Abstract
BTBR T+ tf/J (BTBR) is a genetically homogenous inbred strain of mice that displays abnormal social behaviors, deficits in vocalizations, and high levels of repetitive behaviors, relevant to the three diagnostic symptoms of autism spectrum disorder, leading to the use of this strain as a mouse model of autism. Comprehensive observations of BTBR social behaviors within the home cage during early stages of development have not been conducted. Here we evaluate the home cage behaviors of BTBR in two laboratory environments (NIMH, Bethesda, Maryland versus UC Davis, Davis, California), starting from the day of weaning and continuing into adulthood. Extensive ethogram parameters were scored for BTBR in home cages that contained four BTBR conspecifics, versus home cages that contained four C57BL/6J (B6) conspecifics. BTBR were considerably less interactive than B6 in the home cage at both sites, as measured during the early dark stage of their circadian cycle. A novel home cage behavioral measure, frequency of long interactions, was found to be more frequent and of longer duration in B6 versus BTBR home cages across experimental sites. Significant strain differences in the occurrence of investigative and affiliative behaviors were also seen, however these findings were not fully consistent across the two testing sites. At the end of the 30-day home cage observation period, each seven-week old subject mouse was tested in the three-chambered social approach task. BTBR displayed lack of sociability and B6 displayed significant sociability, consistent with previous reports. Our findings reveal that BTBR engaged in lower levels of some components of spontaneous conspecific social interactions in the home cage environment throughout juvenile development, consistent with their deficits in juvenile and adult sociability as measured in specialized social tasks.
Keywords: inbred strain, home cage observation, social interaction
1. Introduction
The BTBR T+ tf/J (BTBR) inbred strain is considered a mouse model of autism because of its robust, well-replicated, social deficits on a variety of tasks and its strikingly high frequency of repetitive behaviors. In the three-chambered social approach task, BTBR fail to spend more time in the side chamber containing a novel mouse versus in the side chamber containing a novel object and spend less time sniffing the novel mouse as compared to the novel object [1–14]. Further, BTBR initiate significantly fewer reciprocal social interactions both as juveniles and adults, as compared to standard strains such as C57BL/6J (B6) and FVB [1, 2, 4, 6, 11]. BTBR emit fewer ultrasonic vocalizations than control strains in response to social cues, such as male responses to an estrus female, or to female urine [15–18]. These deficits are highly specific, since BTBR have consistently exhibited normal scores in our laboratory on measures of health, reflexes, anxiety-related behaviors, motor functions and sensory abilities [2, 3, 19, 20]. In addition, high levels of repetitive self-grooming and digging are displayed by BTBR in an empty cage or during a social interaction session [2, 4–7, 9, 21, 22]. This inbred strain of mice, therefore, incorporates behavioral abnormalities relevant to the three diagnostic symptoms of autism.
Careful analysis of spontaneous home cage social interactions in BTBR could reveal the presence or absence of reciprocal social interaction abnormalities in the normal housing environment, particularly during the early dark phase of the circadian cycle when mice are most active. The current experiments were designed to evaluate social interactions in the BTBR home cages and B6 home cages, during the juvenile period of development beginning immediately after weaning. We video-recorded home cages of B6 or BTBR mice across 30 days of juvenile development in two different vivarium environments. Videotapes of the early dark hours were scored with an extensive behavioral ethogram, which captured the occurrence of social behavior parameters including sniffing, following, grooming, attack, flee, pushing under and crawling over. During initial observations it was noted that B6 mice would engage in sustained reciprocal interactions lasting at least 10 seconds and sometimes up to minutes in length. These interactions were almost absent in the BTBR cages. Thus, the behavioral parameter ‘long interaction’ was added to the ethogram to measure the occurrence of these interactions in each of the home cages. Our findings at both locations revealed that home cage social interactions by BTBR mice were lower than home cage social interactions in B6 mice, consistent with the previously reported low adult sociability in BTBR as compared to B6.
2. Methods
2.1 Mice
Behavioral observations were conducted at two sites, the National Institute of Mental Health Intramural Research Program in Bethesda, Maryland and the University of California Davis in Davis, California, each utilizing different housing and video recording strategies.
