Abstract
Background
In Sub-Saharan Africa, prevalence estimates of hepatitis C virus (HCV) vary widely.
Objectives
To assess the prevalence of HCV infection among HIV-infected, pregnant women screened for a large clinical trial in Lilongwe, Malawi.
Study design
Plasma from 2041 HIV-infected, pregnant women was screened for anti-HCV IgG using a chemiluminiscent immunometric assay (CIA). Specimens with a signal-cut-off ratio ≥ 1.00 were considered reactive and those with S/Co ratio < 1.00 non-reactive. All CIA-reactive specimens were tested by a recombinant immunoblot assay (RIBA) for anti-HCV and by PCR for HCV RNA.
Results
Of 2041 specimens, 110 (5.3%, 95% CI: 4.5–6.5%) were CIA reactive. Of the 109 CIA reactive specimens available for RIBA testing, 2 (1.8%) were positive, 28 (25.7%) were indeterminate, and 79 (72.5%) were negative. All CIA-reactive specimens were HCV RNA negative (n = 110). The estimated HCV prevalence based on the screening assay alone was 5.3%; based on supplemental RIBA testing, the status of HCV infection remained indeterminate in 1.4% (28/2040, 95% CI: 0.1–2.0) and the prevalence of confirmed HCV infections was 0.1% (2/2040, 95% CI: 0–0.4%).
Conclusions
HCV seroprevalence among HIV-infected, pregnant women in Malawi confirmed by supplemental RIBA HCV 3.0 is low (0.1%); CIA showed a high false-reactivity rate in this population.
Keywords: HIV, HCV, Pregnant women, Malawi
1. Background
Worldwide, approximately 170 million are infected with hepatitis C virus (HCV); 40 million are infected with human immunodeficiency virus type-1 (HIV); and, 4.5 million are infected with both viruses.1,2 Co-infection with HCV and HIV is of particular concern in Sub-Saharan Africa.3 In Malawi, the estimated prevalence of HCV infection varies widely (0.7–16.5%) by subpopulation and whether supplemental testing confirmed screening results.5–10 The only study to date among HIV-infected, pregnant women in Malawi estimated the prevalence of non-confirmed, anti-HCV positivity at 16.5%.4 Only one study in Malawi employed both serologic and supplemental nucleic acid testing for HCV infection, and it reported that only one in 10 HCV screening positive samples was positive for HCV ribonucleic acid (RNA).7 With the high prevalence of HIV-infection and increasing access to life-prolonging, antiretroviral therapy in Sub-Saharan Africa, liver disease is emerging as a major cause of morbidity and mortality among HIV-infected persons, particularly those co-infected with hepatitis.10,11 It is imperative that we understand the limitations of the current assays for detection of HCV infection and more accurately estimate the prevalence of HCV and HIV co-infection.
2. Objectives
The objective was to assess the prevalence of HCV infection using screening and supplemental confirmatory testing among HIV-infected, pregnant women being screened for a large clinical trial in Lilongwe, Malawi.
3. Study design
We analysed plasma specimens from 2041 antiretroviral-naive, HIV-infected, pregnant (<30 weeks gestation) women collected at pre-inclusion screening for the Breastfeeding, Antiretrovirals, and Nutrition (BAN) Study (www.TheBANStudy.org).12,13 The study was approved by the Malawi National Health Sciences Research Committee and institutional review boards at the University of North Carolina at Chapel Hill and the U.S. Centers for Disease Control and Prevention (CDC) in Atlanta, GA. All women provided written, informed consent for specimen storage and future laboratory studies. Specimens were shipped from −80 °C storage in Lilongwe (Malawi) to CDC for testing. All specimens were tested for anti-HCV IgG using a chemiluminescent immunoassay (CIA) (VITROS aHCV; Ortho-Clinical Diagnostics, Inc., Rochester, NY) according to manufacturer’s instructions. Specimens with a signal-to-cutoff (S/Co) ratio ≥ 1.00 were considered reactive, whereas those with an S/Co < 1.00 were considered non-reactive. All CIA-reactive specimens underwent supplemental testing with a recombinant immunoblot assay (RIBA) (RIBA HCV 3.0 SIA; Novartis Inc., New York, NY) and PCR for HCV RNA by a qualitative assay (COBAS AMPLICOR HCV Test v2.0; Roche Molecular Diagnostics, Indianapolis, IN). Using CDC guidelines,14 a specimen was considered (i) anti-HCV negative if it was CIA non-reactive or RIBA negative; (ii) anti-HCV positive if CIA-reactive and RIBA or HCV RNA positive; (iii) furthermore, since there was no possibility to collect a sample for repeat anti-HCV or HCV RNA testing after >1 month all specimens that were CIA-reactive with an indeterminate RIBA and negative HCV RNA result were considered anti-HCV indeterminate. Also according to CDC testing guidelines, specimens with a CIA S/Co ratio of >8.0 (established for Ortho HCV CIA) can be considered HCV positive without supplemental testing,14 and we evaluated the utility of this cut-off for predicting positive RIBA and HCV RNA results in this population. HCV prevalence was estimated using binomial exact methods.
