HIV-associated neurocognitive disorder (HAND) is currently prevalent at epidemic proportions within the HIV-infected patient population [1]. Of the conditions identified as ‘HAND’, the milder conditions of mild neurocognitive disorder (MND) and asymptomatic neurocognitive impairment are more common than the severe condition of HIV-associated dementia in the current era of effective antiretroviral therapy (ART). This has been confirmed by neuropathological studies [2]. The revised criteria for diagnosing HAND do not identify any specific positive laboratory or neuroimaging techniques for making the diagnosis of HAND. Hence, these conditions remain diagnoses requiring the exclusion of other conditions that could present with neurocognitive impairment in the patient population [3,4]. Establishing HAND diagnoses involves a significant differential diagnostic work-up that extends beyond structural neuroimaging to specialized cerebrospinal fluid studies (e.g., Epstein–Barr virus PCR to rule out primary CNS lymphoma, JC virus PCR to rule out progressive multifocal leukoencephalopathy, and cytomegalovirus PCR to rule out cytomegalovirus encephalopathy). In addition, ruling out metabolic encephalopathy requires evaluation of pulmonic, hepatic and renal function. Furthermore, psychiatric disorders (e.g., major depressive disorder and substance use disorders), as well as psychoneurotoxicity of prescribed medications must be excluded.
Structural neuroimaging studies have demonstrated cerebral atrophy in the late stage of HAND (i.e., HIV-associated dementia) dating back prior to the era of effective ART. Morphometric studies using quantitative measures of brain volume, such as the bicaudate ratio (as a measure of basal ganglia volume), also correlate with impaired neuro-cognitive performance. The caudate nucleus volume has been correlated with motor performance in HIV-infected individuals, independently of measures of generalized brain atrophy and immunological disease progression. More recently, maps of cortical thickness have documented thinning of the cerebral cortex to be correlated both with the level of neurocognitive impairment and the CD4 cell count. Reports of hippocampal atrophy in HAND have also accumulated, as have reports of diffuse, periventricular white matter changes. However, no specific pattern of structural changes has emerged by which HAND might be positively diagnosed.
Proton magnetic resonance spectroscopy (MRS) studies of HAND have shown increases in myoinositol (MI) and choline (Cho) (both associated with microglial proliferation) in white matter and the basal ganglia and reduced N-acetyl aspartate (NAA) (associated with neuronal cell death) in the frontal lobe white matter [5]. While most studies have focused on patterns of these three metabolites in terms of absolute concentration or as ratios with creatine, other metabolites may be helpful in the diagnosis of HAND. Glutamate (Glu) is a widespread and abundant (6–12 mM) neurotransmitter that is associated with excitotoxic neuronal cell death, one of the primary mechanisms of neuronal cell death associated with HAND [6]. NAA is related to this mechanism, since NAA binds Glu in neuronal cells (N-acetyl-aspartyl glutamine). With a drop in NAA levels, this binding capacity decreases and therefore more Glu is available to induce excitotoxicity. With regard to HIV infection, few studies have reported on Glu, and these have been focused on the combination of the Glu and glutamine (Gln) into the Glx peaks. The Glx peak in HIV-infected patients appears to be regionally specific and has been reported as decreased in the frontal grey and the frontal white matter and increased in the basal ganglia. Hence, we have focused upon the finer resolution of the Glx peaks by brain region. Glu and Gln are highly related in the brain, as Glu is thought to be taken up by astrocytes, converted to Gln, transported back to the presynaptic neuron and converted back to Glu [7] – posited as the ‘glutamate/glutamine shuttle’ hypothesis [8,9].
For the sole purpose of illustration, MRS data are reported from one subject with the diagnosis of MND versus one HIV-seropositive control subject without neurocognitive impairment. It is important to note that a comparison such as this cannot be substantiated as formally evidence based owing to sources of variability attributable to subject movement, voxel placement and intrinsic variability ≥25% – a particular problem for Glu [10]. The subject with MND is a 64 year-old African–American homosexual man who is a disabled clerical worker having a history of HIV infection since 1987, when he presented with oral candidiasis. He was treated with emtricitabine/tenofovir and boosted lopinavir, a regimen with a CNS penetration rank of seven, which is moderate [11]. The subject’s most recent plasma viral load was nondetectable and his CD4 cell count had consistently been in the range of 600–800 cells/mm3 – the mild level of immunologic disease progression. The subject also had a history of HIV-induced distal sensory polyneuropathy for which he was being treated with gabapentin. He had performed at 1.33 standard deviations (s.d.s) below the mean on the symbol-search task (information processing speed domain), −1.50 s.d.s on the California Verbal Learning Test (total acquisition of words across trials 1–5) (verbal memory domain), −1.33 s.d.s on block design (visuospatial domain) and −2.19 s.d.s on the grooved pegboard test, non-dominant hand (motor domain). He also had an elevated score on the Cognitive Difficulties Scale, demonstrating an impact on his functional status and, overall, qualifying him for the diagnosis of MND.
