Abstract
Chemoresistance is the major obstacle in multiple myeloma (MM) management. We previously showed that macrophages protect myeloma cells, on a cell contact basis, from melphalan or dexamethasone-induced apoptosis in vitro. In this study, we found that macrophage-mediated myeloma drug resistance was also seen with purified macrophages from myeloma patients’ bone marrow (BM) in vitro and was confirmed in vivo using the human myeloma-SCID (severe combined immunodeficient) mouse model. By profiling differentially regulated and paired plasma membrane protein genes, we showed that PSGL-1 (P-selectin glycoprotein ligand-1)/selectins and ICAM-1/CD18 played an important role in macrophage-mediated myeloma cell drug resistance, as blocking antibodies against these molecules or genetic knockdown of PSGL-1 or ICAM-1 in myeloma cells repressed macrophages’ ability to protect myeloma cells. Interaction of macrophages and myeloma cells via these molecules activated Src and Erk1/2 kinases and c-myc pathways and suppressed caspase activation induced by chemotherapy drugs. Thus, our study sheds new light on the mechanism of drug resistance in MM and provides novel targets for improving the efficacy of chemotherapy in patients.
Keywords: macrophage, multidrug resistance, multiple myeloma, PSGL-1
INTRODUCTION
Multiple myeloma (MM), a terminally differentiated plasma cell malignancy in the bone marrow (BM), is still an incurable disease, and chemotherapy is currently one of the most effective treatments for MM.1,2 However, despite the introduction of novel chemotherapy agents and advances of treatment plan, chemoresistance, especially multidrug resistance, remains the major problem in MM management. Drug resistance has been reported for all clinically used MM chemotherapy agents, including conventional chemotherapeutics melphalan, dexamethasone and doxorubicin,3 and novel chemotherapeutics thalidomide, lenalidomide and bortezomib.4,5 Indeed, almost all MM patients have relapsed or refractory tumor after prior treatments.6 Relapsed tumors are usually more resistant to drugs, especially conventional chemotherapy agents, and patients’ median survival after relapse is only about 9 months.7
Myeloma BM plays an essential role in MM drug resistance.8,9 For MM patients, the BM is not only where MM cells accumulate, but it also provides a homing microenvironment, which is important for the tumor development, growth, proliferation, metastasis and chemoresistance. As evidence, primary MM cells isolated from the BM microenvironment exhibit decreased viability ex vivo and increased sensitivity toward cytotoxic agents.10 MM–BM microenvironment is constituted of both cellular and non-cellular components. Direct contact between MM cells to those BM components, and soluble factors secreted by MM–BM cells, both confer MM cell drug resistance. As expected, multiple factors are involved in MM drug resistance within the BM microenvironment, but many details of MM drug resistance are still missing.
Our previous work has shown that MM–BM has increased macrophage (MΦ) infiltration, compared with non-malignant BM.10 In vitro experiments showed that MΦs mediated MM drug resistance to conventional chemotherapeutics melphalan and dexamethasone. The goals of this study were to perform mechanistic studies to further elucidate the mechanism underlying MΦ-mediated MM drug resistance in vitro and in vivo, to identify cell surface molecules on both MM cells and MΦs that were involved in cell interaction, and to investigate signaling pathways in MM cells that confer drug resistance. Such a study may provide new insight into the mechanism of drug resistance in MM and new targets for improving the efficacy of chemotherapy in MM patients.
MATERIALS AND METHODS
Reagents and antibodies
Reagents used included Src kinase inhibitor PP2 (Sigma, St Louis, MO, USA), c-myc inhibitory peptide Int-H1-S6A F8 (Enzo Life Science, Farmingdale, NY, USA), Erk kinase inhibitor U0126 (Cell Signaling, Boston, MA, USA) and CellTrace CFSE (carboxyfluorescein diacetate succinimidyl ester) kit (Invitrogen, Carlsbad, CA, USA). Blocking antibodies were anti-CD18, ICAM-1 and control immunoglobulin G (IgG) (Biolegend, San Diego, CA, USA), anti-P/E-selectin, anti-PSGL-1 and E-selectin–Fc fusion protein (R&D Systems, Minneapolis, MN, USA). Western blotting antibodies against PARP, caspase-3, Akt, pSrc(Y416), pErk(T202/204), Erk and c-myc were purchased from Cell Signaling. Anti-p-Akt(308), PSGL-1 and actin antibodies were from Santa Cruz Biotecnologies (Santa Cruz, CA, USA). Immunohistochemistry antibodies against cleaved PARP were purchased from Cell Signaling, and anti-CD68 antibody was from Dako (Carpinteria, Santa Barbara, CA, USA). Flow cytometry antibodies fluorescein isothiocyanate-CD3, CD14, CD16, CD19, CD20, Annexin V and control IgG were purchased from eBioscience (San Diego, CA, USA) and ACP-CD68 and CD11c were from Biolegend.