4. Results
The women had a median age of 25 (interquartile range (IQR): 22–29, range: 14–45) years, CD4 count of 425 (IQR: 300–571, range: 100–1218) cells/mm3, and alanine aminotransferase (ALT) level of 13 (IQR: 10–15, range: 2–74) IU/L. Overall, the population was of low socioeconomic status, as indicated by a low percentage (18%) with electricity in the home.
Of 2041 specimens, 110 (5.4%, 95% CI: 4.5–6.5%) were CIA reactive (Fig. 1); 98 had S/Co ≤ 8.0, and 12 had S/Co > 8.0. Of the 98 with a S/Co ≤ 8.0, 97 were tested by RIBA; 1 had insufficient volume and was excluded from further analysis. Of the 97, 1 (1.0%) was positive, 27 (27.8%) were indeterminate, and 69 (71.1%) were negative by RIBA; all were HCV RNA negative. Of the 12 samples with S/Co ≤ 8.0, 1 (8.3%) was positive, 1 (8.3%) was indeterminate, and 10 (83.3%) were negative by RIBA; all were HCV RNA negative. The two specimens that tested RIBA positive had CIA S/Co ratios of 15.5 and 4.6. Therefore, a CIA S/Co > 8.0 did not correlate with RIBA and HCV RNA PCR positivity in this population.
Fig. 1.
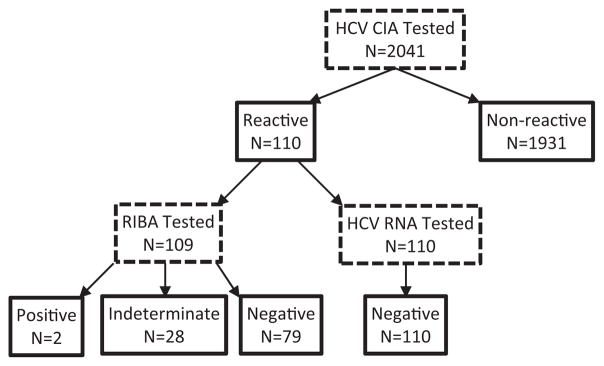
Hepatitis C CIA with supplemental RIBA and HCV RNA testing of 2041 HIV-infected, pregnant women in Malawi.
Overall, of the 109 CIA reactive specimens tested by RIBA, 2 (1.8%) were positive, 28 (25.7%) were indeterminate, and 79 (72.5%) were negative. None of the CIA-reactive specimens tested positive for HCV RNA. The two women who were RIBA positive had a significantly higher median ALT level (29 IU/L) compared to women with indeterminate (12 IU/L) or negative (13 IU/L) RIBA results (p = 0.02).
The RIBA confirmed anti-HCV prevalence among these HIV-infected pregnant women in Malawi was 0.1% (2/2040, 95% CI: 0–0.4%), while 1.4% of participants had anti-HCV indeterminate results. Among the 81 women with reactive CIA and determinate RIBA results, the positive predictive value (PPV) of the CIA for RIBA reactivity was 2.5% (2 of 81, 95% CI: 0.8–8.5%) and the false-positive rate was 97.5% (79 of 81, 95% CI: 91.5–99.2%). The specificity of the CIA assay for evidence of HCV infection among women in this study is uncertain since CIA-non-reactive specimens were not tested by RIBA or HCV RNA. However, following CDC guidelines, we considered CIA-non-reactive specimens (n = 1931) anti-HCV negative and excluded the 28 indeterminate results from the denominator (2040) to estimate the CIA specificity at 96.0% (1931/2012, 95% CI: 95.0–96.8%).
