Abstract
Cell polarity plays an important role in tissue morphogenesis; however, the mechanisms of polarity and their role in mammalian development are still poorly understood. We show here that membrane-associated guanylate kinase protein Dlg5 is required for proper branching morphogenesis and progenitor differentiation in mammalian lung. We found that during lung development Dlg5 functions as an apical-basal polarity protein, which is necessary for the apical maintenance of atypical protein kinase C (aPKC). These results identify Dlg5 as a regulator of apical polarity complexes and uncover the critical function of Dlg5 in branching morphogenesis and differentiation of lung progenitor cells.
Keywords: branching morphogenesis, lung development, cell polarity, apical-basal polarity, Dlg5
Introduction
Cell polarity is important for proper morphogenesis of all mammalian organisms; however, the mechanisms of cell polarity and, even more importantly, the particular role and significance of these mechanisms in the major developmental events are still poorly understood. Significant knowledge about the proteins involved in apical-basal cell polarity was generated using such model organisms as C. elegans and Drosophila (McCaffrey and Macara, 2012; Wodarz and Nathke, 2007). These studies identified atypical PKC (aPKC)/Par3/Par6 proteins as critical members of the apical cell polarity machinery, which localize to the apical membrane domain and are necessary for the establishment and maintenance of the apical membrane domain identity (McCaffrey and Macara, 2009b). In contrast, the Par1, Par4, Dlg, Lgl and Scribble proteins localize to the basolateral membrane domain and are required for basolateral domain formation and maintenance (Yamanaka and Ohno, 2008). In general, the function and the mechanisms of the apical membrane polarity complexes aPKC/Par6/Par3 are understood much better than the function and the mechanisms of the basolateral polarity proteins. Par3 and Par6 are the PDZ (PSD95/Dlg/ZO1) domain-containing molecular adaptor and scaffold proteins, which bind to aPKC, the only enzyme in the apical polarity complex (McCaffrey and Macara, 2009b). aPKC phosphorylates and negatively regulates the function of Par1 and Lgl basolateral polarity proteins (Betschinger et al., 2003; Hurov et al., 2004). Reciprocally, Par1 phosphorylates and negatively regulates the membrane association and cell polarity function of Par3 (Benton and St Johnston, 2003). Dlg is an essential basolateral polarity gene, which genetically interacts with Lgl and Scribble in Drosophila (Bilder et al., 2000; Woods and Bryant, 1991). Dlg is a member of the membrane associated guanylate kinase (MAGUK) proteins. The functional role of Dlg in the regulation of cell polarity remains obscure; however, MAGUK proteins usually function as protein scaffolds that help to cluster multiple transmembrane and accessory proteins to hold together the elements of individual signaling pathways, and it is likely that Dlg performs similar function at the lateral membrane domain (Yamanaka and Ohno, 2008).
Dlg5 is a conserved throughout the Metazoan evolution gene that differs from the Drosophila dlg and mammalian Dlg1-4 because in addition to guanylate kinase and PDZ domains, it contains N-terminal CARD and coiled coil domains (Nechiporuk et al., 2007). Function of Dlg5 in Drosophila has not been investigated. Polymorphism in human Dlg5 protein sequence is associated with predisposition to the Crohn’s disease: however, the mechanisms of Dlg5 in Crohn’s disease are not well understood (Stoll et al., 2004). In renal and mammary epithelial cell lines, knockdown of Dlg5 activates cell migration and promotes TGF-β-mediated epithelial-mesenchymal transition (Sezaki et al., 2012; Smolen et al., 2010). To determine the physiological function of Dlg5 in mammalian organism, we have previously generated and analyzed Dlg5−/− mice (Nechiporuk et al., 2007). We found that Dlg5−/− mice develop brain hydrocephalus and kidney cysts. Biochemical analysis revealed an important function of Dlg5 in facilitating the delivery of N-cadherin to the plasma membrane (Nechiporuk et al., 2007).
In this study we analyzed the role of Dlg5 in developing mammalian lung. The mammalian lung is one of the best-studied examples of a developing organ that undergoes the highly coordinated process of branching morphogenesis coupled with timely progenitor cell differentiation. Together, these events result in the formation of an organ containing branched airways that terminate in millions of functional alveolar sacs enabling adequate lung function (Metzger et al., 2008). Failure of proper lung development can result in neonatal death or chronic pulmonary disease, which is often associated with the enlargement of peripheral airspaces (Bourbon et al., 2009; Snider, 1992). We show here that Dlg5 is required for proper mammalian lung morphogenesis as Dlg5−/− mice display abnormal branching morphogenesis and differentiation of lung progenitor cells and develop completely penetrant lung airspace enlargement and emphysema-like phenotype. We demonstrate that Dlg5−/− lung epithelial cells display prominent apical-basal polarity defects, which may be responsible for the defects in branching and differentiation.
Results
Failure of normal lung morphogenesis and emphysema-like phenotype in Dlg5−/− mice
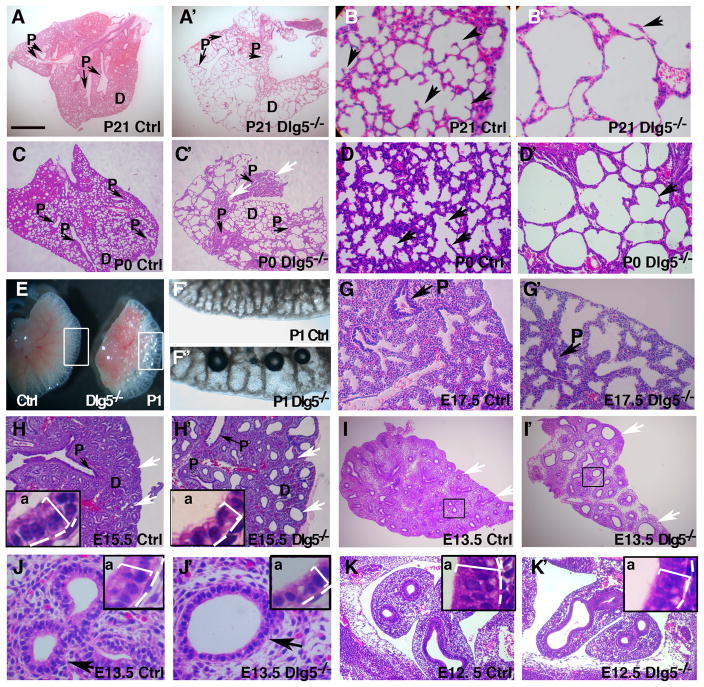
We previously reported that approximately half of the Dlg5−/− mice die perinatally (Nechiporuk et al., 2007). The analysis of the surviving Dlg5−/− adults revealed prominent and completely penetrant lung abnormalities. Therefore, we specifically focused here on the analysis of the role of Dlg5 in murine lung morphogenesis. Histological examination of adult lungs demonstrated an emphysema-like phenotype in Dlg5−/− mice with prominent dilatation of the distal airspaces and an overall decrease in number of alveolar septa (Figure 1A–B′). To assess the origin of these morphological defects, we performed macroscopic and histological analyses of the lungs from Dlg5−/− and wild-type mice at different times of postnatal development. Similar to adult Dlg5−/− animals, newborn Dlg5−/− pups displayed enlarged distal airspaces that contained few alveolar septa and presented with areas of collapsed lung parenchyma (Figure 1C–D′, arrows). The macroscopic analyses of 1-day-old (P1) lungs also revealed the prominent enlargement of distal airspaces in Dlg5−/− pups (Figure 1E–F″).
Figure 1. Emphysema-like phenotype and early developmental defects in Dlg5 −/− lungs.
