Abstract
Background
This study aimed to identify the main risk factors for development of retinopathy of prematurity (ROP) in neonatal intensive care units in Alexandria, Egypt, from January 2010 to January 2012.
Methods
A prospective cohort study was undertaken in infants weighing < 1250 g and maternal postmenstrual age < 32 weeks if there was concern about prolonged exposure to oxygen. The main clinical outcomes were occurrence of any stage of ROP and in particular severe ROP. Perinatal variables considered were: birth weight, gestational age, gender, method of ventilation (nasal continuous airway pressure or intermittent mechanical ventilation), packed red blood cell and/or plasma transfusion, occurrence of sepsis, neonatal indirect hyperbilirubinemia, intraventricular hemorrhage, and patent ductus arteriosus. After obtaining informed consent from the parents, infants at risk were examined for ROP using indirect ophthalmoscopy, ie, RetCam II fundus photography.
Results
The study included 152 infants of mean gestational age 31.02 weeks and mean birth weight 1.229 kg. Seventy-two cases (47.5%) were male and 80 cases (52.5%) were female. Of the cases screened, 100 (65.6%) had no ROP, 52 had ROP of any stage (34.4%), and 27 (18%) had stage 1, five (3.3%) had stage 2, 17 (11.5%) had stage 3, and three (1.6%) had stage 4 disease. No infants had stage 5 ROP. Of all our cases with ROP, 15 (28.6%) had prethreshold disease type 1 that required treatment, comprising 9.8% of all cases screened for ROP. Using stepwise logistic regression analysis, all risk factors studied were found to be significantly associated with the development of ROP, except for neonatal indirect hyperbilirubinemia. Severity of ROP was inversely proportional to birth weight and gestational age.
Conclusion
ROP occurred in 34.4% of all infants screened in the neonatal intensive care units at three obstetric hospitals in Alexandria. The main risk factors for development of threshold ROP by regression analysis were low birth weight, gestational age, method of ventilation, need for packed red blood cell and/or plasma transfusion, occurrence of sepsis, intraventricular hemorrhage, and patent ductus arteriosus but not neonatal indirect hyperbilirubinemia. We suggest that both immaturity and compromised pulmonary function are both important etiological factors in the development of ROP.
Keywords: retinopathy of prematurity, premature infant, neonatal intensive care
Introduction
Retinopathy of prematurity (ROP) is characterized by abnormal vascular development of the retina in premature infants.1 Recent advances in neonatal care have improved the survival rates for premature infants,2 and this has been accompanied by an increase in the incidence of ROP.3–5
ROP is a leading cause of blindness in children,6,7 and accounts for up to 10% of childhood blindness in developed countries.8–10 The pathogenesis of ROP involves vasoconstriction of immature retinal vessels as a result of elevated oxygen levels from supplemental oxygenation.11
The stages of ROP are classified according to the International Classification of Retinopathy of Prematurity.12,13 In stage 1, a demarcation line can be found separating the avascular retina anteriorly from the vascularized retina posteriorly, with abnormal branching of the immediately posterior small vessels; in stage 2, there is a retinal ridge which remains intraretinal; stage 3 shows a ridge with extraretinal fibrovascular proliferation; in stage 4, there is partial retinal detachment; and in stage 5, total retinal detachment can be seen.16 Prethreshold ROP refers to zone 1 ROP of any stage less than threshold; zone 2, stage 2 ROP or greater; zone 2, stage 3 with no plus disease; and zone 2, stage 3 ROP or greater, with fewer than the threshold number of sectors of stage 3 or greater. Threshold severity of ROP is defined as five or more contiguous or eight cumulative clock hours of stage 3 plus ROP in zone 1 or 2. ROP stage identified by the ICROP to have a plus categorization i.e. a plus disease, represents dilatation and tortuosity of blood vessels in the posterior pole.12
An international group of pediatric ophthalmologists and retinal specialists has developed a consensus document that revises some aspects of the International Classification of Retinopathy of Prematurity. The aspects that differ from the original classification include introduction of: the concept of a more virulent form of retinopathy observed in the tiniest babies (aggressive posterior ROP); description of an intermediate level of plus disease (pre-plus) between normal posterior pole vessels and frank plus disease; and a practical clinical tool for estimating the extent of zone 1.13
Several risk factors have been found to be associated with the development of ROP. Of these, lower birth weight, younger gestational age, apnea, blood transfusion, mechanical ventilation, receiving sodium bicarbonate for correction of metabolic acidosis, total parenteral nutrition, intraventricular hemorrhage, and sepsis have been studied. However, with stepwise logistic regression analysis, only birth weight, gestational age, and total parenteral nutrition have been found to be independently associated with development of ROP.14
In this study, we investigated relevant perinatal variables in infants weighing ≤ 1500 g or gestational age ≤ 32 weeks at birth. These included gender, method of ventilation (nasal continuous airway pressure or intermittent mechanical ventilation), packed red blood cell and/or plasma transfusion, occurrence of sepsis, neonatal indirect hyperbilirubinemia, intraventricular hemorrhage, and patent ductus arteriosus.
