Abstract
Background
Cytosine-phosphate-guanine (CpG) oligodeoxynucleotides activate Toll-like receptor 9, leading to induction of proinflammatory cytokines, which play an important role in induction and maintenance of innate and adaptive immune responses. Previously, we have used boron nitride nanospheres (BNNS) as a carrier for delivery of unmodified CpG oligodeoxynucleotides to activate Toll-like receptor 9. However, because CpG oligodeoxynucleotides and BNNS are both negatively charged, electrostatic repulsion between them is likely to reduce the loading of CpG oligodeoxynucleotides onto BNNS. Therefore, the efficiency of uptake of CpG oligodeoxynucleotides is also limited and does not result in induction of a robust cytokine response. To ameliorate these problems, we developed a CpG oligodeoxynucleotide delivery system using chitosan-coated BNNS as a carrier.
Methods
To facilitate attachment of CpG oligodeoxynucleotides onto the BNNS and improve their loading capacity, we prepared positively charged BNNS by coating them with chitosan preparations of three different molecular weights and used them as carriers for delivery of CpG oligodeoxynucleotides.
Results
The zeta potentials of the BNNS-CS complexes were positive, and chitosan coating improved their dispersity and stability in aqueous solution compared with BNNS. The positive charge of the BNNS-CS complexes greatly improved the loading capacity and cellular uptake efficiency of CpG oligodeoxynucleotides. The loading capacity of the CpG oligodeoxynucleotides depended on the molecular weight of chitosan, which affected the positive charge density on the surface of the BNNS. CpG oligodeoxynucleotides loaded onto BNNS-CS complexes significantly enhanced production of interleukin-6 and tumor necrosis factor-α by peripheral blood mononuclear cells compared with CpG oligodeoxynucleotides directly loaded onto BNNS, or when Lipofectamine™ 2000 was used as the carrier. The molecular weight of the chitosan used to coat the BNNS affected the magnitude of cytokine induction by varying the strength of condensation of the CpG oligodeoxynucleotides.
Conclusion
Although the loading capacity of BNNS coated with low molecular weight chitosan preparations was the lowest of all the preparations, they induced the highest levels of cytokines.
Keywords: chitosan, boron nitride nanospheres, CpG oligodeoxynucleotides, drug delivery, cytokines
Introduction
Increasing attention has been paid to immunotherapy using unmethylated cytosine-phosphate-guanine (CpG) dinucleotides.1 These CpG motifs are present in high frequency in bacterial DNA, and stimulate the mammalian innate immune system via activation of pattern-recognition receptor Toll-like receptor 9 (TLR9).1–6 Synthetic short, single-stranded oligodeoxynucleotides that contain CpG motifs are similar to those found in bacterial DNA and stimulate a similar immune response.2,7 Interaction of CpG oligodeoxynucleotides with TLR9 in antigen-presenting cells can activate two distinct signaling pathways in which two transcription factors, ie, NFκB and IRF-7, are translocated to the nucleus, leading to induction of genes encoding proinflammatory cytokines (eg, interleukin [IL]-6 and IL-12) and/or type I interferons (eg, interferon-α), respectively.8 These two types of cytokines play important roles in the induction and maintenance of innate and adaptive immune responses.9 Thus, CpG oligodeoxynucleotides have strong potential as improved vaccines as well as in other types of immunotherapies for allergy, cancer, and infectious diseases. Such modalities have been initiated in humans and numerous animal species.10–13 However, natural CpG oligodeoxynucleotides with phosphodiester backbones are not stable and are prone to nuclease degradation in serum, and much evidence indicates that the biological activity of CpG oligodeoxynucleotides is often transient.14 This severely limits their potential immunotherapeutic application.
Therefore, considerable efforts have been made to optimize their activity. One of the most effective methods is chemical modification of CpG oligodeoxynucleotides.14–16 Substituting the phosphodiester backbone with a phosphorothioate has better immunostimulatory effects due to increased resistance to nuclease degradation.15,16 However, there is concern regarding several severe adverse effects caused by modification of the DNA backbone.17 For example, repeated administration of backbone-modified CpG oligodeoxynucleotides has been associated with a reduced immune response, organ enlargement, and destruction of lymphoid follicles.18 Recently, researchers have focused on searching for formulations and delivery systems to improve the stimulatory activity of CpG oligodeoxynucleotides. Various particles, such as liposomes and inorganic nanoparticles, have been used as carriers to deliver unmodified CpG oligodeoxynucleotides because they improve the stability and efficiency of cellular uptake of CpG oligodeoxynucleotides.19–23
Boron nitride has received increasing attention because of its advantageous properties and structure that is analogous to that of carbon materials.24,25 These properties make boron nitrides suitable for potential applications, ranging from synthesis of composite materials to fabrication of electrical and optical devices.26,27 Further, boron nitrides show good biocompatibility, less toxicity, and more efficient uptake by cells than carbon.28,29 Ciofani et al investigated the cytocompatibility and magnetic properties of boron nitride nanotubes using neuroblastoma cells and demonstrated that these nanotubes are suitable as nanovectors for cell therapy and drug and gene delivery, as well as for other biomedical and clinical applications.30
In our previous study, we used 150 nm boron nitride nanospheres (BNNS) as a carrier for delivering unmodified CpG oligodeoxynucleotides to activate TLR9.31 These BNNS were not cytotoxic and protected unmodified CpG oligodeoxynucleotides from degradation by DNase. Further, BNNS were taken up into endolysosomes, which is particularly advantageous for enhancing TLR9 activity because TLR9 localizes to endolysosomes. However, because CpG oligodeoxynucleotides and BNNS are negatively charged, the electrostatic repulsion between them is likely to reduce the loading of CpG oligodeoxynucleotides onto BNNS. Therefore, the efficiency of CpG oligodeoxynucleotide uptake was also limited and a robust cytokine response was not induced.
To ameliorate these problems, we prepared chitosan-coated BNNS to deliver CpG oligodeoxynucleotides (Figure 1). Chitosan is a naturally occurring polysaccharide derived from chitin, which is composed of glucosamine and N-acetylglucosamine residues.32 Chitosan has beneficial properties, including biocompatibility, biodegradability, and low toxicity.33 In contrast with other biodegradable polymers, chitosan is the only one with cationic characteristics. These cationic characteristics allow chitosan to form stable complexes with many negatively charged nucleic acids via electrostatic interactions.34 Therefore, chitosan has been widely used in many drug and gene delivery systems. For example, Wu et al developed a novel immunoadjuvant by enveloping CpG oligodeoxynucleotides with chitosan nanoparticles and demonstrated that these nanoparticles significantly enhanced cellular and humoral immunity and resistance of mice to infection with Escherichia coli.35 Moreover, chitosan preparations are frequently used as a coating agent to adjust the surface charge of nanoparticles to enable them to bind negatively charged nucleic acid drugs.36
Figure 1.
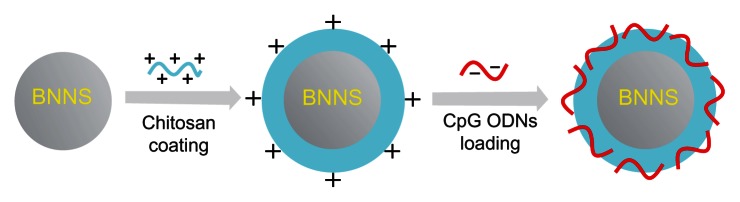
Preparation and application of chitosan-coated boron nitride nanospheres for delivering CpG oligonucleotides into cells.
Note: Negatively charged CpG oligodeoxynucleotides bound to positively charged chitosan-coated boron nitride nanospheres through electrostatic interactions.
Abbreviations: CpG, cytosine-guanine; ODNs, oligonucleotides.
