Abstract
Objective
To compare bupropion to placebo for reducing methamphetamine (MA) use, increasing retention, and reducing the severity of depressive symptoms and MA cravings. A secondary objective compared bupropion to placebo for reducing cigarette smoking among MA dependent participants.
Methods
Following a 2-week, non-medication baseline screening period, 73 treatment-seeking MA dependent participants were randomly assigned to bupropion sustained release (150 mg twice daily; N=36) or placebo (twice daily; N=37) for 12-weeks under double blind conditions. Participants attended clinic thrice weekly to provide urine samples analyzed for MA-metabolite, to complete research measures and assessments, and to receive contingency management and weekly cognitive behavioral therapy sessions.
Results
There were no statistically significant effects for bupropion relative to placebo on MA use verified by urine drug screens, for reducing the severity of depressive symptoms or MA cravings, or on study retention. In a post hoc analysis, there was a statistically significant effect of bupropion treatment on MA use among participants with lighter (0–2 MA-positive urines), but not heavier (3–6 MA-positive urines) MA use during baseline (OR=2.81, 95% CI=1.61–4.93, p<0.001 for MA-free week with bupropion among light users). Bupropion treatment was also associated with significantly reduced cigarette smoking, by almost 5 cigarettes per day (p=0.0002).
Conclusion
Bupropion was no more effective than placebo in reducing MA use in planned analyses, though bupropion did reduce cigarette smoking. Post hoc findings of an effect for bupropion among baseline light, but not heavy, MA users suggests further evaluation of bupropion for light MA users is warranted.
Keywords: bupropion, methamphetamine dependence, randomized clinical trial
1. Introduction
Methamphetamine (MA) is a psycho-stimulant drug whose addictive characteristics, combined with its longer lasting stimulant effects and a cheaper street price than cocaine (Newton et al., 2005), have led to a surge in its abuse within the United States (US). Once only confined to western and rural areas, MA is becoming increasingly available in most US metropolitan areas (National Drug Intelligence Center, 2006). According to The National Household Survey on Drug Abuse, increased availability of MA has more than doubled the number of individuals who have tried MA in their lifetime from 3.8 million in 1994 to 11.7 million in 2004 (Substance Abuse and Mental Health Service Administration, 2005).
Widespread use of MA has led to numerous health care concerns. MA-related emergency room visits increased 54% in the US between 1995 and 2002 (Drug Abuse Warning Network, 2002). The immediate somatic effects of MA intake include increased blood pressure and heart rate (Newton et al., 2005), raising the risk for fatal cardiac rhythm disturbances and cerebral hemorrhaging (Mokhlesi et al., 2004) as well as acute coronary syndrome and myocardial infarction (Chen, 2007; Turnipseed et al., 2003; Wijetunga et al., 2004). Moreover, MA using populations are at high risk of infection with Hepatitis C virus (Gonzales et al., 2006) and HIV (Peck et al., 2005; Shoptaw et al., 2003) and frequently develop severe dental decay and multiple caries (Donaldson and Goodchild, 2006). MA users also have significant rates of co-morbid mood and anxiety disorders and are at significantly higher risk of developing a psychotic disorder than the general population and may continue to experience psychotic symptoms even years after stopping MA use (Zweben et al., 2004). Finally, although one-time MA use has been shown to improve performance on a variety of cognitive tasks (Silber et al., 2006), prolonged use causes numerous cognitive deficits, including decreased functioning of working memory, executive function, and reaction time (Kalechstein et al., 2003). These multiple health-related complications of MA abuse suggest that effective treatments for MA dependence are needed in order to minimize the negative public health effects of MA abuse.
Acute subjective and reinforcing effects of MA include feelings of euphoria, increased energy, and heightened sense of attentiveness (Hart et al., 2001; Newton et al., 2005; Newton et al., 2006). Withdrawal from MA is characterized by acute symptoms that have the opposite effect of the drug itself; depressive symptoms often including intense feelings of dysphoria (Logan, 2002), anxiety (Zweben et al., 2004), and fatigue (Newton et al., 2004). The acute subjective and reinforcing effects of MA are thought to result from MA-induced release of monoamines, including dopamine (DA) and norepinephrine (NE), via a variety of mechanisms. Like other stimulants, MA inhibits the reuptake of DA by the dopamine transporter (DAT) and causes reverse transport of DA into the synapse via DAT, producing increased extra cellular DA and enhanced stimulation of postsynaptic DA receptors (Khoshbouei et al., 2003). MA has also been shown to inhibit monoamine oxidase and to increase the expression of the DA synthesizing enzyme, tyrosine hydroxylase (Sulzer et al., 2005).
While the neurotoxic effects of MA on the dopaminergic system have yet to be fully understood, there is evidence that the withdrawal symptoms from MA use can be attributed to depletions in extra cellular DA concentrations. For example, studies have shown extensive reductions in the density and activity of DAT in the striatum in the days following MA use (Chang et al., 2007). Imaging studies have also shown that hypoactivity in the striatum can be correlated with self-reports of depression and anxiety in recovering MA users (Thompson et al., 2004). In addition, cognitive dysfunction and decreased activity of DA receptors caused by prolonged MA use can contribute to poor impulse control and inability to maintain goal-related behavior (Monterosso et al., 2005). Conceptually, restoring levels of DA (by increasing DA release, preventing reuptake, or slowing degradation after release) to pre-dependence levels may help MA abusers to initiate and/or maintain abstinence, may alleviate withdrawal symptoms, and may prevent relapse (Vocci and Appel, 2007).
Bupropion is a DA and NE reuptake inhibitor of the aminoketone class that has been approved both as an antidepressant and as a smoking cessation drug. Bupropion has relatively few antidepressant-associated side effects, such as sexual dysfunction and sedation (Stahl et al., 2004), has a low abuse potential (Nomikos et al., 1989), and is not fatal when taken in large doses (Shepherd et al., 2004). While bupropion's precise mechanism of action is not known, bupropion binds to DAT and has been shown to increase DA transmission in both the nucleus accumbens and the prefrontal cortex (Rau et al., 2005). By restoring depleted levels of monoamines, bupropion may be effective in ameliorating withdrawal symptoms and cognitive deficits in patients recovering from MA abuse, thereby reducing MA use. A randomized, placebo-controlled trial of bupropion for cocaine dependence found a significant effect for bupropion relative to placebo in reducing cocaine use when provided with a contingency management intervention, but not with a non-contingent voucher program (Poling et al., 2006). Two other randomized placebo- controlled trials of bupropion plus cognitive behavioral therapy failed to find an effect for bupropion in cocaine dependence (Margolin et al., 1995; Shoptaw et al., 2008).
