Abstract
Efforts have been made recently to implement nanoscale surface features on magnesium, a biodegradable metal, to increase bone formation. Compared with normal magnesium, nanostructured magnesium has unique characteristics, including increased grain boundary properties, surface to volume ratio, surface roughness, and surface energy, which may influence the initial adsorption of proteins known to promote the function of osteoblasts (bone-forming cells). Previous studies have shown that one way to increase nanosurface roughness on magnesium is to soak the metal in NaOH. However, it has not been determined if degradation of magnesium is altered by creating nanoscale features on its surface to influence osteoblast density. The aim of the present in vitro study was to determine the influence of degradation of nanostructured magnesium, created by soaking in NaOH, on osteoblast density. Our results showed a less detrimental effect of magnesium degradation on osteoblast density when magnesium was treated with NaOH to create nanoscale surface features. The detrimental degradation products of magnesium are of significant concern when considering use of magnesium as an orthopedic implant material, and this study identified a surface treatment, ie, soaking in NaOH to create nanoscale features for magnesium that can improve its use in numerous orthopedic applications.
Keywords: nanostructured magnesium, degradation, detrimental effects, osteoblasts
Introduction
The direct costs of treating orthopedic fractures are approximately 18 billion dollars per year in the US.1 These costs are high because initial treatment usually requires follow-up. For example, the initial cost for treating hip fractures ranges from $30,000 to $43,000, but the follow-up costs in the subsequent year can add an additional $14,600. Indirect costs include loss of productivity for patients and caregivers, and can amount to billions of dollars.2
Metals are frequently used as orthopedic implants because of their high strength and durability, which makes them suitable for load-bearing applications; however, a disadvantage of using metals is their lack of biodegradability, raising concern about lifelong toxicity.3 Many studies have reported on adverse effects caused by permanent metallic implants due to the ongoing release of metallic wear debris.4,5 Specifically, Böhler et al examined alumina-blasted cementless titanium-based hip implants with various cobalt alloy bearings and found that there were significant increases in the concentrations of cobalt, chromium, titanium, and aluminum in the tissue surrounding the implant.5 In addition, implants in loose sockets allowed alumina particles to accumulate, resulting in early implant failure and pain due to bone necrosis.
As a result of the aforementioned concerns, there has been mounting interest in the use of biodegradable metals such as magnesium, which is the fourth most abundant mineral in the body, in orthopedic implants.6 Clearly, wear debris created from a degradable metal will not represent a persistent source of toxicity, and magnesium plays a major role in human health. Approximately 45% and 5% of magnesium circulates in intracellular and extracellular fluid, respectively, making it the most abundant intracellular cation in the body.6 As a cation, magnesium is a cofactor for over 302 enzymatic reactions and is essential for the synthesis of proteins, antioxidants, carbohydrates, and nucleic acids.7–10 Of extreme importance is its role in maintaining genomic stability. In addition to stabilizing the structure of DNA and RNA, magnesium is a cofactor in the repair process to remove DNA damage caused by external mutagens. during replication.10 Because of the major role played by magnesium in these metabolic pathways, magnesium deficiency causes an increase in susceptibility to oxidative stress.9
Magnesium ions are also needed for the transport of potassium and calcium ions,9 which are minerals essential for bone growth. High levels of magnesium cations ensure adequate levels of calcium in the blood to prevent excessive bone resorption.9 In fact, studies have shown that a magnesium- restricted diet significantly reduces the levels of serum magnesium and osteocalcin, a protein that affects bone formation. 11 Furthermore, low magnesium levels increase bone resorption, as seen by elevated levels of parathyroid hormone and deoxypyridinoline in rats with a magnesium- restricted diet.12 These factors result in a decrease in bone mass and bone strength. Similarly, Toba et al showed that magnesium supplementation promotes bone formation (increases in osteocalcin) and prevents bone resorption (decreases in deoxypyridinoline and parathyroid hormone).13 They also found a reduction in calcium adsorption, which supports the decrease in bone resorption since lower levels of serum calcium stimulate the release of parathyroid hormone.13
Given that magnesium is a mineral essential for bone growth, it can be expected that as a biomaterial it would support bone growth. The impact magnesium has on bone growth was shown further by Hussain et al, who found that adding magnesium calcium phosphate into gelatin sponges containing bone marrow mesenchymal stem cells promoted proliferation and osteogenic differentiation of these cells, and was reflected by higher levels of alkaline phosphatase activity than those seen in sponges containing no magnesium calcium phosphate.15 Magnesium has also been shown to have mechanical properties suitable for several orthopedic applications.9
However, there are some disadvantages to the use of magnesium as a biodegradable orthopedic implant. Specifically, magnesium degrades in aqueous environments according to the following electrochemical reaction to produce magnesium hydroxide and hydrogen:16
| (1) |
Equations 2–4 show the partial reactions:
| (2) |
| (3) |
| (4) |
This hydrogenation results in an alkaline environment that raises the pH, which is harmful to cells and decreases cell viability.16,17 A major research focus is how to slow down the initial corrosion rate during the first 48 hours of implantation in order to allow bone to form on the magnesium which will help to decrease the corrosion process.18–20
Along these lines, efforts have been made recently to develop magnesium with nanoscale surface features that can increase immediate bone formation to decrease or compensate for the negative effects of magnesium corrosion.21 Nanostructured magnesium has unique characteristics compared with its bulk counterpart, including increased grain boundary exposure, surface to volume ratio, surface roughness, and surface energy. Previous studies have shown that treating magnesium with NaOH results in nanoscale surface features that increase osteoblast adhesion, proliferation, alkaline phosphatase activity, and calcium deposition.21 However, it has not been determined as yet if the degradation of magnesium is altered by creating nanoscale surface features on magnesium via soaking in NaOH to influence osteoblast density. Thus, the objective of the present study was to determine the influence of degradation of nanostructured magnesium, created by soaking in NaOH, on the functions of osteoblasts.
