age at recruitment; Pm, peak mass (g) during the chick stage.
. TN is the experimental treatment at the chick stage; Gm, daily growth rate (g d−1) during the chick stage; α, age at recruitment; Pm, peak mass (g) during the chick stage.
| explanatory variables |
Gm*** 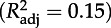
|
Pm*** 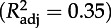
|
|---|---|---|
| intercept | 17.60 (0.48)*** | 423.33 (0.04)*** |
| sex (M) | 1.26 (0.47)** | 30.73 (8.78)*** |
| TN (unfed) | −1.29 (0.37)*** | −25.83 (10.63)* |
| sex (M) : TN (unfed) | 20.84 (11.81)^ |
Levels of statistical significance: ***p < 0.001, **p < 0.01, * p < 0.05, ^ < 0.10.
The best model for peak weight included sex, feeding treatment and their interaction as explanatory variables. Male chicks in fed nests (454.1±20.86 g) had marginally greater peak weight than males in unfed nests (449.1±26.92 g). Female chicks in fed nests (423.3±25.12 g) reached a lower peak weight than males, but greater than female chicks in unfed nests (397.5±25.08 g).
(b). Recruitment age
Seven Cox proportional hazard models had ΔAIC < 2 and no interaction among explanatory variables was included in any of them (table 2). The most important variable associated with age at recruitment was feeding treatment at recruitment. Mean age at recruitment was lower in fed nests (6.14±1.56 year) than in unfed nests (7.21±1.59 year; figure 2). According to the averaged model (table 3), B-chicks tended to recruit earlier than A-chicks both in fed and unfed nests, and singletons tended to recruit later than both A- and B-chicks both in fed and unfed nests. Males tended to recruit earlier than females, and faster growth during the nestling stage tended to increase age at recruitment.
Table 2.
Cox proportional hazard models for age at recruitment with ΔAICc < 2, and the null model. The last row of the table indicates relative importance of variables, computed as the sum of the Akaike weights over all of the models in which the parameter of interest appears. Asterisks (*) denote variables included in the model. Int denotes intercept; R, social rank during the chick stage; TB, experimental treatment at recruitment stage; Gm, daily growth rate (g d−1) during the chick stage. d.f., degrees of freedom; logLik, log-likelihood; AICc, AIC corrected for finite sample size; ΔAICc, difference between AICc of a model and AICc of the best model; weight, ratio of AICc values for each model relative to the whole set of candidate models.
| Int | R | S | TB | Gm | d.f. | logLik | AICc | ΔAICc | weight |
|---|---|---|---|---|---|---|---|---|---|
| * | * | 1 | −405.920 | 813.9 | 0.00 | 0.264 | |||
| * | * | * | * | 2 | −405.152 | 814.4 | 0.54 | 0.202 | |
| * | * | * | 3 | −404.228 | 814.7 | 0.80 | 0.177 | ||
| * | * | * | 4 | −403.318 | 815.0 | 1.14 | 0.149 | ||
| * | * | 2 | −404.228 | 815.7 | 1.85 | 0.105 | |||
| * | * | * | * | 3 | −405.808 | 815.8 | 1.87 | 0.104 | |
| * | 2 | −410.323 | 820.6 | 4.90 | — | ||||
| variable importance | |||||||||
| — | 0.33 | 0.45 | 1 | 0.21 | |||||
Figure 2.
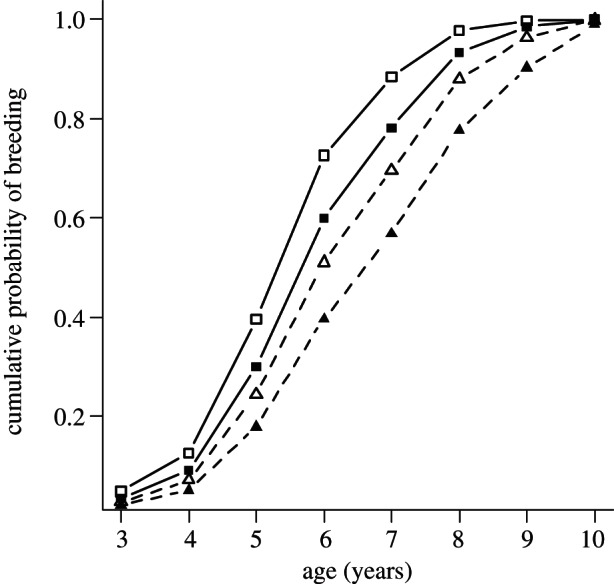
Predictions from the Cox proportional hazard model for age at recruitment of male kittiwakes in relation to their social status during development (A- versus B-chicks) and experimental treatment at recruitment. Kittiwakes recruited earlier in fed nests (A-chicks, solid line with filled squares; B-chicks, solid line with unfilled squares) than in unfed nests (A-chicks, dashed line with filled triangles; B-chicks, dashed line with unfilled triangles). Within treatments at recruitment, B-chicks recruited earlier than A-chicks.