To date, no effective pharmacologic treatments for MA addiction have been identified, although results of a recent clinical trial provide preliminary evidence supporting the efficacy of bupropion for reducing MA use among participants with lighter (MA use on 18 or fewer of the past 30 days) but not heavier (MA use on more than 18 of the last 30 days) MA use at baseline (Elkashef et al., 2007). The aim of this study is to evaluate the efficacy of bupropion compared to placebo as a treatment for MA dependence in the presence of evidence-based behavioral therapies, including contingency management and cognitive behavioral therapy. We hypothesized that participants receiving bupropion would demonstrate statistically significant reductions in MA use over participants receiving placebo. We also expected to see greater treatment retention and larger reductions in depressive symptoms and cravings for MA among participants receiving bupropion compared to those receiving placebo. Because bupropion treatment has been shown to reduce cigarette smoking in other populations, we expected to see reductions in cigarette smoking as well.
2. Methods
2.1. Participants
Study participants were 73 MA dependent outpatients seeking treatment in the Los Angeles area. All participants met the following inclusion criteria: (1) 18 years of age or older, (2) current MA dependence verified by the Structured Clinical Interview for the DSM-IV-TR (SCID; (Spitzer et al., 1995), (3) willing and able to comply with study procedures, (4) willing and able to provide written informed consent and (5) if female and of childbearing potential, not pregnant or lactating, and willing to use an acceptable method of birth control.
Participants met none of the following exclusion criteria: (1) a medical condition that would interfere with safe study participation, such as active tuberculosis, unstable cardiac or liver disease, unstable diabetes, uncontrolled hypertension, symptomatic AIDS diagnosis, or elevated liver enzymes greater than 3 times the upper limit of normal, (2) a current neurologic or major psychiatric disorder not due to substance abuse (e.g., schizophrenia, bipolar or major affective disorder) as assessed by the SCID, (3) current serious suicidal intention or plan, (4) taking a prescription medication that is known to interact with the study medication, (5) current dependence on cocaine, opiates, alcohol, or benzodiazepines, as assessed by the SCID, (6) a history of alcohol dependence within the past 3 years, (7) any history of seizures or a closed head injury, (8) a history of anorexia or bulimia, and (9) a history of sensitivity to bupropion.
2.2. Procedures
Study activities occurred at three clinical research sites in the Los Angeles area (Rancho Cucamonga, Hollywood, and UCLA). All study protocols were approved by two local IRBs (UCLA and Friends Research Institute, Inc.).
2.2.1. Recruitment
Potential study participants were recruited from the community using advertisements for a study of experimental medications for MA dependence. Interested individuals telephoned a toll-free number and if appropriate would schedule an intake visit with a study investigator to discuss the study risks and procedures and initiate the informed consent process.
2.2.2. Design
The study used a randomized, double-blind, placebo-controlled clinical trial design with an active medication condition (bupropion sustained release 150 mg twice daily) and a matching placebo and a psychosocial/behavioral platform of cognitive behavioral therapy (CBT) and contingency management (CM). After completing a two week baseline/screening period for eligibility assessment, including a complete physical exam with blood work and an EKG, eligible participants were randomly assigned to receive either bupropion or placebo, in conjunction with CBT and CM, for 12 weeks. Participants visited the study clinic three times per week (Monday, Wednesday, and Friday) to provide urine samples, to conduct medication exchanges and monitor participant safety, to complete study assessments, and to receive psychosocial/behavioral treatments. At termination, participants underwent a repeat physical examination, including blood work and an EKG, with the study physician, followed by a brief health visit at 30 days after terminating study participation. All study activities were provided free of charge. Other than non-cash vouchers earned as part of the CM intervention, participants were not compensated for participation but did receive $20 both for completing the baseline assessments and the study completion/termination assessments.
2.2.3. Assessments
A battery of measures determined study eligibility, assessed participant safety and documented treatment efficacy. The SCID was used to identify past and current psychiatric and substance use diagnoses and to verify inclusion and exclusion criteria. The ASI-Lite (McLellan et al., 1992) was used to measure the severity of participants' reported addiction- related problems in seven areas of functioning: medical, employment, drug use, alcohol use, legal, family/social, and psychiatric. The Beck Depression Inventory (BDI), a 21-item questionnaire concerning symptoms of depression (Beck, 1967), was used to measure participants' depressive symptoms at baseline and weekly during the medication period. Participants' reports of MA craving over the past 24 hours was measured at baseline and weekly during the trial using a visual analogue scale that ranges from 0 (no craving) to 100 (most intense craving possible). The quantity of participants' self-reported alcohol and drug use, as well as average number of cigarettes smoked per day, were assessed at baseline and weekly during treatment using the Substance Use Inventory (Sobell et al., 1986).
Medication adherence was measured using weekly pill counts justified against reports of medication-taking to calculate the proportion of dispensed medication doses that were taken. Participants met with the study physician each week to receive a one-week supply of medication in blister packaging in exchange for the previous week's blister package with any unused medication and to complete the pill count. Urine samples were collected thrice weekly throughout the study period. All samples between 93–100° F at the time of collection were considered valid. Urine samples were analyzed immediately onsite using radioimmunoassay (Phamatech, Inc, San Diego, CA) for qualitative tests of MA metabolite.
2.2.4. Psychosocial counseling
All participants received a standard counseling program, consisting of weekly individual CBT sessions during the medication phase of the study. Counseling was delivered by a masters-level therapist who received training in the use of the 12-week CBT program and familiarity with its manualized format (Carroll, 1998). To maintain the integrity of the counseling program, counselors met once weekly with the principal investigator (S.S.) to receive corrective feedback and individual clinical supervision.
