Abstract
The Multicenter Anterior Cruciate Ligament (ACL) Revision Study (MARS) group was formed to study a large cohort of revision ACL reconstruction patients. The purpose of this subset analysis study of the MARS database is to describe specific details of femoral tunnel malposition and subsequent management strategies that surgeons chose in the revision setting. The design of this study is a case series. The multicenter MARS database is compiled from a questionnaire regarding 460 ACL reconstruction revision cases returned by 87 surgeons. This subset analysis described technical aspects and operative findings in specifically those cases in which femoral tunnel malposition was cited as the cause of primary ACL reconstruction failure. Of the 460 revisions included for study, 276 (60%) cases cited a specific “technical cause of failure.” Femoral tunnel malposition was cited in 219 (47.6%) of 460 cases. Femoral tunnel malposition was cited as the only cause of failure in 117 cases (25.4%). Surgeons judged the femoral tunnel too vertical in 42 cases (35.9%), too anterior in 35 cases (29.9%), and too vertical and anterior in 31 cases (26.5%). Revision reconstruction involved the drilling of an entirely new femoral tunnel in 91 cases (82.1%). For primary reconstruction, autograft tissue was used in 82 cases (70.1%). For revision reconstruction, autograft tissue was used in 61 cases (52.1%) and allograft tissue in 56 cases (47.9%). Femoral tunnel malposition in primary ACL reconstruction was the most commonly cited reason for graft failure in this cohort. Graft selection is widely variable among surgeons.
Keywords: anterior cruciate ligament, reconstruction, revision, technique
Anterior cruciate ligament (ACL) reconstruction has become the standard of care for patients who sustain an ACL tear and wish to return to their preinjury activity levels.1 Failure of primary ACL reconstruction has been noted in ~0.7 to 10%.2-11 As ACL reconstruction failure occurs infrequently, and few surgeons perform large numbers of ACL revision surgery, the Multicenter ACL Revision Study (MARS) group was formed.12 The purpose of the MARS group is to prospectively collect data to help to identify independent variables and predictors of outcomes to guide treatment strategies for both primary and revision ACL reconstruction.
Review of the literature has shown technical errors to be a major cause of ACL reconstruction failures with various studies reporting rates of 24 to 64%.13-18 Specifically, femoral tunnel malposition has been implicated as the primary cause of failure in 60 to 79% of patients.17,19-22 We hypothesize that MARS surgeons will also identify femoral tunnel malposition at the time of the prior ACL reconstruction as the most common reason for graft failure. The purpose of this subset analysis study of the MARS database is to describe the specific details of femoral tunnel malposition and the subsequent management strategies that surgeons chose in the revision setting.
Methods
MARS is an 87 surgeon, 52 site group enrolling revision ACL reconstructions in a prospective longitudinal cohort.18 The MARS database was compiled from a 48-page questionnaire completed and returned by surgeons who performed revision ACL reconstruction, but may or may not have performed the primary ACL reconstruction for each case submitted. The questionnaire included data related to the cause of primary ACL reconstruction failure and subsequent surgical intervention by the surgeon performing the revision reconstruction. These data include surgical details for both the previous ACL reconstruction and the revision ACL procedure.
Cases in the database were stratified according to the cause of failure for the prior ACL reconstruction as judged by the surgeon performing the revision surgery. We identified the cases with a “technical cause of failure,” as opposed to trauma or biological failure of the graft. These patients were further subdivided to identify those with femoral tunnel malposition as the cause of failure. A cohort was identified for which surgeons cited “femoral tunnel malposition alone” as the cause of failure by excluding cases that involved femoral tunnel malposition in combination with other technical errors.
Details of those cases that had “technical cause of failure” cited and “femoral tunnel malposition alone” make up the study group for this descriptive statistical analysis. Data were examined regarding surgical technique and approach, graft selection, and tunnel position for the prior ACL reconstruction. Further data were analyzed regarding surgical technique and approach, tunnel preparation, graft selection, and bone grafting at the time of revision ACL reconstruction.
Statistical Analysis
As the treatment strategies employed for each of the cases in this study were chosen on an individual basis guided by surgeon preference rather than a uniform decision-making algorithm, the data do not lend itself to hypothesis testing or inferential statistics. For this reason, purely descriptive statistics were employed in this report.
Results
At the time of this review, 460 revision ACL reconstructions were identified in the prospectively gathered MARS database. The MARS questionnaire evaluated the presumed cause of failure of ACL reconstruction in two separate questions: first, the “surgeon’s opinion on cause of failure,” which could be answered “traumatic,” “technical error from prior surgery,” “biologic failure to heal (i.e., tissue stretching),” “combination of above,” “infection,” and “other”; and second, the “cause of technical failure,” which could be answered “femoral tunnel malposition,” “tibial tunnel malposition,” “malalignment (in any plane),” “femoral fixation,” “tibial fixation,” “autograft source,” “allograft source,” “posteromedial laxity,” “posterolateral laxity,” “none,” and “other” (to be answered at the surgeon’s discretion). As both questions were obligatorily answered by filling out the questionnaire (an answer of “technical error from prior surgery” on the first question not requisite for answering the second), the authors elected to focus on the second question, as it appeared more precise for the purposes of this study.