Materials and methods
Creating nanorough magnesium
Magnesium ribbon (Alfa Aesar, Ward Hill, MA) 0.30 cm × 0.015 cm was cut into pieces 1.4 cm in length. NaOH pellets (Fisher Scientific, Fairlawn, NJ) were dissolved in deionized water to produce solutions of 1N, 5N, and 10N concentration. The magnesium pieces were treated with each concentration of NaOH for 20 minutes (1N 20 minutes, 5N20 minutes, and 10N20 minutes [1N20, 5N20, and 10N20, respectively) at room temperature. The samples were then washed several times with phosphate-buffered solution, and the rinsed solution was tested with pH paper to ensure that all the excess base was removed. The magnesium pieces were left to dry in a tissue culture hood and then stored in a desiccator to prevent any potential reaction with moisture from the air. The samples were sterilized by exposure to ultraviolet light.
Material characterization
Scanning electron microscopy
Scanning electron microscopy was performed using a field emission scanning electron microscope (LEO 1530 VP FE-4800, Zeiss, Peabody, MA) to determine if nanoroughness was produced from the NaOH treatment.
Atomic force microscopy
Atomic force microscopy was performed using an Asylum-1 MFP-3D system (Asylum Research, Santa Barbara, CA) to examine the topography of the NaOH-treated magnesium samples. A 1 μm × 1 μm area was scanned, and root mean square values were obtained to quantify the surface roughness.
Electron spectroscopy for chemical analysis
Electron spectroscopy for chemical analysis was performed using a 5500 multitechnique surface analyzer system (Perkin Elmer, Waltham, MA). The surfaces of the NaOH-treated samples and nontreated control were analyzed to determine their chemical composition.
Contact angle measurements
Contact angle measurements to determine surface hydrophilicity were obtained using a Krüss easy drop contact angle instrument (A Krüss Optronic GmbH, Hamburg, Germany) connected to the Drop Shape Analysis program version 1.8. A 2.5 μL drop of water was produced on the surface and contact angles were obtained after a specified time. Measurements were acquired after 60 seconds of drop placement for all treated samples. Contact angles were acquired on three random fields for each sample. All measurements were run in triplicate.
Measurements of pH
The supernatants from the cell studies described in the next column were collected after 4 hours of incubation, and for the proliferation studies they were collected every 24 hours up to 120 hours and tested using a standard pH meter.
Cell studies
Healthy human osteoblasts (CRL-11372, American Type Culture Collection, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium, high glucose (HyClone, Logan, UT; Gibco, Grand Island, NY), supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in a 37°C, humidified 5% CO2 environment. Generation 3 and 4 cells were used for all cell experiments. Polystyrene tissue culture wells without magnesium samples were used as controls. The WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)- 2H-tetrazolium) assay (Roche, Basel, Switzerland) was used to determine the cell density in all samples. For cell adhesion, cells were seeded at 3500 cells/cm2 per substrate and allowed to adhere for 4 hours in a 37°C, humidified 5% CO2 environment. After the 4-hour cell adhesion assay, 100 μL of WST-1 was added to each well.
The present study determined cell density on both the magnesium and the tissue culture polystyrene surrounding the magnesium, so for the WST-1 assay, the magnesium samples were removed from the tissue culture polystyrene at the end of the experiment (then exposed to WST-1 assay reagents) to determine differences in density between the two samples. Determining the osteoblast density on the polystyrene was helpful to determine the effect of magnesium degradation on the osteoblast density on the juxtaposed polystyrene tissue culture plate. For this, the tetrazolium was cleaved into a soluble formazan by glycolytic production of nicotinamide adenine dinucleotide phosphate oxidase in viable cells. After 2 hours of incubation, the solutions were transferred to a clean 96-well plate and absorbance was measured with a microplate reader at 440 nm. All experiments were completed in triplicate and repeated three times.
