Abstract
We used event-related potentials (ERPs) to investigate the time course and distribution of brain activity while adults performed (a) a sequential learning task involving complex structured sequences, and (b) a language processing task. The same positive ERP deflection, the P600 effect, typically linked to difficult or ungrammatical syntactic processing, was found for structural incongruencies in both sequential learning as well as natural language, and with similar topographical distributions. Additionally, a left anterior negativity (LAN) was observed for language but not for sequential learning. These results are interpreted as an indication that the P600 provides an index of violations and the cost of integration of expectations for upcoming material when processing complex sequential structure. We conclude that the same neural mechanisms may be recruited for both syntactic processing of linguistic stimuli and sequential learning of structured sequence patterns more generally.
Keywords: Event-Related Potentials (ERP), Sequential Learning, Implicit Learning, Language Processing, Prediction, P600, LAN
Introduction
Much of human cognition and behavior relies on the ability to make implicit predictions about upcoming events (Barr, 2007). Being able to predict future events is advantageous because it allows the brain to “pre-engage” appropriate sensory or cognitive processes to facilitate upcoming processing. That is, when generating a prediction of what will occur next, the brain activates those neural regions that process the specific type of information expected to be encountered (Barr, 2007). For example, observing the actions of two agents engaging in predictable behaviors enhances visual perception of those agents (Neri, Luu, & Levi, 2006). This mechanism of pre-engagement is more efficient than simply passively waiting until encountering an event before activating potentially relevant neural or cognitive processes.
Prediction and expectation are clearly important in the realm of language processing. For written language, analysis of eye movements shows that predictable words are fixated upon for a much shorter duration or even skipped altogether (e.g., Rayner & Well, 1999), allowing for quicker and more efficient reading comprehension. Spoken language comprehension, too, is remarkably fast and effortless because of its reliance on predictions. Experimental evidence shows that the human language system not only makes ongoing, continuous incremental interpretation of what is being said, but actually anticipates the next items, which can be measured through eye-tracking and brain-based methodologies, such as event-related potentials (ERP) (Federmeier, 2007; Kamide, 2008). The brain actively gathers whatever information is available, even if incomplete, to generate implicit predictions about what will be said next (van Berkum, 2008). In general, such anticipations will result in a processing benefit; however, there is also an associated cost: if the prediction turns out to be wrong, extra resources may be required to “repair” the incorrect commitment (Kamide, 2008).
Just how does the brain know what to expect? Barr (2007) argued that memory for associations, gained through a lifetime of extracting repeating patterns and regularities present in the world, are the “building blocks” used to generate predictions. This kind of incidental learning appears to be ubiquitous in cognition—ranging from perceptual patterns and motor sequences to linguistic structure and social constructs—and typically occurs without deliberate effort or apparent awareness of what is being learned (for reviews, see Cleeremans, Destrebecqz & Boyer, 1998; Clegg, DiGirolamo & Keele, 1998; Ferguson & Bargh, 2004; Perruchet & Pacton, 2006). Via such implicit learning, the brain can learn about the trends and invariances in the environment to help it anticipate upcoming events.
A key component of implicit learning involves the extraction and further processing of discrete elements occurring in a sequence (Conway & Christiansen, 2001). This type of sequential learning1 has been demonstrated across a variety of language-like learning situations, including when segmenting speech (Onnis, Waterfall, & Edelman, 2008; Saffran, Aslin, & Newport, 1996), detecting the orthographic (Pacton, Perruchet, Fayol, & Cleeremans, 2001) and phonotactic (Chambers, Onishi, & Fisher, 2003) regularities of words, constraining speech production errors (Dell, Reed, Adams, & Meyer, 2000), discovering complex word-internal structure between nonadjacent elements (Newport & Aslin, 2004), acquiring gender-like morphological systems (Brooks et al., 1993; Frigo & McDonald, 1998), locating syntactic phrase boundaries (Onnis et al., 2008; Saffran, 2002; Saffran, 2001), using function words to delineate phrases (Green, 1979; Valian & Coulson, 1988), integrating prosodic and morphological cues in the learning of phrase structure (Morgan, Meier, & Newport, 1987), and detecting long-distances relationships between words (Gómez, 2002; Onnis, Christiansen, Chater & Gómez, 2003). Evidence of sequential learning has been found with as little as 2 minutes of exposure (Saffran et al., 1996) and when learners are not explicitly focused on learning the structure of the stimuli (Saffran et al., 1997; though see also Toro, Sinnett & Soto-Faraco, 2005; Turk-Browne, Junge, & Scholl, 2005).
Sequential learning has also been demonstrated in non-language domains, including visual processing (Fiser & Aslin, 2002), visuomotor learning (Hunt & Aslin, 2001), tactile sequence learning (Conway & Christiansen, 2005), and non-linguistic, auditory processing (Saffran, Johnson, Aslin & Newport, 1999). In general, this type of learning has been shown to be fast, robust, and automatic in nature (e.g., Cleeremans & McClelland, 1991; Curran & Keele, 1993; Reed & Johnson, 1994; Saffran et al., 1996; Stadler, 1992). It is even present in non-human primates (e.g., Heimbauer et al., 2010) but in a more limited form (see Conway & Christiansen, 2001, for a review).
A key question in the sequential learning literature pertains to exactly what it is that participants learn in these experiments. Originally, based on Reber’s (1967) artificial grammar learning (AGL) work, it was suggested that participants acquire abstract knowledge of the rules underlying the grammar used to generate the training items. More recent research has increasingly sought to explain sequential learning performance in terms of surface features of the training items, including sensitivity to statistics computed over two- or three-element chunks (e.g., Johnstone & Shanks, 1999; Knowlton & Squire, 1994; Redington & Chater, 1996), conditional probabilities between elements (e.g., Aslin, Saffran & Newport, 1998; Fiser & Aslin, 2002), or overall exemplar similarity (Pothos & Bailey, 2000; Vokey & Brooks, 1992). Nonetheless, it has been suggested that such surface-based learning mechanisms on their own are unable to accommodate certain types of rule-like generalizations, and must therefore be supplemented with separate mechanisms for abstract rule learning (e.g., Marcus, Vijayan, Bandi Rao & Vishton, 1999; Meulemans & Van der Linden, 1997; Peña, Bonnatti, Nespor & Mehler, 2002).
In response, other researchers have sought to demonstrate through computational modeling that a single associative mechanism may suffice for learning both surface regularities and rule-like generalizations (e.g., Altmann & Dienes, 1999; Christiansen, Conway & Curtin, 2000; Redington & Chater, 1996; Seidenberg & Elman, 1999). Thus, although sequential learning accounts relying exclusively on abstract, rule-based knowledge no longer have much theoretical support, the exact nature of what is learned is still under debate (see Perruchet & Pacton, 2006; Pothos, 2007, for recent reviews). What is important for the purpose of the current paper, however, is that sequential learning provides a domain-general mechanism for acquiring predictive relationships between sequence elements, independently of whether such regularities are represented in terms of rules, statistical associations, or some combination between the two. In other words, we interpret sequential learning in terms of Barr’s (2007) framework as providing a mechanism by which to acquire knowledge about the structural regularities of sequential input, upon which the brain can anticipate upcoming elements in a sequence.
Here we ask whether the neural mechanisms involved in generating sequential structural expectations are the same in both language and non-language situations. Although many researchers assume that sequential learning is important for language acquisition and processing (e.g., Gómez & Gerken, 2000; Saffran, 2003), there is very little direct behavioral or neural evidence supporting such a claim. However, recent findings have indicated that individual differences in a non-linguistic sequential learning task are significantly correlated with how well listeners use preceding context to implicitly predict upcoming speech units, as measured by perceptual facilitation in a degraded speech perception task (Conway, Bauernschmidt, Huang, & Pisoni, 2010; Conway, Karpicke, & Pisoni, 2007). Likewise, Misyak, Christiansen and Tomblin (2010) found that individual differences in predicting nonadjacency relations in a sequential learning paradigm correlated with variations in on-line processing of long-distance dependencies in natural language.
In terms of neural data, there is some evidence from ERP studies showing that structural incongruencies in non-language sequential stimuli elicit similar brain responses as those observed for syntactic anomalies in natural language: a positive shift in the electrophysiological response observed about 600 msec after the incongruency, known as the P600 effect (Friederici, Steinhauer, & Pfeifer, 2002; Lelekov, Dominey, & Garcia-Larrea, 2000; Patel et al., 1998). Although encouraging, the similarities in ERPs have been inferred across different subject populations and across different experimental paradigms. Thus, no firm conclusions can be made because there is no study that provides a direct within-subject comparison of the ERP responses to both natural language and the learning of non-linguistic sequential patterns.
In this paper, we investigate the possibility that structural incongruencies in both language and other sequential stimuli will elicit the same electrophysiological response profile, a P600. Specifically, we argue that domain-general sequential learning abilities are used to encode the word order regularities of language, which, once learned, can be used to make implicit predictions about upcoming words in a sentence. Toward this end, the present study includes two crucial characteristics. First, we use a sequential learning task designed to promote participants’ implicit predictions of what element ought to occur next in a sequence; second, we provide a within-subject comparison of the neural responses to structural violations in both the sequential learning task and a language processing task. These two characteristics allow us to directly assess the hypothesis that the learning of sequential information is an important cognitive mechanism involved in language processing. Such a demonstration is important for both theoretical and practical reasons. Of practical import, sequential learning has become a popular method for investigating language acquisition and processing, especially in infant populations (in particular under the guise of “statistical learning”, e.g., Gómez & Gerken, 2000; Saffran, 2003). Providing direct neural evidence linking sequential learning to language processing therefore is necessary for validating this approach to language. Moreover, our study is also of theoretical importance as it addresses issues relating to what extent domain-general cognitive abilities, specifically, sequential learning based expectations, play a role in linguistic processing. Before presenting our ERP study, we first review recent electrophysiological evidence regarding the neural correlates of both language and sequential learning.
ERP Correlates of Natural Language
In ERP studies of syntactic processing, the P600 response was originally observed as an increased late positivity recorded around 600 msec after the onset of a word that is syntactically anomalous (e.g., Hagoort, Brown & Groothusen, 1993; Neville, Nicol, Barss, Forster & Garrett, 1991). Osterhout & Mobley (1995) found a similar P600 pattern for ungrammatical items in a study of agreement violations in language (e.g., ‘The elected officials hope/*hopes to succeed’, and ‘The successful woman congratulated herself/*himself’; see also Allen, Badecker, & Osterhout, 2003; Barber & Carreiras, 2005; Nevins, Dillon, Malhotra, & Phillips, 1998). Additionally, the P600 signature also indexes several other types of syntactic violations. Hagoort et al. (1993) found a late positivity for word order violations (e.g., ‘the expensive *very tulip’). Violations of phrase structure (e.g., ‘My uncle watched about a movie my family’; Friederici et al., 1996; Neville et al., 1991; Silva-Pereyra et al., 2007), pronoun-case marking (e.g., ‘Ray fell down and skinned he knee’; Coulson, King, & Kutas, 1998), and verb subcategorization (e.g., ‘The woman persuaded to answer the door’; Osterhout & Holcomb, 1992) also evoked the P600 effect. Furthermore, Wassenaar and Hagoort (2005) found that word-category violations were also indexed by the P600 (e.g., ‘The lumberjack dodged the vain *propelled on Tuesday’; see also Mueller, Hahne, Fujii, & Friederici, 2005).