2.1.1 Bethesda site
Adult breeding pairs of the inbred strains C57BL/6J(B6) and BTBR T+tf/J (BTBR) were purchased from The Jackson Laboratory (JAX, Bar Harbor, ME) and bred in a conventional vivarium, maintained on a 12:12 light/dark cycle with lights on at 6:00 AM, and at approximately 20°C and 55% humidity. Standard rodent chow and tap water were available ad libitum. All animals were housed in Tecniplast plastic filtertop cages (15cm × 38cm × 12.5cm) on ventilated racks. Housing and procedures were conducted in strict compliance with the National Institute of Health Guidelines for Care and Use of Laboratory Animals and were approved by the National Institute of Mental Health Animal Care and Use Committee.
2.1.2 Davis site
Adult B6 breeding pairs were purchased from JAX West (Sacramento, CA). Adult BTBR breeding pairs were purchased from JAX in Bar Harbor, Maine. Mice were bred in a conventional vivarium at the University of California, Davis. The colony room was maintained on a 12:12 light/dark cycle with lights on at 6:00 AM, and at approximately 20°C and 55% humidity. Standard rodent chow and tap water were available ad libitum. Breeders were housed in Tecniplast plastic filtertop cages (15cm X 38cm X 12.5cm) on conventional, non-ventilated racks. Housing and procedures were conducted in strict compliance with the National Institute of Health Guidelines for Care and Use of Laboratory Animals and were approved by the UC Davis Institutional Animal Care and Use Committee.
2.2 Experimental Groups
Pups were weaned at 21 days of age into B6 home cages or BTBR home cages, each with four same-sex mice per cage. Both male and female home cages were used for observation. Cagemates were taken from two different litters (two mice from each litter). In addition to standard bedding, a Nestlet square and a plastic tube (Bethesda cohort) or plastic igloo (Davis cohort) were provided in each cage for environmental enrichment. Mice from the Bethesda cohort were weaned into Tecniplast cages; identical to the cages they had been reared in. Mice from the Davis cohort were weaned into a Noldus PhenoTyper 3000 chamber (30 cm × 30 cm × 38 cm; Noldus, Leesburg, VA, USA). The Bethesda cohort consisted of three cages of B6 and three cages of BTBR. The Davis cohort consisted of three Phenotyper chambers of B6 and three Phenotyper chambers of BTBR.
For overnight video recording, mice in the Bethesda cohort were transferred to a behavioral testing room on postnatal days 21, 24, 27, 30, 33, 36, 39, 42, 45 and 48. Cages were returned to the colony room before 8 AM on the following morning. At the Davis site mice remained in their Noldus PhenoTyper 3000 chambers within the colony room throughout the duration of the behavioral observation period. At the end of the 30-day observation period, mice were tested on social approach and self-grooming behavioral assays as described below, to compare home cage juvenile social interactions to adult social approach.
2.3 Video Recording
Home cages were recorded using CCTV cameras with night vision capability (420TVL Day/Night Box Security Camera Security Cameras Direct, Luling, TX, USA). Cameras were either positioned to the side of the home cages (Bethesda cohort) or mounted above the cage (Davis cohort). Infrared lights were used to illuminate the room after overhead lighting was turned off at 6:00 PM. Recordings were conducted during the dark phase of the circadian cycle, when mice are most interactive, starting from the first night after weaning. Recordings were continuous from 6:00 PM until 12:00 AM. A total of 10 six-hour video observations were recorded for each cage. During video recording sessions a card containing a unique alphanumeric code was attached to each cage or chamber. Strain differences in coat markings were visible in the Bethesda video recordings and therefore precluded completely blind observations, however the overhead view of the recordings at the Davis site minimized the distinguishing features of the strains and the coding system allowed the observer to remain blind.
2.4 Behavioral Ethogram
The behavioral ethogram (Table 1) was developed after careful reading of a variety of established ethograms of social behaviors of pair housed or group housed mice ([2, 4, 23–26]. The ethogram was intended to capture the occurrence of common and rare behaviors with conceptual relevance to autism spectrum disorders, including social investigation parameters, aggressive interactions and physical proximity to conspecifics. The behavioral measure ‘long interactions’ was defined and scored after noting that B6 mice would engage in bouts of reciprocal interactions that would last for extended amounts of time.
Table 1.