2.2.5. Contingency Management
To increase the likelihood of initiating abstinence from MA use, a contingency management (behavioral reinforcement) intervention was provided (Roll et al., 2006). Non-cash vouchers for goods and services promoting a healthy drug-free lifestyle were earned for MA metabolite-free urine samples, on an escalating schedule for the first 4 weeks after signing consent, then remaining at this level for the remaining 10 weeks prior to discontinuation. The voucher for the initial MA-free sample was worth $3.00. Vouchers increased in value by $1.00 for each consecutive MA-free sample to a maximum of $15.00 at the end of the 4th week and remained at $15.00 for the remainder of the 12 week treatment period. Participants who provided a sample positive for MA-metabolite, or who failed to submit a urine sample, did not receive a voucher for that visit and their subsequent voucher value was reduced to the initial $3.00, with a reset after three consecutive MA-free urine specimens. The behavioral technician provided supportive feedback for samples indicating abstinence and informed the subject of the value of the voucher earned that day and their total voucher balance. The maximum that could be earned for providing MA-metabolite free urine samples at all visits throughout the entire study was $537 in vouchers. Participants who terminated study participation early received vouchers reflecting what they earned to date.
2.2.6. Medication procedures
Bupropion sustained release (SR) 150mg tablets were purchased from the manufacturer (GlaxoSmithKline, Middlesex, UK) and over-encapsulated active medication tablets and matching placebo capsules were prepared by a compounding pharmacy (Inland Compounding Pharmacy, Loma Linda, CA). Doses of study medication were as follows: bupropion SR 150 mg per day for days 1–3 of the first week followed by an increase to 300 mg per day (one 150 mg capsule taken twice daily) until week 12 when the dose was decreased to 150 mg of bupropion SR for the last 3 days. Participants ingested the first dose of study medication under the supervision of the study physician and then were dispensed a 2 week supply of medication in blister packages and instructed in how to self-administer the medication at home. Participants were required to bring the experimental drug packets to the site each visit for pill counts to monitor drug adherence.
2.2.7. Safety
Participants underwent a medical history and physical examination, EKG, and routine laboratory studies during screening and at study termination. Participants' vital signs were measured weekly and study research assistants interviewed participants concerning any adverse events and the use of concomitant medications weekly at clinic visits. Participant suicidal intention was closely monitored using data from the BDI, verbal reports, and during counseling sessions. Participants who showed any signs of suicidal behavior were evaluated by study staff trained to respond with the appropriate steps needed, including other treatment referrals and study discontinuation.
2.3. Data analysis
All analyses used an “intention-to-treat” approach. The primary study outcome was MA use as assessed via urine drug screens and secondary outcomes were treatment retention, depressive symptoms, MA cravings, and adverse events. The following aggregate measures of urine drug screen results were calculated: the Joint Probability Index at six and twelve weeks of treatment (the number of MA-metabolite free urine specimens submitted by participants in each treatment group at that time divided by the number of participants randomized to the treatment group) and the Treatment Effectiveness Score (the sum of the number of MA-free urine samples submitted per participant (Ling et al., 1997), the percentage of samples negative for MA overall, the longest consecutive period of MA abstinence, and the percentage of participants with at least 2 and at least 3 consecutive weeks of MA abstinence. Univariate comparisons of baseline demographic, drug use, and psychiatric characteristics of participants as well as comparisons of missing data rates, aggregate measures of urine drug screen results, medication adherence, and MA craving by treatment group assignment were performed using analysis of variance (ANOVA) for continuous variables and chi square for categorical variables (Tabachnick and Fidell, 2000). The proportion of participants who completed the trial, defined as at least one study visit during week 12 (the final week of the medication treatment period), in each treatment condition was compared using chi square analysis. Study retention was measured as the number of days from the first dose of study medication at the time of randomization to the participant's last study visit during the 12 week medication treatment period. Differences in retention by treatment condition were evaluated using a Kaplan–Meier survival function (Allison and SAS Institute, 1995).
Primary study hypotheses concerning the effect of bupropion versus placebo on treatment outcomes were tested using generalized estimating equation (GEE) models (Zeger and Liang, 1986). The effect of treatment condition on urine drug screen results was examined using a GEE logistic regression model with the dependent variable being a MA-free week, defined as all available urine specimens during the week are MA-metabolite free. A post hoc analysis compared treatment outcomes separately among baseline light MA users, defined as 0–2 of the 6 urine drug screens during the two week baseline/screening period positive for MA-metabolites, and baseline heavy MA users, defined as 3–6 of the 6 urine drug screens during the two week baseline/screening period positive for MA-metabolites. The post hoc analysis comparing potential effects of treatment on urine drug screen results in separate GEE models among baseline heavy versus light MA users was also repeated using self-reported past 30 days MA use to stratify the sample as heavy (MA use on >18 days) versus light (MA use on ≤ 18 days) MA users, as done previously by Elkashef et al. (2007). Effect of treatment condition on continuous measures such as the BDI and MA-craving VAS scale were evaluated using a mixed model approach (Singer, 1998). All analyses were run in SPSS 14.0 (SPSS Incorporated, 2005) and SAS for Windows 9.0 (SAS Institute Incorporated, 2004).
3. Results
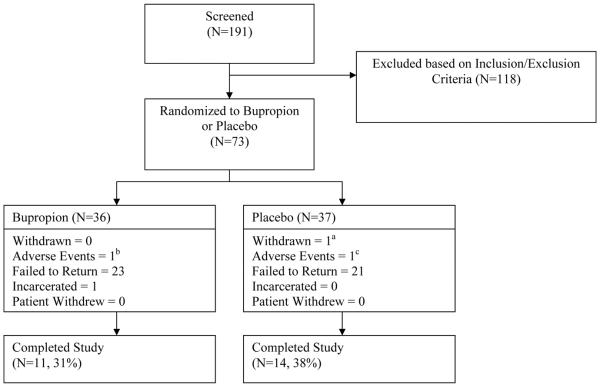
A total of 191 treatment-seeking individuals provided informed consent and entered the 2-week screening period, of which 73 met all inclusion and no exclusion criteria and were randomized into the study (Figure 1). Thirty one percent of participants randomized to the bupropion condition completed the 12-week medication period, defined as at least one study visit during week 12, compared to 38% of participants randomized to placebo (χ2= 0.43, df=1, p=0.512). Reasons for termination prior to study completion in each group are shown in Figure 1. Study completion rates were significantly higher among baseline light MA users (51% versus 17% among baseline heavy MA users, χ2= 9.75, df=1, p=0.002) and males (43% versus 19% among females, χ2= 4.04, df=1, p=0.044), but there were no significant associations between study completion and age, ethnicity, or baseline lifetime MA use or route of MA administration (data not shown). In a post hoc analysis, there were no significant differences in the proportion of participants completing the trial by treatment condition among baseline light MA users (41% for bupropion versus 60% for placebo, χ2= 1.30, df=1, p=0.254) or baseline heavy MA users (21% for bupropion versus 12% for placebo, χ2= 0.56, df=1, p=0.455).