(A–D′) Haematoxylin and eosin (H&E) staining of sections from P21 (A–B′) and P0 (C–D′) wild-type (Ctrl) and Dlg5−/− knockout lungs. Note the enlarged air spaces and alveolar septa depletion in Dlg5−/− lungs. P, proximal airways. Arrows in B–B′ and D–D′ point to alveolar septum. White arrows in C′ point to atelectatic (collapsed) regions in Dlg5−/− lungs.
(E–F′) Gross morphology of P1 wild-type (Ctrl) and Dlg5−/− lungs. Areas in white boxes in E are shown at higher magnification in F′-F″.
(G–K′) H&E staining of sections from E17.5 (saccular stage), E15.5, E13.5 and E12.5 (pseudoglandular stage) wild-type (Ctrl) and Dlg5−/− lungs. White arrows in H–I′ point to the distal epithelial buds. Insets in H–H′, J–J′ and K–K′ show high magnification of the epithelial buds. P, proximal airways, D, distal lung. ‘A’ in insets denotes apical side of the epithelial buds. Note flattening of the epithelium in the Dlg5−/− lungs (insets, square bracket). Bar in A represents 1 mm in A–A′, 0.2 mm in B–B′ and K–K′, 0.6 mm in C–C′, 0.3 mm H–I′, 0.25 mm in D–D′, 0.7 mm in E, 0.16 mm in F–F′, 0.13 mm in G–G′, 40 μm in J–J′.
Since lung defects were already present in newborn mutants, we histologically examined the lungs of Dlg5−/− and wild-type mice at different times during embryonic development. We found that the lung branching pattern was indistinguishable between wild-type and Dlg5−/− embryos at E12.5. However, starting from E13.5 and throughout the subsequent embryogenesis, 100% Dlg5−/− lungs showed a significant decrease in the number, accompanied by a prominent increase in the size of terminal tubules within the developing lung (Figure 1G–K′). We conclude that the initial onset of lung abnormalities in Dlg5−/− mice occurs between E12.5 and E13.5 of development.
Dlg5 is required for lung branching morphogenesis
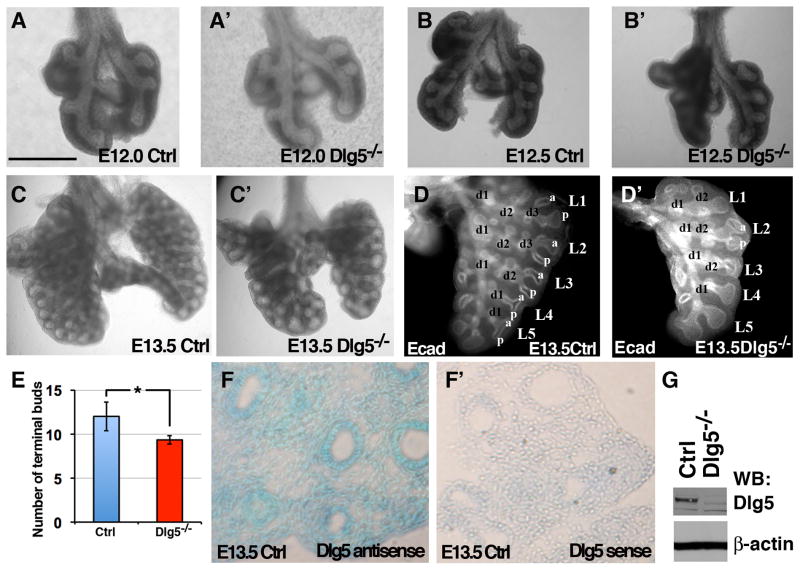
Between days E9.5 and E16.5 of lung development (pseudoglandular stage) primary buds undergo the highly coordinated process of branching morphogenesis that results in the formation of tree-like structures that end with multiple terminal tubules (Morrisey and Hogan, 2010). The histological abnormalities observed in the Dlg5−/− lungs were consistent with a potential defect in branching morphogenesis. To study branching, we analyzed fixed lungs using macroscopic examination and whole-organ immunostaining with anti-E-cadherin antibodies, which facilitates 3-dimensional visualization of the bronchial tree (Metzger et al., 2008). While the comparison of lungs from the Dlg5−/− and wildtype littermate embryos revealed little difference at E12 and E12.5, prominent defects in branching morphogenesis were observed in Dlg5−/− lungs at E13.5 (Figure 2 A–D′). E13.5 Dlg5−/− lungs display delay in branching, with an overall decrease in the number of branches and a prominent dilation of distal tubules (Figure 2 C–D′). Lung branching morphogenesis in the developing mouse embryo is a highly stereotypical process that is comprised of 3 branching modes: domain branching and planar and orthogonal bifurcation of terminal buds (Metzger et al., 2008). Comparison of left lung lobes in E13.5 wildtype and Dlg5−/− littermates show a similar number of L branches (L1–L5); however, all terminal buds are bifurcated (a-p) in the wildtype, but not in the Dlg5−/− lungs (Figure 2D–E, n=4). Moreover, while the domain branching mode results in the formation of D branches in both wildtype and Dlg5−/− lungs, the d3 branch is missing in the high order L1 and L2 branches of the Dlg5−/− embryos (Figure 2D–D′). The decrease in the number of branches and the dilation of terminal buds are consistent with the histological phenotypes observed in E13.5–E15.5 Dlg5−/− lungs (Figure 1H–I′). We conclude that Dlg5 is required for proper lung branching morphogenesis and that the absence of Dlg5 results in an overall defect in branching with dilation of the distal tubules and terminal buds.
Figure 2. Branching morphogenesis defects in Dlg5 −/− lungs.
(A–C′) Gross morphology of E12.0, E12.5 and E13.5 lungs from Dlg5 −/− and control (Ctrl) embryos.
(D–D′) Whole-mount left lobes from E13.5 wild-type (Ctrl) and Dlg5−/− lungs immunostained for E-cadherin (white) to show the airway epithelium. L1–L5, Lateral secondary branches. d1-d3, dorsal ternary branches. a-p, bifurcations of secondary L branches along the A–P axis. Note delayed branching and dilation of the distal tips in Dlg5−/− lungs.
(E) Quantitation of the number of terminal buds in the left lobs in E13.5 wild-type (Ctrl) and Dlg5−/− lungs. * indicates P<0.05. t-test, n=4 for each genotype
(F–F′) In situ hybridization on sections from E13.5 wild-type lungs with Dlg5 antisense and control sense probes.
(G) Western blot analysis of total protein extracts from wild-type (Ctrl) and Dlg5−/− lungs with anti-Dlg5 and anti-β-actin antibodies.
Bar represents 0.5 mm in A–A′ and D–D′, 0.6 mm in B–B′, 1 mm in C–C′, 0.1 mm in E–E′ and 0.65 μm in F–F′.
Epithelial-mesodermal interactions play an important role in the regulation of lung development and defects in either epithelial or mesenchymal tissues can result in an abnormal branching morphogenesis (Morrisey and Hogan, 2010). To determine whether Dlg5 is expressed in epithelial or mesodermal compartments during lung development, we analyzed Dlg5 expression using in situ hybridization. While Dlg5 was expressed throughout the lung tissue, the highest levels of Dlg5 expression were present in the epithelial tubes, the same structures that fail to branch and instead dilate in Dlg5−/− embryos (Figure 2F–G).