Ethogram of Home Cage Observation Parameters
| Long Interaction: Two mice engage in directed social behaviors for at least 10 seconds |
| Investigative Behaviors |
| Anogenital Sniff: Subject mouse sniffs the anogenital region of the partner |
| Nose-to-Nose Sniff: Subject sniffs the head and snout region of the partner |
| Body Sniff: Subject sniffs any other area of the body of the partner |
| Follow: Subject follows the partner around the cage without any fast, sudden or run movements |
| Affiliative behaviors |
| Huddle: Lying flat or standing still, with eyes closed or open, while maintaining close physical contact with the partner. |
| Allogrooming: one mouse grooms the other mouse on any part of the body |
| Push under: Subject pushes underneath the partner’s anterior body area and rests in that position |
| Crawl over: Subject traverses the partner’s body by crawling over the back from one side to the other |
| Crawl under: Subject traverses the partner’s body by crawling under from one side to the other |
| Push past: Subject pushes between the partner and the cage wall |
| Aggressive behaviors |
| Attack: a rushing and leaping approach carried on over the back of the partner, often accompanied by biting attempts |
| Chase: Subject pursues a fleeing partner |
| Aggressive grooming: Subject persistently allogrooms the partner, accompanied by vigorous pulling of the back fur and nipping at the skin of the partner mostly around the nape of the neck; and holding the other mouse down with forepaws |
| Food competition: gross movements directed at the head or snout of a partner with a food item |
| Overtake: One mouse takes over the physical location of another mouse |
| Defensive behavior |
| Submissive upright posture: Subject stands on its hinds legs with head pulled back and body rigid |
| Flee: rapid movements to the opposite side of the cage in response to attacks |
2.5 Video Scoring
Digital video files were analyzed on a Dell laptop computer. A trained observer quantified bouts of defined behaviors in all video files using Noldus Observer 8.0 XT software (Noldus Information Technology, Leesburg, VA, USA). A series of approximately 20 video files were scored initially to determine intra-rater reliability. The observer reached an approximately 90% reliability rate for repeated scoring of the same videos, before beginning formal scoring.
2.6 Adult Behavioral testing
Social approach and self-grooming assays were conducted at the end of the 30-day observation period (postnatal day 51), in dedicated behavioral testing rooms during the standard light phase, usually between 1000 h and 1500 h.
Social approach was assayed in our automated three-chambered apparatus (NIMH Research Services Branch, Bethesda, MD) using methods previously described [2, 5–7, 9, 27–31]. Briefly, the apparatus was a rectangular, three-chambered box made of clear polycarbonate capable of automatically detecting entries between chambers and time spent in each chamber by photocells embedded in the doorways. The test session began with a 10 min habituation session in the center chamber only, followed by a 10 min habituation to all three empty chambers. Following the second habituation phase, a clean novel object (wire cup) was placed in one of the side chambers and a novel mouse was placed in an identical wire cup located in the other side chamber. After both stimuli were positioned, the subject mouse was allowed access to all three chambers for 10 min. Trials were video recorded and time spent sniffing the novel object and time spent sniffing the novel mouse were later scored by a trained observer
Mice were scored for spontaneous self-grooming behaviors as described previously [2, 5, 6, 9]. Briefly, each mouse was given a 10 min habituation period in a clean, empty mouse cage and then video recorded for 10 min. The video recorded session was scored for cumulative time spent grooming all body regions by trained observers using a stopwatch. Differences in color and markings between the inbred strains prevented fully blind ratings. However the distinguishing features of the strain were less visible in the video recordings, which is why this method was chosen over live scoring.
2.7 Statistical Analysis
2.7.1 Home Cage Observations
Analyses of variance (ANOVAs) were used to assess behavioral differences between B6 and BTBR in their home cages. One-way ANOVAs were used to compare the total occurrence of individual behaviors and long interactions across strains. Repeated Measures ANOVA was used to compare the occurrence of long interactions across hours of each night and across days of the observation period. For these analyses strain was the between subject factor, and time or day the within subject factor.
2.7.2 Behavioral tasks
For the automated social approach task, time spent in the two side chambers was compared using within-strain Repeated Measures ANOVAs, with chamber side (novel mouse side vs. novel object side) as the within-group factor. Time spent in the center chamber was included on the graphs for illustrative purposes, but was not included in the statistical analyses. Time spent sniffing was similarly analyzed using within-strain Repeated Measures ANOVAs, with the item being sniffed as the within-group factor (novel mouse vs. novel object). One-Way ANOVA was used to analyze total number of entries to the two side chambers in the social approach task and to analyze time spent self-grooming in an empty cage, across strains.