Materials and methods
The study was performed from January 2010 to January 2012 in infants at risk of ROP in three private hospitals at Alexandria. During this period, 152 infants at risk were examined for ROP, comprising 89% of the total infants at risk in these hospitals.
Eye examinations were performed for all infants who met the criteria set down in the guidelines by the Royal College of Ophthalmologists in 1995. Eligible infants were referred by the attending neonatologist according to the following guidelines: birth weight < 1250 g, maternal postmenstrual age < 32 weeks, and concern about prolonged exposure to oxygen.
The infants were examined at 6 weeks chronological age or 34 weeks postmenstrual age (postmenstrual age = gestational age at time of birth plus chronological age in weeks since birth), whichever was earlier. Fundus examination was done using an indirect ophthalmoscope with a 30 D lens and scleral indentation for examination of retinal periphery.
A history was taken from hospital records, including demographic data, name, gender, gestational age at time of birth and postmenstrual age, birth weight, and perinatal history (including occurrence of sepsis, supplemental oxygen therapy administration, type of respiratory support, and diagnosis of neonatal indirect hyperbilirubinemia, intraventricular hemorrhage, or patent ductus arteriosus).
For eyes with changes as a result of ROP, we classified the condition according to the International Classification of Retinopathy of Prematurity and took fundus pictures using a wide-field retinal camera (RetCam II 120°, Clarity Medical System Inc, Pleasanton, CA, USA) whenever possible. Determination of whether prethreshold disease requiring treatment was present was made according to the results of the Early Treatment for Retinopathy of Prematurity study.16 For eyes with ROP less than prethreshold disease, subsequent examination was scheduled until normal vascularization reached zone 3, progression to prethreshold disease requiring treatment, or death of the baby. Data were retrieved retrospectively and analyzed for medical and neonatal risk factors using logistic regression.
Results
Our cases had a mean gestational age of 31.02 ± 2.13 (range 25–35) weeks. To simplify the statistical analysis, we divided the cases into three groups, ie, ≤28 weeks (15 cases, 10%), 29–30 weeks (47 cases, 30.9%), and >30 weeks (90 cases, 59.2%).
The mean birth weight was 1.329 ± 0.280 (range 0.678–1.896) kg. We divided the cases into four groups, ie, ≤0.750 kg (six cases [3.3%], all of whom developed ROP), 0.751–1.000 kg (20 cases [13.1%]), 1.001–1.500 kg (109 cases [72.1%]), and >1.500 kg (17 cases [11.5%], none of whom developed ROP). Seventy-two cases (47.5%) were male and 80 cases (52.5%) were female. Twenty-seven male infants (37.9%) developed ROP, while 25 female infants (31.2%) developed the disease, and the difference was not statistically significant.
Of the screened cases, the 100 (65.6%) who had no ROP were of mean gestational age 32 weeks and mean birth weight 1.249 kg, and the 52 cases who had ROP (34.4%) and 27 cases (18%) who had stage 1 ROP (Figure 1) were of mean gestational age 30 weeks and mean birth weight 1.103 kg. Five cases (3.3%) who had stage 2 disease (Figure 2) were of mean gestational age 28 weeks and mean birth weight 0.935 kg, 17 cases (11.5%) who had stage 3 disease (Figure 3) were of mean gestational age 31 weeks and mean birth weight 1.192 kg, and three cases (1.6%) who had stage 4 disease were of mean gestational age 25 weeks and mean birth weight 0.700 kg. No infant had stage 5 ROP. All infants who developed ROP had a similar classification in both eyes regarding stage of disease, zone affected, and presence of plus disease.