Several signal transduction pathways including Wnt, FGF, PDGF and BMP signalings have been implicated in the regulation of lung branching morphogenesis (Cardoso and Lu, 2006)(De Langhe and Reynolds, 2008). We therefore analyzed whether Wnt, FGF, PDGF, or BMP signalings are perturbed in Dlg5−/− lungs. Beta-catenin is pivotal for canonical Wnt signaling and Dlg5 can physically interact with β-catenin (Wakabayashi et al., 2003). To determine whether canonical Wnt signaling is affected in Dlg5−/− lungs, we crossed our mutants with TOPGAL mice carrying LacZ reporter for β-catenin (DasGupta and Fuchs, 1999). Staining for LacZ activity did not reveal significant differences between the control and Dlg5−/− lungs (Supplementary Figure 1). Similarly to Wnt pathway, no significant differences in the levels of phosphorylated FGFR2, PDGFα, or their downstream target, ERK1/2, were found between wild-type and Dlg5−/− lungs (Supplementary Figure 2A–C). In addition, we did not find significant differences in the phosphorylation of SMAD1/5, a downstream target and transducer of BMP signaling (Supplementary Figure 2D). In situ hybridizations revealed overall similar levels of Fgf10 mRNA, although we noted that the Fgf10 expression pattern was somewhat disorganized in E13 Dlg5−/− lungs, with lower levels of expression at the distal tips of the mesenchyme between epithelial stalks and somewhat higher level of expression at the interface between the epithelium and mesenchyme (Supplementary Figure 2E–F′). Since FGF10 plays an important role in lung branching morphogenesis (Mertzger, 2008), small perturbations in the pattern of FGF10 expression may contribute to the lung branching morphogenesis defect in Dlg5−/− lungs.
To use a more comprehensive approach and account for any other abnormal transcription, we performed the transcriptional microarray analysis of E13.5 control and the Dlg5−/− lungs (Supplementary Table 1). As expected, Dlg5 was the most downregulated transcript in Dlg5−/− lungs. 29 genes were statistically significantly downregulated and 1079 genes upregulated in Dlg5−/− lungs (Bayes P<0.01). Pathway analysis using Ingenuity software identified the cell cycle and protein ubiquitination as two most significantly affected pathways in Dlg5−/− lungs. However, the analysis of identified changes did not result in finding of any additional signaling pathways that may be responsible for lung morphogenesis defects in Dlg5−/− mice.
Dlg5 is necessary for proper differentiation of alveolar lung progenitor cells
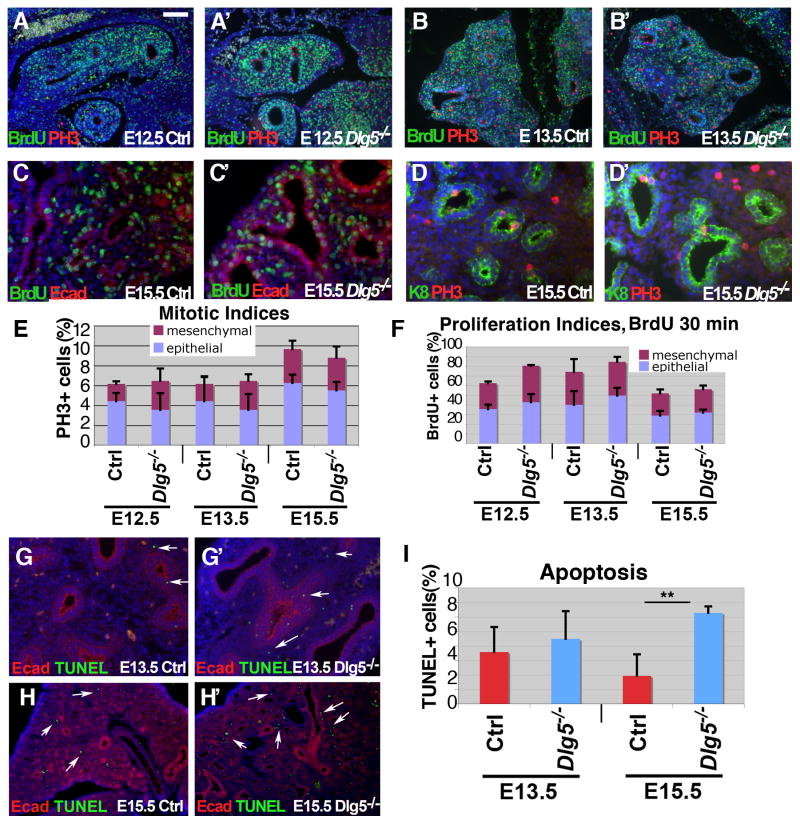
During mammalian lung development, branching morphogenesis is coupled with progenitor cell proliferation, differentiation, and programmed cell death. Defects in any of these processes can potentially result in an abnormal branching morphogenesis. To analyze whether the absence of Dlg5 results in a decreased rate of cell proliferation, which could potentially explain the decreased lung branching phenotype, we performed BrdU incorporation analysis and stained lungs with the mitotic cell marker, phospho-histone H3 (PH3). We did not find significant differences in the proportion of mitotic cells at E12.5, E13.5, or E15.5 between wild-type and Dlg5−/− cells (Figure 3A–B, D–E). In addition, the proportion of cells that stained positive for BrdU was either unchanged or slightly higher in Dlg5−/− lungs (Figure 3A–C′, F). Therefore, the lung morphogenesis defect in Dlg5−/− embryos was not associated with a paucity of cell proliferation. Analysis of apoptosis using TUNEL staining revealed no significant differences between Dlg5−/− and control lungs at E13.5 (Figure 3G–H′); however, there was a small, but statistically significant increase in the proportion of apoptotic cells in E15.5 Dlg5−/− lungs, which localized primarily to the mesenchymal cell compartment (Figure 3I). While the significance of a small increase in the percentage of apoptotic cells in Dlg5−/− lungs is presently unclear, apoptosis is a normal process in the mesenchymal lung compartment that takes place as the mesenchyme is thinned between the pseudoglandular and saccular stages (E16.5–E18.5) of lung morphogenesis (Scavo et al., 1998). Therefore, it is possible that this wave of mesenchymal apoptosis commences earlier in Dlg5−/− lungs.
Figure 3. Proliferation and apoptosis in developing Dlg5−/− lung.
(A–D′) Immunofluorescent stainings of E12.5, E13.5 and E15.5 lung sections from wild-type (Ctrl) and Dlg5−/− embryos with anti-BrdU (green in A–C′), anti-phospho-histone H3 (red, PH3 in A–B′, D–D′), anti-E-cadherin (epithelial cell marker, red in C–C′), anti-keratin 8 (epithelial cell marker, green, K8 in D–D′) antibodies. BrdU was injected into pregnant females 30 mins before euthanasia.
(E–F) Quantitation of the results shown in A–D′. Mitotic and proliferation indexes are determined as percentages of PH3+ and BrdU+ cells in the respective total cell population. Blue columns indicate epithelial and purple columns-mesenchymal cell types. Genotypes and ages are shown. Bars denote standard deviations, n=3–5 for each genotype.
(G–H′) Immunofluorescent stainings of E13.5 and E15.5 lung sections from wild-type (Ctrl) and Dlg5−/− embryos with anti-E-cadherin (Ecad, red) antibodies and TUNEL staining for apoptotic cells (green).
(I) Quantitation of TUNEL stainings shown in G–H′. The graphs show percentages of apoptotic cells in the developing lung; ages and genotypes are shown. ** indicates P <0.05, t-test, n=4
Bar in A represents 0.2 mm in A–B′ and H–H′, 50 mm in C–D′ and 0.1 mm in G–G′.