Table 3.
Model-averaged coefficients (±s.e) for the hazard function for age at recruitment (h(a), Cox proportional hazard model) and relative number of chicks fledged (Fr, ordinary least-squares regression) for sets of best models (ΔAIC < 2). Levels of statistical significance as in table 1. Relative performances of the models for h(a) and Fr are presented in tables 2 and 4, respectively. TN denotes experimental treatment during the chick stage; Gm, daily growth rate (g d−1) during the chick stage; α, age at recruitment; TB, experimental treatment at recruitment; Pm, peak mass (g) during the chick stage.
| explanatory variable |
h(a) 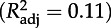
|
Fr
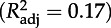
|
|---|---|---|
| intercept | −1.85 (0.90)* | |
| sex (M) | 0.31 (0.25) | −0.153 (0.18)^ |
| TN (unfed) | 1.11 (0.63)* | |
| rank B | 0.34 (0.21) | |
| rank S | −0.13 (0.29) | |
| TB (unfed) | −0.59 (0.19)** | 0.34 (0.21) |
| Gm | −0.03 (0.05) | 0.06 (0.03)^ |
| α | 0.21 (0.07)** | |
| TN (unfed) : α | −0.16 (0.09)^ | |
| TN (unfed) : TB (unfed) | −0.67 (0.29)* | |
| Pm | −0.15 (0.18)* |
(c). Nest selection
Individuals fed as chicks were more likely to recruit into fed nests and individuals unfed as chicks to recruit into unfed nests (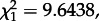 p < 0.01). Twenty out of 57 kittiwakes from fed nests later recruited into unfed nests, whereas 19 out of 56 kittiwakes from unfed nests later recruited into fed nests.
p < 0.01). Twenty out of 57 kittiwakes from fed nests later recruited into unfed nests, whereas 19 out of 56 kittiwakes from unfed nests later recruited into fed nests.
(d). Relative productivity
For relative productivity, 12 different models had ΔAIC < 2 (table 4). According to the averaged linear model (table 3 and figure 3), age at recruitment had a positive effect on relative productivity and was the most important explanatory variable in the model. For recruits younger than 6 years old, individuals from fed nests had lower relative productivity in both fed and unfed nests than those from unfed nests. For older birds, there was no clear difference in relative productivity between birds born in fed or unfed nests. Daily growth and peak mass as chicks had positive and negative relationships with relative productivity, respectively, but the importance of both variables was low (table 4). Across ages at recruitment, kittiwakes born in unfed nests had slightly higher relative productivity when recruiting into fed nests, while kittiwakes born in fed nests had slightly higher relative productivity when recruiting into unfed nests (figure 3).
Table 4.
Models for relative reproductive success Fr with ΔAICc < 2, and the null model. Last row of the table indicates relative importance of variables, computed as the sum of the Akaike weights over all of the models in which the parameter of interest appears. Colon (:) indicates interaction between variables. Asterisks (*) indicate that the variable or interaction is included in the model. Int denotes intercept; TN, experimental treatment at the chick stage; S, sex; TB, experimental treatment at recruitment; Gm, daily growth rate (g d−1) during the chick stage; α, age at recruitment; Pm, peak mass (g) during the chick stage. d.f., degrees of freedom; logLik, log-likelihood; AICc, AIC corrected for finite sample size; ΔAICc, difference between AICc of a model and AICc of the best model; weight, ratio of AICc values for each model relative to the whole set of candidate models.
| Int | TN | S | TB | Gm | Pm | α | TN : TB | TN : α | d.f. | logLik | AICc | ΔAICc | weight |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * | * | * | * | * | * | * | 8 | −110.4416 | 238.323 | 0 | 0.1305 | ||
| * | * | * | * | * | 6 | −112.7763 | 238.376 | 0.053 | 0.127 | ||||
| * | * | * | * | * | * | 7 | −111.8592 | 238.827 | 0.504 | 0.1014 | |||
| * | * | * | * | * | * | 7 | −111.9281 | 238.965 | 0.642 | 0.0946 | |||
| * | * | * | * | * | * | * | * | 9 | −109.6142 | 239.046 | 0.723 | 0.0908 | |
| * | * | * | * | 5 | −114.4219 | 239.426 | 1.103 | 0.0751 | |||||
| * | * | * | * | * | 6 | −113.3917 | 239.607 | 1.284 | 0.0686 | ||||
| * | * | * | * | * | * | 7 | −112.2744 | 239.657 | 1.334 | 0.0669 | |||
| * | * | * | * | * | * | * | 8 | −111.1133 | 239.666 | 1.343 | 0.0666 | ||
| * | * | 3 | −116.7843 | 239.797 | 1.474 | 0.0624 | |||||||
| * | * | * | * | * | * | 7 | −112.3755 | 239.860 | 1.537 | 0.0605 | |||
| * | * | * | 4 | −115.8346 | 240.053 | 1.730 | 0.0549 | ||||||
| * | 2 | −123.924 | 252.00 | 13.680 | — | ||||||||
| variable importance | |||||||||||||
| 0.88 | 0.06 | 0.53 | 0.73 | 0.25 | 1 | 0.53 | 0.65 | ||||||
Figure 3.