A similar process to that described above was used for cell proliferation, except that the cells were seeded at 2000 cells/cm2 per substrate and allowed to proliferate for 24, 72, and 120 hours in a 37°C, humidified 5% CO2 environment. The medium in the wells were removed and replaced with fresh Dulbecco’s modified Eagle’s medium every 24 hours for all substrates. After the specified periods, the samples were washed with phosphate-buffered solution and transferred to fresh medium in a clean 24-well plate. The WST-1 assay was conducted as described above to determine cell viability. Experiments were conducted in triplicate and repeated three times. For all experiments, differences between means were evaluated using the Student t-test.
Results
Characterization of magnesium
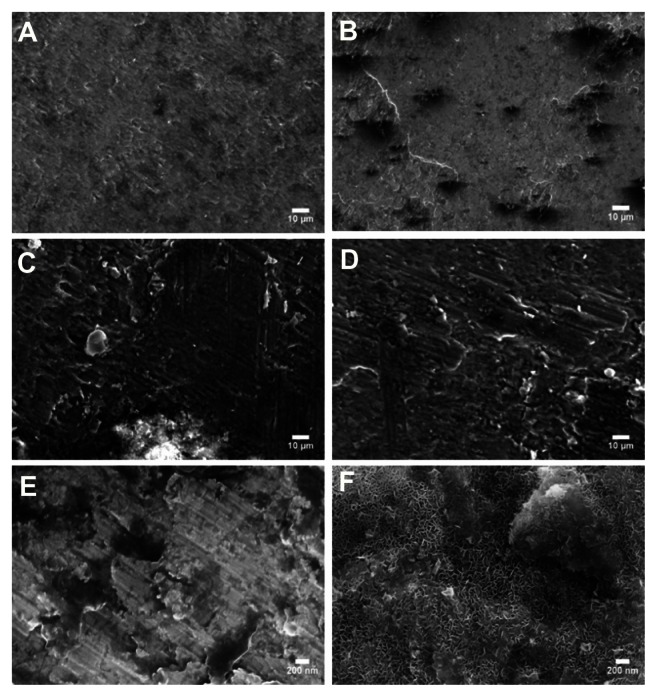
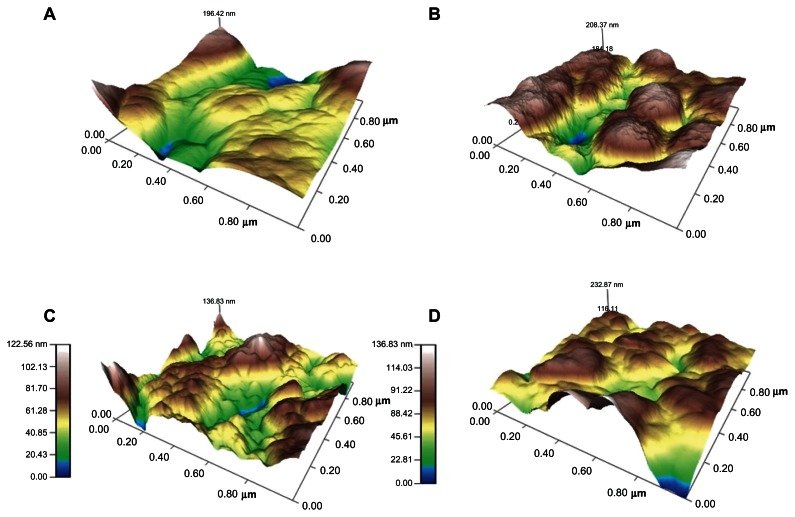
The surfaces of the magnesium samples treated with NaOH were examined using a scanning electron microscope (Figure 1). As expected, increased roughness at the nanoscale level was seen on all the treated samples compared with the controls, and this roughness increased with increasing NaOH concentration. The surface roughness was also examined and quantified by atomic force microscopy, which supported the results of scanning electron microscopy (Table 1 and Figure 2). Root mean square values increased in the treated samples with increasing NaOH concentration. It is important to note that, while NaOH degrades magnesium, because the treatment occurred in a closed container, the change in surface roughness may be due to a combination of degradation and deposition of ions. That is, due to the process followed in this study, after treatment with a strong base, rapid rinsing in phosphate-buffered solution, which causes a rapid pH change, may cause ion precipitation (such as calcium) on the substrate surface.
Figure 1.
Surface morphology of magnesium treated with NaOH viewed under scanning electron microscopy at 2000× for (A) control (untreated), (B) 1N20, (C) 5N20, and (D) 10 N20, and at 80,000× for (E) control (untreated), and (F) 10N20.
Notes: Scale bars represent 10 μm (A–D) and 200 nm (E and F).
Table 1.