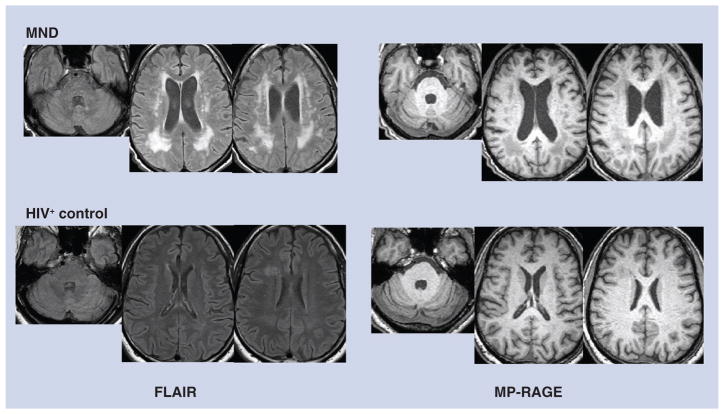
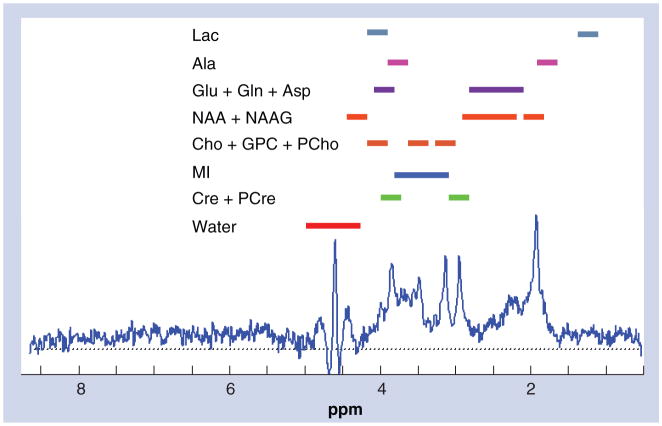
With regard to neuroimaging, axial T1 magnetization-prepared rapid acquisition gradient echo showed some ventricular enlargement but no midline shift or mass effect (Figure 1). Axial fluid attenuated inversion recovery (FLAIR) demonstrated confluent foci of high signal in the peri-ventricular white matter, more prominent posteriorly on both frontoparietal slices (Figure 1), and a small region of the pons adjacent to the aqueduct. The FLAIR image of a HIV-seropositive control subject without neurocognitive impairment is offered for comparison, which suggests very mild white matter edema that could portend future impairment (Figure 1). A schematic sample spectrograph is presented in Figure 2 to display the peaks attributed to different compounds (notably NAA, Cho, Gln and MI). Note that two or more areas of the spectrograph contribute to each compound except MI and water, both of which are broad. Hence, quantitation of peaks alone may not be sufficient to represent the concentration of the compounds in the region.
Figure 1. Structural neuroimaging in mild neurocognitive disorder.
MRI images from the MND patient described in the text (top row) and comparison images from a seropositive control (bottom row). Slices intersecting the pons and lateral ventricles are shown. T2-weighted FLAIR images (left panels) demonstrate periventricular hyperintense white matter lesions that are pronounced in the MND patient. MP-RAGE images (right panels) show these lesions as hypointense due to T1-weighting. Both FLAIR and MP-RAGE also show pronounced ventricular enlargement in the MND patient that is presumably the result of brain atrophy.
FLAIR: Fluid attenuated inversion recovery; MND: Mild neurocognitive disorder;
MP-RAGE: Magnetization prepared rapid acquisition gradient echo.
Figure 2. Sample magnetic resonance spectroscopy spectrograph from an HIV-seronegative control subject.