3. Results
3.1 Occurrence of individual behaviors
Video files from the first two hours of each recorded night (20 hours of video footage per cage) were used to score the occurrence of individual behaviors as defined in the ethogram. In the Bethesda cohort, B6 and BTBR differed significantly on three behaviors. B6 followed their cagemates more than BTBR did (F(1,4) = 11.25, p < .05; Table 2). B6 also chased their cagemates more (F(1,4) = 12.25, p < .05; Table 2) and perhaps as a consequence B6 displayed more submissive postures in the home cage than BTBR mice did (F(1,4) = 19.60, p < .05; Table 2). There was also a tendency for B6 mice to sniff the noses of their cagemates more (F(1,4) = 6.84, p < .06; Table 2) and engage in more food competitions than BTBR mice did (F(1,4) = 6.78, p < .06; Table 2). In the Davis cohort B6 and BTBR only significantly differed on two individual behaviors. B6 mice groomed (i.e., allogroom) their cagemates more than BTBR mice did (F(1,4) = 25.00, p < .05; Table 2). They also displaced (i.e., overtake) their cagemates from their physical location more often than BTBR mice did (F(1,4) = 8.23, p < .05; Table 2).
Table 2.
Strain differences in individual behaviors at each observation site
| Behavior | Bethesda | Davis | ||
|---|---|---|---|---|
| Anogenital Sniff | B6: 13.6 ±2.6 BTBR: 11.0 ±4.1 |
p = .6267 | B6: 22.5 ±3.9 BTBR: 20.1 ±3.1 |
p = .6575 |
| Nose-to-Nose Sniff |
B6: 17.8 ±4.6 BTBR: 5.1 ±1.5 |
p = .0590 | B6: 11.16 ±3.0 BTBR: 6.7 ±2.5 |
p = .2168 |
| Body Sniff | B6: 12.4 ±1.6 BTBR: 13.7 ±3.9 |
p = .7802 | B6: 11.7 ±4.9 BTBR: 6.8 ±0.8 |
p = .4341 |
| Follow | B6: 6.3 ±1.5 BTBR: 1.3 ±0.3 |
p = .0285* | B6: 5.0 ±1.9 BTBR: 1.5 ±0.5 |
p = .1484 |
| Huddle | B6: 3.6 ±1.0 BTBR: 1.2 ±0.3 |
p = .3339 | B6: 2.7 ±1.0 BTBR: 1.0 ±0.2 |
p = .2378 |
| Allogroom | B6: 5.9 ±1.8 BTBR: 2.5 ±1.2 |
p = .1917 | B6: 6.8 ±2.0 BTBR: 0.6 ±0.2 |
p = .0075* |