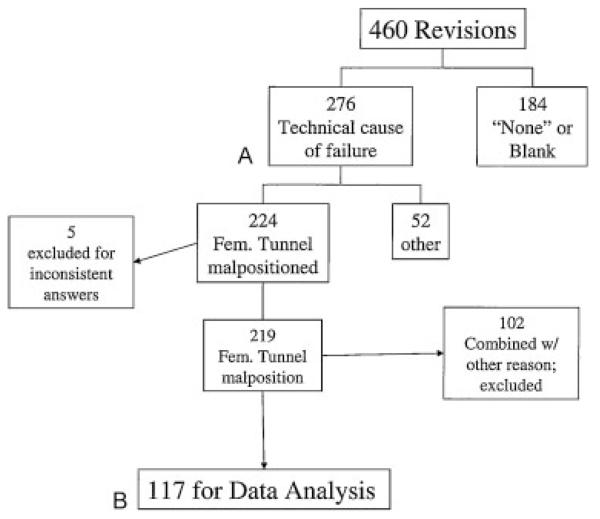
Of the 276 revisions in which a specific technical cause of failure was cited, failure of the primary reconstruction was attributed to femoral tunnel malposition alone in 122 cases (44.2%) or in association with another cause of technical failure in 102 cases (37.0%), combined with “tibial tunnel malposition” in 82 cases (29.7%), “allograft source” in 13 cases (4.7%), “femoral fixation” in 8 cases (2.9%), “malalignment (in any plane)” in 5 cases (1.8%), “posteromedial laxity” in 4 cases (1.4%), and “posterolateral laxity” and “tibial fixation” in 1 case each (0.4%). Of the 122 cases in which femoral tunnel malposition alone was cited as the technical cause of failure, 117 cases consistently defined the tunnel position as abnormal. In the other five cases, the surgeons had judged femoral tunnel malposition to be the cause for failure, but later described the femoral tunnel placement as either “ideal” (n = 3) or did not select an answer choice for this question (left blank; n = 2). These cases were excluded from analysis because of inconsistencies in the data as noted above. A detailed outline of the femoral tunnel malposition cohort stratification is given in Fig. 1. The final cohort for this subset analysis included 117 subjects (68 males and 49 females) with a mean age of 28.7 (range 15 to 57) years.
Figure 1.
(A) Reason for primary reconstruction failure. (B) Femoral tunnel malposition only cohort.
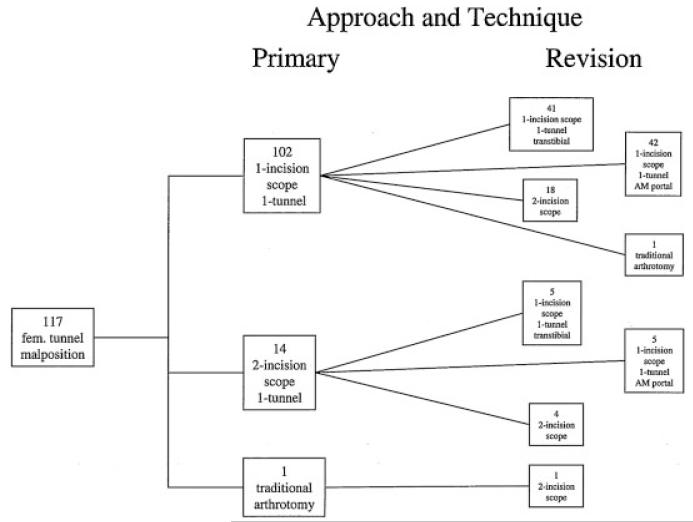
For the 117 cases of femoral tunnel malposition alone, primary reconstruction involved a one-incision arthroscopic single-tunnel approach in 102 cases (87.2%), a two-incision arthroscopic single-tunnel approach in 14 cases (12.0%), and a traditional arthrotomy in 1 case (0.9%) (Fig. 2). For the 102 primary cases in which a one-incision single-tunnel approach was used, preparation of the femoral tunnel for revision ACL reconstruction involved a one-incision arthroscopic transtibial approach in 41 cases (40.2%), a one-incision arthroscopic anteromedial portal drilling technique in 42 cases (41.2%), a two-incision approach in 18 cases (17.6%), and a traditional arthrotomy in 1 case (1.0%). For the 14 cases in which a two-incision approach had been used, preparation of the femoral tunnel for revision ACL reconstruction involved a one-incision arthroscopic transtibial approach in five cases, a one-incision arthroscopic anteromedial portal drilling technique in five cases, a two-incision approach in four cases, and a traditional arthrotomy in one case. For the one primary case that used a traditional arthrotomy, preparation of the femoral tunnel for revision ACL reconstruction involved a two-incision approach (Fig. 2).
Figure 2.
Breakdown of surgical approaches and techniques at primary and revision ACL reconstruction.