MM cells, MΦs and cell (co)cultures
Human MM cell lines ARP-1, ARK, MM.1S, CAG and U266 were maintained in RPMI-1640 medium with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C and 5% CO2. Primary MM cells were isolated from BM aspirates of newly diagnosed patients’ by CD138+ cell sorting.
Human MΦs were generated from peripheral blood monocytes of healthy donors or myeloma patients’ as described previously.10 In brief, 20–25 millions of mononuclear cells from the blood of healthy donors were incubated in 24-well or 6-well plates for 2 h at 37 °C to remove non-adherent cells. The adherent monocytes were incubated for 7 days in medium with macrophage colony-stimulating factor (10 ng/ml) to become MΦ. Primary MM patients’ BM–MΦs were isolated from BM aspirates of myeloma patients’ by CD14+/CD163 + double-positive cell sorting. The sorted cells were cultured in RPMI-1640 complete medium overnight before the experiment.
For MΦ/MM cell coculture with direct contact, MM cells were added directly to MΦs and cocultured for the indicated length of time (usually 24 h). For MΦ/MM cell coculture without direct contact, MM cells were cultured in transwell inserts (0.4-μm pore size, Corning Inc., Corning, NY, USA). In some experiments, MM cells in transwells were cocultured with MM cells and MΦs at bottom of wells to evaluate contact-dependent soluble factors in MM drug resistance. After direct coculture, suspension MM cells were harvested and depleted of contaminating MΦs by magnetic CD14+ cell depletion (Miltenyi Biotec, Bergisch Glabach, Germany) before apoptosis assay, western blotting or microarray analysis. After MΦ depletion, the purity of MM cells is >99%. All studies involving primary cells were approved by the Institutional Review Board at the University of Texas, MD Anderson Cancer Center.
Apoptosis assay and flow cytometry analysis
MM cell apoptosis was measured by Annexin V-binding assay as previously described.10 In some experiments when CFSE-labeled or green fluorescent protein-expressing MM cells were used for apoptosis assay, APC-Annexin V (eBioscience) was used for apoptotic cell detection.
Expression of P-selectin, E-selectin, CD18, PSGL-1 and ICAM-1 was determined by direct immunofluorescence using fluorescein isothiocyanate - or R-phycoerythrin (PE)-conjugated antibodies. After staining, cells were resuspended in phosphate-buffered saline and analyzed by a FACScan flow cytometer (Becton Dickinson, (BD) Company, Franklin Lakes, NJ, USA). Flow cytometry analysis of myeloid dendritic cells (mDCs), plasmacytoid DCs (pDCs) and MΦs was performed as described previously.11,12
Short hairpin RNA (shRNA) for target protein knocking down
PSGL-1 was knocked down in MM cells by shRNA lentiviral infection. PSGL-1 shRNA (target sequence: 5′-GAGGAGTACTGAAGAGTGA-3′) lentivirus was constructed as previously described.13 After lentiviral infection, transduced cells were sorted for green fluorescent protein- expression. Similarly, ICAM-1 shRNA (target sequence 1: 5′-GAACAGAGTGGAAGACATA-3′; and target sequence 2: 5′-GGTTACAGGTTCAGAGATT-3′) lentivirus was constructed and used for ICAM-1 silencing in MM cells.
Microarray analysis for differentially regulated and paired membrane protein gene identification
ARP-1 cells and MΦs were cultured alone or cocultured with direct contact for 24 h. Total RNA was extracted from each type of the cells using TRIzol (Invitrogen). Gene expression profiles were determined by Illumina GeneArray (Illumina, Inc., San Diego, CA, USA). Three biological repeats were included in the microarray analysis.
To identify functional, paired membrane protein genes on MM cells and MΦs, we hypothesized that if a pair of surface protein genes were upregulated under coculture condition, it was likely that this pair of genes were involved in MΦ-mediated drug resistance. Therefore, we first identified differentially upregulated genes in MM and MΦ using the function value (gene, i) = Σ(log2(data(coculture) gene, i) − log2(data(alone) gene, i)). Next we ranked the genes with descending order of values, and genes with largest values ranked at the top of the list. The position of a gene in the ranked list is the score of that gene, for example. the top gene score is 1, the second gene score is 2, and so on. We then compared the ranked gene list with the human surfaceome database,14 and produced a ranked surface-gene list. Next, we extracted each surface protein’s surface interactors using the protein–protein interaction database (www.hprd.org). Finally, we ranked paired surface genes by the sum of each gene’s scores. For example, gene A on MM had score 1, and its interactor gene B on MΦs had score 4, and the sum of scores was 5. The gene pair with the least sum score number ranked at the top of the final list. After removing self-interactions, a total of 1434 pairs of surface gene interactions was ranked and the top 250 genes were used for future analysis.