Figure 1.
Study design flow chart. awithdrawal due to participant becoming pregnant during medication phase; bmedication discontinued after participant developed chest pain; c treatment discontinued and participant referred to higher level of care after hospitalized for suicidality
3.1. Baseline demographic, drug use, and psychiatric characteristics
The baseline characteristics of participants randomized to the bupropion and placebo conditions are shown in Table 1. Participants in the bupropion condition reported more days of cannabis use in the past 30 days than participants in the placebo group, otherwise there were no significant differences.
Table 1.
Participant baseline demographic, drug use, and psychiatric characteristics by treatment condition (bupropion or placebo)
| Measures | Condition | |
|---|---|---|
|
| ||
| Bupropion (n=36) mean (sd) or % | Placebo (n=37) mean (sd) or % | |
| Age (in years) | 34.6 (10.6) | 34.6 (10) |
| Ethnicity | ||
| White | 55.6% | 56.8% |
| Hispanic | 38.9% | 35.1% |
| Black | 2.8% | 2.7% |
| Asian | 0% | 2.7% |
| Other | 2.8% | 2.7% |
| Gender | ||
| Male | 61.1% | 67.6% |
| Female | 38.9% | 32.4% |
| Martial Status | ||
| Married | 25.0% | 32.4% |
| Never married | 47.2% | 45.9% |
| Divorced/separated | 27.8% | 21.6% |
| Education (in years) | 13.1 (2.1) | 12.8 (2.3) |
| Employment | ||
| Full time | 50.0% | 62.2% |
| Part time | 25.0% | 18.9% |
| Unemployed | 19.4% | 18.9% |
| Student/retired/military | 5.6% | 0.0% |
| Income in past 30 days (US$) | 6,181 (13,565) | 2,092 (2,197) |
| Days MA use (in past 30 days) | 15.1 (10.4) | 16.2 (10.8) |
| Years MA use | 11 (9.6) | 8.3 (5.8) |
| Route of MA administration | ||
| Smoking | 58.3% | 70.3% |
| Nasal | 25.0% | 18.9% |
| Injection | 13.9% | 10.8% |
| Oral | 2.8% | 0% |
| Days cocaine use (in past 30 days) | 0.1 (0.4) | 0.1 (0.4) |
| Days cannabis use (in past 30 days)* | 8.8 (11.1) | 2.4 (4.7) |
| Days alcohol use (in past 30 days) | 4.4 (6.5) | 3.6 (5.5) |
| Current cigarette smoker | 81% | 51% |
| Baseline ASI composite scores | ||
| Medical | 0.18 (0.24) | 0.22 (0.29) |
| Employment | 0.38 (0.34) | 0.37 (0.31) |
| Alcohol | 0.10 (0.18) | 0.06 (0.09) |
| Drug | 0.21 (0.10) | 0.20 (0.11) |
| Legal | 0.19 (0.20) | 0.14 (0.22) |
| Family/social | 0.24 (0.23) | 0.20 (0.27) |
| Psychiatric | 0.15 (0.18) | 0.19 (0.21) |
| Beck Depression Inventory score | 17.3 (10.3) | 16.8 (11.3) |
t=3.21, df=71, p=0.002
3.2 Missing data
There was no statistically significant difference between the mean proportion of missing urine drug screen specimens in the bupropion versus the placebo conditions (t= −0.45, df=71, p=0.65). On average, of the 36 possible urine samples during the 12 week treatment period, 52% were missing in the bupropion condition compared to 56% in the placebo condition.
3.3 Medication adherence
Pill count data were missing for 5 participants (2 in the bupropion condition and 3 in the placebo condition) who dropped out of the study prior to returning any medication blister packages for pill counts. Among the remaining participants with pill count data available, participants in the bupropion condition reported taking 85% of the pills dispensed to them compared to 92% in the placebo group, which was not a statistically significant difference (t=−1.41, df=49, p=0.16).
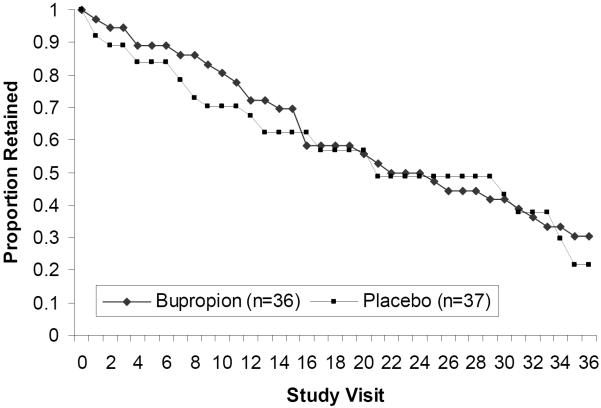
3.4 Retention
Survival analysis results showed that there were no statistically significant differences in retention between participants assigned to receive bupropion versus those assigned to receive placebo as tested via log rank (χ2=0.34, df=1, p=0.56, Figure 2). Participants in the bupropion condition were retained in the study for a mean of 52.6 days (SD=28.1) compared to 50.7 days (SD=31.3) for participants in the placebo condition (t=0.28, df= 71, p=0.783). In a post hoc survival analysis, there were no statistically significant differences in retention between treatment groups among baseline heavy or baseline light MA users (χ2=3.18, df=1, p=0.07 for heavy users and χ2=0.05, df=1, p=0.83 for light users, plots not shown).
Figure 2.
Survival analysis depicting the proportion of participants retained in each treatment condition (bupropion and placebo) throughout the 36 study visits (12 weeks).