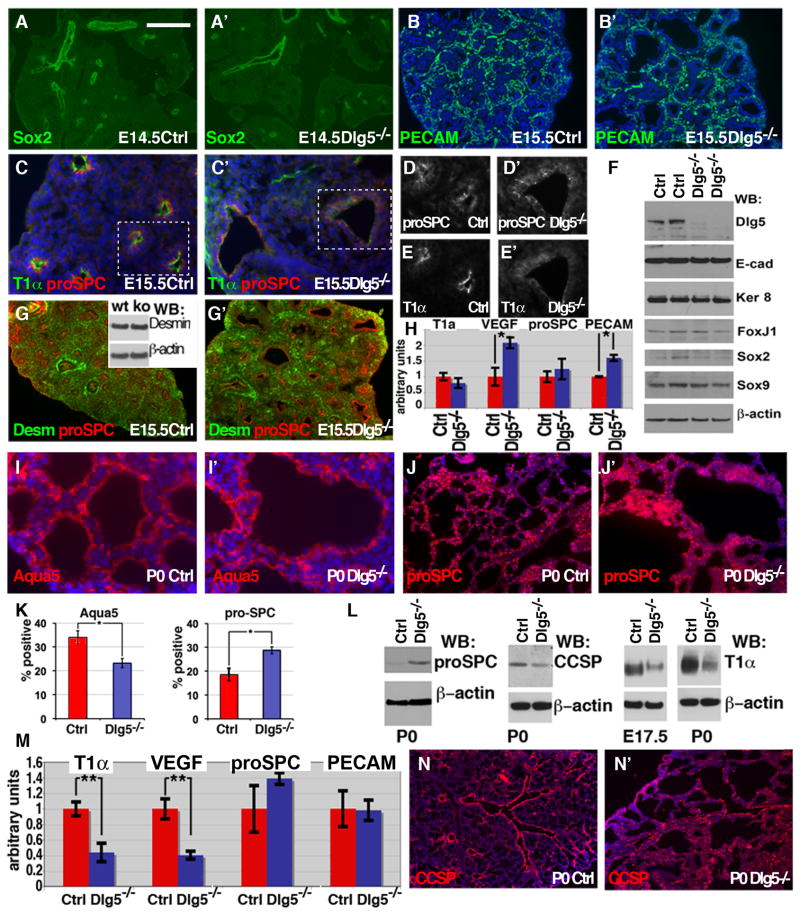
Proper lung morphogenesis also requires progenitor cells to undergo differentiation in a spatially and temporally coordinated manner; therefore, abnormalities in cell differentiation could result in aberrant lung formation. Normally, the tips of the buds contain the rapidly proliferating, nondifferentiated progenitors. As branching morphogenesis proceeds, these cells differentiate to form stalks, which later form the bronchi and bronchioles (Morrisey and Hogan, 2010). The late progenitors are localized to the tips of the buds and form the distal tubules and alveoli. To analyze the early stages of progenitor cell differentiation in Dlg5−/− lungs, we performed indirect immunofluorescence, western blotting, and quantitative RT-PCR for the cell type-specific markers. During early lung development, the cells in the proximal lung epithelium express the transcription factor, Sox2, while the cells in distal epithelium express Sox9 (Rajagopal et al., 2008). Immunostaining and western blot analysis using anti-Sox2 and anti-Sox9 antibodies revealed a normal proximal-distal patterning in the E14.5–E15.5 Dlg5−/− lungs (Figure 4A–A′, F and Supplementary Figure 3). In addition, the overall proportion of epithelial (E-cadherin+) and mesenchymal (Desmin+) cells was also unchanged in Dlg5−/− lungs (Figure 4G–G′, G inset, F).
Figure 4. Dlg5 is required for lung alveolar differentiation program.
(A–A′) Normal epithelial differentiation in E14.5 Dlg5−/− lungs. Immunofluorescent stainings of lung sections from wild-type (Ctrl) and Dlg5−/− embryos with anti-Sox2 antibodies.
(B–B′) Vasculature development in Dlg5−/− lungs. Immunofluorescent stainings of E15.5 lung sections from wild-type (Ctrl) and Dlg5−/− embryos with anti-PECAM (endothelial cell marker) antibody.
(C–E′) Immunofluorescent stainings of lung sections from E15.5 wild-type (Ctrl) and Dlg5−/− embryos with anti-T1α and anti-pro-SPC (both expressed in epithelial lung cells at E15.5) antibodies. Boxed areas in C–C′ are shown with split channels in D–E′. Note failure of apical (luminal) localization of T1α in Dlg5−/− cells in E′.
(F) Western blot analysis of total protein extracts from E15.5 wild-type (Ctrl) and Dlg5−/− lungs with anti-Dlg5, anti-E-cadherin (E-cad), anti-Keratin 8 (Ker 8), anti-FoxJ1, anti-Sox2, anti-Sox9 and anti-β-actin antibodies.
(G–G′) Immunofluorescent stainings of lung sections from E15.5 wild-type (Ctrl) and Dlg5−/− embryos with anti-desmin (green, Desm), anti-pro-SPC (red) antibodies. Western blotting on E15.5 total protein extracts with anti-Desmin and anti-β-actin antibodies is shown in the inset.
(H) qRT-PCR analysis of indicated gene expression in E13.5 wild-type (Ctrl) and Dlg5−/− lungs. Mean values with standard deviations are in arbitrary units with the level of wild-type lung adjusted to 1. * indicates P<0.05, t-test, n=3–4 for each genotype.
(I–J′) Immunohistochemistry staining of newborn (P0) lung sections from wild-type (Ctrl) and Dlg5−/− pups with anti-Aquaporin 5 (I–I′) (AEC1 marker at P0) and anti-proSPC (J–J′) (AEC2 marker at P0) antibodies.
(K) Quantitation of the proportion of AEC1 (Aqua5+) and AEC2 (proSPC+) cells in P0 wild-type (Ctrl) and Dlg5−/− lungs. * indicates P<0.05. Mann-Whitney test, n=4.
(L) Western blot analysis of total protein extracts from E17.5 and P0 wild-type (Ctrl) and Dlg5−/− lungs with anti-pro-SPC (AEC2 marker), CCSP (Clara cell marker), anti-T1α (AEC1 marker) and anti-β-actin antibodies. Note prominent increase in pro-SPC and decrease in T1α levels in Dlg5−/− lungs.
(M) qRT-PCR analysis of indicated gene expression in P0 wild-type (Ctrl) and Dlg5−/− lungs. ** indicates P<0.01. t-test, n=3
(N–N′) Immunofluorescent stainings of lung sections from P0 wild-type (Ctrl) and Dlg5−/− pups with anti-CCSP (Clara cell marker) antibodies.
Bar in A represents 1mm in A–A′, 0.4 mm in B–B′, G–G′, I–J′, N–N′,100 μm in C–E′.
Mesenchymal myofibroblasts are essential for alveolar septa formation (Warburton et al., 2008). To analyze myofibroblasts, we performed immunostaining with anti-α smooth muscle actin antibodies. Both proximal and distal lung in Dlg5−/− mice showed similar to controls distribution of myofibroblasts (Supplementary Figure 4). Examination of the vascular endothelial cells (PECAM+) by immunofluorescent localization revealed normal lung vasculature in the developing mutant embryo (Figure 4B–B′). Interestingly, the relative proportion of vascular cells was increased slightly in Dlg5−/− lungs and this was accompanied by an increase in the expression of VEGF, a major activator of blood vessel formation that is secreted by lung epithelial cells (Millauer et al., 1993) (Figure 4H). Immunostaining and qRT-PCR analysis of expression of pro-SPC (marker of epithelial cells in the early developing lung) revealed no significant differences between E13.5–E15.5 Dlg5−/− and wild-type lungs (Figure 4C–C′, D–D′, G–G′, H). Similarly, we found no differences in the expression levels of proximal airway epithelial cell marker FoxJ1 (marker of ciliated cells) (Figure 4F) (McElroy and Kasper, 2004; Wert et al., 1993). Interestingly, staining with anti-T1α/podoplanin antibodies revealed striking differences between E13.5–E15.5 Dlg5−/− and wild-type lungs. T1α is a marker of epithelial lung cells in early development and a specific marker of type 1 alveolar cells (AEC1) in late development and in the adult lung. While T1α was expressed at similar levels in both mutant and wild-type epithelial cells (Figure 4H), it failed to properly localize to the apical membrane domain in Dlg5−/− cells, indicating potential problems with apical basal polarity in lung epithelial cells (Figure 4E–E′). Overall, we conclude that the early differentiation of progenitor cells is relatively normal in Dlg5−/− lungs.