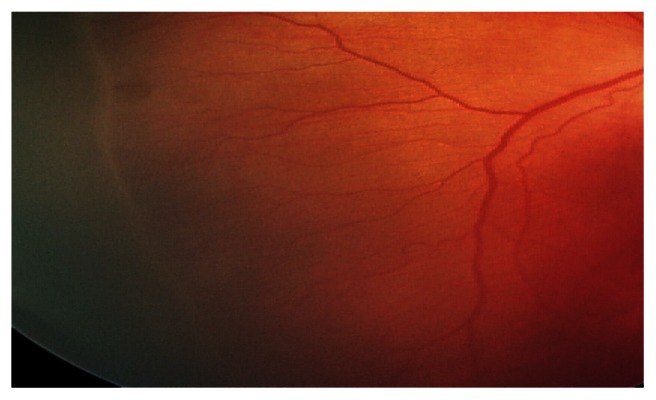
Figure 1.
Stage 1 zone 3 from a baby of birth weight 1200 g and gestational age 32 weeks, with a respiratory illness, neonatal indirect hyperbilirubinemia, and red blood cell transfusion.
Figure 2.
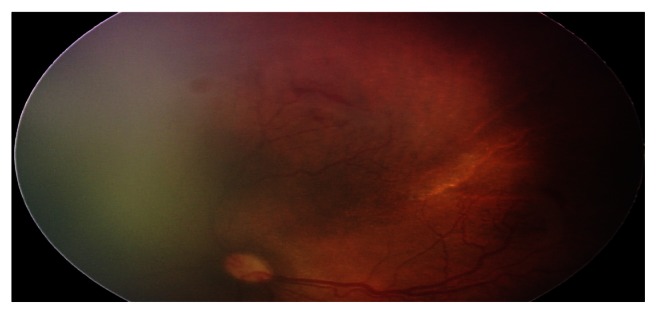
Prethreshold disease (OS stage 2, zone I, preretinal hemorrhages, plus sign).
Figure 3.
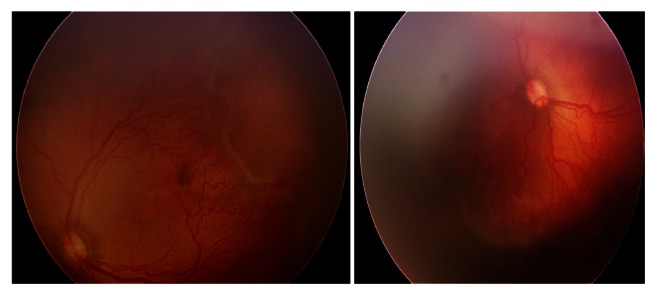
Threshold disease in a baby with OS stage 3, zone I disease, gestational age 30 weeks, and body weight 1000 g.
Note: Risk factors were respiratory distress syndrome, neonatal indirect hyperbilirubinemia, intraventricular hemorrhage, and nasal continuous positive airway pressure until day 5.
Preterm infants of younger gestational age had a significantly (P < 0.05) higher incidence of ROP (Table 1), and preterm infants with smaller birth weight had significantly (P < 0.05) higher incidence of ROP (Table 2).
Table 1.
Distribution of cases of different retinopathy of prematurity stages according to gestational age
| GA (weeks) | No ROP | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| ≤28 | 2 | 13.3 | 5 | 33.3 | 5 | 33.3 | 0 | 0 | 3 | 20 | 0 | 0 | 15 | 100 |
| 29–30 | 30 | 63.2 | 10 | 21 | 0 | 0 | 7 | 15.8 | 0 | 0 | 0 | 0 | 47 | 100 |
| >30 | 68 | 75.5 | 12 | 13.4 | 0 | 0 | 10 | 11.1 | 0 | 0 | 0 | 0 | 90 | 100 |
Abbreviations: GA, gestational age; ROP, retinopathy of prematurity.
Table 2.