3.5 Urine drug screen results
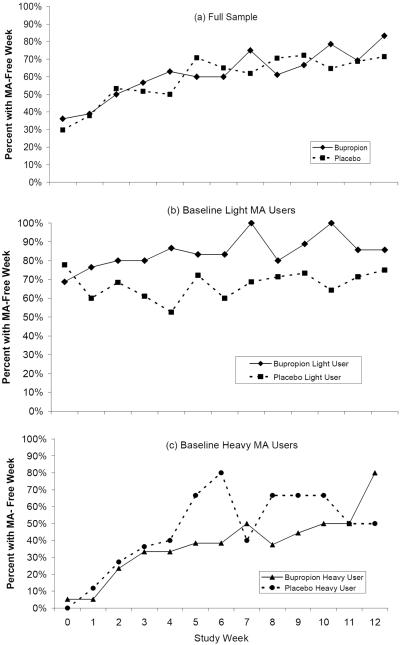
There were no statistically significant differences between participants receiving bupropion and those receiving placebo in planned univariate analyses of aggregate urine drug screen results (Table 2, Full Sample). The proportion of participants with a MA-free week throughout the trial in the bupropion condition was similar to that in the placebo condition (Figure 3, panel a) and there was no significant difference between treatment conditions in the probability of achieving a MA-free week in a GEE model (χ2=0.004, df=71, p=0.95). In a post hoc analysis, there were no significant differences between bupropion and placebo in aggregate urine measures with the sample stratified by baseline heavy- MA use, defined as 3–6 urine drug screens positive for MA-metabolites during the two week baseline period positive (N=36; Table 2, Heavy MA Users) versus light-MA use, defined as 0–2 MA-positive urine drug screens during the two week baseline period (N=37; Table 2, Light MA Users). There was a statistically significant effect for bupropion relative to placebo in a GEE model adjusting for gender and ethnicity with the sample stratified by baseline light versus heavy MA users. Among baseline light MA users, the probability of achieving a MA-free week was significantly higher in the bupropion condition relative to the placebo condition (OR=2.81, 95% CI=1.61–4.93, p<0.001; Figure 3, panel b), while there was no significant difference between conditions among baseline heavy MA users (OR=0.93, 95% CI=0.24–3.53, p=0.91 for bupropion relative to placebo; Figure 3, panel c). In an additional post hoc analysis replicating the analysis of Elkashef et al. (2007), there was no statistically significant effect for bupropion relative to placebo in separate GEE models among participants with baseline heavy MA use (MA use on >18 of the last 30 days) or baseline light MA use (MA use on ≤ 18 of the last 30 days), although the effect for bupropion on a MA-free week among light users was in the predicted direction (OR=1.26, 95%CI 0.77–2.09, p=0.36). The heavy/light user variables defined via baseline urine sample results and baseline self-reported MA use were significantly correlated using Pearson's correlation coefficient (r =0.562, p=0.01).
Table 2.
Aggregate measures of urine drug screen results, depressive symptoms (BDI score), and methamphetamine (MA) cravings (visual analog scale) by treatment condition in the full sample and among baseline heavy (3 or more MA-positive urine drug screens during lead-in) and light (0–2 MA-positive urine drug screens during lead-in) MA users
| Full Sample | Heavy MA Users | Light MA Users | ||||
|---|---|---|---|---|---|---|
| Bupropion (N=36) | Placebo (N=37) | Bupropion (N=19) | Placebo (N=17) | Bupropion (N=17) | Placebo (N=20) | |
| Urine Aggregates | ||||||
| Joint Probability Indexa | ||||||
| Treatment Week 6 | 0.33 | 0.22 | 0.21 | 0.12 | 0.47 | 0.30 |
| Treatment Week 12 | 0.25 | 0.16 | 0.16 | 0.06 | 0.35 | 0.25 |
| Treatment Effectiveness Scoreb | 12.5 | 11.3 | 7.2 | 4.0 | 18.5 | 17.6 |
| MA-negative urine samples, mean, % | 35% | 31% | 20% | 11% | 51% | 49% |
| Longest MA abstinence, mean, days | 18 | 16 | 10 | 5 | 27 | 25 |
| Two consecutive weeks of MA abstinence, % | 39% | 38% | 16% | 18% | 65% | 55% |
| Three consecutive weeks of MA abstinence, % | 28% | 27% | 16% | 6% | 41% | 45% |
| BDI Scores, mean | ||||||
| Baseline | 17.5 | 16.8 | 18.5 | 19.9 | 16.3 | 14.3 |
| Treatment Week 12 | 4.2 | 3.5 | 3.8 | 5.7 | 4.4 | 2.9 |
| MA craving, mean, visual analog scale | ||||||
| Baseline | 53.1 | 45.9 | 66.8 | 60.6 | 37.6 | 33.5 |
| Treatment Week 12 | 22.5 | 27.1 | 22 | 33.3 | 22.9 | 25.5 |
The number of MA-free urine specimens submitted at the final visit in the week divided by the number of participants randomized to the treatment condition.
The average of the sum of MA-free urine specimens provided during the treatment period by participants in each treatment condition.
Figure 3.
Percentage of participants with a methamphetamine (MA)- free week by treatment condition for (a) the full sample and among baseline (b) light-MA users (0–2 MA positive urines during the baseline period) and (c) heavy- MA users (3–6 MA positive urines during the baseline period).
3.6 Depressive symptoms
Depressive symptoms, as measured via the BDI, decreased among participants in both conditions during the treatment period (Table 2, Full Sample). But there was no statistically significant difference in BDI scores during the treatment period between participants in the two treatment conditions in a mixed effects model (t=0.22, df=71, p=0.82). In a post hoc analysis, BDI scores decreased during the treatment period among both heavy and light MA users (Table 2), but there were no statistically significant differences in BDI scores between the two treatment conditions in mixed effects models among heavy (t= −0.86, df=1, p=0.40) or light (t=1.01, df=1, p=0.32) MA users.
3.7 MA craving
Cravings for MA, as measured on a 0 to 100 visual analog scale, decreased among participants in both conditions during the treatment period (Table 2). But there was no statistically significant difference in MA cravings between participants in the two treatment conditions throughout the trial in a mixed effects model (t=0.38, df=71, p=0.70). In a post hoc analysis, MA cravings decreased during the treatment period among both heavy and light MA users (Table 2), but there were no statistically significant differences in MA cravings between the two treatment conditions in mixed effects models among heavy (t=0.67, df=1, p=0.51) or light (t= −0.16, df=1, p=0.88) MA users.