While considerable ERP research has been devoted to different kinds of linguistic violations, recent findings have demonstrated that the P600 can be informative about mechanisms underlying the processing of well-formed sentences as well. For example, P600 responses are observed at the point of disambiguation in syntactically ambiguous sentences in which participants experienced a ‘garden path’ effect (e.g., at ‘was’ in ‘The lawyer charged the defendant was lying’; Osterhout & Holcomb, 1992; see also Gouvea, Phillips, Kazanina and Poeppel, 2010; Kaan & Swaab, 2003; Osterhout, Holcomb, & Swinney, 1994). Moreover, complex syntactic phenomena such as the processing of long-distance dependencies also elicit P600 effects (e.g., when the predicted thematic role of patient associated with ‘who’ has to be integrated with the verb, ‘imitated’, in ‘Emily wondered who the performer in the concert had imitated for the audience’s amusement’; Kaan, Harris, Gibson, & Holcomb, 2000; see also Felser, Clahsen, & Münte, 2003; Phillips, Kazanina, & Abada, 2005).
Although the P600 has traditionally been tied to syntactic processing, the P600 has alosy been elicited in response to semantic violations, such as violations of expectations for thematic roles (e.g., animacy expectations at the verb ‘eat’ in ‘Every morning at breakfast the eggs would eat …’; Kuperberg, Sitnikova, Caplan, & Holcomb, 2003; see also Kim & Osterhout, 2005; Kuperberg et al., 2007), which originally was thought to be the sole purview of the N400 ERP component (Kutas & Hillyard, 1980). Although the debate over the nature of these “semantic” P600 effects has not been settled (see e.g., Bornkessel-Schlesewsky & Schlesewsky, 2008), one possibility is that the P600 and N400 reflect the operation of two competing neural processes: one that computes structural or combinatorial relations primarily relating morpho-syntactic information (P600) and another that makes memory-based, ongoing semantic interpretations of the message (N400) (Federmeier, 2007; Kuperberg, 2007). Thus, from this perspective the P600 is seen primarily as a response to violations of structural and combinatorial expectations, whereas the N400 is more closely tied to violations of expectations relating to semantic interpretation.
It is possible that the sequential expectations associated with the semantic P600 effects may be derived from quite subtle word co-occurrence statistics, including so-called semantic valence tendencies (e.g., that the verb ‘provide’ tends to precede positive words, as in ‘to provide work’, whereas the verb ‘cause’ typically precedes negative words, as in ‘to cause trouble’; Onnis et al., 2008). Violations of expectations based on such rich distributional information, capturing what may otherwise be thought of as pragmatic knowledge, may help explain the presence of late positivities in the comprehension of jokes (e.g., at ‘husband’ in ‘By the time Mary had her fourteenth child, she’d run out of names to call her husband’; Coulson & Lovett, 2004; see also Coulson & Kutas, 2001). Similarly, the P600 effects elicited by metaphor understanding may be attributed to unexpected departures from learned word co-occurrence patterns (e.g., on the final word in ‘The actor says interviews are always a headache’; Coulson & Van Petten, 2002, 2007; see also Kazmerski, Blasko & Dessalegn, 2003). However, ERPs recorded during the processing of statements that were made ironic by prior context (e.g., ‘These artists are fantastic’ in the context of a negative description of an orchestral performance; Regel, Gunter & Friederici, 2011) indicate that the P600 component can also be observed during the successful integration of implicit predictions, similar to the late positivities associated with long-distance dependencies (e.g., Felser et al., 2003; Kaan et al., 2000; Phillips et al., 2005). Consistent with this interpretation, Regel, Coulson and Gunter (2010) found larger P600 effects for ironic utterances spoken by individuals who produced a preponderance of ironic statements, likely resulting in implicit expectations for irony for that speaker.
Given the variety of language situations eliciting the P600, there has been considerable debate over the interpretation of this component. One aspect of this debate relates to the specific psycholinguistic nature of the late positivity. For example, Osterhout, Holcomb and Swinney (1994) suggest that the P600 reflects the cost of reprocessing after experiencing some sort of parsing difficulty. Friederici (1995) views the P600 within a “syntax-first” framework as associated with structural reanalysis of an ungrammatical sentence (or one that appears to be ungrammatical). From a similar serial-parser perspective, Gouvea et al. (2010) propose that the P600 is a multi-process response to the creation as well as potential deletions of syntactic relations resulting in different latencies, durations and amplitudes based on the specific structure being processed. Other recent accounts have stressed the importance of prediction in interpreting the P600 effect. Thus, Kaan et al. (2000) propose that the P600 component is not restricted to reanalysis processes but provides a more general index of the processing cost associated with the integration of syntactic relations predicted by prior sentential context. From the viewpoint of a parallel, unification-based approach, Hagoort (2003, 2009) construes the P600 component as reflecting processes involved in the integration of information in a sentence as it becomes available, both perceptually and retrieved from long-term memory, in order to form a unitary representation.
Another key aspect of the debate over the nature of the P600 pertains to whether this component is specific to psycholinguistic processing, or whether it may reflect more domain-general functions. Coulson, King and Kutas (1998) examined the relationship between the P600 effect and the P300 “odd-ball” response to relatively rare, unexpected events. Specifically, they observed that the amplitude of the P600, similar to the P300, was affected by both the probability of a within-experiment occurrence of syntactic violations and the saliency of the psycholinguistic violation, and concluded that the P600 is part of the broader, domain-general family of P300 components. However, Coulson et al. did not conduct a within-subject comparison with non-linguistic stimuli, which may limit the inferences that can be made from their results (Osterhout & Hagoort, 1999). Moreover, variations in P600 responses may reflect key aspects of the (linguistic) stimuli. For example, Osterhout et al. (1994) noted that the amplitude of the P600 response was modulated by the subcategorization properties of the main verb (e.g., The doctor hoped/forced/believed/charged the patient was lying), indicating sensitivity to frequency information. In addition to syntactic violation probability, sentence complexity also affects the P600 (Gunter, Stowe, & Mulder, 1997). More recent studies have additionally found theoretically interpretable differences in latency, duration or topographical distribution of the P600 relating to differences in the structural regularities under investigation (e.g., Gouvea et al., 2010; Hagoort & Brown, 1994; Kaan et al, 2000; Kaab & Swab, 2003; Rossi, Gugler, Hahne & Friederici, 2005). Although the current study does not address the P300/P600 debate directly, we note that it is possible for the P600 to be domain-general, perhaps relating to structured sequence processing, without necessarily belonging to the P300 family of components (see also Gouvea et al., 2010).
What is important for the perspective that we advocate here is the suggestion that the processes underlying the P600 (and possibly other language-related ERP components) rely to a great extent on predictive processing. That is, much of online language comprehension appears to involve the integration of various lexical, semantic, and syntactic cues to provide an implicit prediction about the next word in a sentence (e.g., Federmeier, 2007; Hagoort, 2009; Kaan et al., 2000; see Kamide, 2008; Pickering & Garrod, 2007, for a review of behavioral evidence). This predictive processing component may be important not just in online language comprehension, but in any kind of task involving information that is distributed in time (Niv & Schoenbaum, 2008), which is the case in many kinds of sequential learning tasks. Indeed, if both language and sequential learning involve similar basic mechanisms for sequential prediction, we would expect similar P600 signatures for both tasks.
ERP Correlates of Sequential Learning
Although there has been some interest in specifying the electrophysiological correlates of implicit or sequence learning generally, very few ERP studies have been conducted using sequential learning tasks that employ structured patterns. The distinction between non-structured and structured sequence learning is not trivial. Non-structured sequence learning involves learning an arbitrary, fixed repeating pattern with no internal structure, such as 3-1-4-2-3-1-4-2. On the other hand, structured sequence learning involves learning a more complex pattern where each element that occurs is not perfectly predictable but is rather determined probabilistically based on what has occurred previously (for further discussion of the distinction between sequence learning of fixed and more complex, structured patterns, see Conway & Christiansen, 2001).
The ERP correlates of fixed sequence learning have been investigated in some depth using the serial reaction time (SRT) task (Nissen & Bullemer, 1987). In the standard version of this task, a visual stimulus is presented in one of four possible locations and the participant is required to press one of four buttons that corresponds to the location of the stimulus. Unbeknownst to the participants, the sequence of responses follows a fixed repeating pattern. Reaction times decrease for the repeating sequence relative to sequences that do not follow the same pattern, indicating that learning has occurred. A number of ERP studies have indicated that this type of perceptual-motor (non-structured) sequence learning is accompanied by N200 and P300 components, which may reflect processes involved in sensitivity to expectancy violations (Eimer, Goschke, Schlaghecken, & Stürmer, 1996; Ferdinand, Mecklinger, & Kray, 2008; Miyawaki, Sato, Yasuda, Kumano, & Kuboki, 2005; Rüsseler, Hennighausen, Münte, & Rösler, 2003; Rüsseler, Hennighausen, & Rösler, 2001; Rüsseler & Rösler, 1999; Rüsseler & Rösler, 2000; Schlaghecken, Stürmer, & Eimer, 2000).
The electrophysiological correlates of structured sequential learning have received much less attention. Structured sequential learning is primarily investigated behaviorally using some sort of variation of the AGL paradigm (Reber, 1967), in which a finite-state “grammar” is used to generate sequences conforming to underlying rules of correct formation. After relatively short exposure to a subset of sequences generated by an artificial grammar, participants are able to discriminate between correct and incorrect sequences with a reasonable degree of accuracy, although they are typically unaware of the constraints that govern the sequences. This paradigm has been used to investigate both implicit learning (e.g., Reber, 1967) and language acquisition (e.g., Gómez & Gerken, 2000).
It is possible that the neural processes recruited during the learning of such complex structured sequential stimuli may be at least partly coextensive with neural processes implicated in language (see also Hoen & Dominey, 2000). If this hypothesis holds, it should be possible to find similar neural signatures to violations in AGL and natural language sequences alike. Indeed, several studies have found natural language-like P600 responses from participants who had learned the sequential structure of an artificial language (e.g., Bahlmann, Gunter, & Friederici, 2006; Friederici et al., 2002; Lelekov et al., 2000; Mueller, Bahlmann, & Friederici, 2008). The P600 was also observed for incongruent musical chord sequences by Patel et al. (1998), who detected no statistically significant differences between the P600 for syntactic and musical structural incongruities. Importantly, none of the AGL studies have used a within-subject design to compare the ERP profiles in sequential learning and language in the manner that Patel et al. (1998) did.
In sum, prior studies suggest that the P600 may reflect the operation of a general neural mechanism that processes sequential patterns and makes implicit predictions about the next items in a sequence, whether linguistic or not. Therefore, we set out to assess ERP responses in adult subjects on two separate tasks, one involving structured sequential learning and the other involving the processing of English sentences. We hypothesized that overlapping neural processes subserve both sequential learning and language processing, and thus anticipated obtaining a similar brain response, the P600, to structural incongruencies in both tasks.
Methods
Participants
Eighteen students (6 male) at Cornell University were paid for their participation. All but one were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). Data from an additional 4 participants were excluded because more than 25% of experimental trials were contaminated due to an excessive number of eye blinks/movements (n=3) or poor data quality (n=1). The age of the remaining participants ranged between 18 and 22 years (M = 19.8). All were native speakers of English, with no history of neurological impairment, and had normal or corrected-to-normal vision.