To analyze the differentiation of progenitor cells during late lung embryogenesis, we performed immunostaining, western blotting, and qRT-PCR analyses of cell type-specific markers in E17.5-P0 animals. During this period of embryonic lung development, the late progenitors at the tips of epithelial tree give rise to two main cell types of the future alveolus: alveolar epithelial type 1 and type 2 cells (AEC1 and AEC2) (Morrisey and Hogan, 2010). The cuboidal AEC2 cells (pro-SPC+) are capable of dividing to self-renew and generate terminally differentiated flat AEC1 cells (T1α+), lining the most surface of the alveoli (Evans et al., 1975; Morrisey and Hogan, 2010). Examining the AEC1 and AEC2 cell type-specific markers revealed a prominent differentiation defect in Dlg5−/− lungs. While AEC2 (pro-SPC+) cells were overrepresented, the terminally differentiated AEC1 cells (Aquaporin 5+ or T1α+ ) were prominently underrepresented in E17.5-P0 Dlg5−/− lungs (Figure 4I–M). In addition, staining for CCSP (a marker of the bronchi and bronchioli Clara cells) revealed disorganization of airways in Dlg5−/− lungs (Figure 4N–N′). These results indicate that Dlg5 is required for the proper differentiation of the late lung progenitor cells and formation of terminally differentiated AEC1 cells.
Dlg5 is required for the apical membrane localization of atypical PKC (aPKC)
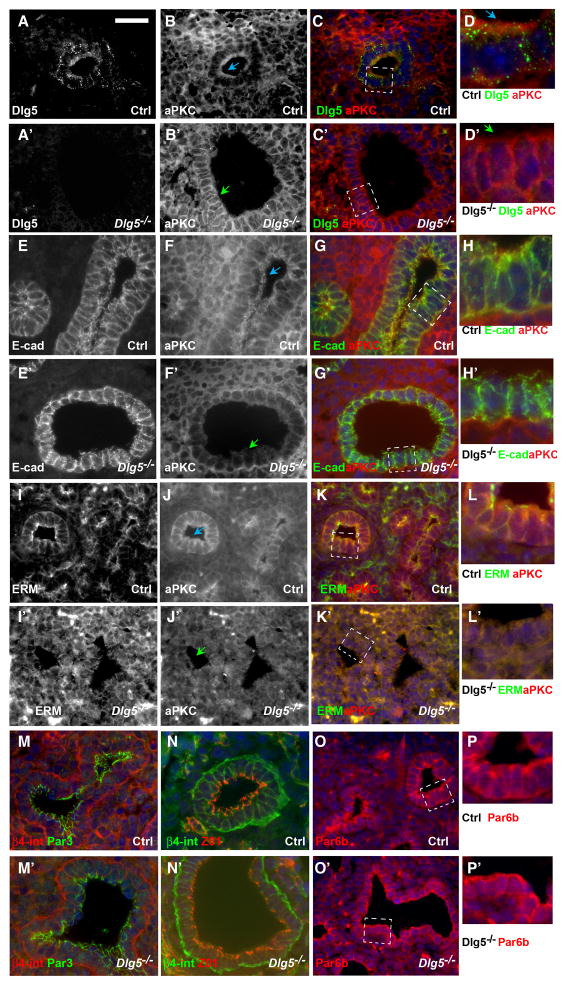
During the pseudoglandular stage of lung development (E9.5–E16.5), the epithelium lining the developing lung tubules is represented by a polarized columnar epithelial cell layer with basally-localized nuclei and a prominent apical membrane domain that faces the lumen. During our histological analysis of the Dlg5−/− lungs, we noticed that in mutant embryonic lungs the tall columnar epithelial cells were replaced by cuboidal cells containing centrally-positioned nuclei (Figure 1, insets in J–K′). These morphological changes and the failure of T1α protein to properly localize to the apical membrane domain suggested potential perturbations in apical-basal cell polarity in Dlg5−/− lungs. To analyze cell polarity, we performed immunofluorescent stainings of sections from E14.5 wild-type and Dlg5−/− lungs using markers of apical-basal cell polarity. We found that the apical membrane domain markers atypical PKCζ (aPKC) and ezrin-radixin-moesin (ERM) proteins failed to localize apically and instead showed basolateral and diffuse cytoplasmic localization in Dlg5−/− epithelial cells (Figure 5B–B′, D–D′, F–F′, I–J′, Supplementary Figure 5B–B′). In addition to significant perturbations in the localization of apical proteins, we observed more diffuse and disorganized staining of the lateral membrane marker E-cadherin, while basal membrane proteins such as β4-integrin remained unchanged (Figure 5E–E′, M–N′). Tight junctions (ZO1+) were also preserved in Dlg5−/− epithelial cells (Figure 5N–N′). Interestingly, while the loss of the apically localized aPKC and ERM proteins in Dlg5−/− lungs was the earliest polarity defect in the mutant lung epithelial cells, this was not a general defect in the localization of apical polarity proteins, because these cells displayed normal apically localized Par3 and Par6b proteins (Figure 5M–M′, O–P′, Supplementary Figure 5).
Figure 5. Dlg5 is required for apical localization of atypical PKC (aPKC) and maintenance of apical-basal cell polarity.
(A–D′) Immunofluorescent stainings of lung sections from E14.5 wild-type (Ctrl) and Dlg5−/− embryos with anti-Dlg5 (A–A′, green in C–D′) and anti-aPKC (B–B′, red in C–D′) antibodies. Note partial co-localization of Dlg5 and aPKC in control cells (A–D) and loss of apical and prominent lateral membrane staining of aPKC in Dlg5 −/− cells (B′, D′).
(E–P′) Immunofluorescent stainings of lung sections from E14.5 wild-type (Ctrl) and Dlg5−/− embryos with anti-E-cadherin (E–E′, green in G–G′, H–H′), anti-aPKC (F–F′, J–J′, red in G–G′, H–H′, K–K′, L–L′), anti-ERM (I–I′, green in K–K′, L–L′), anti-Par3 (green in M–M′), anti-β4-integrin (red in M–M′, green in N–N′), anti-ZO1 (red in N–N′) and anti-Par6b (red in O–P′) antibodies. Note properly polarized localization of basal (β4-integrin), apical (Par6b, Par3) and tight junctional (ZO1) markers in E14.5 Dlg5 −/− cells. Areas in dashed white boxes in C–C′, G–G′, K–K′, O–O′ are shown at higher magnification in D–D′, H–H′, L–L′, P–P′, respectively.
Blue arrows denote properly localized apical aPKC and green arrows denote loss of apical aPKC in Dlg5 −/− cells.
Bar in A represents 67 μm in A–C′, E–G′, I–K′, M–O′, 20 μm in D–D′, H–H′, L–L′, P–P′.
aPKC is a critical apical polarity protein and is the only enzyme with kinase activity among the canonical apical polarity proteins. Upon activation, aPKC becomes phosphorylated at Thr555/563 and Thr410/403. To determine whether Dlg5 regulates the activity of aPKC, we performed western blot analysis of total lung protein extracts with anti-phospho-specific aPKC antibodies. We found no changes in overall activity of aPKC in Dlg5−/− lungs, indicating that Dlg5 is required for proper aPKC localization, but not activation of aPKC (Supplementary Figure 6). Since Dlg5 was required for proper localization of aPKC and ERM proteins, we analyzed whether Dlg5 itself is also asymmetrically localized. Immunofluoresecent stainings of lung sections using anti-Dlg5 antibodies demonstrated that Dlg5 is present in both epithelial and, to a lesser degree, in mesenchymal cells of the developing lung. In epithelial cells Dlg5 localizes to cytoplasmic vesicles at the basolateral domain, as well as to the apical membrane domains, where it overlaps prominently with aPKC (Figure 5A–D′). The specificity of anti-Dlg5 antibodies was confirmed by the absence of staining on the sections from Dlg5−/− lungs. We conclude that Dlg5 is required for the maintenance of apical aPKC and that the loss of Dlg5 results in diffuse and basal-lateral relocalization of aPKC and disruption of the normal apical-basal cell polarity in the developing lung airways.