3.8 Results of Psychosocial and Behavioral Interventions
Participants in the bupropion condition attended an average of 5 of the 12 weekly CBT counseling sessions (SD=3.8) compared to an average of 4 sessions (SD=3.7) for the placebo condition, which is a non-significant difference (t=0.17, df=71, p=0.87). Out of a total possible $537 in vouchers that could be earned contingent on providing MA-metabolite free urine specimens throughout the trial, participants receiving bupropion earned on average $134 (SD=$175) in vouchers, compared to $110 (SD=$153) among participants in the placebo group, which was not a statistically significant difference (t=0.66, df=71, p=0.51).
3.9 Cigarette smoking
Analyses of cigarette smoking were limited to participants who reported cigarette smoking during the trial (48 (65.8%) of the 73 participants randomized). There were no statistically significant differences in the mean number of cigarettes smoked per day between cigarette smokers in the two treatment conditions at baseline. In a mixed effects model, the reduction in the number of cigarettes smoked per day during the trial was significantly greater in the bupropion condition compared to the placebo condition, with participants in the bupropion condition smoking on average almost 5 fewer cigarettes per day compared to participants in the placebo condition (estimate= −4.85, t=−3.26, df=46, p=0.002 for bupropion relative to placebo).
3.10 Adverse events
Three serious adverse events requiring hospitalization occurred during the trial; none were determined to be treatment related. Two HIV positive participants in the bupropion condition were hospitalized for infections (perirectal abscess and cellulitis), which resolved without sequelae allowing them to continue to participate. One participant in the placebo condition was hospitalized for depressive symptoms and suicidal ideation in the setting of continued MA use and was discontinued from study participation and referred to a higher level of care. Experimental medication was also discontinued in one participant in the bupropion condition who developed chest pain requiring treatment in the emergency room but not hospitalization and one participant in the placebo group who became pregnant. The most commonly reported adverse events were headache and nasal congestion/upper respiratory infection symptoms, which occurred in similar rates in the two treatment conditions (Table 3).
Table 3.
Frequency of adverse events reported by treatment condition (bupropion, N=36 and placebo, N=37)
| Bupropion | Placebo | Total | |
|---|---|---|---|
| Headache | 14 | 14 | 28 |
| Nasal congestion/URI | 4 | 10 | 14 |
| Musculoskeletal pain | 6 | 7 | 13 |
| Insomnia | 5 | 6 | 11 |
| Dizziness/Lightheaded | 4 | 1 | 5 |
| Injury | 2 | 3 | 5 |
| Chest Pain | 2 | 1 | 3 |
| Dysphoria | 1 | 2 | 3 |
| Ear pain | 3 | 0 | 3 |
| Flu Symptoms | 1 | 2 | 3 |
| Toothache | 2 | 1 | 3 |
| Diarrhea | 1 | 1 | 2 |
| Fever | 0 | 2 | 2 |
| Heart palpitations | 1 | 1 | 2 |
| Nausea/vomiting | 2 | 0 | 2 |
| Rash/itching | 2 | 0 | 2 |
| Stomach pain | 1 | 1 | 2 |
| Blurry vision | 0 | 1 | 1 |
| Cellulitis | 1 | 0 | 1 |
| Dental pain | 1 | 0 | 1 |
| Dry mouth | 0 | 1 | 1 |
| Eye infection | 0 | 1 | 1 |
| Grogginess | 0 | 1 | 1 |
| Hepatitis | 1 | 0 | 1 |
| Menstrual pain | 0 | 1 | 1 |
| Nausea/vomiting | 1 | 0 | 1 |
| Nausea/vomiting | 1 | 0 | 1 |
| Nosebleed | 1 | 0 | 1 |
| Perirectal abscess | 1 | 0 | 1 |
| Polyuria | 0 | 1 | 1 |
| Pregnancy | 0 | 1 | 1 |
|
| |||
| Total | 58 | 59 | 117 |
4. Discussion
In this randomized, double-blind, placebo-controlled trial of bupropion for the treatment of MA dependence, there were no significant effects for bupropion relative to placebo in planned analyses of MA use, retention, depressive symptoms, and MA-cravings. In a post hoc analysis, bupropion reduced MA use significantly more than placebo among participants with light- but not heavy-MA use as defined by the frequency of MA positive urine drug screens during the baseline period. These findings are consistent with those of a previous trial that found bupropion to be more effective in reducing MA use among male participants with low-to-moderate self-reported MA use at baseline (Elkashef et al., 2007), although we were unable to directly replicate the previous study's findings due to the small sample size in our study. Bupropion also significantly reduced ad libitum cigarette smoking relative to placebo despite the lack of any psychosocial/behavioral treatment targeting cigarette smoking cessation, which is consistent with bupropion's efficacy for smoking cessation (Hughes et al., 2007) and suggests that the failure to detect a main effect for bupropion on MA use was not due to problems with internal validity. Together, results of the two trials of bupropion for MA dependence suggest that larger studies to determine the effectiveness of bupropion in reducing MA use among baseline light-MA users are warranted.
Chronic high-dose MA use produces neurotoxic effects that may be responsible for bupropion's lack of efficacy among heavy- MA users. In preclinical studies, high-dose MA produces neurotoxic changes in striatal dopaminergic cells as well as deficits in striatal tyrosine hydroxylase activity, DA concentrations, and DAT levels (Davidson et al., 2001; Harvey et al., 2000; Robinson et al., 1990; Sabol et al., 2001; Wagner et al., 1980). Clinical imaging studies have also found significant deficits in dopaminergic function among chronic MA users, including reductions in DAT density (Volkow et al., 2001b) and DA receptor occupancy (Volkow et al., 2001a; Volkow et al., 2001c), which are thought to contribute to the dysphoric symptoms that accompany MA dependence and withdrawal. Chronic high-dose MA exposure is thought to produce these neurotoxic effects by impairing the ability of synaptic vesicles to take up DA via disruption of the vesicular proton gradient necessary for VMAT-2 functioning (Sulzer et al., 1995; Sulzer and Rayport, 1990), resulting in accumulation of cytoplasmic DA and the subsequent production of harmful reactive oxygen species (Cubells et al., 1994; Fleckenstein et al., 1997). DA reuptake blockers such as bupropion increase vesicular dopamine uptake via enhancement of VMAT-2 function (Brown et al., 2001; Rau et al., 2005) suggesting that treatment with bupropion may counteract the MA-induced accumulation of cytosolic DA and reactive oxygen species thereby reducing the neurotoxic effects of MA. Yet, while preclinical studies have shown that treatment with the reuptake blockers methylphenidate and bupropion reversed MA-induced reductions in VMAT-2 activity, bupropion did not prevent the long-term deficits in dopaminergic function produced by repeated high-dose MA administration (Rau et al., 2005; Sandoval et al., 2003), which may explain the lack of clinical effect for bupropion among heavy-MA users. Alternatively, bupropion's relatively weak effect in blocking DA reuptake (Argyelan et al., 2005; Meyer et al., 2002) may simply be overwhelmed by chronic high-dose MA use.
