Abstract
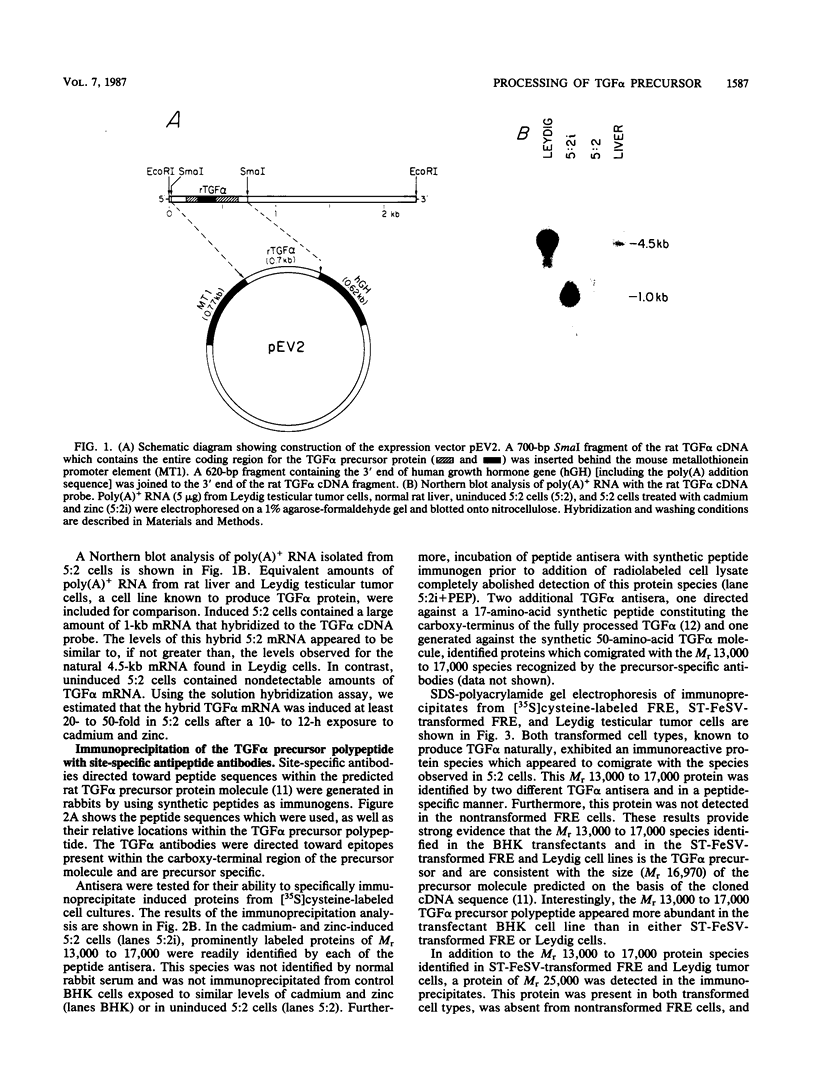
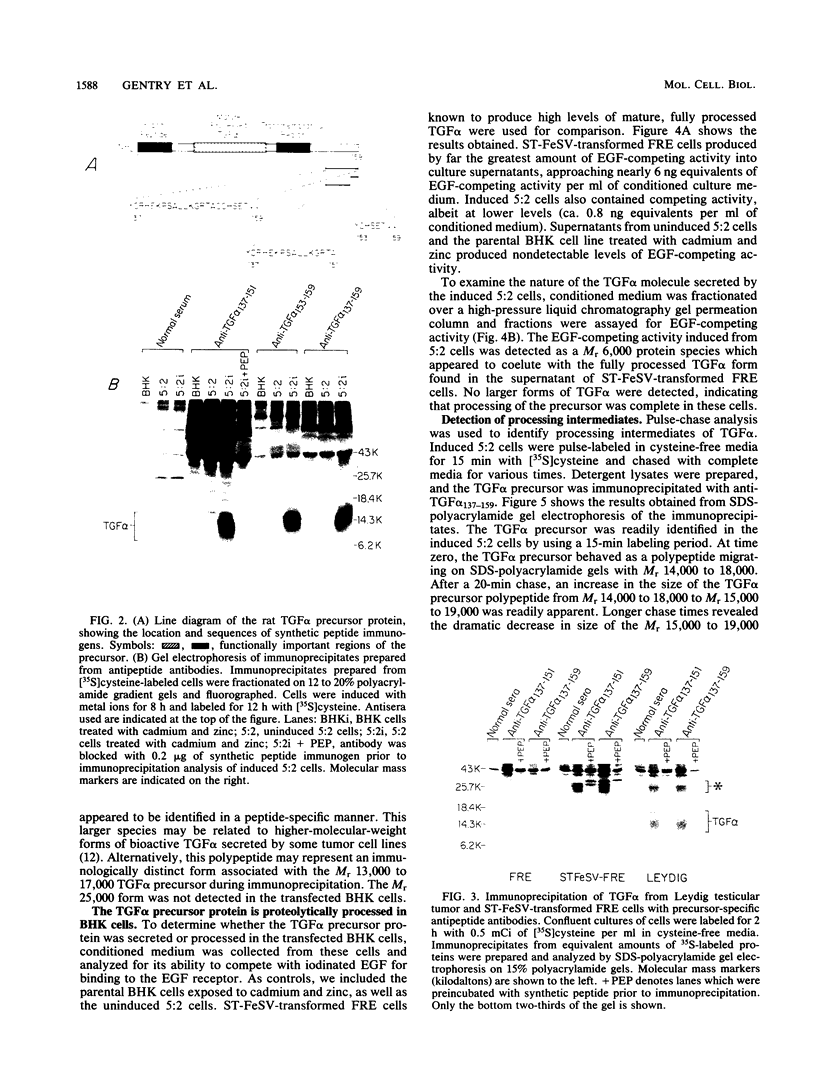
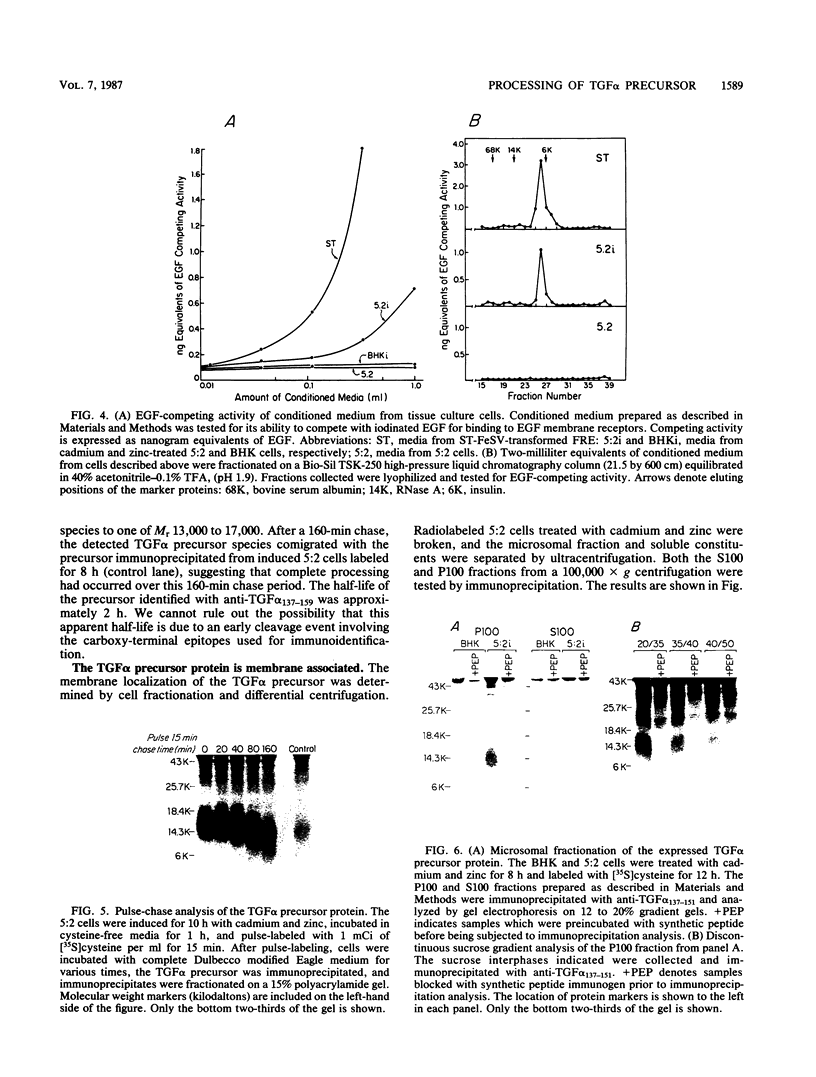
Analysis of a cDNA clone derived from retrovirus-transformed rat fibroblasts has recently suggested that the mature 50-amino-acid form of transforming growth factor alpha (TGF alpha) is derived from a 159-amino-acid transmembrane precursor by proteolytic cleavage. To understand the processing of the TGF alpha precursor molecule in more detail, we have expressed this protein in baby hamster kidney (BHK) fibroblasts under control of the metal-ion-inducible metallothionein promoter and characterized the expressed precursor with site-specific antipeptide antibodies. One of the BHK transfectants, termed 5:2, expressed the TGF alpha mRNA in a cadmium- and zinc-inducible manner. The TGF alpha precursor protein was detected by immunoprecipitation analysis of radiolabeled cell cultures. In the induced 5:2 cells, a polypeptide of Mr 13,000 to 17,000 was readily identified by peptide antisera made to three different regions of the TGF alpha precursor protein. No such protein species were observed in BHK cells treated with cadmium and zinc or in uninduced 5:2 cells. However, two cell lines known to produce TGF alpha naturally, Leydig testicular tumor cells and Snyder-Theilan feline sarcoma virus-transformed Fisher rat embryo fibroblasts, possessed detectable levels of immunologically related Mr 13,000 to 17,000 proteins. Cell fractionation studies indicate that the Mr 13,000 to 17,000 species expressed in induced 5:2 cells is membrane associated, consistent with predictions based on the cDNA sequence of the TGF alpha precursor. Media conditioned by induced 5:2 cells contained epidermal growth factor receptor-competing activity, which, upon size fractionation, was similar in size to the mature processed form of TGF alpha. These data show that these nontransformed BHK cells possess the ability to process the TGF alpha precursor molecule into its native form.