Atomic force microscopic root mean square values of untreated (control) and NaOH treated magnesium
| Substrate | Root mean square value (nm) |
|---|---|
| Control (untreated) magnesium | 32.89 ± 2.64 |
| 1N20 | 26.68 ± 0.55 |
| 5N20 | 34.81 ± 0.70 |
| 10N20 | 37.15 ± 1.54 |
Figure 2.
Surface topography of the magnesium samples scanned by atomic force microscopy for (A) control (untreated), (B) 1 N20, (C) 5 N20, and (D) 10 N20.
The surface wettability of the magnesium samples treated with NaOH was characterized by contact angle measurements. The results showed that all of the NaOH-treated samples were more hydrophilic (ie, had more surface energy) compared with the untreated magnesium (Table 2). Among the nanorough samples, the most aggressive treatment, ie, 10N30, caused a significant decrease in the contact angle compared with the mildest treatment of 1N10 (P < 0.001). As will be described, a change in wettability can alter degradation to influence the behavior of osteoblasts.
Table 2.
Contact angles for the magnesium samples
| Substrate | Contact angle (degrees) |
|---|---|
| Control (untreated) magnesium | 89.8 ± 2.1 |
| 1N20 | 39.5 ± 1.1 |
| 5N20 | 36.1 ± 0.9 |
| 10N20 | 28.4 ± 0.8 |
Lastly, the treated and untreated magnesium samples were analyzed by electron spectroscopy to determine the chemical composition of the surfaces (Table 3). All the samples had peaks for magnesium (Mg2s), oxygen (O1s), and carbon (C1s).21 However, a notable difference between the control and all the treated samples was the carbon peak. The control had two distinct peaks, one at around 289 eV and the other at around 291 eV. Most of the treated samples only had one peak at 289 eV, and for the few that had two peaks, the second peak was much smaller and lower in intensity. One possible explanation for this is that the degradation from NaOH removed some of the carbon from the surface. Another possible explanation is carbon contamination, such as with MgCO3, which could be removed in future studies via an alcohol wash. Another marked difference between the treated and control samples is the increased amount of oxygen on the treated samples, which could also be attributed to treatment with NaOH and formation of oxides. Interestingly, palladium (Pd3d) was also found on some (but not all) of the samples, which most likely was one of the trace metals that remained during the manufacturing process. Even magnesium that is labeled as commercially pure usually only contains 99.95% pure magnesium. Importantly, electron spectroscopy for chemical analysis showed some key chemical differences between the treated and non-treated magnesium, specifically, less carbon and greater oxygen when magnesium was treated with NaOH. However, no sodium was measured, perhaps due to the washing after treatment.
Table 3.
Percent composition of various elements on magnesium samples as determined by electron spectroscopy for chemical analysis
| Substrate | Magnesium | Oxygen | Carbon | Palladium |
|---|---|---|---|---|
| Control (untreated) | 17.72 | 39.42 | 42.68 | |
| 1N20 | 19.89 | 59.95 | 18.82 | 1.34 |
| 5N20 | 20.85 | 60.27 | 18.85 | 0.03 |
| 10N20 | 21.62 | 59.5 | 18.89 |
Change in pH during magnesium degradation
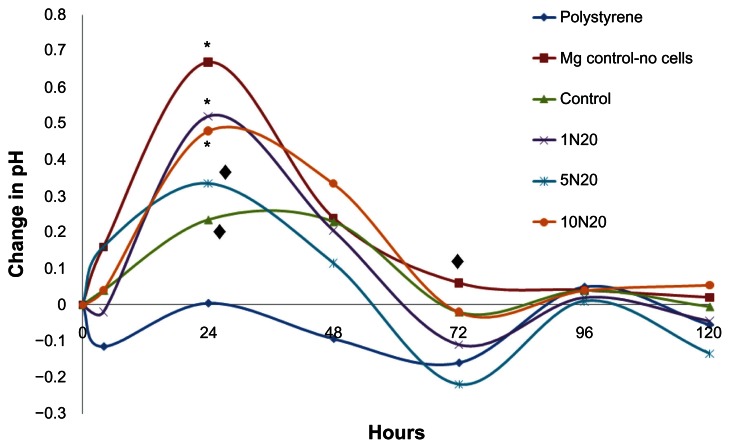
The pH values in the cell culture supernatant from the aforementioned osteoblast adhesion and proliferation experiments were measured to determine the effect that changes in pH had on cell density (Figure 3). pH values were also obtained for the nontreated control sample (magnesium with no cells) to determine the contributing effect of cells on pH levels. The values shown are the differences between the original medium and the culture supernatant after each time point. The cellular supernatant for polystyrene showed a slight decrease in pH, which can be expected because cells generally thrive at slightly alkaline pH levels, so the extracellular pH levels would become lower to maintain equilibrium. The magnesium-no cells showed a significant pH increase as well, while the control with cells did not, suggesting that the presence of cells helped to maintain a pH balance. The other samples did not show a major change in pH after 4 hours. Of interest is the fact that the pH decreased in the 1N20 sample which, as will be discussed, was found to have one of the highest cell viabilities (Figure 4).