Discussion
Dlg5 is an apical-basal cell polarity protein that functions at both apical and basolateral membrane domains
This study demonstrated that in addition to its function at the basolateral membrane domain, Dlg5 localizes to apical membrane of polarized lung epithelial cells and promotes apical localization of aPKC and ERM.
Dlg5 belongs to the Dlg family of MAGUK proteins known for their scaffolding functions in targeting receptors, channels and cell surface molecules to the specialized membrane domains (Funke et al., 2005). The best studied Dlg protein family member, Dlg1, is a critical apical-basal cell polarity protein that uses not yet well understood mechanisms to promote formation of the lateral membrane domain (Yamanaka and Ohno, 2008). Our previous analysis of murine Dlg5 revealed its important role in brain and kidney development (Chang et al., 2010; Nechiporuk et al., 2007). We demonstrated that similar to Dlg1, Dlg5 also has an important function at the lateral membrane domain (Nechiporuk et al., 2007). We found that Dlg5 binds to cadherin-catenin complexes, partially localizes to cadherin-carrying intracellular vesicles and facilitates the cell-surface delivery of cadherins, which promotes the stability of the adherens junctions and the identity of the lateral membrane domain. Consistent with these findings, we observed prominent vesicular localization of Dlg5 and disorganization of E-cadherin-containing lateral membrane domains in Dlg5−/− lung epithelial cells (Figure 5A, D, E–E′, H–H′).
The analysis of the localization of various apical-basal polarity proteins in these cells revealed a very specific defect in localization of aPKC, which was apical in the wild-type cells, but localized to the lateral membrane domain or cytoplasm in Dlg5−/− mutants (Figure 5B–B′, F–F′, J–J′). Interestingly, mislocalization of the apically localized aPKC and Citron kinase and direct interaction between Dlg5 and Citron kinase were previously noted in Dlg5−/− kidney epithelial and neural progenitor cells (Chang et al., 2010; Nechiporuk et al., 2007). Since, while disorganized, the lateral membrane domain was maintained in Dlg5−/− lung epithelial cells, these data suggest that in addition to its function at the lateral membrane domain, Dlg5 may have a more direct role in the maintenance of apical aPKC. Interaction with Par3 plays an important role in targeting of aPKC to the apical membrane domain (Harris and Peifer, 2005; McCaffrey and Macara, 2009a). We found that contrary to aPKC, Par3 remains apically localized in E14.5 Dlg5−/− lung epithelial cells (Supplementary Figure 5). This indicates that Dlg5 may provide a functional link between Par3 and aPKC, as it is necessary for targeting of aPKC to Par3-containing membrane domain. Therefore, Dlg5 has multiple functions in the maintenance of apical-basal cell polarity. While part of Dlg5 protein pool localizes to the vesicles and facilitates the transport of cadherin-catenin complexes to the lateral membrane domain, another part of Dlg5 pool localizes to the apical membrane domain, where it promotes the maintenance of the apical polarity complexes.
Role of Dlg5 in lung progenitor cell differentiation
In addition to a major role in establishing and maintaining apical-basal polarity, Drosophila Dlg1 is also important for regulating the asymmetric cell division of neural progenitor cells (Neumuller and Knoblich, 2009). Drosophila neuroblasts divide asymmetrically to self-renew and generate differentiating daughter cells. In dlg1-mutant fly embryos, neuroblasts fail to asymmetrically localize cell fate determinants and, therefore, generate daughter cells that are unable to differentiate (Ohshiro et al., 2000; Peng et al., 2000). While the function of Drosophila Dlg5 is still unknown, we show here that similar to Drosophila Dlg1 function in differentiation of neuroblasts, mammalian Dlg5 is also important for normal differentiation in lung epithelial cells. In addition to our observed defects in cell polarity in Dlg5−/− embryos, we show that Dlg5 is required for proper differentiation of lung embryonic progenitor AEC2 cells and formation of terminally differentiated AEC1 cells. While it is generally believed that AEC1 cells are formed by self-renewing AEC2 cells, it has not been formally ruled out that both AEC1 and AEC2 cells have a common alveolar progenitor (Morrisey and Hogan, 2010). It is possible that Dlg5 regulates the cell fate decision in these common progenitor cells. Presently, it is not clear how Dlg5 regulates the formation of AEC1 cells, but it is tempting to speculate that the paucity of AEC1 cells and overproduction of AEC2 cells in Dlg5−/− lungs may be the result of the abnormal asymmetric cell division of self-renewing and/or differentiating alveolar progenitors.
In Drosophila, Dlg1 regulates asymmetric cell division by regulating the orientation of the mitotic spindle, to ensure that the cell fate determinants are inherited by only one daughter cell that is then targeted for differentiation (Siegrist and Doe, 2005). This is likely to be achieved via an interaction between Pins and Dlg1, which subsequently recruits Khc73 kinesin and provides a link to astral microtubules (Johnston et al., 2009). Intriguingly, the proper orientation of the mitotic spindle is critical for normal lung morphogenesis (Tang et al., 2011). While the mechanisms responsible for the role of Dlg5 in regulating AEC1 cell production are unknown, it is interesting to note that mammalian Dlg5 interacts with the mitotic kinesin, KIF20A (Taniuchi et al., 2005). In the future, it will be important to determine whether Dlg5 is necessary for proper orientation of the mitotic spindle in asymmetrically dividing progenitor cells.
In recent studies epithelial cell polarity has emerged as a vital factor for lung branching morphogenesis and disruption of apical-basal cell polarity in Eya1−/ − lungs plays a critical role in the regulation of normal lung morphogenesis and differentiation of embryonic epithelial progenitor cells (El-Hashash et al., 2011). Eya1 is a dual function phosphatase and transcription co-activator, which is necessary for the maintenance of cell polarity in the hair cells during inner ear development and in lung epithelial cells during lung morphogenesis (El-Hashash et al., 2011; Zou et al., 2008). Eya1−/− lungs display prominent activation of aPKC, failure of asymmetric localization of Numb and inactivation of Notch signaling (El-Hashash et al., 2011). These changes are significantly different from the phenotype of Dlg5−/− lungs, where we do not see changes in aPKC activity. Moreover, our microarray analysis data demonstrate that the endogenous targets of Notch pathway remain unchanged, indicating the absence of significant impact on the Notch signaling in Dlg5−/− lungs.
The integrin-laminin cell-substratum adhesion system is not a canonical apical-basal polarity mechanism; however, this system has an important role in establishing and maintaining apical-basal cell polarity (Li et al., 2003; Weaver et al., 2002). Intriguingly, there are striking parallels between lung phenotypes in Dlg5−/− embryos and embryos with epithelial-specific knockout of Laminin α5 (Nguyen et al., 2005). Laminin α5, an α chain of Laminin 10/11, is a component of the basement membrane and it is expressed in both embryonic and adult lungs. Similarly to the Dlg5−/− lungs, postnatal pro-SPC-Cre/Lamα5Fl/− lungs show significant dilation of distal lung airspaces and dramatic decrease in expression of the AEC1 marker, T1α. However, unlike Dlg5−/− embryos, Laminin α5 mutants also showed a decrease in the numbers of AEC2 cells. Thus, it appears that the block in alveolar differentiation takes place earlier in pro-SPC-Cre/Lamα5Fl/− lungs. While laminins can play an important role in cell polarity, it is not clear whether apical-basal cell polarity is perturbed in pro-SPC-Cre/Lamα5Fl/− lungs. Similarity between Dlg5−/− and pro-SPC-Cre/Lamα5Fl/− lungs may indicate the important crosstalk between cell polarity and cell-substratum adhesion in tissue morphogenesis. Future analysis of lung phenotypes in additional apical-basal polarity gene mutants will help to determine whether the abnormal branching and distal lung differentiation defects observed in the Dlg5−/− embryos are specific to Dlg5, or they are general phenotypes of abnormal apical-basal polarity pathways.