While this trial did not address cigarette smoking directly, bupropion significantly reduced self-reported cigarette smoking as compared to placebo among MA dependent cigarette smokers in a pre-planned analysis of a secondary outcome. Interestingly, the previous study of bupropion for MA dependence failed to find an effect for bupropion on cigarette smoking relative to placebo (Elkashef et al., 2007), and the reason for this discrepancy between the two studies is not clear. Considering that the prevalence of cigarette smoking among illicit drug users, including MA users, is as high as 70%–90% (Budney et al., 1993; Grant et al., 2004; Kalman et al., 2005; Richter et al., 2002) and that cigarette smoking is associated with poor health outcomes among illicit drug users, above and beyond those found in non-smoking drug users (Hser et al., 1994; Hurt et al., 1996), the identification of smoking cessation treatments effective in MA users is an important public health priority. Our results provide preliminary support for bupropion as a smoking cessation medication among MA users and provide evidence for continued evaluation of bupropion as a medication for co-morbid stimulant abusers who smoke cigarettes.
In contrast to our findings with cigarette smoking, there were no significant effects for bupropion over placebo in reducing depressive symptoms or MA-cravings common to MA-withdrawal despite bupropion's efficacy as an antidepressant (Hansen et al., 2005). This may imply the putative mechanism of action for bupropion as a medication for MA dependence is likely to be independent of reductions in withdrawal symptoms shared by both MA- and nicotine withdrawal, including depressive symptoms, irritability, and difficulty concentrating (Shiffman et al., 2000). Studies suggest that antidepressant treatment is effective among substance abusers for depressive symptoms that persist despite abstinence or in patients with a history of depressive symptoms that pre-date substance use, as compared to transient depressive symptoms related to substance use or withdrawal (Nunes and Levin, 2004). The lack of an effect for bupropion relative to placebo on depressive symptoms may be due to exclusion of participants with non-substance use-related depressive symptoms or to reductions in depressive symptoms in the placebo group as a result of the cognitive behavioral therapy platform.
Findings from this study are limited by the relatively small sample size of this preliminary clinical trial of bupropion for MA dependence and the attrition of participants during the trial, which limit the study's power. But the failure to find a significant effect for bupropion relative to placebo in any of the pre-planned analyses of treatment outcomes suggests that the negative result is likely not due to inadequate power. An additional limitation is that the finding of an effect for bupropion among light-MA users was in a post hoc analysis, although our post hoc findings are similar to those of a previous study that included pre-planned analyses among participants with baseline heavy versus light MA use (Elkashef et al., 2007). There is a strong, but incomplete association between using results of baseline urine drug screens to define heavy/light MA users (0–2 urine samples positive for MA-metabolite versus 3–6 positive samples) and Elkashef et al. who used self-reported past 30 day MA use to define heavy/light users (≤18 days MA use versus >18 days MA use). Coupled with the small sample size and lowered power in the present trial, the differing definitions likely explain the similar, but not identical findings for baseline MA use levels and response to bupropion.
In conclusion, bupropion was no more effective than placebo in reducing MA use in planned analyses of this randomized, double-blind clinical trial, though the medication did reduce cigarette smoking. Bupropion did reduce MA use more than placebo among baseline light-MA users in a post hoc analysis. Further evaluation of bupropion as a treatment for MA dependence among light-MA users is warranted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison PD, SAS Institute . Survival analysis using the SAS system : a practical guide. SAS Institute; Cary, NC: 1995. [Google Scholar]
- Argyelan M, Szabo Z, Kanyo B, Tanacs A, Kovacs Z, Janka Z, Pavics L. Dopamine transporter availability in medication free and in bupropion treated depression: a 99mTc-TRODAT-1 SPECT study. J Affect Disord. 2005;89:115–123. doi: 10.1016/j.jad.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Beck A. Depression. University of Pennsylvania Press; Philadelphia, PA: 1967. [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Cocaine-induced increases in vesicular dopamine uptake: role of dopamine receptors. J Pharmacol Exp Ther. 2001;298:1150–1153. [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Carroll KM. NIDA Therapy Manuals for Drug Addiction. Bethesda, MD: 1998. A cognitive-behavioral approach: treating cocaine addiction. [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction (Abingdon, England) 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chen JP. Methamphetamine-associated acute myocardial infarction and cardiogenic shock with normal coronary arteries: refractory global coronary microvascular spasm. The Journal of invasive cardiology. 2007;19:E89–92. [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Donaldson M, Goodchild JH. Oral health of the methamphetamine abuser. Am J Health Syst Pharm. 2006;63:2078–2082. doi: 10.2146/ajhp060198. [DOI] [PubMed] [Google Scholar]
- Drug Abuse Warning Network . Amphetamine and Methamphetamine Emergency Department Visits, 1995–2002. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2002. [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Wilkins DG, Gibb JW, Hanson GR. Interaction between hyperthermia and oxygen radical formation in the 5-hydroxytryptaminergic response to a single methamphetamine administration. J Pharmacol Exp Ther. 1997;283:281–285. [PubMed] [Google Scholar]
- Gonzales R, Marinelli-Casey P, Shoptaw S, Ang A, Rawson RA. Hepatitis C virus infection among methamphetamine-dependent individuals in outpatient treatment. Journal of substance abuse treatment. 2006;31:195–202. doi: 10.1016/j.jsat.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med. 2005;143:415–426. doi: 10.7326/0003-4819-143-6-200509200-00006. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000;871:259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Prev Med. 1994;23:61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane database of systematic reviews (Online) 2007:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. Jama. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. The Journal of neuropsychiatry and clinical neurosciences. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. The Journal of biological chemistry. 2003;278:12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ. Treatment effectiveness score as an outcome measure in clinical trials. NIDA Res Monogr. 1997;175:208–220. [PubMed] [Google Scholar]