With respect to tunnel malposition, the surgeon judged the femoral tunnel too vertical in 42 cases (35.9%), too anterior in 35 cases (29.9%), too vertical and anterior in 31 cases (26.5%), and compromised due to both position and size in 9 cases (7.7%). Revision reconstruction involved the drilling of an entirely new femoral tunnel in 91 cases (82.1%), the same compromised femoral tunnel aperture in 2 cases (1.7%), a coalescence of a new femoral tunnel with the original (blended) in 16 cases (13.7%), and an added second femoral tunnel in 5 cases (4.3%). Revision reconstruction in the setting of femoral tunnel malposition involved the use of the same optimal tibial tunnel aperture in 60 cases (51.3%), drilling of the same compromised tibial tunnel in 2 cases (1.7%), an entirely new tibial tunnel in 19 cases (16.2%), a “blended” new tibial tunnel aperture in 23 cases (19.7%), and an added second tibial tunnel for a double-bundle revision in 5 cases (4.3%).
Revision reconstruction included the use of autograft in 61 cases (52%) (bone-patellar tendon-bone, BTB, autograft in 29 cases [24.8%]; hamstring [semitendinosus + gracilis] autograft in 22 cases [18.8%]; and hamstring [semitendinosus] autograft in 6 cases [5.1%]) and the use of allograft in 56 cases (BTB allograft in 25 cases [21.4%] and hamstring [semitendinosus] allograft in 1 case [0.9%]). For primary reconstruction, 82 cases (70.1%) had used autograft tissue, 31 cases (26.5%) had used allograft tissue, and 4 cases (3.4%) had used a combination of autograft and allograft tissue. Of the 82 primary autograft cases, 43 revisions (52.4%) used some form of autograft tissue and 39 revisions (47.6%) used allograft tissue. Of the 31 primary allograft cases, 18 revisions (58.1%) used autograft tissue and 13 revisions (41.9%) used allograft tissue.
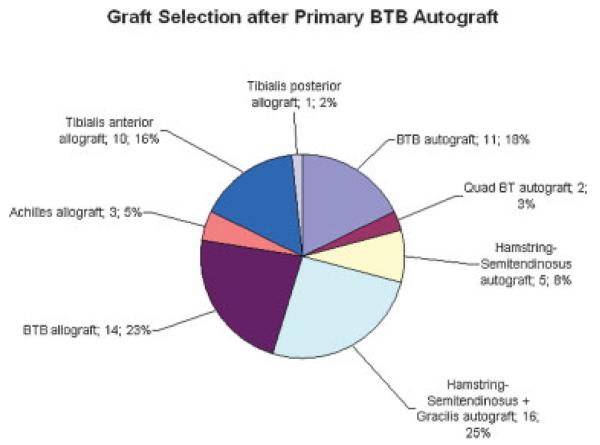
For primary reconstruction, BTB autograft had been used in 61 cases (52.1%), hamstring (semitendinosus + gracilis) autograft in 20 cases (17.1%), BTB allograft in 14 cases (11.5%), and soft tissue allograft in 4 cases (3.4%). Of the primary cases utilizing BTB autograft, revision reconstruction involved the use of autograft tissue in 33 cases (54.1%) and allograft tissue in 28 cases (45.9%): hamstring (semitendinosus + gracilis) autograft in 15 cases (24.6%), BTB allograft in 14 cases (23.0%), BTB autograft in 11 cases (18.0%), tibialis anterior allograft in 10 cases (16.4%), hamstring (semitendinosus) autograft in 5 cases (8.2%), Achilles allograft in 3 cases (4.9%), quad BTB autograft in 2 cases (3.3%), and tibialis posterior allograft in 1 case (1.6%) (Fig. 3).
Figure 3.
Graft selection for revision ACL reconstruction after primary BTB autograft (n = 61).
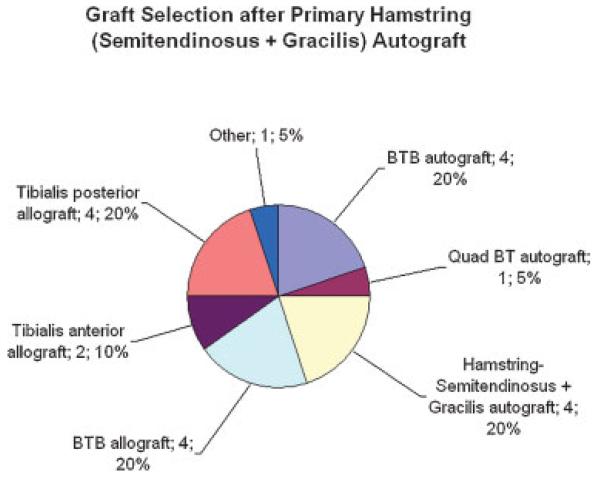
Of the 20 primary cases utilizing hamstring (semitendinosus + gracilis) autograft, revision involved the use of autograft tissue in 10 cases (50%) and allograft tissue in 10 cases (50%): BTB autograft, hamstring (semitendinosus + gracilis) autograft, BTB allograft, and tibialis posterior allograft in 4 cases (20.0%) each, as well as tibialis anterior allograft in 2 cases (10.0%), and quad BT autograft, other autograft, and other allograft in 1 case (5.0%) each (Fig. 4).