This is a sample spectrograph obtained from a 3.0 cm3 voxel of brain tissue centered on the left hippocampus of an HIV-seronegative control subject from this study. It is a short echo time (echo time = 30), localized, single-voxel spectrograph showing proton magnetic resonance signals from various metabolites and neurotransmitters. Signal location assignments are given above the spectrograph.
Cho: Choline; MI: Myoinositol; NAA: N-acetyl aspartate; NAAG: N-acetyl-aspartyl glutamate.
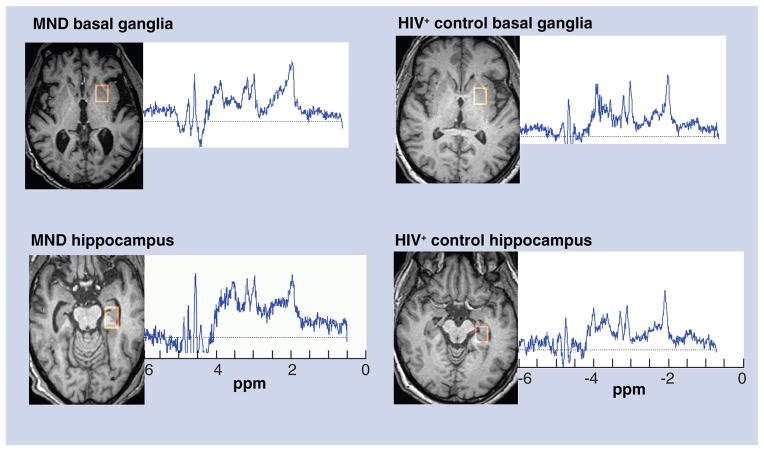
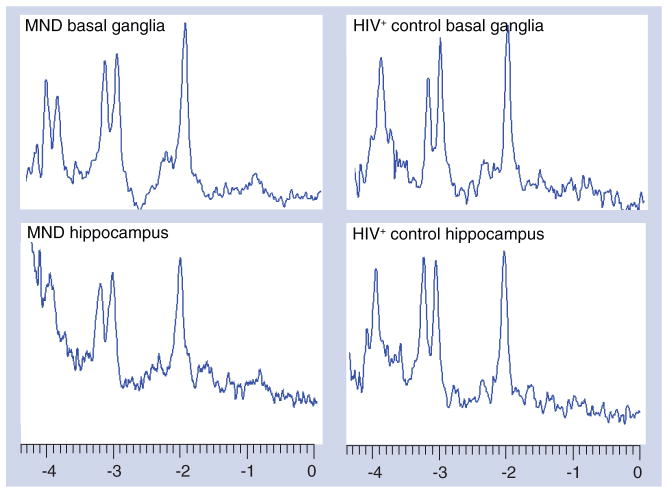
The MRS of the basal ganglia shows a pattern of a slightly decreased NAA peak with an elevated Gln peak, as well as elevated Cho and MI peaks – consistent with MND (Figure 3). A similar pattern was evident in the left hippocampus, with the exception that the NAA peak was not decreased (Figure 3). A newer approach to Glu MRS detection involves obtaining multiple spectra from a particular brain region using different echo times (TE; i.e., a multiple TE [MTE] acquisition) and then averaging the spectra [12]. For the basal ganglia, MTE averages suggested no difference in the NAA peaks between the MND and HIV-positive control subject, while the other single-voxel results were maintained (Figure 4). For the hippocampus, the MTEs showed a decrease in the NAA peak and increased Gln and MI peaks, but no increase in the Cho peak for the MND subject. It has been suggested that different MRS measures may be complementary to one another, such as single-voxel MRS and MRS imaging [13]. Combinations of different types of MRS data together with FLAIR have been used in this report, which suggests that this may be a useful combination with which to diagnose HAND in a proof-positive manner in the future. If verified by future work, the combination of MRS data with FLAIR could offer a sizable benefit in the reduction of labor-intensiveness and in the economic costs involved in the current diagnosis of HAND.
Figure 3. Standard single voxel (single echo time) magnetic resonance spectra.
A short echo time (echo time = 30), localized, single-voxel spectrograph from the MND patient and one from a HIV-seropositive control subject without neurocognitive impairment are presented. The locations which produced the spectra (basal ganglia and hippocampus) are outlined in red on the images to the left of each spectrograph. MND:
Mild neurocognitive disorder.
Figure 4. Multiple echo time magnetic resonance spectroscopy spectra.