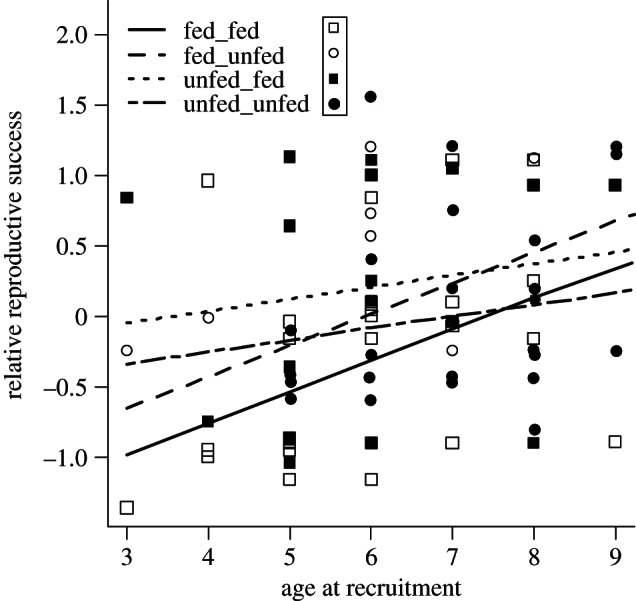
Observations (dots) of relative reproductive success for combinations of treatment as chicks (growing in fed or unfed nests) and treatment at recruitment (recruited into fed or unfed nests) and predictions (lines) for the averaged model (table 3). Predictions are for males with growth and peak mass at their overall means, 17.8 g d−1 and 444 g, respectively. Relative reproductive success is lower and increases with age at recruitment in individuals raised in experimentally fed nests compared with individuals raised in unfed nests. Observations: unfilled squares, growing in fed nests and recruiting into fed nests; unfilled circles, growing in fed nests and recruiting into unfed nests; filled squares, growing in unfed nests and recruiting into fed nests; filled circles, growing in unfed nests and recruiting into unfed nests—filled cicles. Predictions: solid line, growing in fed nests and recruiting into fed nests; dashed lines, growing in fed nests and recruiting into unfed nests; dotted lines, growing in unfed nests and recruiting into fed nests; dot-dash line, growing in unfed nests and recruiting into unfed nests.
4. Discussion
The relative role of early conditions versus conditions at recruitment on reproductive traits can be successfully studied only when conditions at both stages are experimentally controlled. In our study, kittiwakes were likely to recruit earlier in food-supplemented nests. Males tended to recruit earlier than females, B-chicks were more likely to recruit earlier than A-chicks, while singletons tended to recruit later than both A- and B-chicks. Faster body growth during the nestling stage was observed in food-supplemented nests and tended to increase age at recruitment. We did not observe any additional effects of feeding conditions during the nestling stage on age at recruitment. Relative productivity increased with age and was function of both early conditions and conditions at recruitment. Kittiwakes that grew in unfed nests had greater relative productivity than those that grew in food-supplemented nests if younger than 6 years old. Kittiwakes had greater relative productivity when early conditions and conditions at recruitment were mismatched. However, a large amount of variability in productivity remained unexplained.
(a). Growth
The mean growth rate of unfed chicks (17.3±1.90 g) was higher than that reported elsewhere for other North Pacific kittiwake colonies. Kitaysky et al. [24] found that mean growth rates of kittiwakes breeding on the Pribilofs (Alaska) varied between 13 and 16.7 g d−1. Piatt et al. [45] found growth rates at Gull and Barrens colonies (Alaska) similar to those we report in our study. On the contrary, chicks at the Chisik colony (Alaska) grew substantially slower (11–14 g d−1) than those in our study [45]. This suggests that kittiwakes that grew in unfed nests and later recruited into the experimental nests did not experience severe nutritional stress during development. Our dataset confirmed that sexual dimorphism in kittiwakes arises during chick rearing, with males growing faster and reaching a greater peak mass than females [46]. We did not find an effect of social status on growth and peak mass after accounting for feeding conditions and sex. Within feeding treatments (i.e. food-supplemented and control nests), B-chicks had a slower growth and smaller mass at fledging than A-chicks in 1996 and 1997 [47]. This might suggest that B-chicks that recruited into the experimental nests either were of higher quality or were allocating more energy to growth during the nestling phase than B-chicks that died before recruiting or recruited elsewhere.