The molecular weight of chitosan is an important characteristic that influences its physicochemical and biological properties and is thought to play an important role in its ability to form complexes with CpG oligodeoxynucleotides. This property affects the interaction of CpG oligodeoxynucleotides with TLR9. Therefore, we used chitosan preparations with three different molecular weights to coat the surface of BNNS and examined their effects on the loading of CpG oligodeoxynucleotides and on cytokine production. We hypothesized that coating BNNS with chitosan would enhance the loading capacity and cellular uptake of CpG oligodeoxynucleotides, leading to induction of robust cytokine synthesis.
Materials and methods
Preparation of BNNS-CS
Highly purified BNNS were synthesized using a chemical vapor deposition method, as previously reported.37 Chitosans with a molecular mass of 60–120 kDa (CS-L), 110–150 kDa (CS-M), and 140–220 kDa (CS-H) were purchased from Sigma-Aldrich (St Louis, MO, USA) and dissolved in 1% acetic acid to a concentration of 5 mg/mL. A working solution was prepared by diluting these stocks with Milli-Q water (Millipore, Bedford, MA, USA). BNNS (1 mg/mL) were suspended by sonication in Tris-HCl-buffered saline (50 mM HCl, 150 mM NaCl, pH 7.5) containing 0.1% Tween-20. For coating the surfaces, 500 μL BNNS solution was mixed with an equal volume of 0.05 mg/mL chitosan solution, and the suspension was stirred at room temperature for two hours. The chitosan-coated BNNS were then collected by centrifugation at 12,000 for 10 minutes, followed by five washes with Milli-Q water. The BNNS-CS complexes were finally resuspended in phosphate-buffered solution.
Characterization methods
Transmission electron microscopy was performed using a 3000F high-resolution field emission transmission electron microscope (JEOL, Tokyo, Japan) operated at an acceleration voltage of 300 kV. Fourier transform infrared spectra were obtained using a Spectrum GX spectrophotometer (PerkinElmer, Boston, MA, USA) at 4 cm−1 resolution with 32 scans. Zeta potential measurements were conducted using a zeta potential analyzer (LEZA-600, Otsuka Electronics, Hirakata, Japan). Dynamic light scattering measurements were acquired using a Photal DLA-6000DL system (Otsuka Electronics). Thermogravimetric analysis was conducted in an open aluminum cell placed in an SII TG/DTA 6200 system and heated at 5°C per minute.
Cell culture
A 293XL-hTLR9 cell line stably expressing human TLR9 was purchased from InvivoGen (San Diego, CA, USA) and grown in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich) containing 10% (v/v) fetal bovine serum, 50 units/mL penicillin, 50 mg/L streptomycin, 100 μg/mL normocin, and 10 μg/mL blasticidin. Frozen peripheral blood mononuclear cells were purchased from Cellular Technology Limited (Cleveland, OH, USA) and thawed according to the manufacturer’s protocol. Both cell lines were incubated at 37°C in an atmosphere of 5% CO2.
In vitro cytotoxicity assay
The in vitro cytotoxicity of the BNNS and BNNS-CS complexes was investigated using a Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). Ninety-six-well plates were seeded with 5000 cells for 24 hours to allow the cells to adhere, and the cells were then exposed to serial dilutions of BNNS, BNNS-CS complexes, or medium alone (control). After incubation at 37°C for 24 hours in an atmosphere of 5% CO2, 10 μL of Cell Counting Kit-8 solution was added to each well and the cells were incubated for another three hours. Absorbance at 450 nm was then measured using a microplate reader (MTP-880 Lab, Corona Electric, Ibaraki, Japan). Cytotoxicity was expressed as the percentage of viable cells compared with that of untreated control cells.
Preparation of CpG oligodeoxynucleotide-loaded BNNS-CS
Phosphodiester-based class B CpG oligodeoxynucleotides, referred to as CpG oligodeoxynucleotide 2006x3-PD,38 were purchased from Fasmac Inc (Kanagawa, Japan). The CpG oligodeoxynucleotides were diluted to 100 μM in sterile water and stored at −20°C. To prepare the BNNS-CS/CpG oligodeoxynucleotide complexes, 25 μL of CpG oligodeoxynucleotides (20 μM) was incubated with an equal volume of BNNS-CS (2 mg/mL in phosphate-buffered solution at pH 7.4) at room temperature with gentle shaking for one hour. The mixture was then centrifuged at 15,000 rpm for 15 minutes to collect the BNNS-CS/CpG oligodeoxynucleotide complexes. The loading capacity was calculated from the concentration of the unloaded CpG oligodeoxynucleotides in the supernatant; this concentration was measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific Fisher Inc, Waltham, MA, USA). The BNNS-CS/CpG oligodeoxynucleotide complexes were resuspended by adding 50 μL phosphate-buffered solution and used for the following cytokine stimulation experiments.
Cytokine assay
Peripheral blood mononuclear cells were seeded at a density of 5 × 106 cells/mL in RPMI-1640 medium supplemented with 10% fetal bovine serum. The cells were immediately exposed to CS/CpG oligodeoxynucleotides, BNNS/CpG oligodeoxynucleotides, or BNNS-CS/CpG oligodeoxynucleotide complexes. The final concentration of BNNS was approximately 50 μg/mL. For comparison, the cells were also stimulated with Lipofectamine™ 2000-CpG oligodeoxynucleotide complexes (Life Technologies, Carlsbad, CA, USA). After eight and 48 hours of incubation at 37°C, the cell supernatants were collected to determine concentrations of IL-6 and tumor necrosis factor (TNF)-α in the medium by an enzyme-linked immunosorbent assay using the Ready-SET-Go! Human IL-6 and TNF-α kits (eBioscience, San Diego, CA, USA), according to the manufacturer’s protocol.
Cellular uptake of BNNS-CS/CpG oligodeoxynucleotide complexes
Fluorescein isothiocyanate (FITC)-labeled CpG oligodeoxynucleotides were loaded onto BNNS or BNNS-CS to form BNNS/CpG oligodeoxynucleotides or BNNS-CS/CpG oligodeoxynucleotide complexes. The complexes were added to peripheral blood mononuclear cells (8 × 104) seeded in a 35 mm glass bottom Petri dish that had been incubated for 24 hours at 37°C in the presence of 5% CO2. After the cells had been incubated for 24 hours, they were washed twice with phosphate-buffered solution and fixed with 3.7% (v/v) paraformaldehyde. The fixed cells were visualized using an SP5 confocal laser scanning microscope (CLSM, Leica, Nussloch, Germany).
Intracellular localization of CpG oligodeoxynucleotides
The 293XL-hTLR9 cells (8 × 104) were seeded in a 35 mm glass bottom Petri dish and incubated for 24 hours at 37°C in the presence of 5% CO2. BNNS/FITC-CpG oligodeoxynucleotides and BNNS-CS/FITC-CpG oligodeoxynucleotide complexes were then added to the dish at a final concentration of 50 μg/mL. After the cells were incubated for 24 hours, they were washed twice with phosphate-buffered solution and fixed with 3.7% (v/v) paraformaldehyde. The samples were then blocked for one hour at room temperature with 3% bovine serum albumin in phosphate-buffered solution and stained with an anti-lysosome-associated membrane protein-1 (LAMP-1) antibody (Abcam, Cambridge, UK). The cells were visualized using the SP5 confocal laser scanning microscope, and the images were acquired sequentially using separate laser excitation to avoid any overlapping fluorophore signals.