Distribution of cases of different ROP stages according to birth weight (BW)
| No ROP | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||||||||
| No | % | No | % | No | % | No | % | No | % | No | % | No | % | |
| ≤0.750 kg | 1 | 16.7% | 2 | 33.3% | 0 | 0.0% | 0 | 0.0% | 3 | 50.0% | 0 | 0% | 6 | 100% |
| _1.000 kg | 7 | 35.0% | 8 | 40.0% | 3 | 15.0% | 2 | 10.0% | 0 | 0.0% | 0 | 0% | 20 | 100% |
| _1.500 kg | 75 | 68.8% | 17 | 15.6% | 2 | 1.8% | 15 | 13.8% | 0 | 0.0% | 0 | 0% | 109 | 100% |
| >1.500 kg | 17 | 100.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0% | 17 | 100% |
Abbreviations: GA, gestational age; ROP, retinopathy of prematurity.
With regard to risk factors related to young gestational age (Table 3), 95 babies had occurrence of sepsis, 48 (50.5%) of whom developed ROP and 15 (15.8%) required treatment for prethreshold type I ROP, indicating that sepsis was a significant risk factor for development of ROP including severe ROP requiring treatment (P < 0.041). Eighty-five babies had artificial ventilation by nasal positive airway pressure, 42 (49.4%) of whom had ROP changes and 15 (17.6%) required treatment for prethreshold type I ROP (P < 0.022). Thirtyeight babies required intermittent mechanical ventilation, 15 (40%) of whom developed ROP, and seven (18.5%) needed treatment for prethreshold type I ROP (P < 0.013). Therefore, ventilation by either method was a significant risk factor for development of ROP. Neonatal indirect hyperbilirubinemia was diagnosed in 48 babies, 20 (41.6%) of whom developed ROP versus 28 (58%) who did not, and five infants (10.5%) needed treatment for prethreshold type I ROP. Neonatal indirect hyperbilirubinemia was not considered to be a significant risk factor for development of ROP or severe ROP requiring treatment (P = 0.396).
Table 3.
Number and percentage of cases having risk factors for retinopathy of prematurity and disease severity
| Cases (n) | No ROP | ROP not requiring treatment | ROP requiring treatment | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| n | % | n | % | n | % | n | % | ||
| Sepsis | 95 | 62.3 | 47 | 49.47 | 33 | 34.7 | 15 | 15.8 | 0.041* |
| NCPAP | 85 | 55.7 | 43 | 50.5 | 27 | 31.7 | 15 | 17.6 | 0.022* |
| IMV | 38 | 24.6 | 23 | 60.5 | 8 | 21.0 | 7 | 18.5 | 0.013* |
| NIHB | 48 | 31.1 | 28 | 58 | 15 | 31.2 | 5 | 10.5 | 0.396 |
| PDA | 18 | 11.5 | 5 | 27.7 | 10 | 55.5 | 3 | 16.6 | 0.013* |
| IVH | 13 | 8.2 | 4 | 30.7 | 9 | 69.2 | 3 | 32.0 | 0.022* |
| RBC/plasma transfusion | 25 | 16.4 | 8 | 32.0 | 12 | 48.0 | 5 | 20.0 | 0.017* |
Abbreviations: NCPAP, nasal continuous positive airway pressure; RBC, red blood cell; IMV, intermittent mechanical ventilation; NIHB, neonatal indirect hyperbilirubinemia; ROP, retinopathy of prematurity.
Patent ductus arteriosus was found in 18 babies, all of whom were treated with indomethacin and none needed surgical ligation. Thirteen (72%) of these babies developed ROP, while five (27.7%) did not. Three (16.6%) babies needed treatment for prethreshold type I ROP. Patent ductus arteriosus was found to be a significant risk factor for development of ROP, including severe ROP requiring treatment (P < 0.013). Another significant risk factor was intraventricular hemorrhage (P < 0.022), documented in 13 babies, twelve (69.2%) of whom developed ROP. Three (23%) of the babies needed treatment for prethreshold type I ROP.
Twenty-five cases required packed red blood cell and/or plasma transfusion, 17 (68%) of whom developed ROP changes. Five (20%) needed treatment for prethreshold type I ROP. Either type of transfusion was a significant risk factor for ROP (P < 0.017).