(b). Age at recruitment
After accounting for variation in growth rate, we did not detect an additional delayed effect of early feeding conditions on probability of recruiting at a certain age. Although supplementally fed chicks had faster growth on average and were more likely to recruit into supplemented nests, fast growth during development tended to increase age at recruitment, while peak mass before fledging was unrelated to age at recruitment. It is generally assumed that in seabird species with no major post-fledging growth larger individuals are better competitors for mates and access to high-quality breeding sites [19–21,27]. For example, Becker [48] found in common terns that birds recruiting at age 3 reached a higher peak mass as chicks than those recruiting when 4 years or older, and that birds recruiting at age 2 had the highest peak mass. Coulson [27] found that male kittiwakes that recruited at the centre of the colony in North Shields (England) were heavier on average and had greater reproductive success than birds breeding at the edge of the colony. Cam et al. [13] found that A-chicks recruited earlier than B-chicks in kittiwake colonies in Brittany (France), thus suggesting possible developmental constraints for B-chicks, as B-chicks grow typically slower and reach a lower peak mass than A-chicks [27]. In our study, B-chicks were more likely to recruit at a younger age than A-chicks. This observation, combined with the lack of difference in growth (within feeding treatments) between A-chicks and B-chicks that recruited into the experimental nests, may thus suggest the adoption of faster life histories for B-chicks. Overall, our results support the hypothesis of early onset of reproduction induced by the effects of suboptimal early nutritional conditions (lower growth rate during development) or lower social status (B-chicks). We found that birds recruiting into food-supplemented nests initiated reproduction at younger ages when compared with those recruiting into control nests. We cannot exclude that kittiwakes directly benefited from the food supplement and were able to initiate reproduction early. Although only kittiwakes that laid eggs were included in our dataset, it is common for kittiwakes to secure a nest and then fail to lay eggs. Hatch et al. [49] found that laying success is highly variable in Pacific colonies and averages 65 per cent. However, higher laying success (greater than 90%) has been observed in Middleton Island in 1996–1997, with no significant difference between food-supplemented and control nests [23].
(c). Habitat selection
Seabirds might use the presence and/or the abundance of con-specifics at a given location [19,50–52] or their assessment of the local productivity of current residents as cues for choosing breeding sites [21,53–55]. Local productivity of a patch provides valuable information only if habitat quality for breeding is temporally autocorrelated [53,54,56], as it is in our experimental nests. In the absence of constraints, it is expected that natural selection may favour selection of breeding habitat that maximize fitness. This assumes that recruiters are actively selecting the breeding habitat, but this may also be random or subject to constraints (e.g. competition to access the most productive sites, number of future reproductive opportunities) [21].
The earlier age at maturity observed in kittiwakes recruiting into food-supplemented nests seems to indicate that potential recruiters are more likely to secure a nest when high-quality habitat is available, otherwise waiting until the next breeding season. However, behavioural maturity, social and territorial dominance may further modulate habitat selection [21], and the decrease in reproductive opportunities with increasing age may increase the proportion of kittiwakes recruiting into control nests.
In our study, breeders that grew as chicks in supplementally fed nests were more likely to recruit into fed nests and chicks in unfed nests to recruit into unfed nests. The most parsimonious explanation of this pattern is that kittiwakes were recruiting into nest sites in proximity to their natal nests. This simple explanation is supported by the equal proportion (about one-third) shared by the two feeding treatments of kittiwakes that grew in fed (unfed) nests and later recruited into unfed (fed) nests. Since the feeding experiment is on-going, future data may allow us to tease apart the contribution of the phenotype and of natal origins on the choice of recruitment site.
(d). Relative productivity
Relative productivity was initially low and increased with age in breeders that grew in fed nests regardless of their recruitment into fed or unfed nests. Reproductive investment increases throughout the lifespan of many organisms [17], since the value of current to future reproduction increases when the number of opportunities for future reproduction decreases [17,57]. Consequently, older adults are predicted to invest more in current reproduction at the cost of future reproduction.
In contrast, relative productivity changed little with age of recruitment in individuals that grew in unfed nests regardless of their recruitment into fed or unfed nests. We found higher relative productivity of birds that grew in unfed nests if they recruited before 6 years old when compared with birds that grew in fed nests. According to theory, suboptimal early developmental is predicted to lead to early onset of reproduction and increased early reproductive investment; our results are in accordance with this prediction.