Materials
Sequential learning stimuli
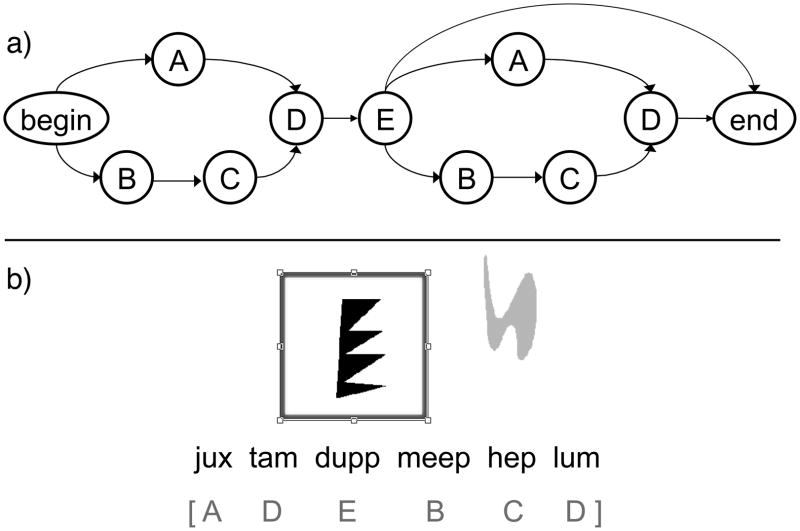
A miniature grammar (see Figure 1.a)—a slightly simplified version of that used by Friederici et al. (2002)—was used to produce a set of sequences containing between three and seven elements. The grammar determined the order of sequence elements drawn from five different categories of stimulus tokens: two categories, A and B, each contained a single token, A and B, respectively; one category, C, consisted of two tokens, C1 and C2; and two sets, D and E, each contained three tokens, D1, D2, D3 and E1, E2, E3, respectively. There were a total of 10 tokens distributed over the five stimulus categories. A sequence was generated by starting at the ‘begin’ state and then following the arrows until the ‘end’ state was reached. For example, the sequence ADEBCD would result from first going to A after the begin state, followed by D and E, and then choosing the lower arrow and visiting states B, C, and D before reaching the end state. At each state (save from the begin and end states) a token is randomly drawn from the relevant stimulus category. Thus, a possible token sequence resulting from the trajectory followed in the above example could be AD2E1BC2D3. The shortest sequence that can be generated has the form ADE (e.g., AD2E1) and the longest BCDEBCD (e.g., BC2D1E3BC1D3).
Figure 1.
a) The artificial grammar used to generate the sequences used in the sequential learning task. The nodes denote stimulus categories and the arrows indicate valid transitions from the beginning node to the end node. b) An example sequence of nonword tokens with its associated visual scene shown here in grey scale (the list of stimulus categories in the square brackets below the nonword sequence is for illustrative purposes only and was not shown to the participants).
To produce the sequences to which the participants were exposed, unique written nonwords were randomly assigned to the ten tokens: jux, dupp, hep, meep, nib, tam, sig, lum, cav, and biff. The specific mapping of nonwords to tokens was randomized separately for each participant in order to avoid potential nonword-related biases. Each nonword sequence was paired with a visual scene (i.e., a kind of reference world), consisting of graphical symbols arranged in specific ways. For example, each D nonword token had a corresponding shape referent; likewise, each E nonword token also had a corresponding referent (circle, octagon, square). The A, B, and C tokens did not have corresponding graphical symbols; instead, these tokens affected the color of the D referent. Thus, a D token preceded by BC1 denoted a green D referent while BC2 resulted in a red D referent; a D token preceded by A meant that the D referent would be black. Note the distributional restriction that A never co-occurs with a C token whereas B is always followed by either C1 or C2. Finally, the position of each graphical symbol was determined in the following manner: E referents always occurred at the center of the screen; D referents appeared either inside the E referent (first occurrence) or outside of the E referent, to the upper right (second occurrence). A possible visual scene for the category sequence ADEBCDis shown in Figure 1.b (in grey scale—along with its possible nonword instantiation).
Sixty sequences were used for the Learning Phase. Each nonword string corresponded to a visual scene consisting of the D and E referents described above. An additional 30 grammatical and 30 ungrammatical sequences were used for the Test Phase. To derive violations for the ungrammatical sequences, tokens of one stimulus category in a grammatical sequence were replaced with tokens from a different stimulus category. Violations never occurred at the beginning or end of a sequence but only at the third and fourth positions in the sequence. The ungrammatical sequences were always accompanied by a “correct” visual scene so that it would generate an implicit expectation for what the correct grammatical sequence should be.
Language stimuli
Two lists, List1 and List2, containing counter-balanced sentence materials were used for the language task, adapted from Osterhout and Mobley (1995). Each list consisted of 60 English sentences, 30 being grammatical and 30 having a violation in terms of subject-noun/verb number agreement (e.g., ‘Most cats likes to play outside’). An additional list of 60 sentences of comparable length to the experimental sentences was used as filler materials, also adapted from Osterhout and Mobley (1995). The filler list had 30 grammatical sentences and 30 sentences that had one of two types of violation: antecedent-reflexive number (e.g., ‘The Olympic swimmer trained themselves for the swim meet’) or gender (e.g., ‘The kind uncle enjoyed herself at Christmas’) agreement. The full set of 120 sentences thus corresponded to a subset of the sentences used in Osterhout and Mobley (1995).
Procedure
Participants were tested individually in a single session, sitting in front of a computer monitor. The participant’s left and right thumbs were each positioned over the left and right buttons of a button box. All participants carried out the sequential learning task first and the language task second.
Sequential learning task
Participants were instructed that their job was to learn an artificial “language” consisting of new words that they would not have seen before and which described different arrangements of visual shapes appearing on the computer screen. The sequential learning task consisted of two phases, a Learning Phase and a Test Phase, with the Learning Phase itself consisting of four sub-phases. We reasoned that participants would only generate strong implicit expectations for upcoming sequence elements if they had learned the task at a high level of proficiency (90+% as in Friederici et al., 2002). Pilot work indicated that in order for participants to learn the sequence regularities well within a short amount of time, we needed to adopt a “starting small” strategy in which participants were gradually exposed to increasingly more complex stimuli (Conway, Ellefson & Christiansen, 2003).
In the first Learning sub-phase, participants were shown D or E tokens, one at a time, with the nonword displayed at the bottom of the screen and its corresponding visual referent displayed in the middle of the screen. Participants could observe the scene for as long as they liked and when they were ready, they pressed a key to continue. All three E tokens but only the three D tokens preceded by A were included (i.e., only the black D referents). These 6 nonwords were presented in random order, 4 times each for a total of 24 trials.
In the second Learning sub-phase, the procedure was identical to the first sub-phase but now the other six D variations were included, those preceded by BC1 or BC2 (i.e., the red and green D referents). The 9 D tokens and 3 E tokens were presented in random order, two times each, for a total of 24 trials.
In the third Learning sub-phase, full sequences were presented to participants, with the nonword tokens presented below the corresponding visual scene. The 60 Learning sequences described above were used for this sub-phase, each presented in random order, 3 times each. Figure 1.b illustrates the presentation of a possible training sequence, “jux tam dupp meep hep lum”, along with its corresponding visual scene (the category sequence, ADEBCD, would, of course, not be seen by the participants but are included here for expositional reasons).
In the fourth and final Learning sub-phase, participants were again exposed to the same 60 Learning sequences but this time the visual referent scene appeared on its own prior to displaying the corresponding nonword tokens. Thus, the visual scene was shown first for 4 sec, and then after a 300 msec pause, the nonword sequence that corresponded to the scene were displayed, one word at a time (duration: 350 msec; ISI: 300 msec). The 60 Learning sequences/scenes were presented in random order. The purpose of presenting the visual scene first was to promote implicit expectations for the upcoming nonword sequences.
In the Test Phase, participants were told that they would be presented with new scenes and sequences from the artificial language. Half of the sequences would correspond to the scenes according to the same rules of the language as before, whereas the other half of the sequences would contain an error with respect to the rules of the language. The participant’s task was to decide which sequences followed the rules correctly and which did not by pressing a button on the response pad. The visual referent scenes were presented first, none of which contained grammatical violations, followed by the nonword sequences (with timing identical to Learning sub-phase 4). Thus, the visual scenes served to ‘prime’ the participants’ expectations for what the sequences should look like (in a similar way to how semantics can create expectations for which word should come next in natural language). After the final token of the sequence was presented, a 1400 msec pause occurred, followed by a test prompt asking for the participant’s response. The 60 Test sequences/scenes were presented in random order, one time each.
Language task
Participants were instructed that they would be presented with English sentences appearing on the screen, one word at a time. Their task was to decide whether each sentence was acceptable or not (by pressing the left or right button), where sentences were considered unacceptable if they contained any type of anomaly and were unlikely to be produced by a fluent English speaker. Before each sentence, a fixation cross was presented for 500 msec in the center of the screen, and then each word of the sentence was presented one at a time for 350 msec, with 300 msec occurring between each word (thus words were presented with a similar duration and ISI as in the sequential learning task). After the final word of the sentence was presented, a 1400 msec pause occurred followed by a test prompt asking the subject to make a button response regarding the sentence’s acceptability. Thus, the presentation and timing of the nonwords/words were identical across the two tasks. Participants received a total of 120 sentences, 60 from List1 or List2 and 60 from the Filler list, in random order.
EEG Recording
The EEG was recorded from 128 scalp sites using the EGI Geodesic Sensor Net (Tucker, 1993) during the Test Phase of the sequential learning task and throughout the language task. Eye movements and blinks were monitored using a subset of the electrodes located at the outer canthi as well as above and below each eye. All electrode impedances were kept below 50 kΩ, as recommended for the Electrical Geodesics high-input impedance amplifiers (Ferree, Luu, Russell & Tucker, 2001). Recordings were made with a 0.1 to 100-Hz bandpass filter and digitized at 250 Hz, initially referenced to the vertex channel. The continuous EEG was segmented into epochs in the interval −100 msec to +900 msec with respect to the onset of the target word that created the structural incongruency.
Prior to beginning the experiment, participants were visually shown a display of the real-time EEG and observed the effects of blinking, jaw clenching, and eye movements, and were given specific instructions to avoid or limit such behaviors throughout the experiment. Trials with eye-movement artifacts (EOG larger than 70 μV) or more than 10 bad channels were excluded from the average. A channel was considered bad if it reached 200 μV or changed more than 100 μV between samples. This resulted in less than 11% of trials being excluded, evenly distributed across conditions. ERPs were baseline-corrected with respect to the 100-msec pre-stimulus interval and re-referenced off-line to linked mastoids2. Separate ERPs were computed for each subject, each condition, and each electrode.