Because the units of measurement for the variables included in this study were different, standardization of the coefficient was done to answer the question of which variables most strongly increased the risk of ROP in this multiple regression analysis. On analysis of the different potential risk factors for ROP (Table 4), it was found that the most significant associated risks were occurrence of sepsis, neonatal indirect hyperbilirubinemia, patent ductus arteriosus, and ventilation using nasal continuous airway pressure.
Table 4.
Multiple logistic regression analysis of different risk factors in relation to incidence of retinopathy of prematurity
| Model | Unstandardized coefficients | Standardized coefficients | t | Significance | |
|---|---|---|---|---|---|
|
|
|
||||
| B | SE | Beta | |||
| (Constant) | −0.169 | 0.112 | −1.503 | 0.139 | |
| Sepsis | 0.380 | 0.117 | 0.388 | 3.247 | 0.002* |
| NCPAP | 0.171 | 0.111 | 0.179 | 1.940 | 0.0430* |
| IMV | 0.024 | 0.126 | 0.021 | 0.188 | 0.852 |
| NIHB | 0.298 | 0.122 | 0.291 | 2.453 | 0.017* |
| PDA | 0.397 | 0.172 | 0.266 | 2.311 | 0.025* |
| IVH | 0.100 | 0.210 | 0.058 | 0.477 | 0.635 |
| RBC transfusion | 0.174 | 0.154 | 0.136 | 1.129 | 0.264 |
Note: Dependent variable is appearance of retinopathy of prematurity.
Abbreviations: SE, standard error; NCPAP, nasal continuous positive airway pressure; RBC, red blood cell; IMV, intermittent mechanical ventilation; NIHB, neonatal indirect hyperbilirubinemia; PDA, patent ductus arteriosus; IVH, intraventicular hemorrhage.
Discussion
ROP represents a major cause of preventable blindness. The research on this disease is still in its early stages. In the current study, the incidence of ROP was 34.4%, which is comparable with the incidence reported in other developing countries. The multicenter Cryotherapy for Retinopathy of Prematurity study15 reported a higher incidence of ROP of 65.8% in babies ≤ 1250 g and 81.6% in babies < 1000 g, while the Early Treatment Retinopathy of Prematurity study reported an incidence of 68%.16
Similar studies have been carried out in other developing countries. Bassiouny17 reported an incidence of 34% in Oman and Maheshwari et al18 reported an incidence of 27% in India. In other study from India, done in 1995, Charan et al19 reported an incidence of 47, 27%. In 2008, Binkhathlan et al20 reported an ROP incidence of 56% in Saudi Arabia, while Al-Amro et al21 reported an incidence of 37.4% in the neonatal intensive care unit at King Khalid University Hospital in Riyadh in 2003. Given that there is no previous similar research reporting the incidence of ROP in Alexandria, we could not compare the incidence of ROP at the present time with that reported in the past to show any trends over time.
The mean gestational age for the babies in the present study was 31.02 ± 2.13 weeks, which is higher than that in other relevant studies. Goble et al14 screened 1611 infants from six centers in Birmingham, UK, and reported a mean gestational age of 29.1 weeks, while Shah et al22 reported on babies with a mean gestational age of 29.7 weeks, which may reflect a higher mortality rate among infants of lower gestational age in our country.
It is clear that a higher incidence and more severe forms of ROP occurred in babies of younger gestational age (86.6% of infants aged < 28 weeks developed ROP versus 24.4% of those aged > 30 weeks) and smaller birth weight (100% of infants < 750 g versus no infants > 1500 g), which is consistent with other reports. The relatively older gestational age and higher birth weight in the group with stage 2 ROP may be explained by the small number of infants in that group.
ROP is a disease of very premature infants in highly developed countries, but in middle-income and developing countries, more mature and larger babies remain at risk of developing this blinding disease.23
None of the infants in our study showed a discrepancy in ROP stage or zone between the two eyes, which again is similar to findings in other studies. The evidence suggests that the rate of progression and severity of ROP between eyes in the same baby is closely related. In the Cryotherapy for Retinopathy of Prematurity study,15 the severity of ROP did not vary between eyes by more than one category in more than 90% of cases (using categories of 1, no ROP; 2, less than prethreshold; 3, prethreshold ROP; and 4, threshold ROP). Over 90% of cases had ROP in the same zone in both eyes. We also found a high degree of concordance between eyes with regard to plus disease.