Ethidium bromide displacement assay
The ethidium bromide (EB) displacement assay was used to determine the binding strength of the CpG oligodeoxynucleotides to chitosan preparations of different molecular weights by monitoring changes in the fluorescence intensity of EB bound to CpG oligodeoxynucleotides as a function of chitosan concentration. CpG oligodeoxynucleotides (100 μL, 10 μg/mL) were treated with 100 μL EB (1 μg/mL) for 10 minutes at room temperature and then with different concentrations of the chitosan preparations and incubated for two minutes; the fluorescence intensity at 570 nm was then measured using the microplate reader. The competitive binding constants (K) were calculated using the equation: I = I0 + K · [CS], where I0 and I are the fluorescence intensities in the absence and presence of the quencher, CS (chitosan), respectively, and [CS] represents the concentration of the chitosan preparation.
Statistical analysis
Statistical analysis was performed using the Student’s t-test. The data are presented as the mean ± standard deviation. Differences were considered to be statistically significant at P < 0.05.
Results and discussion
Preparation and characterization of BNNS-CS complexes
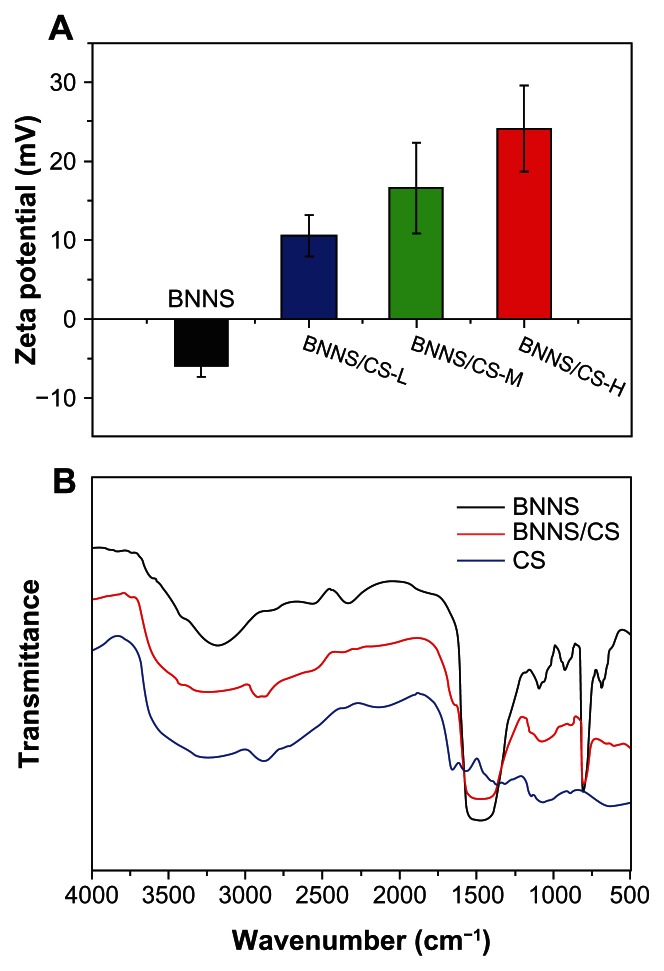
BNNS were synthesized using a chemical vapor deposition method as previously reported.37 They were spherical in shape, and the average size was approximately 150 nm (Figure 2). To obtain a positively charged surface and facilitate attachment of CpG oligodeoxynucleotides onto BNNS, we used chitosan to modify the charge of the BNNS because, to our knowledge, chitosan is the only biodegradable and biocompatible cationic polysaccharide. Figure 3A shows the zeta potentials of the BNNS and BNNS-CS complexes in phosphate-buffered solution, which changed after coating with chitosan. The zeta potential of pure BNNS was −5.98 ± 1.28 mV but became positive in the BNNS-CS complexes. This finding suggests that chitosan coated the surface of BNNS and changed the zeta potential. Further, the surface charge of the BNNS coated with chitosan varied as a function of the molecular weight of chitosan. Thus, the positive charge density increased with increasing molecular weight of chitosan (from 10.55 ± 2.57 mV for BNNS-CS-L complexes to 24.08 ± 5.39 mV for BNNS-CS-H complexes). We assume that these changes in zeta potential were caused by the presence of abundant amino groups in the high molecular weight chitosan preparations.
Figure 2.
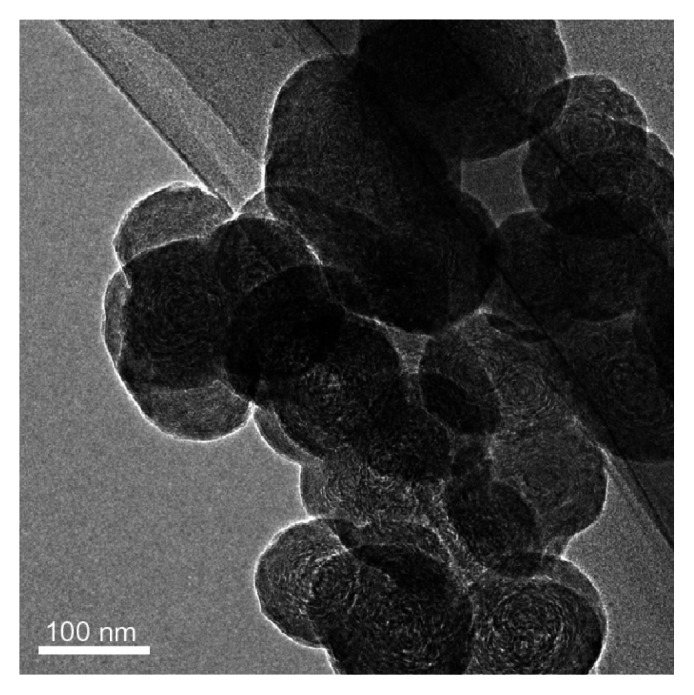
Transmission electron microscopic image of boron nitride nanospheres showing their spherical shape and average size of approximately 150 nm.
Figure 3.
Characterization of BNNS-CS complexes. (A) Zeta potentials in phosphate-buffered solution (pH 7.4) for BNNS and BNNS coated with chitosan preparations of different molecular weights. BNNS coated with chitosan have positively charged surfaces; this charge increases with their molecular weight. (B) Fourier transform infrared spectra of BNNS, CS, and BNNS-CS complexes.
Notes: BNNS-CS complexes clearly display the peaks assigned to both BNNS and CS, which confirmed the success of the coating.
Abbreviations: CS, chitosan; BNNS, boron nitride nanospheres; CS-L, chitosan with a molecular mass of 60–120 kDa; CS-M, chitosan with a molecular mass of 110–150 kDa; CS-H, chitosan with a molecular mass of 140–220 kDa.