Data Analyses
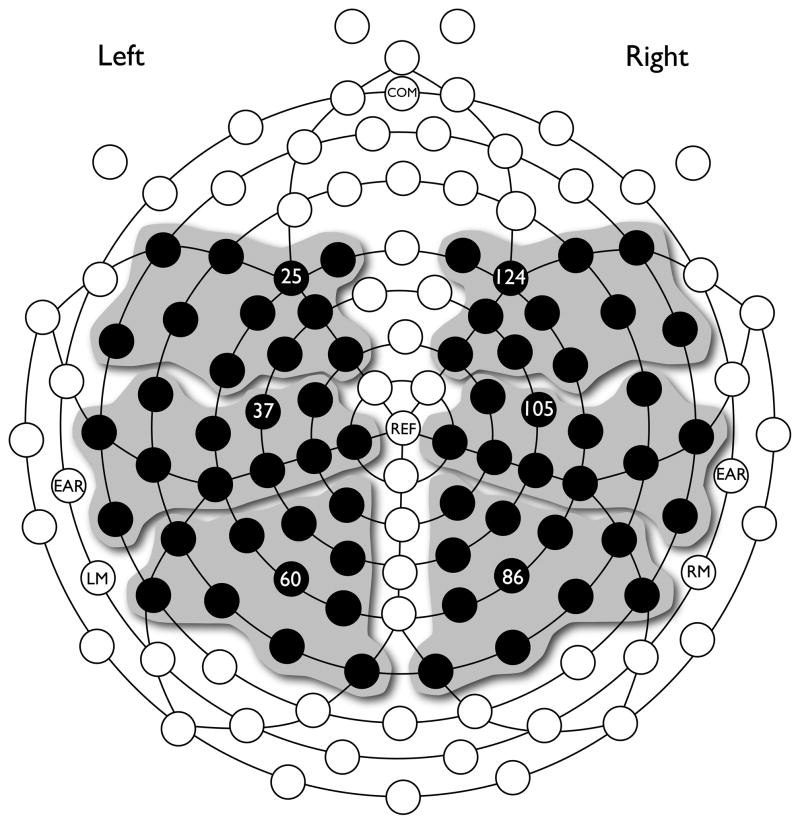
Following Barber and Carreiras (2005), six regions of interest were defined, each containing the means of 11 electrodes: left anterior (13, 20, 21, 25, 28, 29, 30, 34, 35, 36, and 40), left central (31, 32, 37, 38, 41, 42, 43, 46, 47, 48, and 50), left posterior (51, 52, 53, 54, 58, 59, 60, 61, 66, 67, and 72), right anterior (4, 111, 112, 113, 116, 117, 118, 119, 122, 123, and 124), right central (81, 88, 94, 99, 102, 103, 104, 105, 106, 109, and 110), and right posterior (77, 78, 79, 80, 85, 86, 87, 92, 93, 97, and 98). Figure 2 shows the location of these six regions and their component electrodes.
Figure 2.
Schematic representation of the 128 electrode positions in the Geodesic Nets used to record EEG activity (front is up). The six electrode regions used in the analyses are indicated in grey and the six representative electrodes used in Figures 3 and 4 are indicated by their respective numbers. Adapted from Barber and Carreiras (2005).
We performed analyses on the mean voltage within the same three latency windows as in Barber and Carreiras (2005): 300–450, 500–700, and 700–900 msec. Separate repeated-measures ANOVAs were performed for each latency window, with grammaticality (grammatical and ungrammatical), electrode region (anterior, central, and posterior), and hemisphere (left and right) as factors. Geisser-Greenhouse corrections for non-sphericity of variance were applied when appropriate. The description of the results focuses on the effect of the experimental manipulations, effects related to region or hemisphere are only reported when they interact with grammaticality. Results from the omnibus ANOVA are reported first, followed by planned comparisons testing our hypothesis that P600 effects should occur for incongruencies in both the language and the sequential learning conditions (at posterior sites given the typical topographic distribution of P600 responses to violations; cf., Haagort, Brown & Osterhout, 1999; Kaan, 2009). Additional posthoc comparisons with Bonferroni-corrected p-values were conducted to resolve significant interactions not addressed by the planned comparisons.
Results
Grammaticality Judgments
Of the test items in the sequential learning task, participants classified 93.9% correctly. In the language task, 93.5% of the target noun/verb-agreement items were correctly classified. Both levels of classification were significantly better than chance (p’s < .0001) and not different from one another (p > .7).
Event-Related Potentials
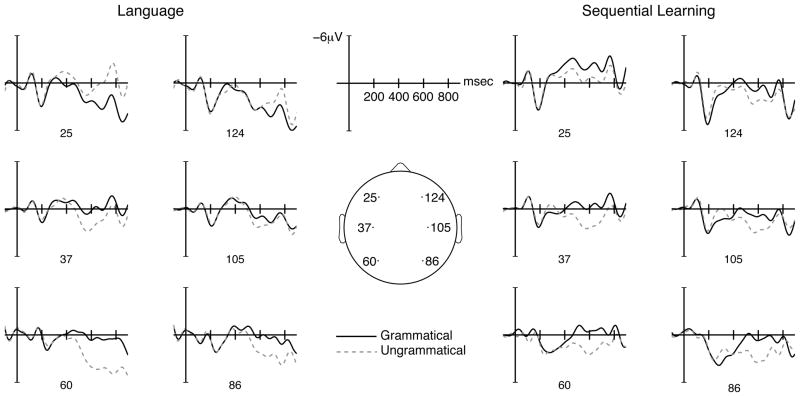
For visualization purposes, EEGLAB (Delorme & Makeig, 2004) was used to smooth the grand average waveforms with a 10 Hz low-pass filter (all statistical analyses, however, involved only unfiltered data). Figure 3 shows the grand average ERP waveforms for grammatical and ungrammatical trials across six representative electrodes (Barber and Carreiras, 2005) for the language (left) and sequential learning (right) tasks. Visual inspection of the ERPs indicates the presence of a left-anterior negativity (LAN) in the language task, but not in the sequential learning task, and a late positivity (P600) at central and posterior sites in both tasks, with a stronger effect in the left-hemisphere and across posterior regions. These observations were confirmed by the statistical analyses reported below.
Figure 3.
Grand average ERPs elicited for target words for grammatical (solid black) and ungrammatical (dashed grey) continuations in the language (left) and sequential learning (right) tasks. The vertical lines mark the onset of the target word. Six electrodes are shown, representative of the left-anterior (25), right-anterior (124), left-central (37), right-central (105), left-posterior (60), and right-posterior (86) regions. Negative voltage is plotted up.
300–450 msec latency window
For the language data, there were no main effects or interactions involving grammaticality. An effect of grammaticality was only found for the left-anterior region, where ungrammatical items were significantly more negative (F(1,17) = 6.071, p < .03), suggesting a LAN. No significant main effects or interactions related to grammaticality were found for the sequential learning data.
500–700 msec latency window
There was a significant interaction between grammaticality and region in the language data (F(2,34) = 5.96, p < .02, ε = .62). This interaction arose due to the differential effect of grammaticality across the anterior and central regions (F(1,17) = 20.48, p < .001). Whereas the negative deflection elicited by the ungrammatical items in the left-anterior region was no longer significant, planned comparisons were significant for the positive wave observed for both posterior regions (left: F(1,17) = 5.13, p < .04; right: F(1,17) = 7.28, p < .02), indicative of a P600 effect.
For the sequential learning data, there was an overall effect of grammaticality (F(1,17) = 10.98, p < .005). The planned comparisons revealed a significant positive deflection across the left- and right posterior regions (F(1,17) = 11.22, p < .005; F(1,17) = 14.66, p < .002), suggesting a P600 effect similar to the one elicited by language.
700–900 msec latency window
A grammaticality × region × hemisphere interaction was found (F(2,34) = 3.66, p < .05, ε = .97) for the language data, along with a grammaticality × region interaction (F(2,34) = 10.09, p < .004, ε = .64). Both interactions were driven by the differential effects of grammaticality on the ERPs in the anterior and central regions (F(1,17) = 25.56, p < .0001), combined with a hemisphere modulation in the three-way interaction (F(1,17) = 4.82, p < .05). Planned comparisons showed that the positive wave continued marginally across left- and right-posterior regions (F(1,17) = 3.70, p = .07; F(1,17) = 3.79, p = .07), and posthoc comparisons indicated that the negative deflection for ungrammatical items reemerged in the left-anterior region (F(1,17) = 12.26, p < .018).
No interactions or main effects involving grammaticality were found for the sequential learning data. In this time window, the positive-going deflection had disappeared across the posterior regions.
Comparison of Language and Sequential Learning
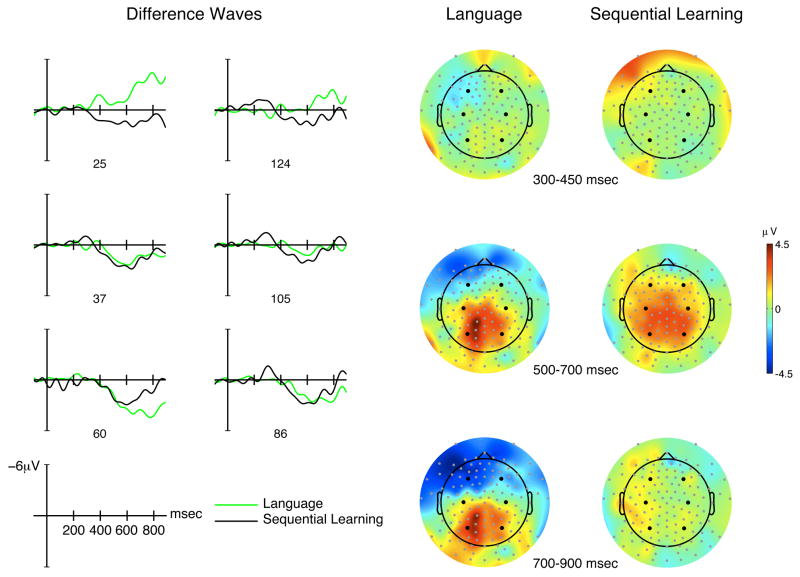
To more closely compare the ERP responses to structural incongruencies in language and sequential learning, we computed ungrammatical-grammatical difference waves for each electrode site. The left-hand side of Figure 4 shows the resulting waveforms for our six representative electrodes. Visual inspection of the difference waves suggests that they were quite similar across the language and sequential learning tasks, except in the anterior region, especially in the left hemisphere, where a negative-going wave can be observed for language starting around 350 msec. To evaluate these observations, we conducted repeated-measures analyses in our three latency windows with task as the main factor.
Figure 4.
Difference waves (ungrammatical minus grammatical) for language (green) and sequential learning (black) are shown on the left for the six representative electrodes. The corresponding topographic maps for the difference waves are shown on the right, averaged within each of the three latency windows. The grey dots show the location of the 128 electrodes with the black dots indicating the six representative electrodes.
350–400 msec latency window
There was no main effect of task (F(1,17) = .43, p = .52), nor any significant interactions with region (F(2,34) = 1.95, p = .17, ε = .66), hemisphere (F(1,17) = 2.34, p = .15), or region × hemisphere (F(2,34) = 1.94, p = .16, ε = .97). However, planned comparisons indicated that the negative-going wave in the left-anterior region for the language task was significantly different from the more positive-going wave in the sequential learning task (F(1,17) = 6.07, p < .03). Otherwise, the difference waves were statistically indistinguishable across the other regions of interest (F’s < .8).
500–700 msec latency window
Again, there was no main effect of task (F(1,17) = 1.61, p = .22), nor any significant interaction with hemisphere (F(1,17) = .05, p = .83). There was, though, a marginal interaction between task and region (F(2,34) = 2.94, p = .085, ε = .73) but this was due to differential task effects in the anterior and central regions (F(1,17) = 4.93, p < .05). Indeed, planned comparisons indicated that only in the left-anterior region was there a significant effect of task due to the LAN-associated negative-going difference wave for the language condition (F(1,17) = 5.87 p < .03). No other effects of task were found (F’s < 1.6).