Figure 4.
Graft selection for revision ACL reconstruction after primary hamstring (semitendinosus + gracilis) autograft (n = 20).
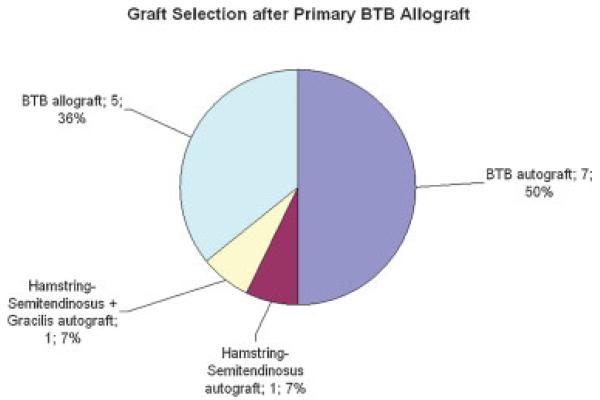
Of the 14 primary cases utilizing BTB allograft, revision reconstruction involved the use of autograft tissue in 8 cases and allograft tissue in 6 cases: BTB autograft in 7 cases, BTB allograft in 5 cases, and hamstring (semitendinosus) and hamstring (semitendinosus + gracilis) autograft for 1 case each (Fig. 5).
Figure 5.
Graft selection for revision ACL reconstruction after primary BTB allograft (n = 14).
Of the 4 primary cases utilizing soft tissue allograft, revision reconstruction involved the use of autograft tissue in 1 case and allograft tissue in 3 cases: hamstring (semitendinosus + gracilis) autograft, Achilles allograft, tibialis anterior allograft, and tibialis posterior allograft in 1 case each.
Revision reconstruction included use of bone graft on the femoral side as a single-staged technique in five cases and dual-staged in nine cases. Of these, 11 cases were in the setting of drilling an entirely new femoral tunnel, while 2 were used with a blended new femoral tunnel, and 1 was used for an added second femoral tunnel. Likewise, revision reconstruction included use of bone graft on the tibial side as a single-staged technique in one case and dual-staged in nine cases.
Discussion
The MARS database represents a benchmark for the study of ACL revision surgery. Longitudinal prospective pooling of data by experienced surgeons at multiple centers holds the potential for tremendous insight into the most current trends in the management of failed primary ACL reconstruction, including revision surgery outcomes and predictors of success.12
This report provides a descriptive analysis of the perceptions of a group of experienced surgeons regarding the mode of primary ACL reconstruction failure. It also outlines their treatment strategies when faced with ACL reconstruction failure in the setting of femoral tunnel malposition.
Choices made in regard to transtibial technique versus anteromedial portal drilling, tunnel position, and graft selection are all important factors to consider. Several authors have recognized that the most common mode of ACL reconstruction failure is technical error with reported rates of 24 to 64%.13-18 In particular, femoral tunnel malposition has been implicated as the primary error in 60 to 79% of patients.17,19-22 This study found that femoral tunnel malposition was judged the reason for failure in almost 50% of the primary ACL revision cases studied. Of primary interest is the means by which surgeons chose to revise ACL reconstructions that had failed as a result of femoral tunnel malposition.
Approach and Technique
Surgeons faced with femoral tunnel malposition were nearly equally distributed between utilizing the transtibial approach compared with anteromedial portal drilling technique for the femoral tunnel. This bimodal distribution illustrates that while many surgeons have modified their approach to use an anteromedial portal drilling technique, many others still feel that the transtibial tunnel technique for femoral tunnel drilling is a viable option in revision surgery.
Although controversial, both transtibial and anteromedial portal femoral tunnel drilling techniques are currently used by surgeons. The anteromedial portal drilling technique has been touted as a more consistent method to create an anatomic femoral tunnel position.23,24 A direct comparison study of 859 primary ACL reconstructions failed to provide strong evidence to support the superiority of one technique over the other.25 These techniques have not been compared thus far in the revision setting, although decision-making in this regard is likely guided by surgeon preference, the degree and type of femoral tunnel malpositioning, and the method of tunnel preparation chosen. The data in this subset analysis suggest that surgeons support a role for either technique when confronted with femoral tunnel malposition.
Femoral Tunnel Placement
In this study, the most common reason for femoral tunnel malposition is vertical and/or anterior misplacement of the tunnel: too vertical occurred in approximately one-third, too anterior in approximately one-third, and a combination in approximately one-third of cases. As noted previously, in the revision setting, there was a nearly identical distribution of transtibial tunnel and anteromedial portal drilling techniques for the creation of the new femoral tunnel.