| Push Under | B6: 5.5 ±1.2 BTBR: 4.4 ±1.8 |
p = .6435 | B6: 3.1 ±0.9 BTBR: 4.0 ±1.0 |
p = .5743 |
| Crawl Over | B6: 11.7 ±2.7 BTBR: 18.9 ±8.9 |
p = .4851 | B6: 10.7 ±2.2 BTBR: 6.7 ±1.2 |
p = .1817 |
| Crawl Under | B6: 0.7 ±0.2 BTBR: 6.0 ±2.0 |
p = .0712 | B6: 7.5 ±3.7 BTBR: 2.8 ±1.1 |
p = .3928 |
| Push Past | B6: 6.9 ±2.2 BTBR: 16.3 ±6.7 |
p = .2516 | B6: 6.7 ±2.1 BTBR: 4.5 ±0.8 |
p = .4024 |
| Attack | B6: 1.3 ±0.3 BTBR: 0.0 ±0.0 |
p = .3739 | B6: 3.3 ±0.6 BTBR: 0.0 ±0.0 |
p = .3739 |
| Chase | B6: 2.3 ±0.7 BTBR: 0.3 ±0.3 |
p = .0249* | B6: 1.3 ±0.3 BTBR: 0.0 ±0.0 |
p = .3739 |
| Aggressive Groom |
B6: 1.6 ±0.6 BTBR: 1.3 ±0.3 |
p = .5185 | B6: 1.0 ±0.1 BTBR: 0.0 ±0.0 |
p = .3739 |
| Submissive Posture |
B6: 5.0 ±1.0 BTBR: 0.3 ±0.3 |
p = .0114* | B6: 3.1 ±1.6 BTBR: 2.6 ±1.3 |
p = .8247 |
| Flee | B6: 0.3 ±0.3 BTBR: 0.0 ±0.0 |
p = .3739 | B6: 3.0 ±1.5 BTBR: 2.6 ±1.3 |
p = .8774 |
| Food competition |
B6: 11.0 ±3.6 BTBR: 1.3 ±0.9 |
p = .0598 | B6: 4.7 ±2.1 BTBR: 1.2 ±0.2 |
p = .1856 |
| Overtake | B6: 27.4 ±14.2 BTBR: 7.0 ±2.5 |
p = .2285 | B6: 10.3 ±2.3 BTBR: 3.0 ±1.0 |
p = .0455* |
3.2 Occurrence of long interactions
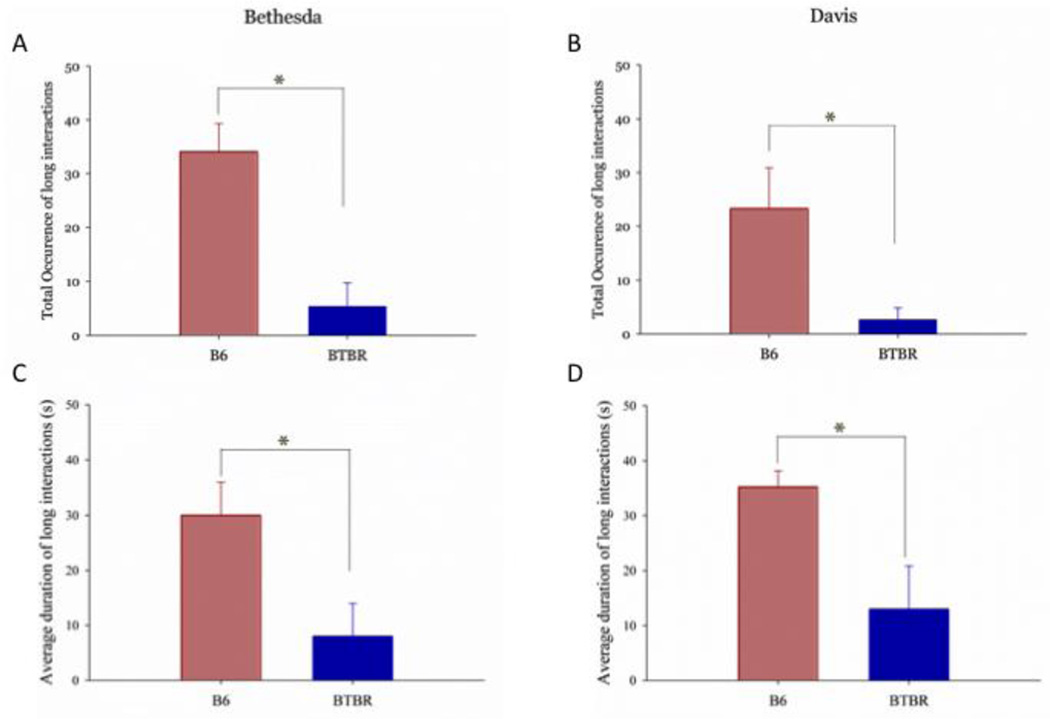
All video files were scanned for the occurrence of long bouts of social interactions (60 hours of video footage per cage), defined as a series of directed social behaviors lasting longer than ten seconds. The total occurrence of social interactions lasting longer than ten seconds, across all recorded sessions, was significantly higher in B6 home cages as compared to the BTBR home cages at both sites (Bethesda: F(1,4) = 17.43, p < .05 Figure 1A; Davis F(1,4) = 7.976, p < .05 Figure 1B). Further, the duration of the long interactions were significantly longer in the B6 home cages as compared to the BTBR home cages both in the Bethesda cohort F(1,4) = 10.02, p < .05 (Figure 1C) and the Davis cohort F(1,4) = 7.08, p < .05 (Figure 1D).
Figure 1.