Our results for Pacific kittiwakes breeding on Middleton Island are in contrast to those previously reported for their Atlantic con-specifics. Compared with their Atlantic counterparts, Pacific kittiwakes (including Middleton birds) have low productivity and high survival [27,58]. This may point to the adoption of different life-history strategies of kittiwakes in their two macro-areas of distribution (fast life histories for Atlantic and slow life histories for Pacific colonies). However, our study is limited to a single Pacific colony, therefore, even within the Pacific Ocean both fast and slow life-history strategies might still be exhibited in different colonies [39].
(e). Predictive adaptive response
Our observations do not support the PAR prediction of higher relative productivity when early development and breeding conditions were matched. We actually observed the opposite pattern: kittiwakes tended to have higher relative productivity when early development and breeding conditions mismatched.
(f). Future work
Our investigation is limited to first breeding, but reproductive success may vary through the lifetime and be habitat-specific. Callum & Coulson [20] found consistent individual differences in reproductive success of kittiwakes living in the North Shields (England) through their whole reproductive lifetime. They found that breeding lifespan was the major determinant of lifetime reproductive success, and individuals with consistent high reproductive output did not suffer from an increase in mortality (‘high-quality individuals’). Aubry et al. [30] found that kittiwakes that recruited early had a smaller risk of post-breeding mortality the more breeding experience they accumulated, and suggested that cumulative breeding attempts can be considered as proxy to individual quality.
Also, in birds in general less than one-third of the potential breeders contribute to the next generation [59]; similar figures have been observed for kittiwakes [20,60]. However, it is still unclear whether the difference between breeders and non-breeders is because of intrinsic quality of birds (‘individual quality hypothesis’) [22]—which can be determined by nutritional histories of individuals during development (at least partially addressed in this study), genetic/epigenetic factors or arise from learned behaviour—or reflects dynamic accumulation of fitness differences [61]. Although this is an important question, long-term monitoring programmes are needed to address it, preferably those focusing on complete reproductive histories and longevity of individuals, multi-generational effects and considering migrants from other colonies.
A challenging yet valuable goal for further studies on Middleton Island would be to focus on productivity over the entire life course of individuals recruiting at different ages within nestling and recruitment habitat combinations. In particular, studies could test whether the productivity of birds that either grew in unfed nests or were B-chicks declines over time and/or the cost of breeding increases (i.e. increased post-breeding mortality). This is expected according to life-history theory and implies possible early-life origins of low-quality phenotypes. In addition, by following kittiwakes through their reproductive life, we could test the consistency across breeding events of the greater relative productivity we observed at recruitment when conditions at birth and at recruitment were mismatched.
In conclusion, our results support the prediction of life-history theory of a younger age at first breeding for kittiwakes that experienced suboptimal natal conditions, as well as greater relative productivity of early recruiting kittiwakes that grew in control nests when compared with those that grew in food-supplemented nests.
Data supporting the results in the article are stored on figshare (http://dx.doi.org/10.6084/m9.figshare.650867; http://dx.doi.org/10.6084/m9.figshare.650868).
Acknowledgements
This work was supported by the North Pacific Research Board (project no. 320, BEST-BSIERP Projects B74, B67 and B77). Many volunteer and student fieldworkers assisted in the field. We thank in particular the several camp leaders who supervised Middleton Island fieldwork in 2 or more years: V. A. Gill, C. Sterne, N. A. Bargmann, A. M. Ramey, J. Kotzerka, T. van Nus and L. Agdere. We thank Will Satterthwaite, Morgan Benowitz-Fredericks and two anonymous reviewers for comments that helped improve the manuscript. Any use of trade names is only for descriptive purposes and does not imply endorsement by the US Government.