700–900 msec latency window
Once more, there was no main effect of task (F(1,17) = .13, p = .72), nor any significant interaction with hemisphere (F(1,17) = .64, p = .44). The interaction between task and region had now reached significance (F(2,34) = 6.42, p < .02, ε = .71). As in the previous latency window, this interaction was driven by differences between the anterior and central regions in task effects (F(1,17) = 8.45, p < .02). This anterior-central difference was especially pronounced in the left-hemisphere, yielding a marginal 3-way interaction (F(2,34) = 2.60, p = .096, ε = .90). Planned comparisons revealed that the only task-related difference was in the left-anterior region (F(1,17) = 6.24, p < .025; all other F’s < 1.96). This suggests that the 3-way interaction and the grammatical × region interaction was due to the differential modulation of task and hemisphere factors in the anterior and central regions, consistent with a sustained LAN effect in the language condition but not in the sequential learning condition.
The right-hand side of Figure 4 shows topographical maps for the difference waves for language and sequential learning, averaged within each of the three latency windows. The maps indicate a similar spatial distribution of scalp activity across the two tasks, except for the gradually emerging anterior negativity in the language task. There are few discernible differences within the first latency window, though a left-anterior negativity can be observed in the language task whereas the sequential learning task involves left-anterior positivity. A P600 effect is visible within the 500–700 window for both tasks but slightly more widespread across central and posterior areas in the sequential learning task, perhaps because of the opposing effect of the increasing LAN in the language task. A somewhat reduced P600 effect continues in the 700–900 latency window for the language task but is absent for the sequential learning task. Thus, the main differences between the two tasks in terms of the distribution of scalp activity across time is the presence of a LAN effect that is visible across left frontal electrodes for the language task, increasing in both strength and spatial extent over time, and a shorter P600 effect for the sequential learning task.
Discussion
This study provides the first direct comparison of electrophysiological brain signatures of structured sequential learning and language processing using a within-subject design. The advantage of such a design is that inter-individual variance is held constant, unlike previous studies that compared neural responses between different individuals participating in different experiments. Following a brief exposure to structured sequences in a sequential learning task that was designed to encourage participants to make implicit predictions of upcoming visual stimuli, our participants showed a P600 signature for sequences that contained structural incongruencies. Crucially, this P600 was statistically indistinguishable from the P600 elicited by syntactic violations in the language task and with similar topographical distributions, consistent with our hypothesis that both tasks likely tap into the same underlying neural processing mechanisms.
The close match between the language and sequential learning P600 effects is particularly remarkable given the difference in the types of violations across the two tasks: the language task involved agreement errors whereas the sequential learning task involved stimulus category violations (loosely similar to a “word” category violation in natural language). Although natural language studies have elicited P600 effects for both types of violations (e.g., Osterhout & Mobley, 1995; Wassenaar & Hagoort, 2005), the difference in violation types might be expected to potentially reduce the similarity of the P600 effect across tasks. Indeed, when Rossi et al. (2005) directly compared P600 responses to both agreement and word category violations in a within-subjects design, they observed a smaller positivity for violations to word category relative to agreement in later processing (800 msec onwards). Thus, the weaker P600 effect we found for the sequential learning task (and which did not reach significance in the 700–900 msec latency window3) may thus be explained by the difference in violation type. In addition, the very brief exposure to the predictive sequential regularities in the sequential learning task likely contribute to the weaker P600 effect observed here—especially when compared to the 20 years or more of exposure that our participants have had with language—given the documented effects of frequency on P600 effects (e.g., Osterhout et al., 1994).
Our P600 results contrast with two previous studies incorporating AGL-like stimuli and which did not find a P600 effect. Baldwin and Kutas (1997) and Carrión and Bly (2007) used artificial grammars in an SRT task and an auditory sequence learning task, respectively, both obtaining P300 effects rather than P600 components. One possible explanation is that the P600 and the P300 may reflect the same underlying component, elicited by improbable task-relevant events whether they are linguistic or not (Coulson et al., 1998). Potential evidence against this viewpoint comes from a study of agrammatic aphasics who show a relatively normal P300 response to unexpected events in a classical tone oddball task but who nonetheless did not always show a P600 response to syntactic anomalies (Wassenaar, Hagoort, & Brown, 1997). Moreover, language impairment in agrammatic aphasia is associated with a breakdown in structured sequential learning abilities (Christiansen, Kelly, Shillcock & Greenfield, 2010). These findings suggest that P300 responses may be associated with basic mechanisms related to the detection of simple contingency violations whereas the P600, in a sequential learning context, reflects expectation violations for more complex, structured input patterns. Even though this account suggests that the P300 and P600 may be distinct components, it leaves open to question whether they reflect entirely different or potentially overlapping neural mechanisms. This perspective is thus consistent with explanations of P600 effects that focus on structural prediction and integration (e.g., Hagoort, 2003, 2009; Kaan et al., 2000)—as long as these are allowed to be domain-general.
It may be possible to interpret the observed P600 effect in the sequential learning task as reflecting some kind of structural reanalysis or revision processes akin to those proposed for syntactic processing (Friederici, 1995; Gouvea et al., 2010; Osterhout et al., 1994). This interpretation would require either that the language system was recruited for the sequential learning task or that a domain-general system was employed in both tasks. However, the notion that the processing of incongruent sequences in a sequential learning task should involve some sort of repair or revisions processes is inconsistent with most current theoretical and computational models of this type of learning (e.g., Altmann & Dienes, 1999; Christiansen et al., 2000; Redington & Chater, 1996; see Perruchet & Pacton, 2006; Pothos, 2007, for reviews). Thus, even though we cannot rule out the possibility that the P600 component obtained in the sequential learning task might reflect repair processes, we find this interpretation unlikely given the past literature on such learning.
Another possibility that might explain the difference between our results and those showing a P300, rather than a P600, in structured sequential learning tasks (Baldwin & Kutas, 1997; Carrión & Bly, 2007), is that our participants displayed better learning for the patterns (e.g., 93% behavioral performance vs. 77% for Carrión & Bly, 2007). Although it is difficult to directly compare learning between our task and that of Baldwin and Kutas (1997), who primarily assessed learning through changes to reaction times, their participants did show poor performance on an explicit prediction questionnaire that assessed their declarative knowledge of the structure. Thus, the differences not only in the tasks that were used, but also in the level of behavioral performance on those tasks compared to ours, make it difficult to make any firm conclusions about the underlying cause of the different ERP results. Future work is needed to systematically explore the ERP correlates of sequential learning across different domains, tasks, and input structures.
The primary difference between the ERP data from the two tasks used in the present study was that we observed a LAN for the language task but not for the sequential learning task. Anterior negativity, primarily in the left hemisphere, is sometimes observed following morphosyntactic anomalies (e.g., Friederici, Pfeifer, & Hahne, 1993; Osterhout & Mobley, 1995), especially following agreement errors (Gouvea et al., 2010; Münte, Matzke & Johannes, 1997). The LAN has been suggested to reflect more automatic processes compared to the P600 (Gunter et al., 1997). An early form of this negativity (ELAN), with an onset around 100–150 msec, has been viewed as an index of an initial parse of phrase structure information (Friederici, 1995). With regard to the sequential learning task, there are several likely reasons why we did not observe a LAN. First, Wassenar and Hagoort (2005) did not obtain a LAN for word category violations with visually presented stimuli, thus paralleling both the modality of presentation and the form of structural incongruency used in the sequential learning task (though Rossi et al., 2005, did report a LAN with auditory presentation). Second, sequential learning over a short period of time may result in less well-learnt category information. Hence, a category violation may be noted more in terms of violation of sequential expectations than in terms of category violations as such, producing a P600 but no LAN. This hypothesis is consistent with ERP data from second-language learners where anterior negativities are generally absent in the context of word category violations, while P600 effects are observed (Hahne, 2001; Weber-Fox & Neville, 1996).4 Moreover, Lau, Stroud, Plesch and Phillips (2006) found that LAN effects were affected by the predictability of the constraints being violated (see also Steinhauer & Connolly, 2008), thus potentially suggesting that the relatively short exposure in the sequential learning task may not have made the constraints sufficiently strong to elicit a LAN. Indeed, the results from the Friederici et al. (2002) AGL study suggest that with extensive training a LAN effect can be obtained. Finally, it is possible that overlap with a late positive shift may mask a smaller negative component to syntactic incongruency relating to weakly learned word categories (cf. the sequential learning topographic distribution in Figure 4). In contrast, Münte et al. (1997) found that the kind of agreement violations used in our language task is a strong LAN elicitor in that such a negativity but no P600 effect was observed for agreement violations in sentences containing German pseudo-words (e.g., ‘Some globbies higgles the vlinch’, English gloss, where higgle is incorrectly marked for 3rd person singular present tense).
Although our language results are largely similar to those reported by Osterhout and Mobley (1995) with similar materials, only in our study was it observed that the LAN continued late into the 700–900 msec latency window. The exact cause of this discrepancy is unclear but we note that such sustained anterior negativity (SAN) has also been observed elsewhere, as in the current study, for agreement violations (Lau et al., 2006). Bilateral sustained anterior negativities have additionally been found for phrase-structure violations in Chinese adults (Ye, Luo, Friederici & Zhou, 2006) and German 10-to-11-year old children (Jentschke & Koelsch, 2009) as well as in studies investigating the processing of long-distance dependencies (e.g., Phillips et al., 2005; Fiebach, Schlesewsky & Friederici, 2002). Similarly, comprehending jokes can result in sustained left-anterior negativity (Coulson & Kutas, 2001; Coulson & Lovett, 2004; Coulson & Williams, 2005) as can responses to irony (Regel et al., 2011). Fiebach et al. propose that the sustained anterior negativities may be associated with working memory processes (see also Kluender & Kutas, 1993, for a similar interpretation of LAN). However, given that the agreeing verb followed directly after the noun in our language task, this seems to be a less likely explanation of the SAN here. Alternatively, the SAN may be due to subvocalizations, which have been associated with larger and sustained frontal negativities (King & Kutas, 1995), though the lack of memory load afforded by the sentences in the current study would seem to argue against this option as well. Research specifically targeting early and late anterior negativities is needed to further determine the nature of the SAN and its relationship to the (E)LAN as well as to syntactic processing more generally.
Conclusion
The present findings have important theoretical and practical implications regarding the nature of the neural mechanisms recruited during language learning and processing. The results suggest that brain areas responsible for processing words in sequences are at least partly coextensive with brain areas responsible for processing other types of complex sequential information such as sequences of sounds, visual objects, or events in general. More precisely, we propose that the neural underpinnings of language may be part of a broader family of neural mechanisms that the brain recruits when extracting and integrating sequential information (in any domain) in order to make implicit predictions about the next element in a sequence (c.f., Barr, 2007).