Failure to position, the tunnel in the optimal anatomic position has been noted to cause graft impingement. A tunnel positioned too vertically can also impinge on the PCL, altering native tension and force profiles.26,27 Osseous landmarks have been identified to aid in discerning the anatomic location of the femoral footprint, including the lateral intercondylar ridge (running proximal to distal) and the lateral bifurcate ridge (between anteromedial and posterolateral bundle attachment sites).28 These landmarks offer reliability as a target for tunnel position on the femoral side with both single-bundle and double-bundle ACL reconstruction techniques, but may be less apparent when performing revision surgery.
Revision Tunnel Preparation
Revision reconstruction in this series involved the drilling of an entirely new femoral tunnel in over 80% of the cases. In the opinion of most MARS surgeons, a malpositioned primary femoral tunnel often necessitates the drilling of an entirely new tunnel.
We also found that in this subset analysis of 117 cases of femoral tunnel malposition, ~50% of the time, surgeons used the same tibial tunnel aperture. This demonstrates that in at least 50% of these particular cases, the tibial tunnel was felt to be in the correct position. This subset analysis did not specifically address tibial tunnel position, and therefore the authors cannot comment further on these elements of the data.
Graft Selection
Based on the MARS data, graft selection for primary ACL reconstruction and revision ACL reconstruction is highly variable among surgeons. The most common graft sources for primary ACL reconstruction used for these 117 cases involved autograft in two-thirds of cases and allograft in the remainder.
Surgeon preference for the revision setting was also highly variable. In cases that utilized a BTB autograft for primary reconstruction, the most common revision graft sources were hamstring autograft (24.6%) and BTB allograft (23%). In cases that utilized a hamstring autograft for primary reconstruction, surgeon preference was equally divided (20%) for BTB autograft, BTB allograft, soft tissue allograft, and contralateral hamstring autograft. In cases that utilized a BTB allograft for primary reconstruction, surgeon preference was for BTB autograft in 50% of cases, and repeat BTB allograft in 35.7%. In cases that utilized a soft tissue allograft for primary reconstruction, surgeon preference was equally divided (25%) between hamstring autograft, Achilles allograft, tibialis anterior allograft, and tibialis posterior allograft.
A recent meta-analysis29 of clinical outcomes of primary ACL reconstruction comparing autograft versus allograft demonstrated no significant difference in outcomes in the short term between either source. Most of the included studies investigated BTB graft sources, limiting generalization to the myriad graft sources observed in the MARS database. Also, the studies included in this meta-analysis did not stratify their cohorts or evaluate data in a multivariate model to assess the effects of any confounding variables. More recently, Borchers et al demonstrated higher failure rates with the use of soft tissue (tibialis anterior) allograft in young high-demand athletes.30
Bone Grafting
Revision reconstruction included the use of bone grafting on the femoral side in only 12% of the cases in this subset analysis—single-staged in five cases and dual-staged in nine cases. While low in general, this rate is higher than previously reported (≤4%).17,31 The specific reason for adding bone graft to tunnels was not elucidated as a result of the questionnaire’s design, and therefore, we cannot comment on the factors that led to this choice on the part of the revising surgeon. One reason may be that a severely malpositioned tunnel allows creation of an entirely new femoral tunnel without the need for bone grafting. It is also possible that with modern methods of secure graft fixation, the use of bone graft in the revision setting may become even less frequent.
Strengths and Weaknesses
The strengths of this article include that data were obtained from the largest prospectively gathered multicenter database regarding ACL revision reconstruction to the authors’ knowledge. In addition, the MARS surgeons were recruited based on expertise and experience in ACL reconstruction surgery, and we believe that the data compiled can therefore be considered expert opinion. Furthermore, the database is highly detailed, incorporating many facets of revision ACL reconstruction technique.
A weakness of this article includes its descriptive statistical analysis based on surgeons’ subjective perceptions of the cause of failure. Subsequent management strategies were based on individual surgeon preference and decision-making and not on any uniform treatment algorithm. The goal of this article is not to provide the reader with a single best management strategy, but simply to inform the reader of current trends in ACL revision reconstruction in the pool of surgeons participating in the MARS group. The time from primary reconstruction to revision reconstruction was not cataloged in the portion of the study questionnaire provided for analysis. This information would have added much to the understanding of the relationship of femoral tunnel malposition as the cause of technical failure compared with other causes available as answer choices in this questionnaire.
Conclusion
This descriptive, subset analysis of revision ACL reconstruction reveals that graft selection is widely variable among surgeons. The MARS database identifies technical error as the most common reason for primary ACL reconstruction failure and femoral tunnel malposition as the most common technical error. Surgeons prefer drilling of an entirely new femoral tunnel in the vast majority of revision procedures using both transtibial and anteromedial portal techniques. These observations underscore the importance of understanding the ACL femoral footprint anatomy and the technique of femoral tunnel placement at the time of primary ACL reconstruction.