In the current study, we investigated seven risk factors related to the development of ROP, including the severe form of the disease, ie, prethreshold type I or worse requiring treatment. These risk factors included sepsis, nasal continuous positive airway pressure, intermittent mechanical ventilation, neonatal indirect hyperbilirubinemia, intraventricular hemorrhage, patent ductus arteriosus, and red blood cell and/or plasma transfusion. We found that all these factors were significantly (P < 0.05) related to the occurrence of ROP and development of the severe form of the disease, except for neonatal indirect hyperbilirubinemia (P < 0.396), which coincides with the findings of other researchers.
Unfortunately, because of lack of sufficient data, we were not able to investigate other comorbidities found elsewhere to have a significant association with ROP,17 including bronchopulmonary dysplasia and necrotizing enterocolitis, and these need further study in our country.
In a prospective cohort study by Fortes Filho et al,24 467 infants weighing ≤ 1500 g or gestational age ≤ 32 weeks at birth were divided into three groups according to gestational age: group 1, comprising infants of gestational age ≤ 28 weeks at birth; group 2, comprising infants of gestational age 29–31 weeks at birth; and group 3, comprising infants of gestational age ≥ 32 weeks at birth. Mean birth weight and gestational age in the total cohort was 1216.5 ± 278.3 g and 30.3 ± 2.2 weeks, respectively. Only birth weight and volume of blood transfusion were identified to be significant risk factors for the occurrence of any stage of ROP in all three groups. Gestational age, twin status, and use of erythropoietin were statistically significant risk factors in group 1, and only gestational age and need for blood transfusion were statistically significant risk factors in group 2. In group 3, use of oxygen in mechanical ventilation, occurrence of sepsis, and need for blood transfusion were statistically significant risk factors for ROP. Logistic regression determined that patients in groups 2 and 3 were less likely to develop ROP than patients in group 1.
In a study reported by Shah et al,22 in which preterm infants were examined according to the guidelines published by the Royal College of Ophthalmologists and retinopathy was graded following the International Classification of ROP, the incidence of ROP among very low birth weight infants was 29.2%. ROP was more common in smaller, more immature, and sicker infants. The median age at onset of ROP was 35 (range 31–40) weeks postmenstrual age. Infants of gestational age < 30 weeks or birth weight < 1000 g were at considerable risk for threshold ROP. In that study, the main risk factors for development of threshold ROP by regression analysis were maternal pre-eclampsia, birth weight, and presence of pulmonary hemorrhage, duration of ventilation, and continuous positive pressure ventilation.
In the current study, no infants with birth weight > 1500 g developed ROP changes, while 25% of those with a gestational age > 30 weeks and having other risk factors developed ROP, the oldest of them had a gestational age of 33 weeks. Therefore, we recommend adopting a birth weight limit of 1500 g and gestational age of 33 weeks as the upper limit for an ROP screening protocol in our country. This is comparable with the screening protocols used in other countries.
We suggest that immaturity, low birth weight, occurrence of sepsis, and compromised pulmonary function requiring assisted ventilation, intraventricular hemorrhage, patent ductus arteriosus, and red blood cell and/or plasma transfusion are important risk factors for the development of ROP. Judicious use of ventilation and oxygen therapy is the only promising strategy available at the present time for reducing the incidence and severity of ROP in these high-risk infants.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chang SY, Shu JL, Feng LH, Wen MH, Jorn HL. Retinopathy of prematurity: screening, incidence and risk factor analysis. Chin Med J (Taipei) 2001;64:706–712. [PubMed] [Google Scholar]