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bringman T. S., Lindquist P. B., Derynck R. Different transforming growth factor-alpha species are derived from a glycosylated and palmitoylated transmembrane precursor. Cell. 1987 Feb 13;48(3):429–440. doi: 10.1016/0092-8674(87)90194-2. [DOI] [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Lawton A. Characterization of site-specific antibodies to the erbB gene product and EGF receptor: inhibition of tyrosine kinase activity. Virology. 1986 Jul 30;152(2):421–431. doi: 10.1016/0042-6822(86)90144-3. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., Harrod J., Gowen M., D'Souza S., Smith D. D., Winkler M. E., Derynck R., Mundy G. R. Human recombinant transforming growth factor alpha stimulates bone resorption and inhibits formation in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2228–2232. doi: 10.1073/pnas.83.7.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbotson K. J., Twardzik D. R., D'Souza S. M., Hargreaves W. R., Todaro G. J., Mundy G. R. Stimulation of bone resorption in vitro by synthetic transforming growth factor-alpha. Science. 1985 May 24;228(4702):1007–1009. doi: 10.1126/science.3859011. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Kelly B., Davis R. J., Massagué J. Biologically active precursor for transforming growth factor type alpha, released by retrovirally transformed cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6307–6311. doi: 10.1073/pnas.83.17.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Rochford R., Todaro G. J., Villarreal L. P. Developmental expression of rat transforming growth factor-alpha mRNA. Mol Cell Biol. 1985 Dec;5(12):3644–3646. doi: 10.1128/mcb.5.12.