Despite the lack of statistical differences between the P600 difference waves for our two tasks and the similarity in topographical distribution, it is conceivable that the tasks may be subserved by different underlying neural generators. However, given evidence suggesting that language problems in aphasia (e.g., Christiansen et al., 2010; Hoen at al., 2003) and specific language impairment (e.g., Plante, Gómez & Gerken, 2002; Tomblin, Mainela-Arnold & Zhang, 2007) are associated with deficits in sequential learning, we find this possibility unlikely. We interpret our results as suggesting that the P600 component is not language-specific (see also e.g., Kaan, 2009; Kuperberg, 2007)—rather, it is a broader index of violations and the cost integration of expectations based on sequential learning processes. More generally, we construe language processing within a constrained-based theoretical perspective (e.g., MacDonald, Pearlmutter & Seidenberg, 1994; Tanenhaus & Trueswell 1995), within which sequential learning provides a key source of predictive constraints. In this regard, we see the connectionist model of Crocker, Knoeferle and Mayberry (2010) as a potentially promising first step toward providing a computational account of P600 and LAN effects within a prediction-based, multiple-constraint satisfaction framework, consistent with our sequential learning based approach to the P600.
In summary, there are two different potential theoretical consequences of our results: either the language system was recruited to deal with the (verbalizable) material in the sequential learning task, or a domain-general system was employed in both language and sequential learning. Importantly, both theoretical scenarios validate the application of sequential learning paradigms to the study of language acquisition and processing, underscoring the sequential learning approach as a fruitful way of studying language. Although further research is required to decide between the two theoretical scenarios, we find that evolutionary considerations provide preliminary support for the domain-general perspective (e.g., Christiansen & Chater, 2008). We therefore conclude that the neural processes involved in the prediction of temporally unfolding events, based on knowledge of sequential regularities, form an important aspect of language processing and comprehension.
Acknowledgments
This research was supported by Human Frontiers Science Program grant RGP0177/2001-B to MHC, by NIDCD grant R03DC9485 to CMC, and by NICHD grant 5R03HD051671-02 to LO. We are grateful for the helpful comments from Stewart McCauley and two anonymous reviewers.
Footnotes
Findings relating to sequential learning are variously published under different headings such as “statistical learning”, “artificial language learning”, or “artificial grammar learning”, largely for historical reasons. However, as we see these studies as relating to the same underlying implicit learning mechanisms (Conway & Christiansen, 2006; Perruchet & Pacton, 2006), we prefer the term ‘sequential learning’ as it highlights the sequential nature of the stimuli and its potential relevance to language processing.
We additionally analyzed the data re-referenced to average reference and obtained qualitatively similar results.
In contrast to our results, Friederici et al. (2002) found a reliable P600 effect in the 700–900 msec interval for an artificial language learning task using similar stimuli as here. We see at least two factors that may contribute to this discrepancy: 1) The participants in Friederici et al.’s study spent many hours during the learning phase of this study compared to the 30 minutes of exposure that our participants received; 2) Friederici et al. used a more language-like learning situation in which participants were playing a computerized board game in pairs using utterances from the artificial language with explicit feedback on incorrect language use, whereas our participants only received passive exposure to the sequences and associated visual referents. Thus, the participants in the Friederici et al. study not only received more than 10 times the exposure compared to our participants, but they were also actively trained and received feedback on their use of the language. Together, these factors likely explain why we obtained a weaker P600 effect in our study.
More recently, Mueller et al. (2005) did report a broadly distributed negativity for word category violations in miniature-Japanese learning task but this was observed even in untrained control participants, perhaps suggesting that the negativity in the trained participants may be related to other nonsyntactic factors.
Contributor Information
Morten H. Christiansen, Cornell University
Christopher M. Conway, Saint Louis University
Luca Onnis, University of Hawaii.
References
- Allen M, Badecker W, Osterhout L. Morphological analysis in sentence processing: An ERP study. Language & Cognitive Processes. 2003;18:405–430. [Google Scholar]
- Altmann GTM, Dienes Z. Rule learning by seven-month-old infants and neural networks. Science. 1999;284:875. doi: 10.1126/science.283.5398.77. [DOI] [PubMed] [Google Scholar]
- Aslin RN, Saffran JR, Newport EL. Computation of conditional probability statistics by 8-month-old infants. Psychological Science. 1998;9:321–324. [Google Scholar]
- Bahlmann J, Gunter TC, Friederici AD. Hierarchical and linear sequence processing: An electrophysiological exploration of two different grammar types. Journal of Cognitive Neuroscience. 2006;18:1829–1842. doi: 10.1162/jocn.2006.18.11.1829. [DOI] [PubMed] [Google Scholar]
- Baldwin KB, Kutas M. An ERP analysis of implicit structured sequence learning. Psychophysiology. 1997;34:74–86. doi: 10.1111/j.1469-8986.1997.tb02418.x. [DOI] [PubMed] [Google Scholar]
- Barber H, Carreiras M. Grammatical gender and number agreement in Spanish: An ERP comparison. Journal of Cognitive Neuroscience. 2005;17:137–153. doi: 10.1162/0898929052880101. [DOI] [PubMed] [Google Scholar]
- Barr M. The proactive brain: Using analogies and associations to generate predictions. Trends in Cognitive Sciences. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky I, Schlesewsky M. An alternative perspective on “semantic P600” effects in language comprehension. Brain Research Reviews. 2008;59:55–73. doi: 10.1016/j.brainresrev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Brooks PJ, Braine MDS, Catalano L, Brody RE, Sudhalter V. Acquisition of gender-like noun subclasses in an artificial language: The contribution of phonological markers to learning. Journal of Memory and Language. 1993;32:76–95. [Google Scholar]
- Carrión RE, Bly BM. Event-related potential markers of expectation violation in an artificial grammar learning task. NeuroReport. 2007;18:191–195. doi: 10.1097/WNR.0b013e328011b8ae. [DOI] [PubMed] [Google Scholar]
- Chambers KE, Onishi KH, Fisher C. Infants learn phonotactic regularities from brief auditory experience. Cognition. 2003;87:B69–B77. doi: 10.1016/s0010-0277(02)00233-0. [DOI] [PubMed] [Google Scholar]
- Christiansen MH, Chater N. Language as shaped by the brain. Behavioral & Brain Sciences. 2008;31:489–558. doi: 10.1017/S0140525X08004998. [DOI] [PubMed] [Google Scholar]
- Christiansen MH, Conway CM, Curtin S. A connectionist single-mechanism account of rule-like behavior in infancy. The Proceedings of the 22nd Annual Conference of the Cognitive Science Society; Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 83–88. [Google Scholar]
- Christiansen MH, Kelly ML, Shillcock RC, Greenfield K. Impaired artificial grammar learning in agrammatism. Cognition. 2010;116:382–393. doi: 10.1016/j.cognition.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Cleeremans A, Destrebecqz A, Boyer M. Implicit learning: News from the front. Trends in Cognitive Sciences. 1998;2:406–416. doi: 10.1016/s1364-6613(98)01232-7. [DOI] [PubMed] [Google Scholar]
- Cleeremans A, McClelland JL. Learning the structure of event sequences. Journal of Experimental Psychology: General. 1991;120:235–253. doi: 10.1037//0096-3445.120.3.235. [DOI] [PubMed] [Google Scholar]
- Clegg BA, DiGirolamo GJ, Keele SW. Sequence learning. Trends in Cognitive Sciences. 1998;2:275–281. doi: 10.1016/s1364-6613(98)01202-9. [DOI] [PubMed] [Google Scholar]
- Conway CM, Bauernschmidt A, Huang SS, Pisoni DB. Implicit statistical learning in language processing: Word predictability is the key. Cognition. 2010;114:356–371. doi: 10.1016/j.cognition.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CM, Christiansen MH. Modality-constrained statistical learning of tactile, visual, and auditory sequences. Journal of Experimental Psychology. 2005;31:24–39. doi: 10.1037/0278-7393.31.1.24. [DOI] [PubMed] [Google Scholar]
- Conway CM, Christiansen MH. Statistical learning within and between modalities: Pitting abstract against stimulus specific representations. Psychological Science. 2006;17:905–912. doi: 10.1111/j.1467-9280.2006.01801.x. [DOI] [PubMed] [Google Scholar]
- Conway CM, Christiansen MH. Sequential learning in non-human primates. Trends in Cognitive Sciences. 2001;5:539–546. doi: 10.1016/s1364-6613(00)01800-3. [DOI] [PubMed] [Google Scholar]
- Conway CM, Ellefson MR, Christiansen MH. When less is less and when less is more: Starting small with staged input. Proceedings of the 25th Annual Conference of the Cognitive Science Society; Mahwah, NJ: Lawrence Erlbaum; 2003. pp. 270–275. [Google Scholar]
- Conway CM, Karpicke J, Pisoni DB. Contribution of implicit sequence learning to spoken language processing: Some preliminary findings from normal-hearing adults. Journal of Deaf Studies and Deaf Education. 2007;12:317–334. doi: 10.1093/deafed/enm019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson S, King JW, Kutas M. Expect the unexpected: Event-related brain response to morphosyntactic violations. Language and Cognitive Processes. 1998;13:21–58. [Google Scholar]
- Coulson S, Kutas M. Getting it: Human event-related brain response to jokes in good and poor comprehenders. Neuroscience Letters. 2001;316:71–74. doi: 10.1016/s0304-3940(01)02387-4. [DOI] [PubMed] [Google Scholar]
- Coulson S, Lovett C. Handedness, hemispheric asymmetry, and joke comprehension. Cognitive Brain Research. 2004;19:275–288. doi: 10.1016/j.cogbrainres.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Coulson S, Van Petten C. Conceptual integration and metaphor: An ERP Study. Memory & Cognition. 2002;30:958–968. doi: 10.3758/bf03195780. [DOI] [PubMed] [Google Scholar]
- Coulson S, Van Petten C. A special role for the right hemisphere in metaphor comprehension: An ERP Study. Brain Research. 2007;1146:128–145. doi: 10.1016/j.brainres.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Coulson S, Williams RF. Hemispheric asymmetries and joke comprehension. Neuropsychologia. 2005;43:128–141. doi: 10.1016/j.neuropsychologia.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Crocker MW, Knoeferle P, Mayberry MR. Situated sentence processing: The coordinated interplay account and a neurobehavioral model. Brain and language. 2010;112:189–201. doi: 10.1016/j.bandl.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Curran T, Keele SW. Attentional and Nonattentional Forms of Sequence Learning. Journal of Experimental Psychology. 1993;19:189–202. [Google Scholar]
- Dell GS, Reed KD, Adams DR, Meyer AS. Speech errors, phonotactic constraints, and implicit learning: A study of the role of experience in language production. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2000;26:1355–1367. doi: 10.1037//0278-7393.26.6.1355. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Eimer M, Goschke TT, Schlaghecken F, Stürmer B. Explicit and implicit learning of event sequences: Evidence from event-related brain potentials. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1996;22:970–987. doi: 10.1037//0278-7393.22.4.970. [DOI] [PubMed] [Google Scholar]