Immunohistochemistry analyses
Formalin-fixed, paraffin-embedded sections of tumors from tumor-bearing SCID (severe combined immunodeficient) mice were used for immunohistochemistry analyses to detect CD68-expressing MΦs or cleaved PARP (apoptotic tumor cells) as described previously.15
MΦ-mediated MM chemoresistance in vivo
Six- to eight-week-old female SCID mice were housed and monitored in the MD Anderson Cancer Center animal research facility. All experiments had been approved by the Institutional Animal Care and Use Committee at the University of Texas, MD Anderson Cancer Center. Mice were subcutaneously inoculated in the right flank with one million ARP-1 and two million monocytes suspended in 100 μl phosphate-bufferd saline. For some experiments, monocytes were labeled with CFSE (Invitrogen) before inoculation. In control mice, ARP-1 cells only (1 million) were subcutaneously inoculated.
After palpable tumor developed (tumor diameter ≥5 mm), the presence and number of MΦs in the tumors were determined. Specifically, tumors developed after ARP-1/monocytes or ARP-1 inoculations were harvested for immunohistochemistry staining for human CD68 or flow cytometry analysis for CFSE+ MΦs.
Some mice were treated with intraperitoneal injections of melphalan (100 μg per mouse every 3 days) for 15 days. Injections of DMSO served as a control. Five mice were included in each treatment group. In some experiment, mice were intraperitoneally injected with PSGL-1-neutralizing antibody or control IgG (100 μg per mouse, every 3 days), and treated with melphalan as described above. Tumor sizes were measured daily and blood samples were collected every 5 days. Tumor burdens were evaluated by measuring tumor size and detecting circulating human kappa chain by ELISA (Bethyl Laboratories Inc., Montgomery, AL, USA).
Statistical analysis
All data are shown as mean±s.d. The Student t-test was used to compare various experimental groups. A P value <0.05 was considered significant.
RESULTS
MΦ-mediated MM drug resistance is cell contact-dependent
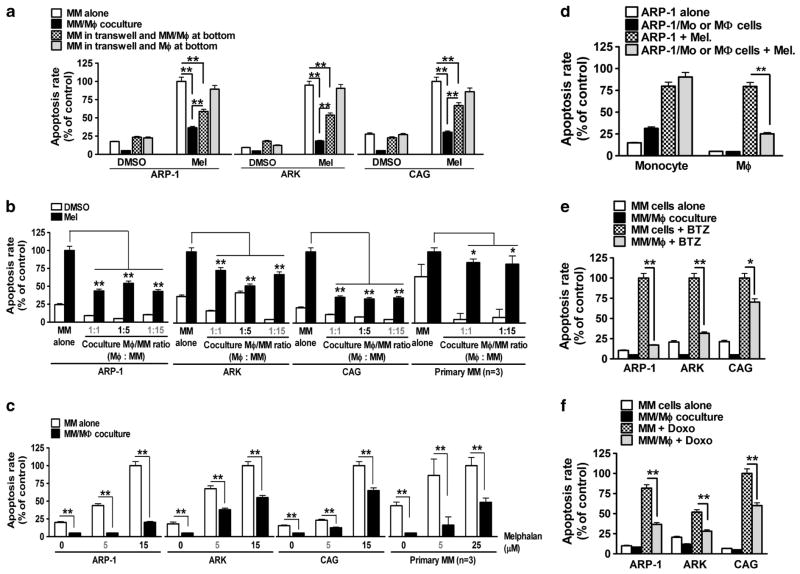
To test MΦ-mediated MM drug resistance, MM cells ARP-1, ARK or CAG, cocultured with monocyte-derived MΦs from blood donors, were treated with or without the apoptotic agent, melphalan. As shown in Figure 1a, direct cell contact between MM cells and MΦs, but not separated by transwell inserts, conferred MM cell drug resistance (P<0.01, compared with melphalan-treated MM cell culture alone). Interestingly, drug resistance could also be partially induced in MM cells placed on transwell inserts in the coculture of MM cells and MΦs together in the bottoms, indicating that bystander MM cells could also be protected by soluble factors derived from MM/MΦ interaction (Figure 1a; P<0.01, compared with melphalan-treated MM cell culture alone or MM/MΦ coculture). Next, we showed that MΦs protected MM cells ARP-1, ARK or CAG with a wide range of MΦ/MM ratios (Figure 1b; P<0.01, compared with melphalan-treated MM cell culture alone), and at different doses of melphalan treatment (Figure 1c). In addition, we showed that only MΦs, but not monocytes, protected MM cells from melphalan-induced cell apoptosis (Figure 1d). We extended our experiment to other cytotoxic agents such as doxorubicin and bortezomib. Our results showed that, under the coculture condition, MΦs protected all tested MM cells from the chemotherapeutics-induced apoptosis (Figures 1e and f). We do not believe that fewer apoptotic cells were detected in the cocultures because MΦs engulfed apoptotic myeloma cells as our previous studies showed that similar percentages of apoptotic myeloma cells were detected in cocultures with MΦs treated with or without cytochalasin D, which inhibited the endocytosis ability of MΦs.10 Overall, our findings suggested that MΦs conferred MM multidrug resistance on a cell contact-dependent basis.