To confirm further the presence of a chitosan layer on the BNNS, Fourier transform infrared spectra were determined, and Figure 3B shows bands at 2927 cm−1 (C–H stretch) and 1644 cm−1 (amide I band, C=O stretch of the acetyl group), which correspond to the characteristic stretching and bending vibrations, respectively, of chitosan.39 The BNNS spectrum showed a strong band at around 1400 cm−1, along with a less intense band at 780 cm−1, which are assigned to the (B-N) and (B-N-B) vibrations, respectively.37 These data show that the BNNS-CS complexes clearly have the peak characteristic of both BNNS and chitosan, and demonstrate the presence of chitosan in the BNNS-CS complexes, thereby confirming the success of coating. Therefore, we conclude that the negatively charged CpG oligodeoxynucleotides were able to bind electrostatically to positively charged BNNS-CS complexes. The size of biomaterials alters TLR9-mediated cytokine profiles because it affects their properties, such as the efficiency of cellular uptake and intracellular trafficking.9
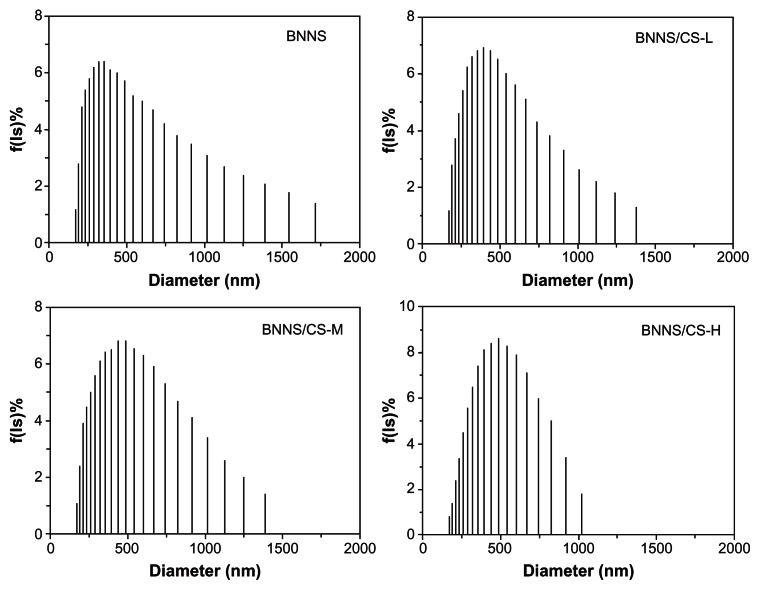
We next determined the size distributions of the BNNS with and without chitosan coating by dynamic light scattering. Compared with pure BNNS, which have an average diameter of approximately 360 nm, coating with chitosan on the surface may have resulted in partial aggregation of the BNNS without significantly increasing their size. The dynamic light scattering profiles showed major peaks centered around 393 nm, 438 nm, and 491 nm for the BNNS coated with CS-L, CS-M, and CS-H, respectively (Figure 4). This finding might be due to the relatively low concentration of chitosan included in the BNNS suspension.
Figure 4.
Size distribution of BNNS coated by chitosan preparations with different molecular weights was determined by dynamic light scattering.
Note: Coating with chitosan did not significantly increase the size of the BNNS.
Abbreviations: CS, chitosan; BNNS, boron nitride nanospheres; CS-L, chitosan with a molecular mass of 60–120 kDa; CS-M, chitosan with a molecular mass of 110–150 kDa; CS-H, chitosan with a molecular mass of 140–220 kDa.
The dispersity and stability of nanoparticles are crucial characteristics for their application as carriers in drug delivery systems. In our experiment, we observed that the chitosan coating significantly improved the dispersity and stability of BNNS in aqueous solution. Although the exact mechanism involved is unknown, interaction of the amino groups in chitosan with the BNNS surface may play an important role. Amino functional groups from chitosan adsorbed onto the BNNS surface led to good dispersion, and chitosan molecules can act as polycationic surfactants and improve the dispersion of the BNNS.40,41 Further, adsorption of the chitosan triggers entropic repulsion among the chitosan-coated BNNS and stabilizes their dispersion, which is expected to be the most likely stabilization mechanism.42 Therefore, the chitosan coating should enhance the ability of BNNS to serve as a carrier.
Cytotoxicity of BNNS-CS complexes
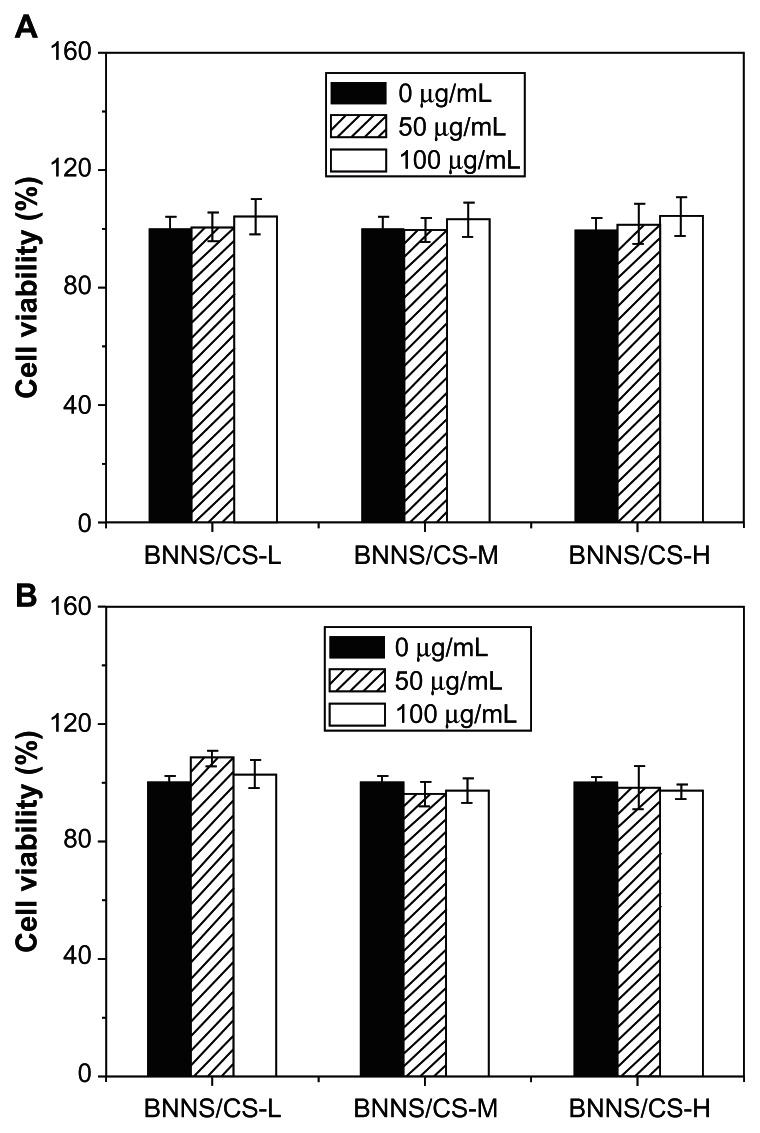
Investigation of the biological safety of drug delivery vehicles is critical for drug delivery systems.43 Although previous studies have demonstrated that BNNS and chitosan are biocompatible and suitable for drug delivery, it is still necessary to investigate the safety of the BNNS-CS complexes, because in the present study we used acetic acid to dissolve the chitosan preparations. Here, human peripheral blood mononuclear cells and 293XL-hTLR9 were used to study the in vitro cytotoxicity of BNNS-CS complexes measured using a water-soluble tetrazolium cell proliferation assay. Although high molecular weight chitosan preparations are associated with potential cytotoxicity, BNNS coated with chitosan of different molecular weights showed similar biocompatibility and no detectable cytotoxicity when added to cultures of human peripheral blood mononuclear cells or 293XL-hTLR9 cells (Figure 5). These results suggest that BNNS-CS complexes are safe and suitable as vehicles for drug delivery in vitro.
Figure 5.
Cytotoxicity of BNNS coated with chitosan preparations with different molecular weights. (A) Peripheral blood mononuclear cells. (B) 293XL-hTLR9 cells.
Notes: These BNNS did not show cytotoxicity up to a concentration of 100 μg/mL. The cells were incubated with increasing concentrations of BNNS-CS complexes, and cell viability was measured using a water-soluble tetrazolium salt assay. The data are presented as the mean ± standard deviation (n = 5).
Abbreviations: CS, chitosan; BNNS, boron nitride nanospheres; CS-L, chitosan with a molecular mass of 60–120 kDa; CS-M, chitosan with a molecular mass of 110–150 kDa; CS-H, chitosan with a molecular mass of 140–220 kDa.