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. C., Rose T. M., Webb N. R., Todaro G. J. Cloning and sequence analysis of a cDNA for rat transforming growth factor-alpha. Nature. 1985 Feb 7;313(6002):489–491. doi: 10.1038/313489a0. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Hargreaves W. R., Twardzik D. R., Todaro G. J. Detection of larger polypeptides structurally and functionally related to type I transforming growth factor. Proc Natl Acad Sci U S A. 1985 Jan;82(2):356–360. doi: 10.1073/pnas.82.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. J., Hammer R. E., Goodman R. H., Habener J. F., Palmiter R. D., Brinster R. L. Tissue-specific posttranslational processing of pre-prosomatostatin encoded by a metallothionein-somatostatin fusion gene in transgenic mice. Cell. 1985 May;41(1):211–219. doi: 10.1016/0092-8674(85)90075-3. [DOI] [PubMed] [Google Scholar]
- Manger R., Rasheed S., Rohrschneider L. Localization of the feline sarcoma virus fgr gene product (P70gag-actin-fgr): association with the plasma membrane and detergent-insoluble matrix. J Virol. 1986 Jul;59(1):66–72. doi: 10.1128/jvi.59.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Twardzik D. R., De Larco J. E., Stephenson J. R., Todaro G. J. Transforming growth factors produced by retrovirus-transformed rodent fibroblasts and human melanoma cells: amino acid sequence homology with epidermal growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4684–4688. doi: 10.1073/pnas.80.15.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Marquardt H., Todaro G. J., Gallis B., Casnellie J. E., Bornstein P., Krebs E. G. Transforming growth factor and epidermal growth factor stimulate the phosphorylation of a synthetic, tyrosine-containing peptide in a similar manner. J Biol Chem. 1982 Dec 25;257(24):14628–14631. [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Todaro G. J., Fryling C., Stephenson J. R. Human transforming growth factors induce tyrosine phosphorylation of EGF receptors. Nature. 