- 2.Gong A, Anday E, Boros S, et al. American Exosurf Neonatal Study Group I. One-year follow-up evaluation of 260 premature infants with respiratory distress syndrome and birth weights of 700 to 1350 grams randomized to two rescue doses of synthetic surfactant or air placebo. J Pediatr. 1995;126:S68–S74. doi: 10.1016/s0022-3476(95)70010-2. [DOI] [PubMed] [Google Scholar]
- 3.Valentine PH, Jackson JC, Kalina RE, Woodrum DE. Increased survival of low birth weight infants: impact on the incidence of retinopathy of prematurity. Pediatrics. 1989;84:442–445. [PubMed] [Google Scholar]
- 4.Gibson DL, Sheps SB, Uh SH, Schechter MT, McCormick AQ. Retinopathy of prematurity-induced blindness: birth weight-specific survival and the new epidemic. Pediatrics. 1990;6:405–412. [PubMed] [Google Scholar]
- 5.Schalij-Delfos NE, Cats BP. Retinopathy of prematurity: the continuing threat to vision in preterm infants. Acta Ophthalmol Scand. 1997;75:72–75. doi: 10.1111/j.1600-0420.1997.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 6.Nissenkorn I, Wijsenbeek Y, Cohen S. Etiology of blindness in children in Israel in recent years. Acta Concilium Ophthalmologicum. 1987;25:742–744. [Google Scholar]
- 7.Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1999;3:26–32. doi: 10.1016/s1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 8.Goggin M, O’Keefe M. Childhood blindness in the Republic of Ireland: a national survey. Br J Ophthalmol. 1991;75:425–449. doi: 10.1136/bjo.75.7.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleck BW, Dangata Y. Causes of visual handicap in the Royal Blind School, Edinburgh, 1991–1992. Br J Ophthalmol. 1994;78:421. doi: 10.1136/bjo.78.5.421-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson DL, Sheps SB, Schechter MT, Wiggins S, McCormick AQ. Retinopathy of prematurity: a new epidemic? Pediatrics. 1989;83:486–492. [PubMed] [Google Scholar]
- 11.Patz A. Current concepts of the effects of oxygen on the developing retina. Curr Eye Res. 1984;3:159–163. doi: 10.3109/02713688408997197. [DOI] [PubMed] [Google Scholar]
- 12.An international classification of retinopathy of prematurity. Prepared by an International Committee. Br J Ophthalmol. 1984;68:690–697. doi: 10.1136/bjo.68.10.690. [No authors listed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 14.Goble RR, Jones HS, Fielder AR. Are we screening too many babies for retinopathy? Eye. 1997;11:509–514. doi: 10.1038/eye.1997.136. [DOI] [PubMed] [Google Scholar]
- 15.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1988;106:471–479. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 16.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 17.Bassiouny MR. Risk factors associated with retinopathy of prematurity: a study from Oman. J Trop Pediatr. 1996;42:355–358. doi: 10.1093/tropej/42.6.355. [DOI] [PubMed] [Google Scholar]
- 18.Maheshwari R, Kumar H, Paul VK, Singh M, Deorari AK, Tiwari HK. Incidence of retinopathy of prematurity in a tertiary care newborn unit in New Delhi. Natl Med J India. 1996;9:211–214. [PubMed] [Google Scholar]
- 19.Charan R, Dogra MR, Gupta A, Narang A. The incidence of retinopathy of prematurity in neonatal intensive care unit. Indian J Ophthalmol. 1995;43:123–126. [PubMed] [Google Scholar]
- 20.Binkhathlan AA, Almahmoud LA, Saleh MJ, Srungeri S. Retinopathy of prematurity in Saudi Arabia: incidence, risk factors, and the applicability of current screening criteria. Br J Ophthalmol. 2008;92:167–169. doi: 10.1136/bjo.2007.126508. [DOI] [PubMed] [Google Scholar]
- 21.Al-Amro SA, Al-Kharfi TM, Thabit AA, Al-Mofada SM. Retinopathy of prematurity at a university hospital in Riyadh, Saudi Arabia. Saudi Med J. 2003;24:720–784. [PubMed] [Google Scholar]
- 22.Shah VA, Yeo CL, Ling YL, Ho LY. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore. Ann Acad Med Singapore. 2005;34:169–178. [PubMed] [Google Scholar]
- 23.Gilbert C, Fielder A, Gordillo L, et al. International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate and high levels of development: implications for screening programs. Pediatrics. 2005;115:e518–e525. doi: 10.1542/peds.2004-1180. [DOI] [PubMed] [Google Scholar]
- 24.Fortes Filho JB, Eckert GU, Valiatti FB, Dos Santos PG, da Costa MC, Procianoy RS. The influence of gestational age on the dynamic behavior of other risk factors associated with retinopathy of prematurity (ROP) Graefes Arch Clin Exp Ophthalmol. 2010;248:893–900. doi: 10.1007/s00417-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]