This figure shows data obtained using multiple echo times that are averaged from the basal ganglia and hippocampus – for both the subject with MND and the HIV-seropositive control subject without neurocognitive impairment.
MND: Mild neurocognitive disorder.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Disclaimer
The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute of Mental Health or the National Institute of Biomedical Imaging and Bioengineering.
Informed consent
Subjects were recruited at the Cedars-Sinai Medical Center/UCLA (CA, USA). This protocol was approved by the local institutional review board at both institutions, and the subjects provided their written informed consent for the collection and use of MRI images and magnetic resonance spectroscopy data.
Financial & competing interest disclosure
This work has been supported by the NIH grants MH 75658 to K Goodkin and EB 00822 to AA Maudsley. The authors have no other relevant affiliations of financial involvement in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was used in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.McArthur JC, Brew B. HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. 2010;24 (9):1367–1370. doi: 10.1097/QAD.0b013e3283391d56. [DOI] [PubMed] [Google Scholar]
- 2.Neuenburg JK, Brodt HR, Herndier BG, et al. HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(2):171–177. doi: 10.1097/00126334-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 3▪.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders (HAND) Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. Sets forth the revision of the historical HIV-associated neurocognitive disorder criteria for the era of effective antiretroviral therapy, and added the new condition of ‘asymptomatic neurocognitive impairment’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodkin K, Fernandez F, Forstein F, et al. A perspective on the proposal for neurocognitive disorder criteria in DSM-5 as applied to HIV-associated neurocognitive disorders (HAND) Neuropsychiatry. 2011;1(5):431–440. doi: 10.2217/npy.11.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪▪.Chang L, Lee PL, Yiannoutsos CT, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23(4):1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. HIV neuroasymptomatic subjects had higher myoinositol. Dementia was associated with further increased myoinositol and with increased choline and decreased N-acetyl aspartate. [DOI] [PubMed] [Google Scholar]
- 6.Goodkin K, Aronow A, Baldwin G, et al. Neurocognitive disorders in the HAART era. In: Goodkin K, Shapshak P, Verma A, editors. The Spectrum of Neuro-AIDS Disorders: Pathophysiology, Diagnosis, and Treatment. American Society for Microbiology (ASM) Press; WA, USA: 2009. pp. 3–27. [Google Scholar]
- 7.Rothman DL, Sibson NR, Hyder F, et al. In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate-glutamine neurotransmitter cycle and functional neuroenergetics. Philos Trans R Soc Lond B: Biol Sci. 1999;354:1165–1167. doi: 10.1098/rstb.1999.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimmich GA, Roussie JA, Randles J. Aspartate aminotransferase isotope exchange reactions: implications for glutamate/glutamine shuttle hypothesis. Am J Physiol Cell Physiol. 2002;282 (6):1404–1413. doi: 10.1152/ajpcell.00487.2001. [DOI] [PubMed] [Google Scholar]
- 9.Shank RP, Campbell GL. Alpha-ketoglutarate and malate uptake and metabolism by synaptosomes: further evidence for an astrocyte-to-neuron metabolic shuttle. J Neurochem. 1984;42(4):1153–1161. doi: 10.1111/j.1471-4159.1984.tb12724.x. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan R, Sailasuta N, Hurd R, et al. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3T. Brain. 2005;128:1016–1025. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- 11.Letendre S, FitzSimons C, Ellis R, et al. Correlates of CSF viral loads in 1221 volunteers of the CHARTER cohort. Neuropathogenesis: clinical correlates and impact of ART. Presented at: the 17th Annual Conference on Retrovirology and Opportunistic Infections (CROI); San Francisco, CA, USA. 16–19 February 2010. [Google Scholar]
- 12.Hurd R, Sailasuta N, Srinivasan R, et al. Measurement of brain glutamate using TE-averaged PRESS at 3T. Mag Res Med. 2004;51(3):435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- 13▪▪.Sacktor N, Skolasky RL, Ernst T, et al. A multicenter study of two magnetic resonance spectroscopy techniques in individuals with HIV dementia. J Mag Res Imaging. 2005;21(4):325–333. doi: 10.1002/jmri.20272. This study showed that the combination of standard single-voxel short TE magnetic resonance spectroscopy measures and brother regional coverage by magnetic resonance spectroscopy imaging might be useful. [DOI] [PubMed] [Google Scholar]