1981 Jul 16;292(5820):259–262. doi: 10.1038/292259a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Lindquist P. B., Bringman T. S., Goeddel D. V., Derynck R. Expression in rat fibroblasts of a human transforming growth factor-alpha cDNA results in transformation. Cell. 1986 Jul 18;46(2):301–309. doi: 10.1016/0092-8674(86)90747-6. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Sherwin S. A., Twardzik D. R., Bohn W. H., Cockley K. D., Todaro G. J. High-molecular-weight transforming growth factor activity in the urine of patients with disseminated cancer. Cancer Res. 1983 Jan;43(1):403–407. [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardzik D. R., Sherwin S. A., Ranchalis J., Todaro G. J. Transforming growth factors in the urine of normal, pregnant, and tumor-bearing humans. J Natl Cancer Inst. 1982 Oct;69(4):793–798. [PubMed] [Google Scholar]
- Twardzik D. R., Todaro G. J., Marquardt H., Reynolds F. H., Jr, Stephenson J. R. Transformation induced by Abelson murine leukemia virus involves production of a polypeptide growth factor. Science. 1982 May 21;216(4548):894–897. doi: 10.1126/science.6177040. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Todaro G. J., Reynolds F. H., Jr, Stephenson J. R. Similar transforming growth factors (TGFs) produced by cells transformed by different isolates of feline sarcoma virus. Virology. 1983 Jan 15;124(1):201–207. doi: 10.1016/0042-6822(83)90307-0. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Unkeless J. C. Partial purification of a lipoprotein with 5'-nucleotidase activity from membranes of rat liver cells. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1050–1057. doi: 10.1073/pnas.61.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]