References
- 1.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Kitaysky AS, Kitaiskaia EV, Piatt J, Wingfield JC. 2003. Benefits and costs of increased levels of corticosterone in seabird chicks. Horm. Behav. 43, 140–149 10.1016/S0018-506X(02)00030-2 (doi:10.1016/S0018-506X(02)00030-2) [DOI] [PubMed] [Google Scholar]
- 3.Kitaysky AS, Kitaiskaia EV, Piatt JF, Wingfield JC. 2006. A mechanistic link between chick diet and decline in seabirds? Proc. R. Soc. B 273, 445–450 10.1098/rspb.2005.3351 (doi:10.1098/rspb.2005.3351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMillen CI, Robinson JS. 2005. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633 10.1152/physrev.00053.2003 (doi:10.1152/physrev.00053.2003) [DOI] [PubMed] [Google Scholar]
- 5.Langley-Evans SC. 2007. Developmental programming of health and disease. Proc. Nutr. Soc. 65, 97–105 10.1079/PNS2005478 (doi:10.1079/PNS2005478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348 10.1016/S0169-5347(99)01639-0 (doi:10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 7.Criscuolo F, Monaghan P, Nasir L, Metcalfe NB. 2008. Early nutrition and phenotypic development: ‘catch-up’ growth leads to elevated metabolic rate in adulthood. Proc. R. Soc. B 275, 1565–1570 10.1098/rspb.2008.0148 (doi:10.1098/rspb.2008.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starck JM, Ricklefs RE. 1998. Avian growth and development. Oxford, UK: Oxford University Press [Google Scholar]
- 9.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645 10.1098/rstb.2007.0011 (doi:10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saether B-E. 1997. Environmental stochasticity and population dynamics of large herbivores: a search for mechanisms. Trends Ecol. Evol. 12, 143–149 10.1016/S0169-5347(96)10068-9 (doi:10.1016/S0169-5347(96)10068-9) [DOI] [PubMed] [Google Scholar]
- 11.Olsson O. 1997. Effect of food availability on fledging condition and post-fledging survival in king penguin chicks. Polar Biol. 18, 161–165 10.1007/s003000050172 (doi:10.1007/s003000050172) [DOI] [Google Scholar]
- 12.Thompson PM, Ollason JC. 2001. Lagged effects of ocean climate change on fulmar population dynamics. Nature 413, 417–420 10.1038/35096558 (doi:10.1038/35096558) [DOI] [PubMed] [Google Scholar]
- 13.Cam E, Monnat J-Y, Hines JE. 2003. Long-term fitness consequences of early conditions in the kittiwake. J. Anim. Ecol. 72, 411–424 10.1046/j.1365-2656.2003.00708.x (doi:10.1046/j.1365-2656.2003.00708.x) [DOI] [Google Scholar]
- 14.Nevoux M, Weimerskirch H, Barbraud C. 2010. Long- and short-term influence of environment on recruitment in a species with highly delayed maturity. Oecologia 162, 383–392 10.1007/s00442-009-1482-y (doi:10.1007/s00442-009-1482-y) [DOI] [PubMed] [Google Scholar]
- 15.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260 10.1016/S0169-5347(01)02124-3 (doi:10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 16.Brakefield PM, et al. 2005. What are the effects of maternal and pre-adult environments on ageing in humans, and are there lessons from animal models? Mech. Ageing Dev. 126, 431–438 10.1016/j.mad.2004.07.013 (doi:10.1016/j.mad.2004.07.013) [DOI] [PubMed] [Google Scholar]
- 17.Roff D. 2002. Life history evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- 18.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 19.Becker PH, Bradley JS. 2007. The role of intrinsic factors for the recruitment process in long-lived birds. J. Ornithol. 148, 377–384 10.1007/s10336-007-0157-x (doi:10.1007/s10336-007-0157-x) [DOI] [Google Scholar]
- 20.Callum TS, Coulson JC. 1990. Reproductive success of Kittiwake gulls, Rissa tridactyla. In Reproductive success: studies of individual variation in contrasting breeding systems (ed. Clutton-Brock TH.), p. 548 Chicago, IL: University of Chicago Press [Google Scholar]
- 21.Aubry LM, Cam E, Monnat J. 2009. Habitat selection, age-specific recruitment, and reproductive success in a long-lived seabird, the black-legged kittiwake. In Modeling demographic processes in marked populations (eds Thomson DL, Cooch EG, Conroy MJ.), pp. 365–392 Boston, MA: Springer [Google Scholar]