Figure 3.
Changes in pH values of the culture media at 4, 24, 48, 72, 96, and 120 hours of osteoblast proliferation on polystyrene, magnesium controls without cells (Mg-control-no cells), untreated magnesium controls as well as magnesium treated with 1N20, 5N20, and 10N20.
Note: A decrease in pH was observed for the polystyrene (◆P < 0.05). A significant increase in pH was observed at 24 hours for all samples except the polystyrene (*P < 0.001; ◆P < 0.01). As expected, by 72 hours, only the Mg-control-no cells showed a significant increase in pH (P < 0.01). Values represent the difference between the original pH of the medium and the pH obtained after 4 hours for n = 3.
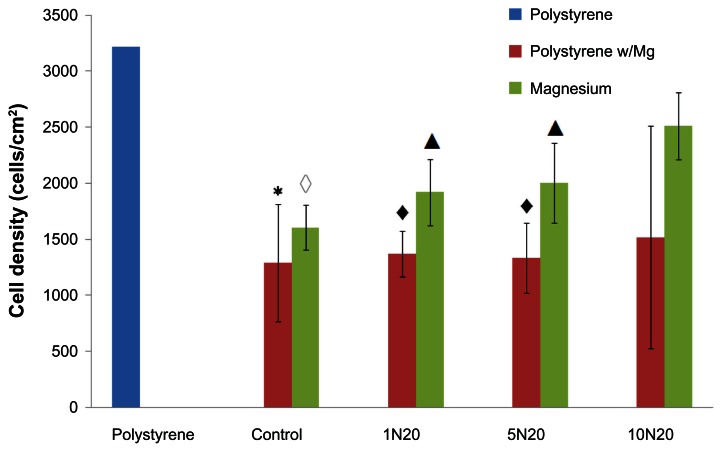
Figure 4.
Greater osteoblast adhesion density on polystyrene when cultured in the presence of NaOH-treated nanostructured magnesium than untreated control magnesium.
Notes: Compared with the polystyrene substrate alone, all the other samples except 10N20 decreased osteoblast adhesion (*P < 0.05; ◆P < 0.01; ◇P < 0.001; ▲P < 0.02). Values represent the mean ± standard deviation for n = 3.
However, the greatest change in pH occurred after 24 hours. As expected, after 24 hours, the magnesium-no cells showed the greatest increase in pH. Among the magnesium substrates, all of the samples had increased pH values (P < 0.01 for 5N20 and 10N20, P < 0.001 for control and 1N20). Of interest is the fact that the control sample produced the least change in pH compared with the treated 1N20, 5N20, and 10N20 samples (P < 0.01, P < 0.05, and P < 0.01, respectively). The pH change for the 5N20 sample was significantly less than that observed for the 1N20 and 10N20 samples (P < 0.01).
At 48 hours, the pH values were still all significantly higher (P < 0.05), except for the cells on polystyrene, which again decreased. The changes in pH for the magnesium samples became smaller and actually decreased at 72 hours for all samples except the magnesium-no cells. This substrate resulted in a significant increase (P < 0.05), showing again that the presence of cells helped to maintain pH levels. For the other samples, a decrease in pH was observed for the polystyrene, 1N20, and 5N20 (P < 0.01, P < 0.05, and P < 0.01, respectively). Little change was observed after 96 hours, suggesting that degradation had slowed. After 120 hours, there was also relatively no change in pH, except for an observed decrease for the 5N20 sample (P < 0.05). Such results demonstrated a clear change in degradation and pH values for the untreated versus NaOH-treated magnesium samples.
Osteoblast density in the presence of magnesium
Most importantly, our results showed an increased osteoblast density on magnesium treated with 10 N20 compared with any of the other magnesium samples (Figure 4). Specifically, the cell density on the polystyrene substrate was around 3200 cells/cm2 compared with the cell density on magnesium, which ranged from 1605 cells/cm2 (control) to 2515 cells/cm2 (10N20 treatment). Also shown is the osteoblast density on polystyrene in the presence of magnesium in order to demonstrate the effect of magnesium degradation on the surrounding cell density. As expected, the cell density on the magnesium control, 1N20, and 5N20 were all significantly lower than the cell adhesion on the polystyrene substrate without the magnesium (P < 0.001, P < 0.02, and P < 0.02, respectively). However, importantly, no significant difference was observed for the cell density on the polystyrene alone compared with the polystyrene in the presence of 10N20 magnesium. This result suggests for the first time that after 4 hours, magnesium treated with 10N20 did not have an adverse effect on the density of surrounding cells, whereas all other magnesium samples did have an adverse effect.