Materials and methods
Mouse strains, histological and macroscopic lung analyses
Generation of Dlg5 knockout mice was previously described (Nechiporuk et al., 2007). TOPGAL β-catenin reporter mice were previously described (DasGupta and Fuchs, 1999). Mice were maintained on C57BL/6J background and the embryonic age was determined by considering the morning of the vaginal plug as E0.25. Lungs from embryos, newborn and adults were dissected, fixed for 1 hour in 4% paraformaldehyde (PFA) in PBS at 4°C, embedded in paraffin wax, sectioned at 5–7 microns and stained with hematoxylin and eosin. For lung branching analyses E11.5–E13.5 lungs were dissected, fixed in 4% PFA and stained with rabbit anti-E-cadherin antibody followed by immunofluorescence-conjugated secondary antibodies as described previously (Harris et al., 2006). BrdU incorporation analyses were performed as described (Klezovitch et al., 2004).
Immunofluorescence and antibodies
Immunofluorescent staining were performed as described (Klezovitch et al., 2004). The following antibodies were used: anti-keratin 8 (Troma-1, Developmental Studies Hybridoma Bank (DSHB), 1:10 dilution), anti-pro-SPC (Chemicon), anti-T1α antibody (antibody 8.1.1, DSHB, 1:100), anti-phospho-histone3 antibody (Upstate, 1:100), mouse α2 integrin (Pharmingen, 1:100), anti-α5 integrin (Pharmingen, 1:100), anti-ERM (Ezrin/Radixin/Moesin, Millipore, 1:100), anti-β1 integrin (Pharmingen), anti-fibronectin and anti-β4 integrin (gift from Dr. William Carter, FHCRC, 1:500 and 1:100, respectively), anti-mouse PECAM (Pharmingen,1:100), anti-Clara cell secretory protein (CCSP, Cell Signaling, 1:100), anti-α smooth muscle actin (Sigma, 1:800), anti-desmin (Abcam, 1:500), anti-Pard6b (Santa Cruz, 1:100), anti-Par3 (Upstate, 1:100), anti-aPKC (Santa Cruz, 1:100). Rabbit polyclonal anti-Dlg5 antibodies were previously described (Nechiporuk et al., 2007). Species-appropriate FITC- or Texas Red–conjugated secondary antibodies were used at 1:200 dilution (Jackson Immunoresearch). To determine apoptosis, TUNNEL staining was performed on paraffin-embedded lung sections using TdT FragEL DNA fragmentation kit (Oncogene Research). In situ hybridization analysis of Dlg5 expression was performed as previously described (Nechiporuk et al., 2007). In situ hybridization probe against FGF10 was kindly provided by Dr. Bridgit L.M. Hogan and whole mount in situ was performed as previously described (Sasaki and Hogan, 1993).
Western blotting
Embryonic lungs were lysed in buffer containing phosphate buffered saline, (pH 7.4), 1% IgPal, 1mM EDTA, phosphatase and proteinase inhibitors. Western blotting was performed using the flowing antibodies: anti-phospho ERK1/2, anti-total Erk1/2, anti-β-actin (Sigma), anti-phospho SMAD1/5, anti-total SMAD5, anti-phospho-aPKC (Cell Signaling), anti-PDGFRa, anti-Sox9, anti-FGFRa, anti-MMP13 and anti-aPKC (Santa Cruz), anti-Par3 (Upstate), anti-desmin, anti-Par6 (Abcam), anti-proSPC (Chemicon), anti-T1α/podoplanin (Antibody 8.1.1, DSHB), keratin 8 (DSHB), anti-E-cadherin (Zymed), anti-Sox2 (Chemicon), and Dlg5 (Nechiporuk et al., 2007) antibodies at 1:1000 dilution.
RNA analyses
For microarray analysis, total RNA from E13.5 Dlg5−/− and wild-type mouse lungs was extracted using TRIZOL (Invitrogen) and contaminating DNA was removed by treatment with RNAse-free DNAse (Ambion). Three RNA pools for each genotype, each containing RNAs extracted from three independent embryos, were analyzed using six Affymetrix GeneChip Mouse Genome 430 2.0 arrays that detect 39,000 transcripts. For qRT-PCR, RNA was reverse transcribed using Superscript II (Invitrogen). qPCR was performed in triplicates using SYBR-green PCR master mix (PE Applied Biosystems) and quantities were determined according to the standard curve. Relative abundance of specific mRNAs was normalized using 18S mRNA. Primer sets: proSPC: 5′-ggtcctgatggagagtccac-3′ and 5′-gatgagaaggcgtttgaggt-3′, PECAM: 5′-atggatgctgttgatggtga-3′ and 5′-gctggtgctctatgcaagc-3′, Vegf: 5′-aaaaacgaaagcgcaagaaa-3′ and 5′-tttctccgctctgaacaagg-3′, T1α: 5′-cagtgttgttctgggttttgg-3′ and 5′-ggttgcattggaaagatgct-3′.
Supplementary Material
RNAs from Dlg5−/+ and Dlg5−/− lungs were analyzed by Affymetrix expression arrays. Relative fold change is calculated with respect to heterozygous brains. Bayes. p is the P value obtained using the CyberT Bayesian statistical framework.
Whole mount LacZ staining of TOPGAL transgenic lungs from E12.5 and E13.5 wild-type (Ctrl) and Dlg5−/− embryos.
(A) Western blot analyses of total protein extracts from E13.5 and E15.5 wild-type (Ctrl) and Dlg5 −/− lungs with anti-phospho-ERK1/2 (pERK1/2), anti-total-ERK (ERK1/2) and anti-β-actin antibody.
(B–C) Western blot analysis of total proteins (input) and phospho-tyrosine immunoprecipitates (IP:pTyr) from proteins extracted from E13.5 wild-type (Ctrl) and Dlg5−/− lungs with anti-FGFR2, anti-PDGFRα and anti-β-actin antibody.
(D) Western blot analyses of total protein extracts from E13.5 and E15.5 wild-type (Ctrl) and Dlg5 −/− lungs with anti-phospho-SMAD1/5 (pSMAD), anti-total-SMAD5 and anti-β-actin antibody.
(E–F′) Whole mount in situ hybridization analyses of Fgf10 mRNA from E13.0 (E–E′) and E13.5 (F–F′) wild-type (Ctrl) and Dlg5−/− lungs. Arrows indicate distal tips of the mesenchyme between epithelial stalks. Bars represent 0.5 mm in E–E′ and 0.35 mm in F–F′.
Immunofluorescent stainings of lung sections from E14.5 wild-type (Ctrl) and Dlg5−/− embryos with anti-Sox9 antibody, green. Arrows indicate Sox9+ distal lung branches. Bar represents 1mm.
Immunofluorescent staining of E15.5 lungs with anti-α smooth muscle actin (αSMA, green) and E-cadherin (E-cad, red) antibodies. Arrows indicate proximal lung branches, arrowheads indicate distal lung branches. Bar represents 67 μm.
Immunofluorescent stainings of lung sections from E14.5 wild-type (Ctrl) and Dlg5−/− embryos with anti-Par3 (A–A′, green in C–C′) and anti-aPKC (B–B′, red in C–C′) antibodies. Bar in A represents 67 μm.
Western blot analysis of total protein extracts from E14.5 Dlg5−/+ and Dlg5−/− lungs with anti-Par3a, anti-Par6b, total aPKC, anti-phosphoThr555/563-aPKC, anti-phosphoThr403/410-aPKC and anti-β-actin antibodies.
Highlights.
Dlg5 is necessary for lung branching morphogenesis.