BNNS-CS complexes as carriers for delivery of CpG oligodeoxynucleotides
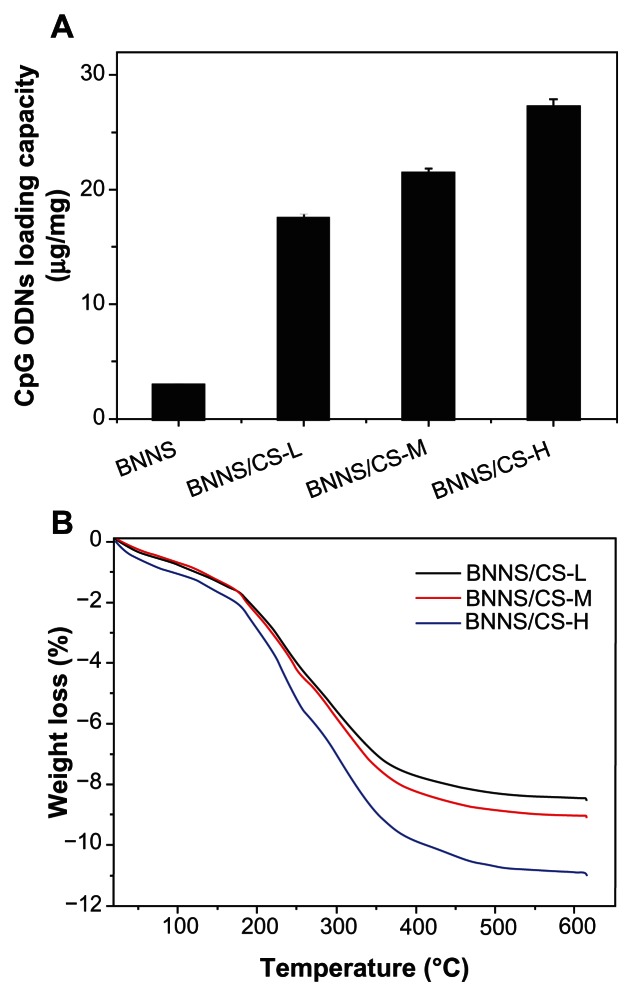
Next, we used BNNS-CS to bind CpG oligodeoxynucleotides so as to obtain BNNS-CS/CpG oligodeoxynucleotide complexes. The negatively charged phosphate groups of the CpG oligodeoxynucleotide backbone should interact with the amine group of chitosan and cause adsorption of CpG oligodeoxynucleotides onto BNNS-CS. As expected, the maximum loading capacity of CpG oligodeoxynucleotides dramatically increased for the BNNS-CS complexes. We found that approximately 17.5, 21.4, and 27.2 μg CpG oligodeoxynucleotides/mg nanoparticles bound to BNNS coated with CS-L, CS-M, and CS-H, respectively. These values are more than six times higher than those for CpG oligodeoxynucleotides loaded directly onto BNNS (Figure 6A). This result may be attributed to the positively charged BNNS surfaces obtained after chitosan coating; negatively charged CpG oligodeoxynucleotides could easily bind to BNNS-CS complexes via electrostatic interactions. Further, the maximum capacity for loading CpG oligodeoxynucleotides increased with increasing molecular weights of chitosan (Figure 6A) because the BNNS coated with higher molecular weight chitosan preparations had a higher positive charge density, allowing them to bind greater amounts of negatively charged CpG oligodeoxynucleotides. The amounts of chitosan coated on the surface of BNNS was investigated further by thermogravimetric analysis. Weight loss from the chitosan preparations was estimated as 8.5%, 9.1%, and 10.9% for CS-L, CS-M, and CS-H, respectively (Figure 6B). Therefore, the ratio of chitosan on the BNNS surface and the loading capacity of CpG oligodeoxynucleotides were similar for all three chitosan preparations. This finding suggests that the CS/BNNS coverage ratio is not involved in the loading capacity of CpG oligodeoxynucleotides.
Figure 6.
Analyses of the loading capacity of CpG oligodeoxynucleotides on BNNS coated with chitosan preparations of different molecular weights and amounts of chitosan coated on the surfaces of BNNS. (A) Loading capacity of CpG oligodeoxynucleotides on BNNS and BNNS-CS complexes (μg CpG oligodeoxynucleotides loaded on 1 mg BNNS). (B) Thermogravimetric analysis curves for BNNS coated with CS.
Note: The data are presented as the mean ± standard deviation (n = 3).
Abbreviations: CpG, cytosine-guanine; CS, chitosan; BNNS, boron nitride nanospheres; CS-L, chitosan with a molecular mass of 60–120 kDa; CS-M, chitosan with a molecular mass of 110–150 kDa; CS-H, chitosan with a molecular mass of 140–220 kDa.
The stability of electrostatically formed nanocomplexes is critical for their successful application. Therefore, the stability of the BNNS-CS/CpG oligodeoxynucleotide complexes was determined by incubating the complexes under conditions that correspond to the physiological environment in TLR9-localized endolysosomes. After 48 hours, release of CpG oligodeoxynucleotides from the BNNS-CS complexes was barely detectable (data not shown). This finding suggests that the BNNS-CS/CpG oligodeoxynucleotide complexes are very stable for at least 48 hours and that CpG oligodeoxynucleotides could not be released easily from the BNNS-CS complexes because of the stronger binding of the CpG oligodeoxynucleotides to BNNS-CS complexes.
The evidence indicates that intracellular drug delivery significantly influences the efficiency of a drug delivery system.14 After endocytosis, the CpG oligodeoxynucleotides in the endosome or lysosome can activate TLR9; this initiates synthesis of multiple cytokines and chemokines, which further modulates the immune response. Therefore, enhancement of cellular uptake of CpG oligodeoxynucleotides is vital for inducing more effective immune responses.
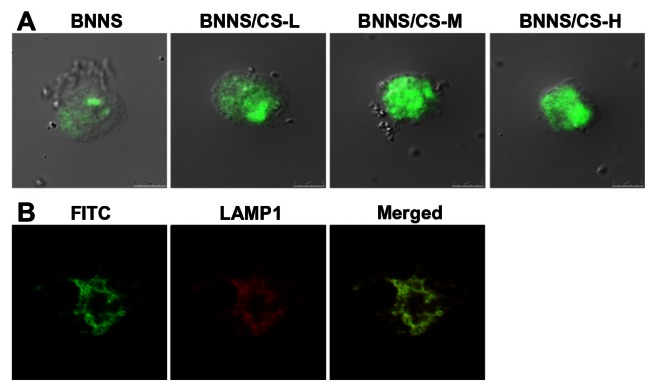
To study the cellular uptake efficiency of CpG oligodeoxynucleotides using BNNS-CS complexes as carriers, FITC-labeled CpG oligodeoxynucleotides were loaded onto BNNS or BNNS-CS to form BNNS/CpG oligodeoxynucleotides or BNNS-CS/CpG oligodeoxynucleotide complexes. The complexes were then incubated with peripheral blood mononuclear cells for 24 hours and observed using CLSM. Green fluorescence emitted by the BNNS/CpG oligodeoxynucleotides and BNNS-CS/CpG oligodeoxynucleotides was observed in the cells (Figure 7A). Further, under the same conditions, the fluorescence intensity emanating from the cells incubated with BNNS-CS/CpG oligodeoxynucleotide complexes was much stronger than that from cells incubated with BNNS/CpG oligodeoxynucleotide complexes; this finding indicates that higher levels of CpG oligodeoxynucleotides were internalized into the peripheral blood mononuclear cells when BNNS-CS complexes were used as carriers. However, no fluorescence was observed in cells incubated with free CpG oligodeoxynucleotides. Therefore, these findings indicate that the BNNS-CS complexes significantly enhanced the efficiency of cellular uptake of CpG oligodeoxynucleotides and could be expected to facilitate interaction of CpG oligodeoxynucleotides with TLR9.
Figure 7.
Cellular uptake and intracellular localization of CpG oligodeoxynucleotides. (A) Cellular uptake of CpG oligodeoxynucleotides delivered by BNNS and BNNS coated with CS-L, CS-M, and CS-H. (B) CpG oligodeoxynucleotides loaded onto BNNS-CS complexes were localized to LAMP-1-positive endosomes.
