Abstract
Cyanoguanidine is an inexpensive commodity chemical and it is found to be a useful reagent for the direct Friedel-Crafts carboxamidation of arenes. The reaction works best in an excess of Brønsted superacid, an observation suggesting the involvement of a superelectrophilic intermediate. Theoretical calculations indicate that the most stable diprotonated species involves protonation at the guanidine and cyano nitrogen atoms.
Keywords: benzamide, superacid, superelectrophile, Friedel-Crafts, carboxamidation
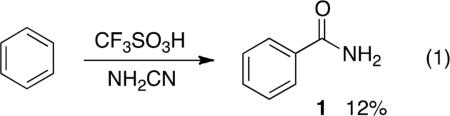
Benzamide compounds are useful building blocks in organic synthesis and they are sub-structures in a variety of pharmaceutical agents.1 Benzamides have been synthesized by hydrolysis of aromatic nitriles, interconversions of carboxylic acid derivatives, rearrangement of oximes, aminocarbonylation, and other routes.2 Aromatic amides have also been prepared by the Friedel-Crafts-type reactions of isocyanates, especially 2° amides.3 Primary benzamides have been previously synthesized using trimethylsilylisocyanate and chlorosulfonylisocyanate – two reagents that are not readily accessible and likely exhibit high levels of toxicity.4 We recently reported a low yield (12%) synthesis of benzamide by the superacid-catalyzed reaction of cyanamide with benzene (eq 1).5 Despite being a direct route to
 |
benzamide, this chemistry was not particularly useful as most of the cyanamide is consumed in side-reactions. Nevertheless, the results prompted us to search for alternative reagents that might provide a route to benzamides by electrophilic aromatic substitution. In this Letter, we report the direct conversion of arenes to the benzamide derivatives by electrophilic aromatic substitution.
Cyanoguanidine (or dicyanodiamide, 2) is an inexpensive, crystalline solid that is used as a feedstock chemical in various industries. We sought to use this material as a substrate in electrophilic aromatic substitution chemistry, primarily because of its similarities to cyanamide. We also reasoned that the guanidinium groups would be easily protonated and consequently it should activate the adjacent cyano group or nitrilium ion for use in Friedel-Crafts chemistry. Our initial experiment involved reacting cyanoguanidine (2) with benzene in superacidic CF3SO3H (eq 2). In a reaction at 25°C, a mixture of benzamide
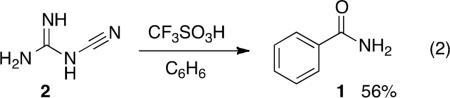 |
(1) and benzonitrile were obtained. By increasing the reaction temperature to 60°C and using 10 equivalents of CF3SO3H, no benzonitrile is observed and the benzamide product 1 can be isolated in 56% yield. Although the conversion has not been fully optimized, it was observed that similar yields could be obtained with as little as 5 equivalents of CF3SO3H. With less acid, the product yield drops considerably. Both H2SO4 and CF3CO2H (in excess quantities) were also reacted with compound 2 in the presence of benzene, but no benzamide product was formed. Presumably, any byproducts or the starting material 2 were lost in the aqueous phase during workup.
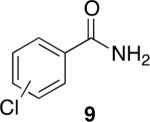
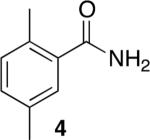
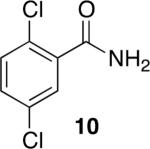
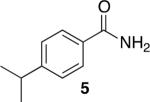
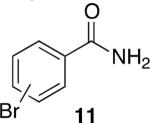
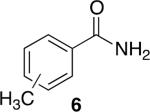
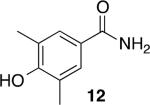
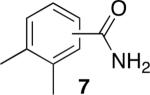
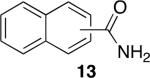
Using compound 2, a series of aromatic primary amides were synthesized from electrophilic aromatic substitution (Table 1).6 Alkyl-substituted benzenes were generally converted to the amides in good overall yields. In the case of toluene and ortho-xylene, mixtures of regioisomers were formed (6 and 7). For the mono-substituted benzenes, the para regioisomer is the major product (6–9, 11). With para-dichlorobenzene, product 10 may be isolated in 10% yield. Although the yield for this conversion is low, para-dichlorobenzene is a moderately deactivated arene, so product formation indicates that compound 2 generates a reactive electrophile in superacid. Despite the high degree of activation of 2,6-dimethylphenol, product 12 could only be isolated in 11% yield. This may be due to protonation of the phenol by the superacid,7 greatly decreasing its reactivity towards electrophilic attack. Naphthalene gave product 13 in good yield, although the reaction leads to the mixture of regioisomers.
Table 1.