- Logan B. Methamphetamine-Effects on human performance and behavior. Forensic Sci Rev. 2002;14:133. [PubMed] [Google Scholar]
- Margolin A, Kosten TR, Avants SK, Wilkins J, Ling W, Beckson M, Arndt IO, Cornish J, Ascher JA, Li SH, Bridge P. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40:125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of substance abuse treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology. 2002;163:102–105. doi: 10.1007/s00213-002-1166-3. [DOI] [PubMed] [Google Scholar]
- Mokhlesi B, Garimella PS, Joffe A, Velho V. Street drug abuse leading to critical illness. Intensive care medicine. 2004;30:1526–1536. doi: 10.1007/s00134-004-2229-1. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- National Drug Intelligence Center . National Drug Threat Assessment. U.S. Department of Justice; Washington, D.C.: 2006. [Google Scholar]
- Newton TF, De La Garza R, 2nd, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacology, biochemistry, and behavior. 2005;82:90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology. 1989;2:273–279. doi: 10.1016/0893-133x(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- Peck JA, Shoptaw S, Rotheram-Fuller E, Reback CJ, Bierman B. HIV-associated medical, behavioral, and psychiatric characteristics of treatment-seeking, methamphetamine-dependent men who have sex with men. J Addict Dis. 2005;24:115–132. doi: 10.1300/J069v24n03_10. [DOI] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Archives of general psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Rau KS, Birdsall E, Hanson JE, Johnson-Davis KL, Carroll FI, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Bupropion increases striatal vesicular monoamine transport. Neuropharmacology. 2005;49:820–830. doi: 10.1016/j.neuropharm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Richter KP, Ahluwalia HK, Mosier MC, Nazir N, Ahluwalia JS. A population-based study of cigarette smoking among illicit drug users in the United States. Addiction. 2002;97:861–869. doi: 10.1046/j.1360-0443.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yew J, Paulson PE, Camp DM. The long-term effects of neurotoxic doses of methamphetamine on the extracellular concentration of dopamine measured with microdialysis in striatum. Neurosci Lett. 1990;110:193–198. doi: 10.1016/0304-3940(90)90810-v. [DOI] [PubMed] [Google Scholar]
- Roll JM, Petry NM, Stitzer ML, Brecht ML, Peirce JM, McCann MJ, Blaine J, MacDonald M, DiMaria J, Lucero L, Kellogg S. Contingency management for the treatment of methamphetamine use disorders. The American journal of psychiatry. 2006;163:1993–1999. doi: 10.1176/ajp.2006.163.11.1993. [DOI] [PubMed] [Google Scholar]
- Sabol KE, Roach JT, Broom SL, Ferreira C, Preau MM. Long-term effects of a high-dose methamphetamine regimen on subsequent methamphetamine-induced dopamine release in vivo. Brain Res. 2001;892:122–129. doi: 10.1016/s0006-8993(00)03244-3. [DOI] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopaminergic deficits. J Pharmacol Exp Ther. 2003;304:1181–1187. doi: 10.1124/jpet.102.045005. [DOI] [PubMed] [Google Scholar]
- SAS Institute Incorporated . SAS for Windows. Version 9.1.3 SAS Institute Incorporated; Cary, NC: 2004. [Google Scholar]
- Shepherd G, Velez LI, Keyes DC. Intentional bupropion overdoses. The Journal of emergency medicine. 2004;27:147–151. doi: 10.1016/j.jemermed.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Kao UH, Wang PC, Bholat MA, Ling W. Bupropion Hydrochloride Versus Placebo, in Combination with Cognitive Behavioral Therapy, for the Treatment of Cocaine Abuse/Dependence. J Addict Dis. 2008;27:13–23. doi: 10.1300/J069v27n01_02. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Peck J, Reback CJ, Rotheram-Fuller E. Psychiatric and substance dependence comorbidities, sexually transmitted diseases, and risk behaviors among methamphetamine-dependent gay and bisexual men seeking outpatient drug abuse treatment. Journal of psychoactive drugs. 2003;35(Suppl 1):161–168. doi: 10.1080/02791072.2003.10400511. [DOI] [PubMed] [Google Scholar]
- Silber BY, Croft RJ, Papafotiou K, Stough C. The acute effects of d-amphetamine and methamphetamine on attention and psychomotor performance. Psychopharmacology. 2006;187:154–169. doi: 10.1007/s00213-006-0410-7. [DOI] [PubMed] [Google Scholar]
- Singer J. Using SAS PROC MIXED to Fit Multilevel Models, Hierarchical Models, and Individual Growth Models. Journal of Educational and Behavioral Statistics. 1998;23:323–355. [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: utility for alcohol research. Addictive behaviors. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbbon M, First M. The Structured Clinical Interview for DSM-IV. American Psychiatric Press; Washington, D.C.: 1995. [Google Scholar]
- SPSS Incorporated . SPSS for Windows. Version 14.0 SPSS Incorporated; New York: 2005. [Google Scholar]
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159–166. doi: 10.4088/pcc.v06n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Service Administration . National Survey on Drug Use and Health Report: Methamphetamine Use, Abuse, and Dependence. US Department of Health and Human Services; Rockville, MD: 2005. [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5:797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Progress in neurobiology. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. Allyn and Bacon; Boston, MA: 2000. [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnipseed SD, Richards JR, Kirk JD, Diercks DB, Amsterdam EA. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. The Journal of emergency medicine. 2003;24:369–373. doi: 10.1016/s0736-4679(03)00031-3. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction (Abingdon, England) 2007;102(Suppl 1):96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001c;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wijetunga M, Bhan R, Lindsay J, Karch S. Acute coronary syndrome and crystal methamphetamine use: a case series. Hawaii medical journal. 2004;63:8–13. 25. [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M. Psychiatric symptoms in methamphetamine users. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]