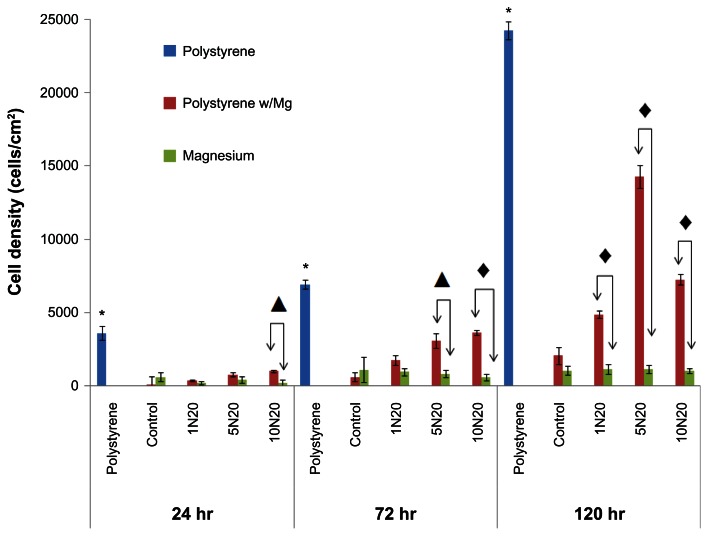
After longer time periods, the results were similar in that treating magnesium with NaOH at higher concentrations for longer periods of time minimized the negative influence of magnesium degradation on surrounding osteoblasts (Figure 5). Specifically, as expected, polystyrene alone had osteoblast densities of 3610, 6935, and 24231 cells/cm2 after 24, 72, and 120 hours, respectively, which was higher than for the polystyrene in the presence of any magnesium for all time periods (P < 0.001). This shows that magnesium degradation had an adverse effect on osteoblast proliferation. However, after 72 and 120 hours, osteoblast density was again greater on the polystyrene with magnesium treated with stronger concentrations and times of NaOH, indicating less of a detrimental effect of magnesium degradation on surrounding osteoblasts upon exposure to greater NaOH concentrations; with the caveat that osteoblast density was greater on the polystyrene in the presence of magnesium treated with 5 N compared with 10 N NaOH for 20 minutes, which requires further investigation. Lastly, in contrast with the adhesion results, no difference in osteoblast density after 48, 72, and 120 hours was observed on the magnesium treated with different concentrations and times of NaOH, which requires further investigation, given that previous studies have demonstrated greater alkaline phosphatase and calcium deposition by osteoblasts on magnesium treated with greater concentrations and time of NaOH.21 It is known that osteoblasts slow down proliferation as they begin to secrete extracellular matrix proteins, which may account for this observation.1
Figure 5.
Greater osteoblast density on polystyrene when cultured in the presence of NaOH-treated nanostructured magnesium than untreated control magnesium.
Notes: Cell density on the polystyrene substrates was significantly greater than on the other substrates for all proliferation times (*P < 0.05). Values represent the mean ± standard error of the mean for n = 3; ▲P < 0.05; ◆P < 0.001.
Discussion
As mentioned, one of the greatest challenges when using magnesium as a biomaterial stems from its fast corrosion rate that creates the reaction: Mg(s) + 2 H2O(aq) ↔ Mg(OH)2(s) + H2(g). This releases OH− ions resulting in an alkaline pH which can clearly be detrimental to cell viability. Numerous studies have highlighted the fact that degradation of magnesium may be a clinical problem, but modification of the magnesium surface can minimize such effects by promoting early cellular events.3,22,23 Specifically, one study created two types of magnesium surfaces, ie, a commercial magnesium that contained an oxide layer via a natural reaction with air (labeled Mg_O) and a smoother polished magnesium (labeled Mg_P).3 As with other studies, it was reported that the same cell culture medium as used here, ie, Dulbecco’s modified Eagle’s medium, degrades magnesium. Of interest was the fact that the Mg_O produced surface pitting within 72 hours, while the Mg_P did not start to form pits until after 7 days. The study also showed formation of oxides after incubation with Dulbecco’s modified Eagle’s medium, with small particulate matter and formation of needle-like structures along the edges.
Alternatively, treating magnesium with HNO3 has been shown to increase degradation, because the H+ ions from the acid can react with the OH− ions produced as magnesium degrades, thus promoting more formation of OH− and increased degradation.1,3 This has also been shown by the increased degradation of magnesium in simulated body fluid, which contains large amounts of tris-HCl, and so more H+ to consume the OH−. Lastly, it has been shown that increased grain boundaries and structural defects promote corrosion if impurities accumulate in these areas.17
As evident in this study, treating magnesium samples with NaOH resulted in increased formation of an oxide layer. This layer can be beneficial because it provides a protective coating that slows degradation to decrease the impact of detrimental magnesium degradation products. Although not accomplished with NaOH treatment, other studies have demonstrated that oxide layers on magnesium can slow the degradation of magnesium.1,3 Specifically, one study examined the degradation of three different magnesium samples, ie, magnesium that was heat-treated to produce a surface oxide layer, heat-treated magnesium that was strained to 9% to mimic natural body stresses, and polished magnesium.3 The authors found that the stained magnesium with an oxide layer degraded faster than the non-strained magnesium with an oxide layer, which makes sense because the oxide layer was weakened and more prone to corrosion attack when strained. However, more importantly, the potential protection of this oxide layer was demonstrated because the non-strained magnesium had a slower degradation rate than the polished magnesium.