Dlg5 is required for proper differentiation of distal lung progenitor cells.
Loss of the apical-basal cell polarity of epithelial progenitors is one of the primary defects in Dlg5−/− lungs. 4. Dlg5 is necessary for the apical membrane domain maintenance of aPKC.
Acknowledgments
We thank Dr. Ross Metzger for the analysis and comments on lung phenotype in Dlg5−/− embryos, Drs. Philippe Brulet, Rolf Kemler, Andrew Farr and Developmental Studies Hybridoma Bank for the gift of antibodies; Dr. Bridgit Hogan for in situ FGF10 probe. This work was supported by NIH R01 CA131047 and R01 CA098161 to V. Vasioukhin. T. Nechiporuk was partially supported by the Chromosome Metabolism and Cancer Training Grant NIH T32 CA09657.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellusci S, et al. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–78. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- Betschinger J, et al. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–30. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Bilder D, et al. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–6. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bostrom H, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–73. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Bourbon JR, et al. Bronchopulmonary dysplasia and emphysema: in search of common therapeutic targets. Trends Mol Med. 2009;15:169–79. doi: 10.1016/j.molmed.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–24. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Chang Y, et al. Discs large 5 is required for polarization of citron kinase in mitotic neural precursors. Cell Cycle. 2010;9:1990–7. doi: 10.4161/cc.9.10.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Reynolds SD. Wnt signaling in lung organogenesis. Organogenesis. 2008;4:100–8. doi: 10.4161/org.4.2.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hashash AH, et al. Eya1 controls cell polarity, spindle orientation, cell fate and Notch signaling in distal embryonic lung epithelium. Development. 2011;138:1395–407. doi: 10.1242/dev.058479. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Evans MJ, et al. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–50. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Funke L, et al. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annual review of biochemistry. 2005;74:219–45. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- Harris KS, et al. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A. 2006;103:2208–13. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol. 2005;170:813–23. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurov JB, et al. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Current biology : CB. 2004;14:736–41. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Johnston CA, et al. Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell. 2009;138:1150–63. doi: 10.1016/j.cell.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klezovitch O, et al. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–71. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, et al. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003;4:613–24. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 2009a;23:1450–60. doi: 10.1101/gad.1795909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. Widely conserved signaling pathways in the establishment of cell polarity. Cold Spring Harbor perspectives in biology. 2009b;1:a001370. doi: 10.1101/cshperspect.a001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. Cold Spring Harbor perspectives in biology. 2012. Signaling Pathways in Cell Polarity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy MC, Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J. 2004;24:664–73. doi: 10.1183/09031936.04.00096003. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, et al. The branching programme of mouse lung development. Nature. 2008;453:745–50. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–46. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechiporuk T, et al. Failure of epithelial tube maintenance causes hydrocephalus and renal cysts in Dlg5−/− mice. Dev Cell. 2007;13:338–50. doi: 10.1016/j.devcel.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumuller RA, Knoblich JA. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 2009;23:2675–99. doi: 10.1101/gad.1850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NM, et al. Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev Biol. 2005;282:111–25. doi: 10.1016/j.ydbio.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, et al. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 2000;408:593–6. doi: 10.1038/35046087. [DOI] [PubMed] [Google Scholar]
- Peng CY, et al. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- Rajagopal J, et al. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–34. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- Scavo LM, et al. Apoptosis in the development of rat and human fetal lungs. Am J Respir Cell Mol Biol. 1998;18:21–31. doi: 10.1165/ajrcmb.18.1.2744. [DOI] [PubMed] [Google Scholar]
- Sezaki T, et al. Role of Dlg5/lp-dlg, a Membrane-Associated Guanylate Kinase Family Protein, in Epithelial-Mesenchymal Transition in LLc-PK1 Renal Epithelial Cells. PLoS ONE. 2012;7:e35519. doi: 10.1371/journal.pone.0035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–35. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Smolen GA, et al. A genome-wide RNAi screen identifies multiple RSK-dependent regulators of cell migration. Genes & development. 2010;24:2654–65. doi: 10.1101/gad.1989110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider GL. Parker B. Francis Lecture. Animal models of chronic airways injury. Chest. 1992;101:74S–79S. doi: 10.1378/chest.101.3_supplement.74s. [DOI] [PubMed] [Google Scholar]
- Stoll M, et al. Genetic variation in DLG5 is associated with inflammatory bowel disease. Nature genetics. 2004;36:476–80. doi: 10.1038/ng1345. [DOI] [PubMed] [Google Scholar]
- Tang N, et al. Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science. 2011;333:342–5. doi: 10.1126/science.1204831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi K, et al. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res. 2005;65:105–12. [PubMed] [Google Scholar]
- Wakabayashi M, et al. Interaction of lp-dlg/KIAA0583, a membrane-associated guanylate kinase family protein, with vinexin and beta-catenin at sites of cell-cell contact. The Journal of biological chemistry. 2003;278:21709–14. doi: 10.1074/jbc.M211004200. [DOI] [PubMed] [Google Scholar]
- Warburton D, et al. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc Am Thorac Soc. 2008;5:703–6. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–16. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wert SE, et al. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol. 1993;156:426–43. doi: 10.1006/dbio.1993.1090. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–24. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–64. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- Zou D, et al. Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet. 2008;17:3340–56. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNAs from Dlg5−/+ and Dlg5−/− lungs were analyzed by Affymetrix expression arrays. Relative fold change is calculated with respect to heterozygous brains. Bayes. p is the P value obtained using the CyberT Bayesian statistical framework.
Whole mount LacZ staining of TOPGAL transgenic lungs from E12.5 and E13.5 wild-type (Ctrl) and Dlg5−/− embryos.
(A) Western blot analyses of total protein extracts from E13.5 and E15.5 wild-type (Ctrl) and Dlg5 −/− lungs with anti-phospho-ERK1/2 (pERK1/2), anti-total-ERK (ERK1/2) and anti-β-actin antibody.
(B–C) Western blot analysis of total proteins (input) and phospho-tyrosine immunoprecipitates (IP:pTyr) from proteins extracted from E13.5 wild-type (Ctrl) and Dlg5−/− lungs with anti-FGFR2, anti-PDGFRα and anti-β-actin antibody.
(D) Western blot analyses of total protein extracts from E13.5 and E15.5 wild-type (Ctrl) and Dlg5 −/− lungs with anti-phospho-SMAD1/5 (pSMAD), anti-total-SMAD5 and anti-β-actin antibody.
(E–F′) Whole mount in situ hybridization analyses of Fgf10 mRNA from E13.0 (E–E′) and E13.5 (F–F′) wild-type (Ctrl) and Dlg5−/− lungs. Arrows indicate distal tips of the mesenchyme between epithelial stalks. Bars represent 0.5 mm in E–E′ and 0.35 mm in F–F′.
Immunofluorescent stainings of lung sections from E14.5 wild-type (Ctrl) and Dlg5−/− embryos with anti-Sox9 antibody, green. Arrows indicate Sox9+ distal lung branches. Bar represents 1mm.
Immunofluorescent staining of E15.5 lungs with anti-α smooth muscle actin (αSMA, green) and E-cadherin (E-cad, red) antibodies. Arrows indicate proximal lung branches, arrowheads indicate distal lung branches. Bar represents 67 μm.
Immunofluorescent stainings of lung sections from E14.5 wild-type (Ctrl) and Dlg5−/− embryos with anti-Par3 (A–A′, green in C–C′) and anti-aPKC (B–B′, red in C–C′) antibodies. Bar in A represents 67 μm.
Western blot analysis of total protein extracts from E14.5 Dlg5−/+ and Dlg5−/− lungs with anti-Par3a, anti-Par6b, total aPKC, anti-phosphoThr555/563-aPKC, anti-phosphoThr403/410-aPKC and anti-β-actin antibodies.