- 22.Cam E, Aubry L. 2011. Early development, recruitment and life history trajectory in long-lived birds. J. Ornithol. 152, 187–201 10.1007/s10336-011-0707-0 (doi:10.1007/s10336-011-0707-0) [DOI] [Google Scholar]
- 23.Gill VA, Hatch SA. 2002. Components of productivity in black-legged kittiwakes Rissa tridactyla: response to supplemental feeding. J. Avian Biol. 33, 113–126 10.1034/j.1600-048X.2002.330201.x (doi:10.1034/j.1600-048X.2002.330201.x) [DOI] [Google Scholar]
- 24.Kitaysky AS, Hunt GL, Flint EN, Rubega MA, Decker MB. 2000. Resource allocation in breeding seabirds: responses to fluctuations in their food supply. Mar. Ecol. Progr. Ser. 206, 283–296 10.3354/meps206283 (doi:10.3354/meps206283) [DOI] [Google Scholar]
- 25.Wingfield JC, Kitaysky AS. 2002. Endocrine responses to unpredictable environmental events: stress or anti-stress hormones? Integr. Comp. Biol. 42, 600–609 10.1093/icb/42.3.600 (doi:10.1093/icb/42.3.600) [DOI] [PubMed] [Google Scholar]
- 26.Brewer JH, O'Reilly KM, Dean Kildaw S, Loren Buck C. 2008. Interannual variation in the adrenal responsiveness of black-legged kittiwake chicks (Rissa tridactyla). Gen. Comp. Endocrinol. 156, 361–368 10.1016/j.ygcen.2008.01.010 (doi:10.1016/j.ygcen.2008.01.010) [DOI] [PubMed] [Google Scholar]
- 27.Coulson JC. 2011. The kittiwake. London, UK: Poyser [Google Scholar]
- 28.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Chapman & Hall [Google Scholar]
- 29.Forslund P, Pärt T. 1995. Age and reproduction in birds: hypotheses and tests. Trends Ecol. Evol. 10, 374–378 10.1016/S0169-5347(00)89141-7 (doi:10.1016/S0169-5347(00)89141-7) [DOI] [PubMed] [Google Scholar]
- 30.Aubry LM, Cam E, Koons DN, Monnat J-Y, Pavard S. 2011. Drivers of age-specific survival in a long-lived seabird: contributions of observed and hidden sources of heterogeneity. J. Anim. Ecol. 80, 375–383 10.1111/j.1365-2656.2010.01784.x (doi:10.1111/j.1365-2656.2010.01784.x) [DOI] [PubMed] [Google Scholar]
- 31.Marchetti K, Price T. 1989. Differences in the foraging of juvenile and adult birds: the importance of developmental constraints. Biol. Rev. 64, 51–70 10.1111/j.1469-185X.1989.tb00638.x (doi:10.1111/j.1469-185X.1989.tb00638.x) [DOI] [Google Scholar]
- 32.Wooller RD, Coulson JC. 1977. Factors affecting the age of first breeding of the kittiwake Rissa tridactyla. Ibis 125, 567–574 [Google Scholar]
- 33.Gluckman P. 2005. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 20, 527–533 10.1016/j.tree.2005.08.001 (doi:10.1016/j.tree.2005.08.001) [DOI] [PubMed] [Google Scholar]
- 34.Rickard IJ, Lummaa V. 2007. The predictive adaptive response and metabolic syndrome: challenges for the hypothesis. Trends Endocrinol. Metab. 18, 94–99 10.1016/j.tem.2007.02.004 (doi:10.1016/j.tem.2007.02.004) [DOI] [PubMed] [Google Scholar]
- 35.Desprez M, Pradel R, Cam E, Monnat J-Y, Gimenez O. 2011. Now you see him, now you don't: experience, not age, is related to reproduction in kittiwakes. Proc. R. Soc. B 278, 3060–3066 10.1098/rspb.2011.0189 (doi:10.1098/rspb.2011.0189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maunder JH, Threlfall W. 1972. The breeding biology of the black-legged kittiwake in Newfoundland. The Auk 89, 789–816 10.2307/4084109 (doi:10.2307/4084109) [DOI] [Google Scholar]
- 37.Helfenstein F, Danchin E, Wagner RH. 2004. Assortative mating and sexual size dimorphism in black-legged kittiwakes. J. Avian Biol. 27, 350–354 [Google Scholar]
- 38.Jodice PGR, Roby DD, Turco KR, Suryan RM, Irons DB, Piatt JF, Shultz MT, Roseneau DG, Kettle AB. 2008. Growth of black-legged kittiwake Rissa tridactyla chicks in relation to delivery rate, size, and energy density of meals. Mar. Ornithol. 114, 107–114 [Google Scholar]
- 39.Kitaysky AS, Piatt JF, Hatch SA, Kitaiskaia EV, Benowitz-Fredericks ZM, Shultz MT, Wingfield JC. 2010. Food availability and population processes: severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Func. Ecol. 24, 625–637 10.1111/j.1365-2435.2009.01679.x (doi:10.1111/j.1365-2435.2009.01679.x) [DOI] [Google Scholar]
- 40.Akaike HAI. 1974. A new look at the statistical model identification. IEEE Trans. Autom. Control 19, 716–723 10.1109/TAC.1974.1100705 (doi:10.1109/TAC.1974.1100705) [DOI] [Google Scholar]
- 41.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 42.Cox DR. 1972. Models and life-tables regression. J. R. Stat. Soc. Ser. B 34, 187–220 [Google Scholar]