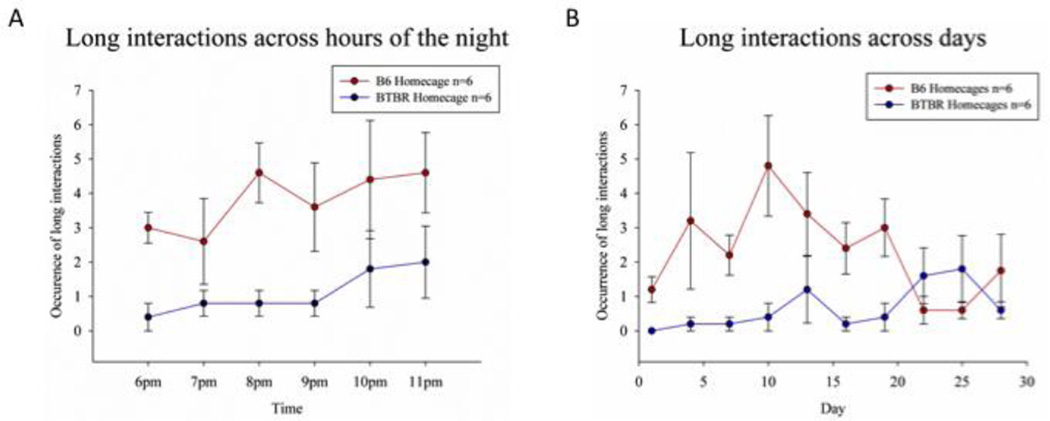
To further investigate how the occurrence of long interactions developed during the observation period, data from both sites were pooled. The occurrence of long interaction bouts did not significantly differ across hours of the night (F(5, 40) = 1.67, p > .1) though there was a tendency for more interactions to occur in the later hours (Figure 2A). There was no significant interaction between strain and hour of the night for the frequency of long interactions (F(5, 40) = .36, p > .1). Occurrence of long interactions did not significantly differ across the 30-day observation period (F(9,81) = 1.12, p > .1; Figure 2B), nor was there a significant interaction between observation night and strain. However, the number of long interactions initiated by B6 across observation nights did appear to differ from BTBR in that B6 initiated more long interactions during the first two weeks after weaning and BTBR displayed the most interactions during the last week of the observation period.
Figure 2.
3.3 Sociability
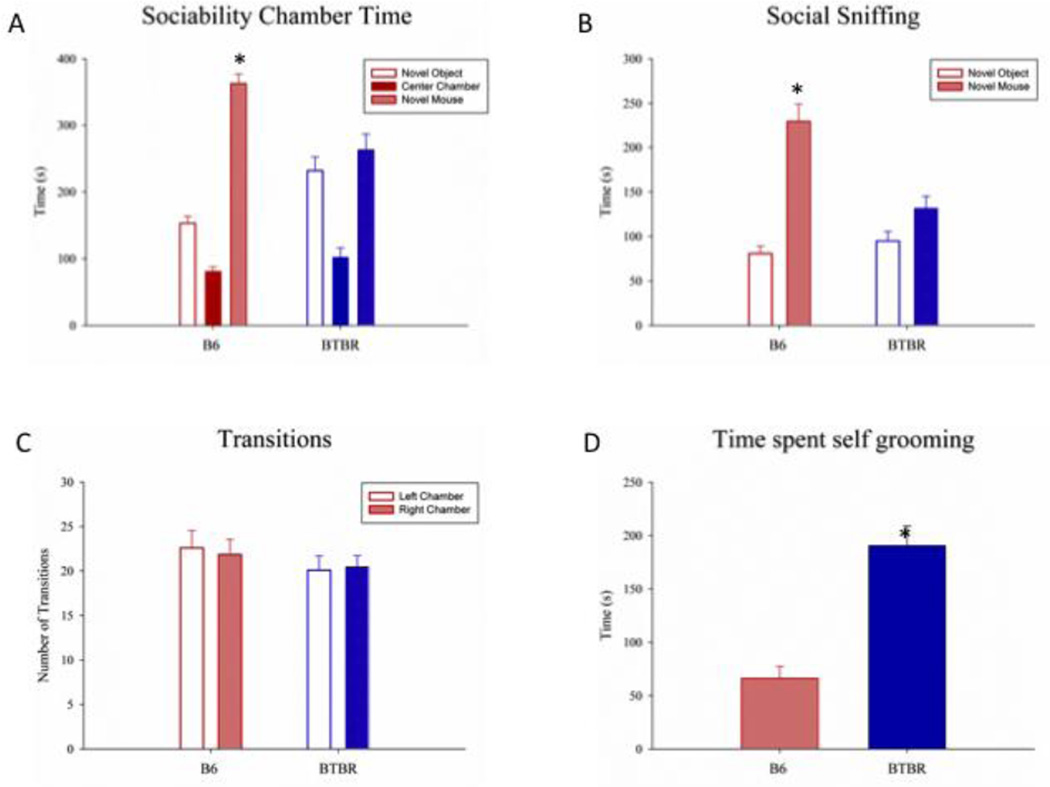
Figure 3 shows social approach and self-grooming in B6 and BTBR mice upon completion of the home cage observations. Data was pooled across sites, as the experimental methods were essentially identical. As shown in figure 3A, B6 subjects spent significantly more time in the chamber containing the novel mouse than in the chamber containing the novel object (F(1,19) = 74.65, p < .001) and more time sniffing the novel mouse as compared to the novel object (F(1,19) = 46.18, p < .001; Figure 3B), meeting the definition of sociability in this assay. In contrast, BTBR failed to spend more time with the novel mouse (F(1,19) = 4.07, p > .05; Figure 3A) and approximately equal time sniffing the mouse or the object (F(1,19) = .52, p > .100; 3B), meeting the definition of lack of sociability, consistent with previous reports [1–14]. There was no significant difference between B6 and BTBR in the number of transitions between chambers made during the sociability phase of the social approach task (F(1,38) = .84, p > .100; Figure 3C), indicating normal locomotion and exploration. Neither strain displayed an innate side preference for the right or left chamber (B6: F(1,19) = .31, p > .100; BTBR: F(1,19) = .05, p > .100; Figure 3C).
Figure 3.
3.4 Self Grooming
As shown in figure 3D, BTBR spent significantly more time self-grooming in an empty cage as compared to B6 (F(1,36) = 27.82, p < .001), consistent with previous reports [2, 4–7, 9, 21, 22].