5. Discussion
The prevalence of HCV was 5.3% by CIA and 0.1% by supplemental RIBA among this population of HIV-infected, pregnant women in Malawi. The only other study in Malawi that used supplementary testing with RIBA and HCV RNA reported a HCV prevalence of 6.8% by CIA and 0.7% by confirmed HCV RNA among blood donors.7 These estimates of HCV prevalence in Malawi are consistent with other studies in Sub-Saharan Africa that used supplemental testing.4,15–17 Together these studies indicate that most HCV prevalence estimates from Sub-Saharan Africa based on screening assays are overestimated. Continued HCV screening without supplemental confirmatory testing of blood donations would result in discarding uninfected blood, and the disclosure of test results may cause unnecessary despair to those misdiagnosed and the permanent withdrawal of uninfected donors from the small donor pool. Future efforts should focus on improving the HCV testing algorithm in Sub-Saharan Africa.
Our data does not support the use of a CIA S/Co ratio ≥ 8.0 for Vitros CIA to screen for HCV infection among HIV-infected, pregnant women in Malawi. Current CDC HCV testing guidelines suggest that CIA-reactive samples with a S/Co ratio ≥ 8.0 for Vitros CIA may be reported as anti-HCV positive without further supplemental testing.14 Although this criterion would reduce screening costs, our study indicates that in such low prevalence settings, S/Co ratio thresholds established for various assays would result in false-positive results and may not reflect true HCV infections.
Our study had some limitations. The number of CIA reactive specimens that gave indeterminate RIBA results was high, but this observation has been noted elsewhere in settings of low HCV seroprevalence16; to determine HCV status in persons with such results another sample should be collected for repeat anti-HCV or for HCV RNA testing > 1 month later. Intermittent viremia is known to occur in HCV infection,14 which could lead to a negative single HCV RNA test. The CDC guidelines are based on studies conducted among different populations in the United States and thus may not be fully applicable to the population of HIV-infected, pregnant women in Malawi. Furthermore, the main BAN trial was not designed to answer questions about HCV and lacks data on history of blood transfusion and injection drug use, which are rare in Malawi.
In conclusion, HCV seroprevalence among HIV-infected, pregnant women in Malawi is low when confirmed by supplemental RIBA HCV 3.0. The CIA showed a high false-reactivity rate. Given the high cost of supplemental testing by RIBA and nucleic acid tests, there is a need to evaluate the performance of available anti-HCV assays (including available rapid tests) in various populations in Malawi and other African settings, including the general population, blood donors, and risk groups.
Acknowledgments
Funding
The study was supported by grants from the Prevention Research Centers Special Interest Project of the Centers for Disease Control and Prevention (SIP 13-01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944-01), the National Institute of Allergy and Infectious Diseases (NIAID P30-AI50410), the University of North Carolina Center for AIDS Research (P30-AI50410), the NIH Fogarty AIDS International Training and Research Program (DHHS/NIH/FIC 2-D43 Tw01039-06) and Fogarty International Clinical Research Fellows Program (R24 TW007988), and Ad Astra Fellowship from University College Dublin, Ireland. The Call to Action PMTCT program was supported by the Elizabeth Glaser Pediatric AIDS Foundation Call to Action and International Leadership Awards, UNICEF, World Food Programme, Malawi Ministry of Health, Johnson and John-son, and USAID. The non-government funders had no role in the design, implementation, analysis and interpretation of the data.
We are grateful to the following: BAN Study Team at University of North Carolina Chapel Hill, Centers for Disease Control and Prevention, Atlanta, and UNC Project team in Lilongwe including Linda Adair, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Margaret Bentley, Brian Bram-son, Emily Bobrow, Nicola Boyle, Sal Butera, Charles Chasela, Charity Chavula, Joseph Chimerang’ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chi-udzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, Denise Jamieson, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C. King, Rod-ney Knight, Athena P. Kourtis, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Cep-pie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Charles van der Horst, Esther Waalberg, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, Elizabeth Widen, and Chifundo Zimba. Finally and most especially, all the women and infants that have agreed to participate in the study.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Competing interest
None to declare.