Amide products (3–13) from the direct electroph aromatic substitution of arenes with cyanoguanidine (2) CF3SO3H.
| Product | Yielda (ratio of isomers) | Product | Yielda (ratio of isomers) |
|---|---|---|---|
|
67% |
|
47% (o:p, 2.2:7.8)b |
|
80% |
|
10% |
|
90% |
|
47% (o:p, 2.5:7.5)b |
|
89% (o:p, 3:7)b |
|
11% |
|
88% (α:β, 3:7)b |
|
86% (α:β, 8:2)b |
|
89% (o:p, 1:3)c |
Isolated yield.
Product ratio determined by GC-FID.
Product ratio determined by isolation of regioisomers by flash chromatography.
In order to probe the mechanism of this conversion, we conducted experimental, spectroscopic, and theoretical studies. For example, substituted cyanoguanidines are readily prepared from dimethyl N-cyanodithioiminocarbonate (14, eq 3).8 We
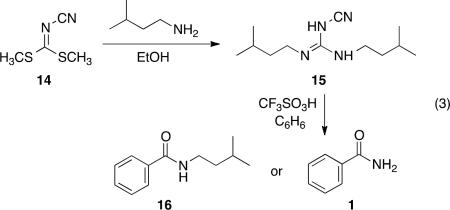 |
reasoned that the Friedel-Crafts chemistry is likely occurring at the cyano group, and therefore, reaction with the alkyl-substituted derivative 15 should provide benzamide 1. If the electrophilic reaction occurs at the guanidium carbon however, the product should be the secondary amide 16. When compound 15 is reacted with benzene in CF3SO3H, only benzamide 1 is observed in the product mixture, suggesting that the Friedel-Crafts chemistry occurs at the cyano group. In spectroscopic studies, solutions of compound 2 were prepared by dissolving 2 in d4-methanol, CF3CO2H, and CF3SO3H. 13C NMR spectra were obtained, however the results were inconclusive. The acidic solutions gave very complex 13C NMR spectra. This may be the result of multiple reactions and equilibria, or given the tendency for nitrilium ions to form 1,3,5-triazines,9 the complex spectra may be the result of slow trimerization reactions. In our synthetic reactions, triazine products were never observed. However, this may be a consequence of the water solubility of the triazine products from 2 (likely lost upon aqueous workup).
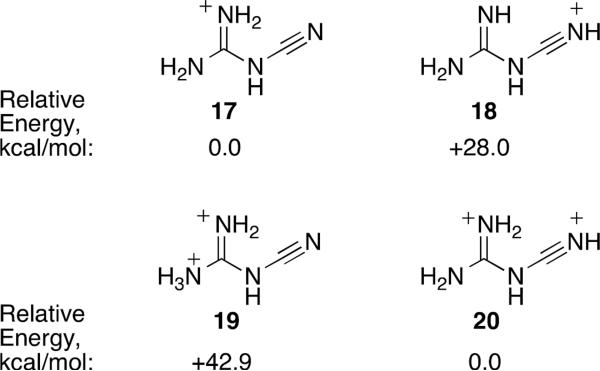
Theoretical calculations were done to explore the protonated structures that could arise from cyanoguanidine 2 in acidic media.10 Calculations were done at the B3LYP 6-311G** level,11 energies are corrected for ZPE, and all structures were characterized as true minima (zero imaginary frequencies) by frequency calculations. Initial protonation is thought to occur at the guanidine group (Figure 1). Thus, cation 17 is about 28 kcal/mol more stable than the cyano protonated species 18. This observation is certainly a consequence of the stability of the guanidinium cation. Nevertheless, electrophilic aromatic substitution with nitriles – the Houben-Hoesch reaction – generally occurs through a nitrilium ion (via protonation of the nitrile). This suggests the involvement of a diprotonated species in the reactions of 2 with arene nucleophiles. Earlier studies by Olah and coworkers showed that guanidine itself could be diprotonated in superacid.12 Although double protonation at the guanidine group (19) is found to be a stable minimum on the potential energy surface, a significantly more stable dication (20) is formed by protonation at the guanidine and cyano nitrogen atoms. Presumably, ion 19 is destabilized by the proximity of the two positive charges and the loss of resonance interactions with the –NH2 group. We propose that superelectrophile 20 is the key intermediate in the conversions to the benzamide products.13 In accord with this suggestion, the LUMO level of 20 is such that reaction with benzene appears to be favorable. Calculations show cyanoguanidine (2) with a LUMO at 0.00509 eV, monocation 17 with a LUMO at −0.021873 eV, and dication 20 with a LUMO at −0.4320 eV. For comparison, the HOMO of benzene is calculated to be at −0.25636 eV.
Figure 1.
Calculated relative energies from optimized structures 17/18 and 19/20 (B3LYP 6-311G** level).