3. Discussion
The BTBR inbred strain provides a useful model of autism because these mice display robust and well-replicated behavioral phenotypes analogous to the three diagnostic criteria for autism: impaired social interactions, communication deficits and increased repetitive behaviors. Adult BTBR display social behavior deficits in multiple paradigms and across several laboratories, including the three-chambered social approach task, reciprocal interactions, and semi-naturalistic group environments [1–14]. We conducted detailed analysis of social interactions of BTBR in the home cage environment to further determine the early developmental trajectory of social abnormalities in this inbred strain of mice.
Between 21–51 days of age BTBR mice generally displayed less social interaction in their home cage as compared to home cages of B6 mice, a standard inbred strain with high adult sociability. Specifically, BTBR engaged in significantly fewer bouts of long interactions, as compared to B6. This behavioral variable is, to our knowledge, a novel behavioral measurement that revealed a robust difference between the two strains, and this difference was consistent across the Bethesda and UC Davis testing sites. As this variable can reliably be identified from video scoring it may provide an opportunity to evaluate the effectiveness of treatments and interventions with minimal disruption/handling of the mice.
BTBR also exhibited significantly fewer bouts of individual social behaviors than B6, including following chasing and submissive postures in the Bethesda cohort, and allogroming and overtake in the Davis cohort. Other behaviors tended to be lower in BTBR home cages as compared to B6 home cages including nose-to-nose sniffs and food competitions (Bethesda cohort). The pattern of behavior displayed by BTBR within the home cage, including reduced level of interactions and less initiation of investigative and affiliative behaviors, is consistent with the behavioral abnormalities seen from this strain using standardized behavioral test paradigms. Thus, our findings indicate that low sociability in BTBR begins at juvenile ages and is consistent across diverse social settings. However, the precise patterns of individual social behaviors appear to be sensitive to the testing environment, emphasizing the importance of standardizing and validating behavioral testing procedures across laboratories and under a variety of conditions.