- Elliott LL. Verbal auditory closure and the speech perception in noise (SPIN) test. Journal of Speech, Language, and Hearing Research. 1995;38:1363–1376. doi: 10.1044/jshr.3806.1363. [DOI] [PubMed] [Google Scholar]
- Federmeier K. Thinking ahead: The role and roots of prediction in language comprehension. Psychophysiology. 2007;44:491–505. doi: 10.1111/j.1469-8986.2007.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand NK, Mecklinger A, Kray J. Error deviance processing in implicit and explicit sequence learning. Journal of Cognitive Neuroscience. 2008;20:629–642. doi: 10.1162/jocn.2008.20046. [DOI] [PubMed] [Google Scholar]
- Felser C, Clahsen H, Münte TF. Storage and integration in the processing of filler-gap dependencies: An ERP study of topicalization and wh-movement in German. Brain and Language. 2003;87:345–354. doi: 10.1016/s0093-934x(03)00135-4. [DOI] [PubMed] [Google Scholar]
- Ferguson MJ, Bargh JA. How social perception can automatically influence behavior. Trends in Cognitive Sciences. 2004;8:33–39. doi: 10.1016/j.tics.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Luu PL, Russell J, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Friederici AD. Separating syntactic memory costs and syntactic integration costs during parsing: the processing of German wh-questions. Journal of Memory and Language. 2002;47:250–272. [Google Scholar]
- Fiser J, Aslin RN. Statistical learning of higher-order temporal structure from visual shape sequences. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2002;28:458–467. doi: 10.1037//0278-7393.28.3.458. [DOI] [PubMed] [Google Scholar]
- Friederici AD. The time course of syntactic activation during language processing: A model based on neuropsychological and neurophysiological data. Brain and Language. 1995;50:259–281. doi: 10.1006/brln.1995.1048. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Hahne A, Mecklinger A. Temporal structure of syntactic parsing: early and late event-related brain potential effects. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:1219–1248. doi: 10.1037//0278-7393.22.5.1219. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Pfeifer E, Hahne A. Event-related brain potentials during natural speech processing: Effects of semantic, morphological and syntactic violations. Cognitive Brain Research. 1993;1:183–192. doi: 10.1016/0926-6410(93)90026-2. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Steinhauer K, Pfeifer E. Brain signatures of artificial language processing: Evidence challenging the critical period hypothesis. Proceedings of the National Academy of Sciences. 2002;99:529–534. doi: 10.1073/pnas.012611199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigo L, McDonald JL. Properties of phonological markers that affect the acquisition of gender-like subclasses. Journal of Memory and Language. 1998;39:218–245. [Google Scholar]
- Gómez RL. Variability and detection of invariant structure. Psychological Science. 2002;13:431–436. doi: 10.1111/1467-9280.00476. [DOI] [PubMed] [Google Scholar]
- Gómez RL, Gerken LA. Infant artificial language learning and language acquisition. Trends in Cognitive Sciences. 2000;4:178–186. doi: 10.1016/s1364-6613(00)01467-4. [DOI] [PubMed] [Google Scholar]
- Gouvea AC, Phillips C, Kazanina N, Poeppel D. The linguistic processes underlying the P600. Language and Cognitive Processes. 2010;25:149–188. [Google Scholar]
- Green TRG. Necessity of syntax markers: 2 experiments with artificial languages. Journal of Verbal Learning and Verbal Behavior. 1979;18:481–496. [Google Scholar]
- Gunter TC, Stowe LA, Mulder G. When syntax meets semantics. Psychophysiology. 1997;34:600–676. doi: 10.1111/j.1469-8986.1997.tb02142.x. [DOI] [PubMed] [Google Scholar]
- Hagoort P. How the brain solves the binding problem for language: A neurocomputational model of syntactic processing. NeuroImage. 2003;20:S18–S29. doi: 10.1016/j.neuroimage.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hagoort P. Reflections on the neurobiology of syntax. In: Bickerton D, Szathmáry E, editors. Biological foundations and origin of syntax. Strüngmann Forum Reports. Vol. 3. Cambridge, MA: MIT Press; 2009. pp. 279–296. [Google Scholar]
- Hagoort P, Brown CM. Brain responses to lexical ambiguity resolution and parsing. In: Clifton C Jr, Frazier L, Rayner K, editors. Perspectives on sentence processing. Hillsdale, NJ: Erlbaum; 1994. pp. 45–80. [Google Scholar]
- Hagoort P, Brown CM, Groothusen J. The Syntactic Positive Shift (SPS) as an ERP measure of syntactic processing. Language and Cognitive Processes. 1993;8:439–484. [Google Scholar]
- Hahne A. What’s different in second-language processing? Evidence from event-related brain potentials. Journal of Psycholinguistic Research. 2001;30:251–266. doi: 10.1023/a:1010490917575. [DOI] [PubMed] [Google Scholar]
- Heimbauer LA, Conway CM, Christiansen MH, Beran MJ, Owren MJ. Grammar rule-based sequence learning by rhesus macaques (Macaca mulatta) American Journal of Primatology. 2010;72:65. [Google Scholar]
- Hoen M, Dominey PF. ERP analysis of cognitive sequencing: A left anterior negativity related to structural transformation processing. Neuroreport. 2000;11:3187–3191. doi: 10.1097/00001756-200009280-00028. [DOI] [PubMed] [Google Scholar]
- Hoen M, Golembiowski M, Guyot E, Deprez V, Caplan D, Dominey PF. Training with cognitive sequences improves syntactic comprehension in agrammatic aphasics. NeuroReport. 2003;14:495–499. doi: 10.1097/00001756-200303030-00040. [DOI] [PubMed] [Google Scholar]
- Hunt RH, Aslin RN. Statistical learning in a serial reaction time task: Access to separable statistical cues by individual learners. Journal of Experimental Psychology: General. 2001;130:658–680. doi: 10.1037//0096-3445.130.4.658. [DOI] [PubMed] [Google Scholar]
- Jentschke S, Koelsch S. Musical training modulates the development of syntax processing in children. NeuroImage. 2009;47:735–744. doi: 10.1016/j.neuroimage.2009.04.090. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Shanks DR. Two mechanisms in implicit artificial grammar learning? Comment on Meulemans and Van der Linden (1997) Journal of Experimental Psychology: Learning, Memory and Cognition. 1999;25:524–531. [Google Scholar]
- Kaan E. Fundamental syntactic phenomena and their putative relation to the brain. In: Bickerton D, Szathmáry E, editors. Biological foundations and origin of syntax. Strüngmann Forum Reports. Vol. 3. Cambridge, MA: MIT Press; 2009. pp. 117–133. [Google Scholar]
- Kaan E, Harris A, Gibson E, Holcomb P. The P600 as an index of syntactic integration difficulty. Language and Cognitive Processes. 2000;15:159–201. [Google Scholar]
- Kaan E, Swaab TY. Electrophysiological evidence for serial sentence processing: a comparison between non-preferred and ungrammatical continuations. Cognitive Brain Research. 2003;17(3):621–635. doi: 10.1016/s0926-6410(03)00175-7. [DOI] [PubMed] [Google Scholar]
- Kalikow DN, Stevens KN, Elliott LL. Development of a test of speech intelligibility in noise using sentence materials with controlled word predictability. Journal of the Acoustical Society of America. 1977;61:1337–1351. doi: 10.1121/1.381436. [DOI] [PubMed] [Google Scholar]
- Kamide Y. Anticipatory processes in sentence processing. Language and Linguistic Compass. 2008;2:647–670. [Google Scholar]
- Kazmerski VA, Blasko DG, Dessalegn BG. ERP and behavioral evidence of individual differences in metaphor comprehension. Memory & Cognition. 2003;31:673–689. doi: 10.3758/bf03196107. [DOI] [PubMed] [Google Scholar]
- Kim A, Osterhout L. The independence of combinatory semantic processing: Evidence from event-related potentials. Journal of Memory and Language. 2005;52:205–255. [Google Scholar]
- King JW, Kutas M. Who did what and when? Using word- and clause-related ERPs to monitor working memory usage in reading. Journal of Cognitive Neuroscience. 1995;7:378–397. doi: 10.1162/jocn.1995.7.3.376. [DOI] [PubMed] [Google Scholar]
- Kluender R, Kutas M. Bridging the gap: Evidence from ERPs on the processing of unbound dependencies. Journal of Cognitive Neuroscience. 1993;5:196–214. doi: 10.1162/jocn.1993.5.2.196. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR. The information acquired during artificial grammar learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:79–91. doi: 10.1037//0278-7393.20.1.79. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR. Neural mechanisms of language comprehension: Challenges to syntax. Brain Research. 2007;1146:23–49. doi: 10.1016/j.brainres.2006.12.063. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Kreher DA, Sitnikova T, Caplan DN, Holcomb PJ. The role of animacy and thematic relationships in processing active English sentences: Evidence from event-related potentials. Brain and Language. 2007;100:223–237. doi: 10.1016/j.bandl.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Caplan D, Holcomb PJ. Electrophysiological distinctions in processing conceptual relationships within simple sentences. Cognitive Brain Research. 2003;17:117–129. doi: 10.1016/s0926-6410(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potential reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Lau E, Stroud C, Plesch S, Phillips C. The role of structural prediction in rapid syntactic analysis. Brain and Language. 2006;98:74–88. doi: 10.1016/j.bandl.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Lelekov T, Dominey PF, Garcia-Larrea L. Dissociable ERP profiles for processing rules vs instances in a cognitive sequencing task. NeuroReport. 2000;11:1–4. doi: 10.1097/00001756-200004070-00043. [DOI] [PubMed] [Google Scholar]
- MacDonald MC, Pearlmutter NJ, Seidenberg MS. The lexical nature of syntactic ambiguity resolution. Psychological Review. 1994;101:676–703. doi: 10.1037/0033-295x.101.4.676. [DOI] [PubMed] [Google Scholar]
- Marcus GF, Vijayan S, Bandi Rao S, Vishton PM. Rule Learning by Seven-Month-Old Infants. Science. 1999;283:77–80. doi: 10.1126/science.283.5398.77. [DOI] [PubMed] [Google Scholar]
- Meulemans T, Van der Linden M. Associative chunk strength in artificial grammar learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23:1007–1028. [Google Scholar]
- Misyak JB, Christiansen MH, Tomblin JB. Sequential expectations: The role of prediction-based learning in language. Topics in Cognitive Science. 2010;2:138–153. doi: 10.1111/j.1756-8765.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Sato A, Yasuda A, Kumano H, Kuboki T. Explicit knowledge and intention to learn in sequence learning: An event-related potential study. NeuroReport. 2005;16:705–708. doi: 10.1097/00001756-200505120-00010. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Meier RP, Newport EL. Structural packaging in the input to language learning: Contributions of prosodic and morphological marking of phrases to the acquisition of language. Cognitive Psychology. 1987;19:498–550. doi: 10.1016/0010-0285(87)90017-x. [DOI] [PubMed] [Google Scholar]
- Mueller JL, Hahne A, Fujii Y, Friederici AD. Native and nonnative speakers’ processing of a miniature version of Japanese as revealed by ERPs. Journal of Cognitive Neuroscience. 2005;17:1229–1244. doi: 10.1162/0898929055002463. [DOI] [PubMed] [Google Scholar]
- Mueller JL, Bahlmann J, Friederici AD. The role of pause cues in language learning: The emergence of event-related potential related to sequence processing. Journal of Cognitive Neuroscience. 2008;20:892–905. doi: 10.1162/jocn.2008.20511. [DOI] [PubMed] [Google Scholar]