MARS Study Group Collaborators
| Michael J. Stuart, M.D. | Mayo Clinic Rochester |
| Joseph A. Morgan, M.D | Mayo Clinic Rochester |
| Diane L. Dahm, M.D. | Mayo Clinic Rochester |
| Bruce A. Levy, M.D. | Mayo Clinic Rochester |
| Laura J. Huston, M.S | Vanderbilt University |
| Amanda K. Haas, M.A. | Washington University, St. Louis |
| Kurt P. Spindler, M.D. | Vanderbilt University |
| Rick W. Wright, M.D. | Washington University, St. Louis |
| John P. Albright, M.D. | University of Iowa Hospitals and Clinics |
| Christina R. Allen, M.D. | University of California, San Francisco |
| Annunziato (Ned) Amendola, M.D. | University of Iowa Hospitals and Clinics |
| Allen F. Anderson, M.D. | Tennessee Orthopaedic Alliance |
| Jack T. Andrish, M.D. | Cleveland Clinic |
| Christopher C. Annunziata, M.D. | Commonwealth Orthopaedics and Rehab |
| Robert A. Arciero, M.D. | University of Connecticut Health Center |
| Bernard R. Bach, Jr., M.D. | Rush University Medical Center |
| Champ L. Baker III, M.D. | The Hughston Clinic |
| Arthur R. Bartolozzi, M.D. | 3B Orthopaedics, University of Pennsylvania Health System |
| Keith M. Baumgarten, M.D. | Orthopedic Institute |
| Jeffery R. Bechler, M.D. | University Orthopedic Associates, LLC |
| Jeffrey H. Berg, M.D. | Town Center Orthopaedic Associates |
| Geoffrey Bernas, M.D. | State University of New York at Buffalo |
| Stephen F. Brockmeier, M.D. | Perry Orthopedics and Sports Medicine |
| Robert H. Brophy, M.D. | Washington University, St. Louis |
| Charles A. Bush-Joseph, M.D. | Rush University Medical Center |
| J. Brad Butler, V, M.D. | Orthopedic and Fracture Clinic |
| John D. Campbell, M.D. | Bridger Orthopaedic and Sports Medicine |
| James L. Carey, MD, M.P.H. | Vanderbilt University |
| James E. Carpenter, M.D. | University of Michigan |
| Brian J. Cole, M.D. | Rush University Medical Center |
| Daniel E. Cooper, M.D. | W.B. Carrell Memorial Clinic |
| Jonathan M. Cooper, D.O. | HealthPartners Specialty Clinic |
| Charles L. Cox, M.D. | Vanderbilt University |
| R. Alexander Creighton, M.D. | University of North Carolina Medical Center |
| Tal S. David, M.D. | Arthroscopic and Orthopedic Sports MedicineAssociates |
| Thomas M. DeBerardino, M.D. | University of Connecticut Health Center |
| Warren R. Dunn, M.D., M.P.H. | Vanderbilt University |
| David C. Flanigan, M.D. | The Ohio State University |
| Robert W. Frederick, M.D. | The Rothman Institute/Thomas Jefferson University |
| Theodore J. Ganley, M.D. | The Children’s Hospital of Philadelphia |
| Charles J. Gatt, Jr., M.D. | University Orthopedic Associates, LLC |
| Steven R. Gecha, M.D. | Princeton Orthopaedic Associates |
| James Robert Giffin, M.D. | Fowler Kennedy Sports Medicine Clinic-University of Western Ontario |
| Donald B. Goodfellow. M.D. | Case Western Reserve University |
| Sharon L. Hame, M.D. | David Geffen School of Medicine at UCLA |
| Jo A. Hannafin, M.D., Ph.D. | Hospital for Special Surgery |
| Christopher D. Harner, M.D. | University of Pittsburgh Medical Center |
| Norman Lindsay Harris, Jr., M.D. | Orthopaedic Associates of Aspen and Glenwood |
| Keith S. Hechtman, M.D. | UHZ Sports Medicine Institute |
| Elliott B. Hershman, M.D. | Lenox Hill Hospital |
| Rudolf G. Hoellrich, M.D. | Slocum Research and Education Foundation |
| Timothy M. Hosea, M.D. | University Orthopedic Associates, LLC |
| David C. Johnson, M.D. | National Sports Medicine Institute |
| Timothy S. Johnson, M.D. | National Sports Medicine Institute |
| Morgan H. Jones, M.D. | Cleveland Clinic |
| Christopher C. Kaeding, M.D. | The Ohio State University |
| Thomas E. Klootwyk, M.D. | Methodist Sports Medicine Center-The Orthopedic Specialists |
| Brett (Brick) A. Lantz, M.D. | Slocum Research and Education Foundation |
| C. Benjamin Ma, M.D. | University of California, San Francisco |
| G. Peter Maiers II, M.D. | Methodist Sports Medicine Center-The Orthopedic Specialists |
| Barton Mann, Ph.D. | AOSSM |
| Robert G. Marx, M.D. | Hospital for Special Surgery |
| Matthew J. Matava, M.D. | Washington University, St. Louis |
| Gregory M. Mathien, M.D. | Knoxville Orthopedic Clinic |
| David R. McAllister, M.D. | David Geffen School of Medicine at UCLA |
| Eric C. McCarty, M.D. | University of Colorado Denver School of Medicine |
| Robert G. McCormack, M.D. | University of British Columbia |