The proposed mechanism involves further steps that include formation of the new C-C bond to give intermediate 21 (Figure 2). Based on the observed products, we suggest cleavage of the C-N bond to give protonated benzonitrile (22) and the guanidinium cation. Previous studies by Shudo and coworkers suggested that the nitrilium ion 22 may itself react with triflic acid to give the adduct with triflate anion (23).14 Hydrolytic work up of the reaction mixture then provides the benzamide (1).
Figure 2.
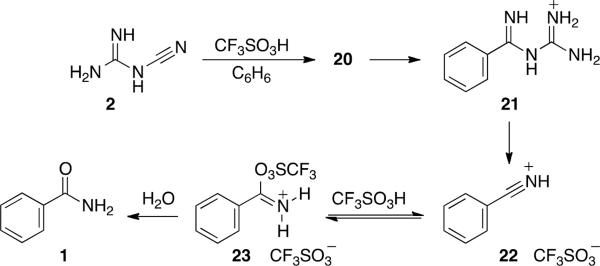
Proposed mechanism for the conversion of 2 to benzamide products.
In conclusion, we have found that cyanoguanidine 2 is a useful reagent for the direct Friedel-Crafts carboxamidation of arenes. The reaction works best in an excess of Brønsted superacid, an observation suggesting the involvement of a superelectrophilic intermediate. Theoretical calculations indicate that the most stable diprotonated species involves protonation at the guanidine and cyano nitrogen atoms.
Acknowledgments
We gratefully acknowledge the support of the NIH-National Institute of General Medical Sciences (GM085736-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.a) Bréthous L, Garcia-Delgado N, Schwartz J, Bertrand S, Bertrand D, Reymond J-L. J. Med. Chem. 2012;55(10):4605–4618. doi: 10.1021/jm300030r. [DOI] [PubMed] [Google Scholar]; b) Mao W, Ning M, Liu Z, Zhu Q, Leng Y, Zhang A. Bioorg. Med. Chem. 2012;20:2982–2991. doi: 10.1016/j.bmc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Wu X-F, Neumann H, Beller M. Chem. Asian J. 2010;5:2168–2172. doi: 10.1002/asia.201000418. [DOI] [PubMed] [Google Scholar]; and references cited therein.
- 3.a) Gauvreau D, Dolman SJ, Hughes G, O'Shea PD, Davies IW. J. Org. Chem. 2010;75:4078. doi: 10.1021/jo1004197. [DOI] [PubMed] [Google Scholar]; b) Effenberger F, Gleiter R. Chem. Ber. 1964;97:472. [Google Scholar]
- 4.a) Kozyukov VP, Kozyukov Vik. P., Muzovskaya EV, Mironov VF. Zhur. Obs. Khim. 1989;59:1202–1203. [Google Scholar]; b) Graf R. German Pat. DE 1010958 (1957) [Google Scholar]
- 5.Raja E, Klumpp DA. Tetrahedron. 2011;67:4494–4497. doi: 10.1016/j.tet.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.General synthetic procedure: cyanoguanidine 2 (0.084 g, 1 mmol) is suspended in 2 mL of neat aromatic substrate (alternatively, the arene may be suspended in CH2Cl2) and freshly distilled triflic acid (0.5 mL, 6 mmol) is slowly added. The mixture is stirred at 60°C for 2 hr, after which 1 mL of cold water is added to the solution. The mixture is then stirred overnight. Product isolation is accomplished with basification of the mixture using 10 M NaOH and extraction of the mixture twice with chloroform. The organic extracts are washed with water, and then brine, and dried with anhydrous MgSO4. Crude products may be further purified by silica gel column chromatography (hexanes : ethyl acetate).
- 7.Olah GA, Prakash GKS, Molnar A, Sommer JM. Superacids. 2nd Ed. John Wiley & Sons Inc.; New York: 2009. [Google Scholar]
- 8.Zhao Y, Li Y, Wang S, Li Z. ARKIVOC. 2009;11:152–164. [Google Scholar]
- 9.Pankratov VA, Chesnokova AE. Russ. Chem. Rev. 1989;58:879–890. [Google Scholar]
- 10.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J. Fox, D. J. Gaussian, Inc.; Wallingford CT: 2009. [Google Scholar]
- 11.(a) Becke AD. Phys. Rev. A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]; (b) Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]; (c) Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]; (d) Krishnan R, Binkley JS, Seegar R, Pople JA. J. Chem. Phys. 1980;72:650–654. [Google Scholar]
- 12.Olah GA, Prakash GKS, Rasul G. J. Phys. Chem. C. 2008;112:7895–7899. [Google Scholar]
- 13.Olah GA, Klumpp DA. Superelectrophiles and Their Chemistry. John Wiley & Sons Inc.; New York: 2008. [Google Scholar]
- 14.Yato M, Ohwada T, Shudo K. J. Am. Chem. Soc. 1991;113:691–692. [Google Scholar]