The NaOH treatment used in the present study produced an oxide layer and served another important purpose of creating nanoscale surface features known to promote osteoblast function,24–27 which compensated for the detrimental magnesium degradation products. As mentioned already, previous studies have reported increased alkaline phosphatase activity and calcium deposition by osteoblasts on magnesium treated with increasing times and concentrations of NaOH.21
The most important finding of the present study was the osteoblast density observed on the polystyrene in the presence of magnesium samples, providing evidence of the influence of magnesium degradation on surrounding osteoblasts. After 72 hours, the cell density was significantly higher compared with that at 24 hours for the 1N20, 5N20, and 10N20. The cells continued to proliferate in a manner similar to that observed for cells seeded onto standard polystyrene (with no magnesium) and an increase was again observed after 120 hours compared with 72 hours for the 1N20, 5N20, and 10N20 (P < 0.001). This suggests that while magnesium degradation limited growth on their actual surface (perhaps because they were secreting extracellular matrix proteins), the cells were still able to proliferate in the area immediately surrounding the degradation site. This observation is supported by other studies that have found increased bone growth in the area surrounding a degrading magnesium scaffold.22 Specifically, Witte et al22 studied the in vivo morphology of magnesium rods implanted into guinea pigs and found that one rod had severe degradation and pitting, but had direct contact with newly formed bone. This contrasted sharply with osteoblasts on polystyrene in the presence of control magnesium. That is, while the osteoblasts on the polystyrene in the presence of nanorough magnesium continued to proliferate, the cells on the polystyrene in the presence of untreated magnesium controls showed much slower proliferation, again demonstrating the harmful effects of magnesium degradation products on osteoblasts. Our study demonstrated a method of compensating for such harmful degradation products by treating magnesium with NaOH.
Conclusion
Previous studies have shown that one method of increasing nanosurface roughness on magnesium is via soaking magnesium in NaOH. This study demonstrates for the first time a less detrimental effect of magnesium degradation on osteoblast density when magnesium is treated with NaOH. Given that the detrimental degradation products of magnesium are significant concerns when considering use of magnesium as an orthopedic implant material, this study identified a surface treatment (soaking in NaOH to create nanoscale features and an oxide layer) for magnesium that can improve its use for numerous orthopedic applications.
Acknowledgment
The authors would like to thank the Hermann Foundation for funding this research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Liu H. Bioinspired nanocomposites for orthopedic applications. In: Webster TJ, editor. Nanotechnology for the Regeneration of Hard and Soft Tissues. Toh Tuck Link, Singapore: World Scientific Publishing Company; 2007. [Google Scholar]
- 2.Smith R. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. [PubMed] [Google Scholar]
- 3.Liu H. Biodegradable metals and responsive biosenors for musculosketal applications. In: Webster TJ, editor. Nanotechnology Enabled In Situ Sensors for Monitoring Health. New York, NY: Springer Science; 2011. [Google Scholar]
- 4.Wolner C, Nauer GE, Trummer J, Putz V, Tschegg S. Possible reasons for the unexpected bad biocompatibility of metal-on-metal hip implants. Mat Sci Eng. 2006;26:34–40. [Google Scholar]
- 5.Bohler M, Kanz F, Schwarz B, et al. Adverse tissue reactions to wear particles form Co-alloy articulations, increased by alumina-blasting particle contamination from cementless Ti-based total hip implants – a report of seven revisions with early failure. J Bone Joint Surg Br. 2002;84B:128–136. doi: 10.1302/0301-620x.84b1.11324. [DOI] [PubMed] [Google Scholar]
- 6.Arnaud MJ. Update on the assessment of magnesium status. Br J Nutr. 2008;99:S24–S36. doi: 10.1017/S000711450800682X. [DOI] [PubMed] [Google Scholar]
- 7.Fox CH, Timm EA, Smith SJ, Touyz RM, Bush EG, Wallace PK. A method for measuring intracellular free magnesium concentration in platelets using flow cytometry. Magnes Res. 2007;20:200–207. [PubMed] [Google Scholar]