| Bruce S. Miller, M.D., M.S. | University of Michigan |
| Ali R. Motamedi, M.D. | Richmond Bone and Joint Clinic |
| Carl W. Nissen, M.D. | Connecticut Children’s Medical Center |
| Daniel F. O’Neill, M.D., Ed.D. | The Alpine Clinic |
| LTC Brett D. Owens, M.D. | Keller Army Community Hospital-United States Military Academy |
| Richard D. Parker, M.D. | Cleveland Clinic |
| Mark L. Purnell, M.D. | Orthopaedic Associates of Aspen and Glenwood |
| Arun J. Ramappa, M.D. | Beth Israel Deaconess Medical Center |
| Michael A. Rauh, M.D. | State University of New York at Buffalo |
| Arthur Rettig, M.D. | Methodist Sports Medicine Center-The Orthopedic Specialists |
| Jon K. Sekiya, M.D. | University of Michigan |
| Kevin G. Shea, M.D. | Intermountain Orthopedics |
| Orrin H. Sherman, M.D. | NYU Hospital for Joint Diseases |
| James R. Slauterbeck, M.D. | University of Vermont College of Medicine |
| Matthew V. Smith, M.D. | Washington University, St. Louis |
| LTC. Steven J. Svoboda, M.D. | Keller Army Community Hospital-United States Military Academy |
| Timothy N. Taft, M.D. | University of North Carolina Medical Center |
| COL Joachim J. Tenuta, M.D. | Keller Army Community Hospital-United States Military Academy |
| Edwin M. Tingstad, M.D. | Inland Orthopaedics/Washington State University |
| Armando F. Vidal, M.D. | University of Colorado Denver School of Medicine |
| Darius G. Viskontas, M.D. | Royal Columbian Hospital |
| Richard A. White, M.D. | University of Missouri-Columbia |
| James S. Williams, Jr., M.D. | Cleveland Clinic |
| Michelle L. Wolcott, M.D. | University of Colorado Denver School of Medicine |
| Brian R. Wolf, M.D. | University of Iowa Hospitals and Clinics |
| James J. York, M.D. | Chesapeake Orthopaedics and Sports Medicine Center |
References
- 1.Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE. Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med. 2005;33(10):1579–1602. doi: 10.1177/0363546505279913. [DOI] [PubMed] [Google Scholar]
- 2.Dahm DL, Wulf CA, Dajani KA, Dobbs RE, Levy BA, Stuart MA. Reconstruction of the anterior cruciate ligament in patients over 50 years. J Bone Joint Surg Br. 2008;90(11):1446–1450. doi: 10.1302/0301-620X.90B11.21210. [DOI] [PubMed] [Google Scholar]
- 3.Fox JA, Pierce M, Bojchuk J, Hayden J, Bush-Joseph CA, Bach BR., Jr. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy. 2004;20(8):787–794. doi: 10.1016/j.arthro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Getelman MH, Friedman MJ. Revision anterior cruciate ligament reconstruction surgery. J Am Acad Orthop Surg. 1999;7(3):189–198. doi: 10.5435/00124635-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Greis PE, Johnson DL, Fu FH. Revision anterior cruciate ligament surgery: causes of graft failure and technical considerations of revision surgery. Clin Sports Med. 1993;12(4):839–852. [PubMed] [Google Scholar]
- 6.Johnson DL, Harner CD, Maday MG, Fu FH. Revision anterior cruciate ligament surgery. In: Fu FH, Harner CD, Vince KG, editors. Knee Surgery. Williams & Wilkins; Baltimore: 1994. [Google Scholar]
- 7.Koh J. Computer-assisted navigation and anterior cruciate ligament reconstruction: accuracy and outcomes. Orthopedics. 2005;28(10, Suppl):s1283–s1287. doi: 10.3928/0147-7447-20051002-16. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Seong SC, Jo CH, Han HS, An JH, Lee MC. Anterior cruciate ligament reconstruction with use of autologous quadriceps tendon graft. J Bone Joint Surg Am. 2007;89(Suppl 3):116–126. doi: 10.2106/JBJS.G.00632. [DOI] [PubMed] [Google Scholar]
- 9.Ménétrey J, Duthon VB, Laumonier T, Fritschy D. “Biological failure” of the anterior cruciate ligament graft. Knee Surg Sports Traumatol Arthrosc. 2008;16(3):224–231. doi: 10.1007/s00167-007-0474-x. [DOI] [PubMed] [Google Scholar]
- 10.Williams RJ, III, Hyman J, Petrigliano F, Rozental T, Wickiewicz TL. Anterior cruciate ligament reconstruction with a four-strand hamstring tendon autograft. Surgical technique. J Bone Joint Surg Am. 2005;87(Pt 1, Suppl 1):51–66. doi: 10.2106/JBJS.D.02805. [DOI] [PubMed] [Google Scholar]
- 11.Wolf RS, Lemak LJ. Revision anterior cruciate ligament reconstruction surgery. J South Orthop Assoc. 2002;11(1):25–32. [PubMed] [Google Scholar]
- 12.Spindler KP. The Multicenter ACL Revision Study (MARS): a prospective longitudinal cohort to define outcomes and independent predictors of outcomes for revision anterior cruciate ligament reconstruction. J Knee Surg. 2007;20(4):303–307. doi: 10.1055/s-0030-1248065. [DOI] [PubMed] [Google Scholar]