Overnight behavioral observations did not appear to affect adult sociability of the B6 or BTBR mice housed in the single strain cages. B6 displayed sociability, as defined as significantly more time spent in the chamber with the novel mouse versus the chamber with the novel object and significantly more time spent sniffing the novel mouse versus the novel object in the three-chambered social approach task, whereas BTBR did not, consistent with previous publications [1, 2, 4, 6, 11]. As described in the supplementary materials, we also conducted a pilot study to evaluate the home cage behaviors of BTBR and B6 mice housed together, as this B6-BTBR mixed-strain rearing condition had previously been shown to rescue the sociability deficits typically seen in BTBR [32], however results across the two sites were inconsistent.
Our descriptive investigation of home cage scoring offers a naturalistic method to detect social deficits in BTBR at an early age, and to follow social scores across the juvenile and young adult developmental period, without otherwise disrupting the robust well-replicated phenotypes previously described in this strain. The observed phenotypic characteristics of home cage behaviors in BTBR are relevant to the clinical symptoms of autism, and further support the use of BTBR as a practical mouse model. The home cage paradigm may prove useful for the evaluation of behavioral and pharmacological interventions at the juvenile stage in BTBR and other mouse models of autism spectrum disorders.
Supplementary Material
Highlights.
BTBR T+tf/J (BTBR) mice display low levels of social interactions in the home cage
Frequency and duration of long interactions are reduced in BTBR home cages
Home cage observations did not disrupt the expected sociability of BTBR or C57/B6J
Acknowledgements
Supported by the National Institute of Mental Health Intramural Research Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behavioural brain research. 2007;176(1):21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McFarlane HG, et al. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, brain, and behavior. 2008;7(2):152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 3.Moy SS, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behavioural brain research. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pobbe RL, et al. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behavioural brain research. 2010;214(2):443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverman JL, et al. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(4):976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. The European journal of neuroscience. 2009;29(8):1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M, et al. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism research : official journal of the International Society for Autism Research. 2011;4(1):17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang M, et al. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Frontiers in behavioral neuroscience. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2007;25(8):515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacology, biochemistry, and behavior. 2011;97(3):586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Defensor EB, et al. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behavioural brain research. 2011;217(2):302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pobbe RL, et al. General and social anxiety in the BTBR T+ tf/J mouse strain. Behavioural brain research. 2011;216(1):446–451. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverman JL, et al. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Science translational medicine. 2012;4(131):131ra51. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, et al. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiology & behavior. 2012 doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roullet FI, Wohr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behavioural brain research. 2011;216(1):19–28. doi: 10.1016/j.bbr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scattoni ML, et al. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PloS one. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes, brain, and behavior. 2011;10(1):44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes, brain, and behavior. 2011;10(1):35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moy SS, et al. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behavioural brain research. 2008;188(1):178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverman JL, et al. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171(4):1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amodeo DA, et al. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behavioural brain research. 2012;227(1):64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson BL, et al. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes, brain, and behavior. 2011;10(2):228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terranova ML, Laviola G, Alleva E. Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Developmental psychobiology. 1993;26(8):467–481. doi: 10.1002/dev.420260805. [DOI] [PubMed] [Google Scholar]
- 24.Terranova ML, et al. A description of the ontogeny of mouse agonistic behavior. Journal of comparative psychology. 1998;112(1):3–12. doi: 10.1037/0735-7036.112.1.3. [DOI] [PubMed] [Google Scholar]
- 25.Winslow JT. Mouse social recognition and preference. Current protocols in neuroscience / editorial board, Jacqueline N. Crawley … [et al.] 2003 doi: 10.1002/0471142301.ns0816s22. Chapter 8: p. Unit 8 16. [DOI] [PubMed] [Google Scholar]
- 26.Grant EC, Mackintosh JH. A Comparison of the Social Postures of Some Common Laboratory Rodents. Behaviour. 1963;21(3/4):246–259. [Google Scholar]
- 27.Chadman KK, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism research : official journal of the International Society for Autism Research. 2008;1(3):147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain pathology. 2007;17(4):448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moy SS, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behavioural brain research. 2008;191(1):118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman JL, et al. Sociability and motor functions in Shank1 mutant mice. Brain research. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Current protocols in neuroscience / editorial board, Jacqueline N. Crawley … [et al.] 2011 doi: 10.1002/0471142301.ns0826s56. Chapter 8: p. Unit 8 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang M, et al. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism research : official journal of the International Society for Autism Research. 2011;4(1):17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.