- 8.Saris NEL, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium – an update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 9.Okuma T. Magnesium and bone strength. Nutrition. 2001;17:679–680. doi: 10.1016/s0899-9007(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 10.Hartwig A. Role of magnesium in genomic stability. Mutat Res. 2001;475:113–121. doi: 10.1016/s0027-5107(01)00074-4. [DOI] [PubMed] [Google Scholar]
- 11.Creedon A, Flynn A, Cashman K. The effect of moderately and severely restricted dietary magnesium intakes on bone composition and bone metabolism in the rat. Br J Nutr. 1999;82:63–71. doi: 10.1017/s0007114599001130. [DOI] [PubMed] [Google Scholar]
- 12.Belluci MM, Giro G, del Barrio RAL, Pereira RMR, Marcantonio E, Orrico SRP. Effects of magnesium intake deficiency on bone metabolism and bone tissue around osseointegrated implants. Clin Oral Implants Res. 2011;22:716–721. doi: 10.1111/j.1600-0501.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- 13.Toba Y, Kajita Y, Masuyama R, Takada Y, Suzuki K, Aoe S. Dietary magnesium supplementation affects bone metabolism and dynamic strength of bone in ovariectomized rats. J Nutr. 2000;130:216–220. doi: 10.1093/jn/130.2.216. [DOI] [PubMed] [Google Scholar]
- 14.Del Barrio RAL, Giro G, Belluci MM, et al. Effect of severe dietary magnesium deficiency on systemic bone density and removal torque of osseointegrated implants. Int J Oral Maxillofac Implants. 2010;25:1125–1130. [PubMed] [Google Scholar]
- 15.Hussain A, Bessho K, Takahashi K, Tabata Y. Magnesium calcium phosphate as a novel component enhances mechanical/physical properties of gelatin scaffold and osteogenic differentiation of bone marrow mesenchymal stem cells. Tissue Eng Part A. 2012;18:768–774. doi: 10.1089/ten.TEA.2011.0310. [DOI] [PubMed] [Google Scholar]
- 16.Zheng YF, Gu XN, Xi YL, Chai DL. In vitro degradation and cytotoxicity of Mg/Ca composites produced by powder metallurgy. Acta Biomater. 2010;6:1783–1791. doi: 10.1016/j.actbio.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Salunke P, Shanov V, Witte F. High purity biodegradable magnesium coating for implant application. Mater Sci Eng. 2011;176:1711–1717. [Google Scholar]
- 18.Gu XN, Zhou WR, Zheng YF, et al. Corrosion fatigue behaviors of two biomedical Mg alloys – AZ91D and WE43 – in simulated body fluid. Acta Biomater. 2010;6:4605–4613. doi: 10.1016/j.actbio.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Willumeit R, Fischer J, Feyerabend F, et al. Chemical surface alteration of biodegradable magnesium exposed to corrosion media. Acta Biomater. 2011;7:2704–2715. doi: 10.1016/j.actbio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Han G, Kim YC, et al. Effects of impurities on the biodegradation behavior of pure magnesium. Met Mater Int. 2009;15:955–961. [Google Scholar]
- 21.Weng L, Webster TJ. Increased osteoblast functions on nanostructured magnesium. Nanotechnology. 2012;23:485105. doi: 10.1088/0957-4484/23/48/485105. [DOI] [PubMed] [Google Scholar]
- 22.Witte F, Ulrich H, Palm C, Willbold E. Biodegradable magnesium scaffolds: Part II: peri-implant bone remodeling. J Biomed Mater Res A. 2007;81A:757–765. doi: 10.1002/jbm.a.31293. [DOI] [PubMed] [Google Scholar]
- 23.Atrens A, Liu M, Zainal Abidin NI. Corrosion mechanism applicable to biodegradable magnesium implants. Mater Sci Eng B. 2011;176:1609–1636. [Google Scholar]
- 24.Price RL, Ellison K, Haberstroh KM, Webster TJ. Nanometer surface roughness increases select osteoblast adhesion on carbon nanofiber compacts. J Biomed Mater Res A. 2004;70A:129–138. doi: 10.1002/jbm.a.30073. [DOI] [PubMed] [Google Scholar]
- 25.Price R, Haberstroh K, Webster T. Enhanced functions of osteoblasts on nanostructured surfaces of carbon and alumina. Med Biol Eng Comput. 2003;41:372–375. doi: 10.1007/BF02348445. [DOI] [PubMed] [Google Scholar]
- 26.Liu HN, Slamovich EB, Webster TJ. Increased osteoblast functions on nanophase titania dispersed in poly-lactic-co-glycolic acid composites. Nanotechnology. 2005;16:S601–S608. doi: 10.1088/0957-4484/16/7/038. [DOI] [PubMed] [Google Scholar]
- 27.Zhang LJ, Ramsaywack S, Fenniri H, Webster TJ. Enhanced osteoblast adhesion on self-assembled nanostructured hydrogel scaffolds. Tissue Eng Part A. 2008;14:1353–1364. doi: 10.1089/ten.tea.2006.0436. [DOI] [PubMed] [Google Scholar]