- 13.Carson EW, Anisko EM, Restrepo C, Panariello RA, O’Brien SJ, Warren RF. Revision anterior cruciate ligament reconstruction: etiology of failures and clinical results. J Knee Surg. 2004;17(3):127–132. doi: 10.1055/s-0030-1248210. [DOI] [PubMed] [Google Scholar]
- 14.Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851–860. doi: 10.1177/0363546507312381. [DOI] [PubMed] [Google Scholar]
- 15.Jaureguito JW, Paulos LE. Why grafts fail. Clin Orthop Relat Res. 1996;(325):25–41. doi: 10.1097/00003086-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DL, Swenson TM, Irrgang JJ, Fu FH, Harner CD. Revision anterior cruciate ligament surgery: experience from Pittsburgh. Clin Orthop Relat Res. 1996;325(325):100–109. doi: 10.1097/00003086-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Uribe JW, Hechtman KS, Zvijac JE, Tjin-A-Tsoi EW. Revision anterior cruciate ligament surgery: experience from Miami. Clin Orthop Relat Res. 1996;(325):91–99. doi: 10.1097/00003086-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Wright RW, Huston LJ, Spindler KP, et al. MARS Group Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2010;38(10):1979–1986. doi: 10.1177/0363546510378645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harner CD, Giffin JR, Dunteman RC, Annunziata CC, Friedman MJ. Evaluation and treatment of recurrent instability after anterior cruciate ligament reconstruction. Instr Course Lect. 2001;50:463–474. [PubMed] [Google Scholar]
- 20.Kato Y, Ingham SJ, Kramer S, Smolinski P, Saito A, Fu FH. Effect of tunnel position for anatomic single-bundle ACL reconstruction on knee biomechanics in a porcine model. Knee Surg Sports Traumatol Arthrosc. 2010;18(1):2–10. doi: 10.1007/s00167-009-0916-8. [DOI] [PubMed] [Google Scholar]
- 21.Garofalo R, Djahangiri A, Siegrist O. Revision anterior cruciate ligament reconstruction with quadriceps tendon-patellar bone autograft. Arthroscopy. 2006;22(2):205–214. doi: 10.1016/j.arthro.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 22.Taggart TF, Kumar A, Bickerstaff DR. Revision anterior cruciate ligament reconstruction: a midterm patient assessment. Knee. 2004;11(1):29–36. doi: 10.1016/S0968-0160(02)00087-X. [DOI] [PubMed] [Google Scholar]
- 23.Dargel J, Schmidt-Wiethoff R, Fischer S, Mader K, Koebke J, Schneider T. Femoral bone tunnel placement using the transtibial tunnel or the anteromedial portal in ACL reconstruction: a radiographic evaluation. Knee Surg Sports Traumatol Arthrosc. 2009;17(3):220–227. doi: 10.1007/s00167-008-0639-2. [DOI] [PubMed] [Google Scholar]
- 24.Gavriilidis I, Motsis EK, Pakos EE, Georgoulis AD, Mitsionis G, Xenakis TA. Transtibial versus anteromedial portal of the femoral tunnel in ACL reconstruction: a cadaveric study. Knee. 2008;15(5):364–367. doi: 10.1016/j.knee.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Alentorn-Geli E, Lajara F, Samitier G, Cugat R. The transtibial versus the anteromedial portal technique in the arthroscopic bone-patellar tendon-bone anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1013–1037. doi: 10.1007/s00167-009-0964-0. [DOI] [PubMed] [Google Scholar]
- 26.Colvin AC, Shen W, Musahl V, Fu FH. Avoiding pitfalls in anatomic ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(8):956–963. doi: 10.1007/s00167-009-0804-2. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson WW, III, Johnson DL. “Vertical grafts”: a common reason for functional failure after ACL reconstruction. Orthopedics. 2007;30(3):206–209. doi: 10.3928/01477447-20070301-17. [DOI] [PubMed] [Google Scholar]
- 28.Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23(11):1218–1225. doi: 10.1016/j.arthro.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Carey JL, Dunn WR, Dahm DL, Zeger SL, Spindler KP. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg Am. 2009;91(9):2242–2250. doi: 10.2106/JBJS.I.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case-control study. Am J Sports Med. 2009;37(12):2362–2367. doi: 10.1177/0363546509340633. [DOI] [PubMed] [Google Scholar]
- 31.Noyes FR, Barber-Westin SD. Revision anterior cruciate ligament surgery: experience from Cincinnati. Clin Orthop Relat Res. 1996;(325):116–129. doi: 10.1097/00003086-199604000-00013. [DOI] [PubMed] [Google Scholar]