Notes: CpG oligodeoxynucleotides were labeled with FITC (green) and LAMP-1 antibodies were stained with PE-Cy5 (red) and imaged by confocal microscopy.
Abbreviations: CpG, cytosine-guanine; CS, chitosan; BNNS, boron nitride nanospheres; CS-L, chitosan with a molecular mass of 60–120 kDa; CS-M, chitosan with a molecular mass of 110–150 kDa; CS-H, chitosan with a molecular mass of 140–220 kDa; LAMP-1, lysosome-associated membrane protein-1; FITC, fluorescein isothiocyanate.
We next investigated the intracellular localization of CpG oligodeoxynucleotides using the BNNS-CS complexes as carriers. LAMP-1 was chosen to identify the endolysosome vesicles in 293XL-hTLR9 cells. Using confocal microscopy, we observed that CpG oligodeoxynucleotides loaded onto BNNS-CS complexes were localized in LAMP-1-positive endolysosome (Figure 7B). These results suggest that chitosan-coated BNNS are efficient carriers for intracellular delivery of CpG oligodeoxynucleotides. Therefore, we expect that delivery of CpG oligodeoxynucleotides to endolysosomes will lead to activation of cell signaling pathways, such as that mediated by NFκB, and will amplify induction of proinflammatory cytokines.
Cytokine induction by BNNS-CS/CpG oligodeoxynucleotide complexes
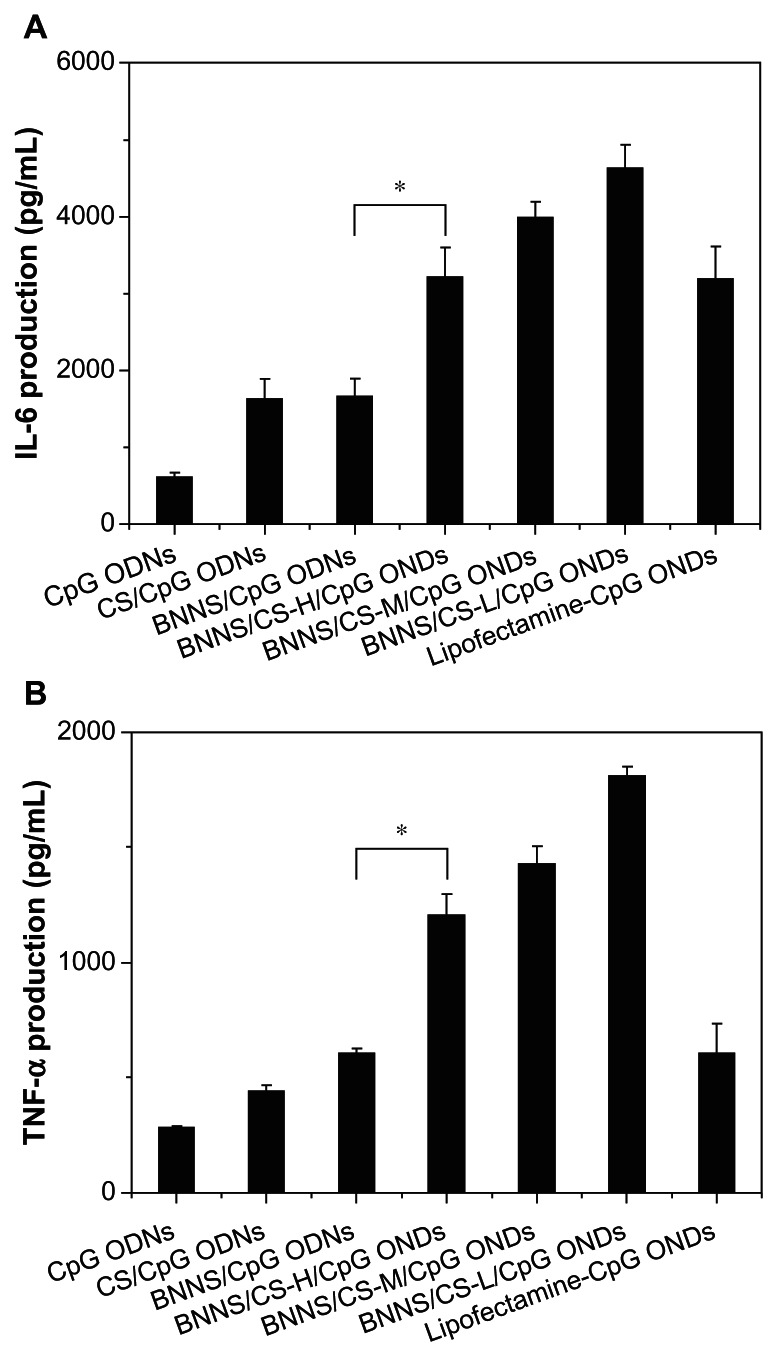
Figure 8 shows the levels of cytokines induced by treating peripheral blood mononuclear cells with CpG oligodeoxynucleotides delivered by BNNS and BNNS coated by chitosan preparations with different molecular weights. As expected, BNNS-CS/CpG oligodeoxynucleotide complexes induced much higher levels of IL-6 (>3226 pg/mL) and TNF-α (>1205 pg/mL) than CpG oligodeoxynucleotides loaded directly onto BNNS (1666 pg/mL and 605 pg/mL, respectively). This finding can be attributed to the higher loading capacity and enhanced cellular uptake of CpG oligodeoxynucleotides using BNNS-CS complexes as the carrier. When we used a BNNS-binding peptide as a linker molecule for CpG oligodeoxynucleotide delivery in a previous study, it improved the loading capacity of CpG oligodeoxynucleotides onto BNNS and significantly enhanced cytokine production.44
Figure 8.
Cytokine induction in peripheral blood mononuclear cells stimulated with CpG ODNs loaded onto BNNS and BNNS-CS complexes (50 μg/mL). (A) IL-6 production and (B) TNF-α production.
Notes: The data are presented as the mean ± standard deviation (n = 3). *P < 0.05.
Abbreviations: CS, chitosan; BNNS, boron nitride nanospheres; CS-L, chitosan with a molecular mass of 60–120 kDa; CS-M, chitosan with a molecular mass of 110–150 kDa; CS-H, chitosan with a molecular mass of 140–220 kDa; CpG, cytosine-guanine; ODNs, oligodeoxynucleotides.
Further, although chitosan itself has been used frequently as a polymeric carrier in many drug and gene delivery systems, in the present study, BNNS-CS/CpG oligodeoxynucleotide complexes induced approximately two-fold higher amounts of cytokines than CS/CpG oligodeoxynucleotides because of the improved cellular uptake of CpG oligodeoxynucleotides. Although Lipofectamine 2000, a commercially available transfection reagent, enhances transfection efficiency, we have shown here that BNNS-CS/CpG oligodeoxynucleotide complexes induced even higher levels of cytokines than those achieved using Lipofectamine-CpG oligodeoxynucleotide complexes. Although the capacity for loading CpG oligodeoxynucleotides increased with increasing molecular weight of the chitosan preparations, BNNS coated with low molecular weight chitosan induced the highest levels of IL-6 and TNF-α production, which decreased when the molecular weight of the chitosan preparations increased.