- Münte TF, Matzke M, Johannes S. Brain activity associated with syntactic incongruencies in words and pseudo-words. Journal of Cognitive Neuroscience. 1997;93:318–329. doi: 10.1162/jocn.1997.9.3.318. [DOI] [PubMed] [Google Scholar]
- Neri P, Luu JY, Levi DM. Meaningful interactions can enhance visual discrimination of human agents. Nature Neuroscience. 2006;9:1186–1192. doi: 10.1038/nn1759. [DOI] [PubMed] [Google Scholar]
- Neville H, Nicol J, Barss A, Forster KI, Garrett MI. Syntactically-based sentence processing classes: Evidence from event-related brain potentials. Journal of Cognitive Neuroscience. 1991;3:151–165. doi: 10.1162/jocn.1991.3.2.151. [DOI] [PubMed] [Google Scholar]
- Nevins A, Dillon B, Malhotra S, Phillips C. The role of feature-number and feature-type in processing Hindi verb agreement violations. Brain Research. 2007;1164:81–94. doi: 10.1016/j.brainres.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Newport EL, Aslin RN. Learning at a distance I: Statistical learning of non-adjacent dependencies. Cognitive Psychology. 2004;48:127–162. doi: 10.1016/s0010-0285(03)00128-2. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Niv Y, Schoenbaum G. Dialogues on prediction errors. Trends in Cognitive Sciences. 2008;12:265–272. doi: 10.1016/j.tics.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onnis L, Christiansen MH, Chater N, Gómez R. Reduction of uncertainty in human sequential learning: Evidence from artificial grammar learning. Proceedings of the 25th Annual Conference of the Cognitive Science Society; Mahwah, NJ: Lawrence Erlbaum; 2003. pp. 886–891. [Google Scholar]
- Onnis L, Farmer TA, Baroni M, Christiansen MH, Spivey M. Generalizable distributional regularities aid fluent language processing: The case of semantic valence tendencies. Italian Journal of Linguistics. 2008;20:125–152. [Google Scholar]
- Onnis L, Waterfall H, Edelman S. Learn locally, act globally: Learning language from variation set cues. Cognition. 2008;109:423–430. doi: 10.1016/j.cognition.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout L, Hagoort P. A superficial resemblance does not necessarily mean you are part of the family: Counterarguments to Coulson, King, & Kutas (1998) in the P600/SPS–P300 debate. Language and Cognitive Processes. 1999;14:1–14. [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related brain potentials elicited by syntactic anomaly. Journal of Memory and Language. 1992;31:785–806. [Google Scholar]
- Osterhout L, Holcomb PJ, Swinney DA. Brain potentials elicited by garden-path sentences: Evidence of the application of verb information during parsing. Journal of Experimental Psychology: Learning, Memory and Cognition. 1994;20:786–803. doi: 10.1037//0278-7393.20.4.786. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Mobley LA. Event-related potentials elicited by failure to agree. Journal of Memory and Language. 1995;34:739–773. [Google Scholar]
- Pacton S, Perruchet P, Fayol M, Cleeremans A. Implicit learning out of the lab: The case of orthographic regularities. Journal of Experimental Psychology: General. 2001;130:401–426. doi: 10.1037//0096-3445.130.3.401. [DOI] [PubMed] [Google Scholar]
- Patel AD, Gibson E, Ratner J, Besson M, Holcomb PJ. Processing syntactic relations in language and music: An event-related potential study. Journal of Cognitive Neuroscience. 1998;10:717–733. doi: 10.1162/089892998563121. [DOI] [PubMed] [Google Scholar]
- Peña M, Bonnatti L, Nespor M, Mehler J. Signal-driven computations in speech processing. Science. 2002;298:604–607. doi: 10.1126/science.1072901. [DOI] [PubMed] [Google Scholar]
- Perruchet P, Pacton S. Implicit learning and statistical learning: One phenomenon, two approaches. Trends in Cognitive Sciences. 2006;10:233–238. doi: 10.1016/j.tics.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Phillips C, Kazanina N, Abada S. ERP effects of the processing of syntactic long-distance dependencies. Cognitive Brain Research. 2005;22:407–428. doi: 10.1016/j.cogbrainres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Pickering MJ, Garrod S. Do people use language production to make predictions during comprehension? Trends in Cognitive Sciences. 2007;11:105–110. doi: 10.1016/j.tics.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Plante E, Gómez R, Gerken L. Sensitivity to word order cues by normal and language/learning disabled adults. Journal of Communication Disorders. 2002;35:453–462. doi: 10.1016/s0021-9924(02)00094-1. [DOI] [PubMed] [Google Scholar]
- Pothos EM. Theories of artificial grammar learning. Psychological Bulletin. 2007;133:227–244. doi: 10.1037/0033-2909.133.2.227. [DOI] [PubMed] [Google Scholar]
- Pothos EM, Bailey TM. The role of similarity in artificial grammar learning. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2000;26:847–862. doi: 10.1037//0278-7393.26.4.847. [DOI] [PubMed] [Google Scholar]
- Rayner K, Well AD. Effects of contextual constraint on eye movements in reading: A further examination. Psychonomic Bulletin & Review. 1999;3:504–509. doi: 10.3758/BF03214555. [DOI] [PubMed] [Google Scholar]
- Reber AS. Implicit learning of artificial grammars. Journal of Verbal Learning and Behavior. 1967;6:855–863. [Google Scholar]
- Reed J, Johnson P. Assessing implicit learning with indirect tests: Determining what is learned about sequence structure. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:585–594. [Google Scholar]
- Redington M, Chater N. Transfer in artificial grammar learning: A reevaluation. Journal of Experimental Psychology: General. 1996;125:123–138. [Google Scholar]
- Regel S, Coulson S, Gunter TC. The communicative style of a speaker can affect language comprehension? ERP evidence from the comprehension of irony. Brain Research. 2010;1311:121–135. doi: 10.1016/j.brainres.2009.10.077. [DOI] [PubMed] [Google Scholar]
- Regel S, Gunter TC, Friederici AD. Isn’t it ironic? An electrophysiological exploration of figurative language processing. Journal of Cognitive Neuroscience. 2011;23:277–293. doi: 10.1162/jocn.2010.21411. [DOI] [PubMed] [Google Scholar]
- Rossi S, Gugler MF, Hahne A, Friederici AD. When word category information encounters morphosyntax: An ERP study. Neuroscience Letters. 2005;384:228–233. doi: 10.1016/j.neulet.2005.04.077. [DOI] [PubMed] [Google Scholar]
- Rüsseler J, Hennighausen E, Münte TF, Rösler F. Differences in incidental and intentional learning of sensorimotor sequences as revealed by event-related brain potentials. Cognitive Brain Research. 2003;15:116–126. doi: 10.1016/s0926-6410(02)00145-3. [DOI] [PubMed] [Google Scholar]
- Rüsseler J, Hennighausen E, Rösler F. Response anticipation processes in the learning of a sensorimotor sequence: Evidence from the lateralized readiness potential. Journal of Psychophysiology. 2001;15:95–105. [Google Scholar]
- Rüsseler J, Rösler F. Representation and learning of structure in perceptuo-motor event-sequences. In: Friederici A, Menzel R, editors. Learning: Rule extraction and representation. New York: Walter de Gruyter; 1999. pp. 117–138. [Google Scholar]
- Rüsseler J, Rösler F. Implicit and explicit learning of event sequences: Evidence for distinct coding of perceptual and motor representations. Acta Psychologica. 2000;104:45–67. doi: 10.1016/s0001-6918(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Saffran JR. Words in a sea of sounds: The output of infant statistical learning. Cognition. 2001;81:149–169. doi: 10.1016/s0010-0277(01)00132-9. [DOI] [PubMed] [Google Scholar]
- Saffran JR. Constraints on statistical language learning. Journal of Memory and Language. 2002;47:172–196. [Google Scholar]
- Saffran JR. Statistical learning: Mechanisms and constraints. Current Directions in Psychological Science. 2003;12:110–114. [Google Scholar]
- Saffran JR, Newport EL, Aslin RN, Tunick RA, Barrueco S. Incidental language learning: Listening (and learning) out of the corner of your ear. Psychological Science. 1997;8:101–105. [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Johnson EK, Aslin RN, Newport EL. Statistical learning of tone sequences by human infants and adults. Cognition. 1999;70:27–52. doi: 10.1016/s0010-0277(98)00075-4. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F, Stürmer B, Eimer M. Chunking processes in the learning of event sequences: Electrophysiological indicators. Memory & Cognition. 2000;28:821–831. doi: 10.3758/bf03198417. [DOI] [PubMed] [Google Scholar]
- Schvaneveldt RW, Gómez RL. Attention and probabilistic sequence learning. Psychological Research. 1998;61:175–190. [Google Scholar]
- Seidenberg MS, Elman JL. Networks are not ‘hidden rules’. Trends in Cognitive Sciences. 1999;3:288–289. doi: 10.1016/s1364-6613(99)01355-8. [DOI] [PubMed] [Google Scholar]
- Stadler MA. Statistical structure and implicit serial learning. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1992;18:318–327. [Google Scholar]
- Silva-Pereyra J, Conboy BT, Klarman L, Kuhl PK. Grammatical processing without semantics? An event-related brain potential study of preschoolers using jabberwocky sentences. Journal of Cognitive Neuroscience. 2007;19:1050–1065. doi: 10.1162/jocn.2007.19.6.1050. [DOI] [PubMed] [Google Scholar]
- Steinhauer K, Connolly JF. Event-related potentials in the study of language. In: Stemmer B, Whitaker HA, editors. Handbook of the neuroscience of language. New York: Elsevier; 2008. pp. 91–104. [Google Scholar]
- Tanenhaus MK, Trueswell JC. Sentence comprehension. In: Miller J, Eimas P, editors. Speech, language, and communication. San Diego, CA: Academic Press; 1995. pp. 217–262. [Google Scholar]
- Tomblin JB, Mainela-Arnold E, Zhang X. Procedural learning in adolescents with and without specific language impairment. Language Learning and Development. 2007;3:269–293. [Google Scholar]
- Toro JM, Sinnett S, Soto-Faraco S. Speech segmentation by statistical learning depends on attention. Cognition. 2005;97:B25–B34. doi: 10.1016/j.cognition.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: The geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87:145–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Junge JA, Scholl BJ. The automaticity of visual statistical learning. Journal of Experimental Psychology: General. 2005;134:522–564. doi: 10.1037/0096-3445.134.4.552. [DOI] [PubMed] [Google Scholar]
- Valian V, Coulson S. Anchor points in language learning: The role of marker frequency. Journal of Memory and Language. 1988;27:71–86. [Google Scholar]
- Van Berkum JJA. Understanding sentences in context: What brain waves can tell us. Current Directions in Psychological Science. 2008;17:376–380. [Google Scholar]
- Vokey JR, Brooks LR. Salience of item knowledge in learning artificial grammar. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;20:328–344. doi: 10.1037//0278-7393.20.6.1504. [DOI] [PubMed] [Google Scholar]
- Wassenaar M, Brown CM, Hagoort P. ERP effects of subject–verb agreement violations in patients with Broca’s aphasia. Journal of Cognitive Neuroscience. 2005;16:553–576. doi: 10.1162/089892904323057290. [DOI] [PubMed] [Google Scholar]
- Wassenaar M, Hagoort P. Word-category violations in patients with Broca’s aphasia: An ERP study. Brain and Language. 2005;92:117–137. doi: 10.1016/j.bandl.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Weber-Fox CM, Neville HJ. Maturational constraints on functional specializations for language processing: ERP and behavioral evidence in bilingual speakers. Journal of Cognitive Neuroscience. 1996;8:231–256. doi: 10.1162/jocn.1996.8.3.231. [DOI] [PubMed] [Google Scholar]
- Ye Z, Luo YJ, Friederici AD, Zhou X. Semantic and syntactic processing in Chinese sentence comprehension: Evidence from event-related potentials. Brain Research. 2006;1071:186–196. doi: 10.1016/j.brainres.2005.11.085. [DOI] [PubMed] [Google Scholar]