- 43.Sandvik H, Erikstad KE, Fauchald P, Tveraa T. 2008. High survival of immatures in a long-lived seabird: insights from a long-term study of the Atlantic puffin (Fratercula arctica). The Auk 125, 723–730 10.1525/auk.2008.07059 (doi:10.1525/auk.2008.07059) [DOI] [Google Scholar]
- 44.R Development Core Team 2011. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 45.Piatt JF. 2002. Response of seabirds to fluctuationsin forage fish density. Final Report to Exxon Valdez Oil Spill Trustee Council (Restoration Project 00163M) and Mineral Management Service (Alaska OCS Region). Alaska Science Center, US Geological Survey, Anchorage, Alaska
- 46.Merkling T, Leclaire S, Danchin E, Lhuillier E, Wagner RH, White J, Hatch SA, Blanchard P. 2012. Food availability and offspring sex in a monogamous seabird: insights from an experimental approach. Behav. Ecol. 23, 751–758 10.1093/beheco/ars023 (doi:10.1093/beheco/ars023) [DOI] [Google Scholar]
- 47.Gill VA, Hatch SA, Lanctot RB. 2002. Sensitivity of breeding parameters to food supply in black-legged kittiwakes Rissa tridactyla. Ibis 144, 268–283 10.1046/j.1474-919X.2002.00043.x (doi:10.1046/j.1474-919X.2002.00043.x) [DOI] [Google Scholar]
- 48.Becker PH. 1999. Whose young win? Parental quality and recruitment in seabirds. In Proc. 22 Int. Ornithol. Congr. Durban (eds Adams NJ, Slotow RH.). Johannesburg, South Africa: BirdLife South Africa [Google Scholar]
- 49.Hatch SA. 1993. Status and ecology of kittiwakes Rissa tridactyla and R. brevirostris in the North Pacific. In The status, ecology and conservation of marine birds in the North Pacific (eds Vermeer K, Briggs KT, Morgan KH, Siegal-Causey D.), pp. 140–153 Ottawa, Canada: Canadian Wildlife Service Special Publication [Google Scholar]
- 50.Kiester AR. 1979. Conspecifics as cues: a mechanism for habitat selection in the Panamanian grass anole. Behav. Ecol. Sociobiol. 5, 323–330 10.1007/BF00292522 (doi:10.1007/BF00292522) [DOI] [Google Scholar]
- 51.Shields WM, Crook JR, Hebblethwaite ML, Wiles-Ehmann SS. 1988. Ideal free coloniality in the swallows. In The ecology of social behavior (ed. Slobodchikoff CN.), pp. 189–228 San Diego, CA: Academic Press [Google Scholar]
- 52.Stamps JA. 1988. Conspecific attraction and aggregation in territorial species. Am. Nat. 131, 329–347 10.1086/284793 (doi:10.1086/284793) [DOI] [Google Scholar]
- 53.Boulinier T, Danchin E. 1997. The use of conspecific reproductive success for breeding patch selection in terrestrial migratory species. Evol. Ecol. 11, 505–517 10.1007/s10682-997-1507-0 (doi:10.1007/s10682-997-1507-0) [DOI] [Google Scholar]
- 54.Danchin E, Boulinier T, Massot M. 1998. Conspecific reproductive success and breeding habitat selection: implications for the study of coloniality. Ecology 79, 2415–2428 10.1890/0012-9658(1998)079[2415:CRSABH]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[2415:CRSABH]2.0.CO;2) [DOI] [Google Scholar]
- 55.Parejo D, Oro D, Danchin E. 2006. Testing habitat copying in breeding habitat selection. Ibis 148, 146–154 10.1111/j.1474-919X.2006.00494.x (doi:10.1111/j.1474-919X.2006.00494.x) [DOI] [Google Scholar]
- 56.Switzer PV. 1997. Past reproductive success affects future habitat selection. Behav. Ecol. Sociobiol. 40, 307–312 10.1007/s002650050346 (doi:10.1007/s002650050346) [DOI] [Google Scholar]
- 57.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press [Google Scholar]
- 58.Hatch SA, Roberts BAYD, Fadely BS. 1993. Adult survival of Black-legged Kittiwakes Rissa tridactyla in a Pacific colony. Ibis 135, 247–254 10.1111/j.1474-919X.1993.tb02841.x (doi:10.1111/j.1474-919X.1993.tb02841.x) [DOI] [Google Scholar]
- 59.Newton I. 1989. Lifetime reproduction in birds. San Diego, CA: Academic Press Inc [Google Scholar]
- 60.Moreno J. 2003. Lifetime reproductive success in seabirds: interindividual differences and implications for conservation. Sci. Marina 67, 7–12 [Google Scholar]
- 61.Steiner UK, Tuljapurkar S, Orzack SH. 2010. Dynamic heterogeneity and life history variability in the kittiwake. J. Anim. Ecol. 79, 436–444 10.1111/j.1365-2656.2009.01653.x (doi:10.1111/j.1365-2656.2009.01653.x) [DOI] [PMC free article] [PubMed] [Google Scholar]