To investigate further the effect of molecular weight on the chitosan preparations on induction of cytokines, we analyzed cellular uptake of FITC-labeled CpG oligodeoxynucleotides loaded onto BNNS coated with the three different chitosan preparations. Uptake of all the complexes was similar (Figure 7A), indicating that differences in cellular uptake cannot account for the effect of molecular weight. These findings suggest that the molecular weight of the chitosan preparations influenced the induction of cytokines. This finding is consistent with that of another study demonstrating that low molecular weight preparations of chitosan are more efficient for transfection than high molecular weight preparations.45
The molecular weight of the chitosan preparation plays an important role in the formation of complexes with CpG oligodeoxynucleotides and affects interaction of CpG oligodeoxynucleotides with TLR9. Ma et al reported that the binding affinity and binding constant of chitosan for plasmid DNA were significantly influenced by the molecular weight of the chitosan preparation, which increased by almost an order of magnitude with an increase from 7 kDa to 153 kDa in molecular weight.46 Danielsen et al found that chitosan could compact plasmid DNA into various well defined geometries, reflecting the strengths of various chitosan-DNA interactions, which were strongly dependent on the molecular weight of chitosan.47
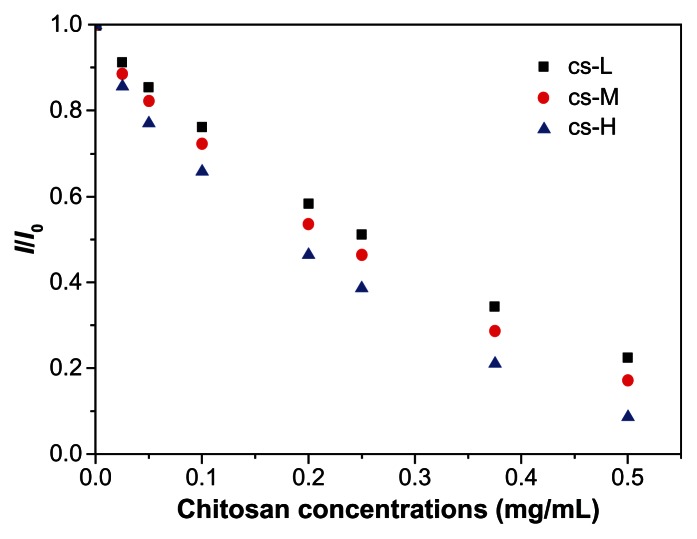
In this study we investigated the binding strength between CpG oligodeoxynucleotides and the chitosan preparations with three different molecular weights by using the EB displacement assay. The emission intensity of EB is greatly enhanced when it intercalates into DNA.48 Accordingly, adding a second DNA-binding ligand would quench EB emission through replacement of the DNA-bound EB because the ligand would have a stronger binding affinity for DNA than would EB.49 As shown in Figure 9, emission of DNA-bound EB reduced on addition of chitosan. The competitive binding constants (K) were approximately 105 M−1 and followed the order CS-H > CS-M > CS-L (Table 1). This finding clearly indicates that the higher molecular weight chitosan preparation has a stronger binding affinity for CpG oligodeoxynucleotides. Therefore, increased cytokine induction by BNNS coated with CS-L is likely caused by enhanced interaction of CpG oligodeoxynucleotides with TLR9, which can be attributed to the loose condensation of CpG oligodeoxynucleotides. In contrast, CS-M and CS-H may complex CpG oligodeoxynucleotides more tightly because of their higher positive charge density, thereby diminishing the interaction between CpG oligodeoxynucleotides and TLR9.
Figure 9.
Plot of I/I0 versus [CS] for analyzing fluorescence quenching by CpG oligodeoxynucleotide-EB using chitosan preparations with different molecular weights.
Notes: CS preparations with higher molecular weights had higher affinities for CpG oligodeoxynucleotides, which more strongly quenches the fluorescence of CpG oligodeoxynucleotides-EB.
Abbreviations: CpG, cytosine-guanine; CS, chitosan; BNNS, boron nitride nanospheres; CS-L, chitosan with a molecular mass of 60–120 kDa; CS-M, chitosan with a molecular mass of 110–150 kDa; CS-H, chitosan with a molecular mass of 140–220 kDa; EB, ethidium bromide.
Table 1.
Competitive binding constants for the interaction between chitosan and CpG oligodeoxynucleotides, determined by fluorescence quenching
| Chitosan | CS-L | CS-M | CS-H |
|---|---|---|---|
| K(M−1) | 3.3 × 105 | 4.8 × 105 | 7.2 × 105 |
Abbreviations: CpG, cytosine-guanine; K, competitive binding constants; CS-L, chitosan with molecular weights of 60–120 kDa; CS-M, chitosan with a molecular mass of 110–150 kDa; CS-H, chitosan with a molecular mass of 140–220 kDa.
Conclusion
We report here the preparation of chitosan-coated BNNS as a carrier for delivering class B CpG oligodeoxynucleotides into cells. The chitosan coating shifted the surface zeta potential of BNNS from negative to positive. The BNNS-CS complexes showed good dispersity and stability in aqueous solution when coated with chitosan. Compared with CpG oligodeoxynucleotides directly loaded onto BNNS, BNNS-CS complexes greatly improved the loading capacity and cellular uptake efficiency of CpG oligodeoxynucleotides because of their positive charge. The loading capacity of the CpG oligodeoxynucleotides depended on the molecular weight of the chitosan preparation, which affected the positive charge density on the surface of BNNS. CpG oligodeoxynucleotides loaded onto BNNS-CS complexes localized within LAMP-1-positive endolysosomes and significantly enhanced the production of IL-6 and TNF-α by peripheral blood mononuclear cells compared with CS/CpG oligodeoxynucleotide complexes and CpG oligodeoxynucleotides directly loaded onto BNNS. These cytokine levels significantly exceeded those detected when Lipofectamine 2000 was used as the carrier. We assume that this is caused by the higher loading capacity and enhanced cellular uptake of CpG oligodeoxynucleotides. Further, the molecular weight of the chitosan preparation used to coat the BNNS affected the induction of cytokines by varying the strength of condensation of the CpG oligodeoxynucleotides. Higher cytokine levels were detected for CpG oligodeoxynucleotides loaded onto BNNS coated with a lower molecular weight chitosan preparation, although its loading capacity was the smallest. We anticipate that these findings will open a new avenue for functionalization of BNNS as a carrier for delivery of nucleic acid therapies, such as CpG oligodeoxynucleotides and small interfering RNA.