Ethical approval
The study was a sub-study of the Breastfeeding, Antiretrovirals, and Nutrition (BAN) study and was approved by the National Health Sciences Research Committee (NHSRC), the University of North Carolina Chapel Hill, and Centers for Disease Control and Prevention.
References
- 1.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13(17):2436–41. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(1 Suppl):S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Modi A, Field J. Viral hepatitis and HIV in Africa. AIDS Rev. 2007;9:25–39. [PubMed] [Google Scholar]
- 4.Ahmed SD, Cuevas LE, Brabin BJ. Seroprevalence of hepatitis B and C and HIV in Malawian pregnant women. J Infection Dis. 1998;37(3):248–51. doi: 10.1016/s0163-4453(98)91983-1. [DOI] [PubMed] [Google Scholar]
- 5.Sutcliffe S, Taha TE, Kumwenda NI, Taylor E, Liomba GN. HIV-1 prevalence and herpes simplex virus 2, hepatitis C virus, and hepatitis B virus infections among male workers at a sugar estate in Malawi. J Acquir Immune Defic Syndrome. 2002;31(1):90–7. doi: 10.1097/00126334-200209010-00012. [DOI] [PubMed] [Google Scholar]
- 6.Maida MJ, Costello DC, Hoffman I, Cohen MS, Kumwenda M, Vernazza PL. Prevalence of Hepatitis C infection in Malawi and lack of association with sexually transmitted diseases. Eur J Epidemiol. 2000;16:1183–4. doi: 10.1023/a:1010920426795. [DOI] [PubMed] [Google Scholar]
- 7.Candotti D, Mundy C, Kadewele G, Nkhoma W, Bates I, Allain JP. Serological and molecular screening for viruses in blood donors from Ntcheu, Malawi: high prevalence of HIV-1 subtype C and of markers of hepatitis B and C viruses. J Medical Virol. 2001;65(1):1–5. [PubMed] [Google Scholar]
- 8.Nyirenda M, Beadsworth MBJ, Stephany P, Hart CA, Hart IJ, Munthali C. Prevalence of infection with hepatitis B and C virus and coinfection with HIV in medical inpatients in Malawi. J Infect. 2008;57(1):72–7. doi: 10.1016/j.jinf.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Moore E, Beadsworth MB, Chaponda M, Mhango B, Faragher B, Njala J, et al. Favourable one-year ART outcomes in adult Malawians with hepatitis B and C co-infection. J Infect. 2010;61(2):155–63. doi: 10.1016/j.jinf.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Thio CL, Seaberg EC, Skolasky R, Phair J, Visscher B, Munoz A, et al. HIV-1, Hepatitis B virus, and risk of liver-related mortality in the Multicenter AIDS Cohort Study (MACS) Lancet. 2002;360(9349):1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 11.Agwale SM, Tanimoto LW, Chad Odama LL, Odama L, Leung K, Duey D, et al. Prevalence of HCV coinfection in HIV-infected individuals in Nigeria and characterization of HCV genotypes. J Clin Virol. 2004;31(Suppl 1):3–6. doi: 10.1016/j.jcv.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30(1):24–33. doi: 10.1016/j.cct.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chasela CS, Jamieson DJ, Kayira D, Kayira D, Hosseinipour M, Kourtis AP, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alter MJ, Kuhnert WL, Finelli L. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52(RR-3):1–13. [PubMed] [Google Scholar]
- 15.Rouet F, Chaix ML, Inwoley A, Msellati P, Viho I, Combe P, et al. HBV and HCV prevalence and viraemia in HIV-positive and HIV-negative pregnant women in Abidjan, Cote d’Ivoire: the ANRS 1236 study. J Med Virol. 2004;74(1):34–40. doi: 10.1002/jmv.20143. [DOI] [PubMed] [Google Scholar]
- 16.Hladik W. Prevalence and screening costs of hepatitis C virus among Ugandan blood donors. Trop Med Int Health. 2006;11(6):951–4. doi: 10.1111/j.1365-3156.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 17.Pirillo ME, Bassani L, Germinario EAP, Mancini MG, Vyankandondera J, Okong P, et al. Seroprevalence of hepatitis B and C viruses among HIV-infected pregnant women in Uganda and Rwanda. J Med Virol. 2007;79(12):1797–801. doi: 10.1002/jmv.21007. [DOI] [PubMed] [Google Scholar]