Acknowledgment
This work was supported by Grants-in-Aid for Scientific Research (C-22560777, 23-01510) from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4(4):248–257. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 2.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 3.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61(3):195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 5.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–368. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 6.Bauer S, Kirschning CJ, Häcker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98(16):9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996;93(7):2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Chen HC, Sun B, Tran KK, Shen H. Effects of particle size on toll-like receptor 9-mediated cytokine profiles. Biomaterials. 2011;32(6):1731–1737. doi: 10.1016/j.biomaterials.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv Drug Delivery Rev. 2009;61(3):248–255. doi: 10.1016/j.addr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca DE, Kline JN. Use of CpG oligonucleotides in treatment of asthma and allergic disease. Adv Drug Deliv Rev. 2009;61(3):256–262. doi: 10.1016/j.addr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Murad YM, Clay TM. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic applications in cancer. BioDrugs. 2009;23(6):361–375. doi: 10.2165/11316930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 14.Mutwiri GK, Nichani AK, Babiuk S, Babiuk LA. Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides. J Control Release. 2004;97(1):1–17. doi: 10.1016/j.jconrel.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Kurreck J. Antisense technologies – improvement through novel chemical modifications. Eur J Biochem. 2003;270(8):1628–1644. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal S, Zhao QY. Antisense therapeutics. Curr Opin Chem Biol. 1998;2(4):519–528. doi: 10.1016/s1367-5931(98)80129-4. [DOI] [PubMed] [Google Scholar]
- 17.Sheehan JP, Lan HC. Phosphorothioate oligonucleotides inhibit the intrinsic tenase complex. Blood. 1998;92(5):1617–1625. [PubMed] [Google Scholar]
- 18.Heikenwalder M, Polymenidou M, Junt T, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10(2):187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Meng W, Li X, Gao H, Hanagata N. Design of mesoporous silica/cytosine-phosphodiester-guanine oligodeoxynucleotide complexes to enhance delivery efficiency. J Phys Chem C. 2010;115(2):447–452. [Google Scholar]
- 20.Wilson KD, de Jong SD, Tam YK. Lipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacy. Adv Drug Deliv Rev. 2009;61(3):233–242. doi: 10.1016/j.addr.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Bianco A, Hoebeke J, Godefroy S, et al. Cationic carbon nanotubes bind to CpG oligodeoxynucleotides and enhance their immunostimulatory properties. J Am Chem Soc. 2004;127(1):58–59. doi: 10.1021/ja044293y. [DOI] [PubMed] [Google Scholar]
- 22.Rattanakiat S, Nishikawa M, Funabashi H, Luo D, Takakura Y. The assembly of a short linear natural cytosine-phosphate-guanine DNA into dendritic structures and its effect on immunostimulatory activity. Biomaterials. 2009;30(29):5701–5706. doi: 10.1016/j.biomaterials.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 23.Standley SM, Mende I, Goh SL, et al. Incorporation of CpG oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific CD8 T-cell immunity. Bioconjug Chem. 2006;18(1):77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 24.Chopra NG, Luyken RJ, Cherrey K, et al. Boron-nitride nanotubes. Science. 1995;269(5226):966–967. doi: 10.1126/science.269.5226.966. [DOI] [PubMed] [Google Scholar]
- 25.Zhi CY, Bando Y, Tang CC, Xie RG, Sekiguchi T, Golberg D. Perfectly dissolved boron nitride nanotubes due to polymer wrapping. J Am Chem Soc. 2005;127(46):15996–15997. doi: 10.1021/ja053917c. [DOI] [PubMed] [Google Scholar]
- 26.Gao ZH, Zhi CY, Bando Y, Golberg D, Serizawa T. Isolation of individual boron nitride nanotubes via peptide wrapping. J Am Chem Soc. 2010;132(14):4976–4977. doi: 10.1021/ja910244b. [DOI] [PubMed] [Google Scholar]
- 27.Golberg D, Bando Y, Tang CC, Zhi CY. Boron nitride nanotubes. Adv Mater. 2007;19(18):2413–2432. [Google Scholar]
- 28.Chen X, Wu P, Rousseas M, et al. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J Am Chem Soc. 2009;131(3):890–891. doi: 10.1021/ja807334b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonsson E, Simonsen L, Karlsson M, Larsson R. The hollow fiber model: a new method for in vivo-evaluation of antitumor effect, toxicity and pharmacokinetics of new anticancer drugs. Ann Oncol. 1998;9:44. [Google Scholar]
- 30.Ciofani G, Raffa V, Menciassi A, Cuschieri A. Boron nitride nanotubes: an innovative tool for nanomedicine. Nano Today. 2009;4(1):8–10. [Google Scholar]
- 31.Zhi CY, Meng WJ, Yamazaki T, et al. BN nanospheres as CpG ODN carriers for activation of Toll-like receptor 9. J Mater Chem. 2011;21(14):5219–5222. [Google Scholar]
- 32.Agrawal P, Strijkers GJ, Nicolay K. Chitosan-based systems for molecular imaging. Adv Drug Deliv Rev. 2010;62(1):42–58. doi: 10.1016/j.addr.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Bernkop-Schnürch A, Dünnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 2012;81(3):463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Wu KY, Wu M, Fu ML, et al. A novel chitosan CpG nanoparticle regulates cellular and humoral immunity of mice. Biomed Environ Sci. 2006;19(2):87–95. [PubMed] [Google Scholar]
- 36.Ravi Kumar MNV, Bakowsky U, Lehr CM. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials. 2004;25(10):1771–1777. doi: 10.1016/j.biomaterials.2003.08.069. [DOI] [PubMed] [Google Scholar]
- 37.Tang CC, Bando Y, Huang Y, Zhi CY, Golberg D. Synthetic routes and formation mechanisms of spherical boron nitride nanoparticles. Adv Funct Mater. 2008;18(22):3653–3661. [Google Scholar]
- 38.Meng WJ, Yamazaki T, Nishida Y, Hanagata N. Nuclease-resistant immunostimulatory phosphodiester CpG oligodeoxynucleotides as human Toll-like receptor 9 agonists. BMC Biotechnol. 2011;11:88. doi: 10.1186/1472-6750-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soares DCF, Ferreira TH, Ferreira CA, Cardoso VN, de Sousa EMB. Boron nitride nanotubes radiolabeled with 99 mTc: preparation, physicochemical characterization, biodistribution study, and scintigraphic imaging in Swiss mice. Int J Pharm. 2012;423(2):489–495. doi: 10.1016/j.ijpharm.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Yan LY, Poon YF, Chan-Park MB, Chen Y, Zhang Q. Individually dispersing single-walled carbon nanotubes with novel neutral pH water-soluble chitosan derivatives. J Phys Chem C. 2008;112(20):7579–7587. [Google Scholar]
- 41.Ozarkar S, Jassal M, Agrawal AK. Improved dispersion of carbon nanotubes in chitosan. Fibers Polym. 2008;9(4):410–415. [Google Scholar]
- 42.Shvartzman-Cohen R, Levi-Kalisman Y, Nativ-Roth E, Yerushalmi-Rozen R. Generic approach for dispersing single-walled carbon nanotubes: the strength of a weak interaction. Langmuir. 2004;20(15):6085–6088. doi: 10.1021/la049344j. [DOI] [PubMed] [Google Scholar]
- 43.Zhu YF, Ikoma T, Hanagata N, Kaskel S. Rattle-type Fe(3)O(4)@SiO(2) hollow mesoporous spheres as carriers for drug delivery. Small. 2010;6(3):471–478. doi: 10.1002/smll.200901403. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Yamazaki T, Zhi C, Hanagata N. Identification of a boron nitride nanosphere-binding peptide for the intracellular delivery of CpG oligodeoxynucleotides. Nanoscale. 2012;4(20):6343–6350. doi: 10.1039/c2nr31189e. [DOI] [PubMed] [Google Scholar]
- 45.Strand SP, Lelu S, Reitan NK, Davies CD, Artursson P, Varum KM. Molecular design of chitosan gene delivery systems with an optimized balance between polyplex stability and polyplex unpacking. Biomaterials. 2010;31(5):975–987. doi: 10.1016/j.biomaterials.2009.09.102. [DOI] [PubMed] [Google Scholar]
- 46.Ma PL, Lavertu M, Winnik FM, Buschmann MD. New insights into chitosan-DNA interactions using isothermal titration microcalorimetry. Biomacromolecules. 2009;10(6):1490–1499. doi: 10.1021/bm900097s. [DOI] [PubMed] [Google Scholar]
- 47.Danielsen S, Vårum KM, Stokke BT. Structural analysis of chitosan mediated DNA condensation by AFM: influence of chitosan molecular parameters. Biomacromolecules. 2004;5(3):928–936. doi: 10.1021/bm034502r. [DOI] [PubMed] [Google Scholar]
- 48.Karidi K, Garoufis A, Tsipis A, Hadjiliadis N, den Dulk H, Reedijk J. Synthesis, characterization, in vitro antitumor activity, DNA-binding properties and electronic structure (DFT) of the new complex cis-(Cl,Cl) Ru(II)Cl2(NO+)(terpy) Cl. Dalton Trans. 2005;7:1176–1187. doi: 10.1039/b418838a. [DOI] [PubMed] [Google Scholar]
- 49.Dhar S, Nethaji M, Chakravarty AR. Steric protection of a photosensitizer in a N,N-bis 2-(2-pyridyl)ethyl -2-phenylethylamine-copper(II) bowl that enhances red light-induced DNA cleavage activity. Inorg Chem. 2005;44(24):8876–8883. doi: 10.1021/ic0505246. [DOI] [PubMed] [Google Scholar]