Figure 1.
MΦs mediate MM multidrug resistance. Shown are percentages of melphalan (mel; 15 μM)-induced apoptotic MM cells in (a) different culture conditions; culture alone, transwell coculture with MΦs, direct coculture with MΦs, and transwell coculture with ARP-1/MΦs; (b) direct coculture with MΦs with different MΦ/MM ratios; and (c) direct coculture with MΦs after different doses of melphalan (5–15 μM) treatment. (d) Melphalan (15 μM)-induced apoptotic ARP-1 cells in direct cocultures with healthy donor monocytes (ex vivo precultured for 1 day) or MΦs. (e) Percentages of bortezomib (BTZ, 10 nM)-induced apoptotic ARP-1, ARK and CAG cells in direct cocultures with MΦs. (f) Percentages of doxorubicin (Doxo, 25 ng/ml)-induced apoptotic ARP-1, ARK and CAG cells in direct cocultures with MΦs. Summarized results from three independent experiments are shown. *P<0.05, **P<0.01.
MΦs may be important cells in an MM–BM microenvironment
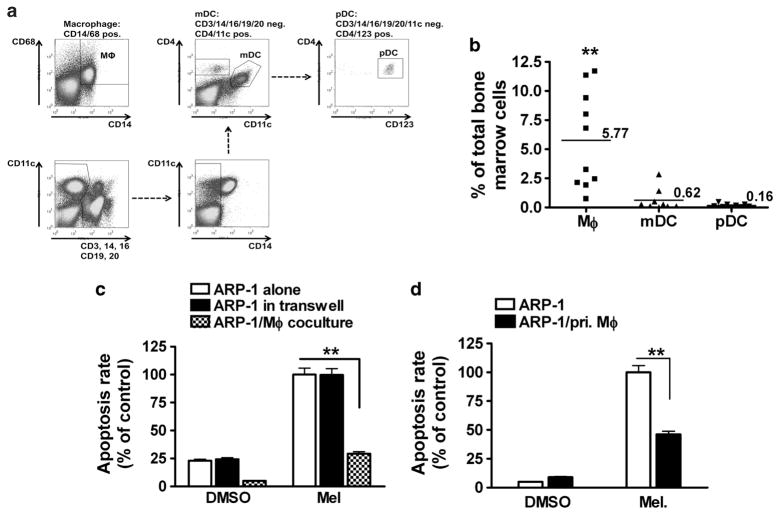
Others have shown that various types of BM cells, such as mDCs, pDCs and osteoclasts, promote MM cell proliferation and/or drug resistance.10,16–18 To investigate their clinical significance, the numbers of pDCs, mDCs and MΦs in the BM of MM patients’ were examined. As shown in Figure 2a (a representative result from a patients’) and Figure 2b (summarized data of 10MM patients’s), the percentages of BM-infiltrating MΦs were significantly higher than those of mDCs or pDCs, suggesting that MΦs, as abundant in the BM, may be important in protecting MM cells against chemotherapy in vivo.
Figure 2.
MΦ is an effective protector cell in MM–BM. (a) A representative flow cytometry analysis showing the percentages of mDCs, pDCs and MΦs in the BM aspirate of a MM patients’; and (b) summarized results of the percentages of these cells in the BM aspirates of 10 MM patients’ samples. Numbers indicate the mean percentages. Also shown are percentages of melphalan (15 μM)-induced apoptotic ARP-1 cells in cocultures (transwell coculture or direct coculture) with (c) MM patients’ monocyte-derived MΦs and (d) primary CD14+/CD163+ MΦs (primary MΦs; pri. MΦs) isolated from the BM of MM patients’s. In (c) and (d), the representative results using primary MM samples from one out of three patients’s are shown. Summarized results from three independent experiments are shown. *P<0.05, **P<0.01.
Next, we used monocyte-derived MΦs from MM patients’ and examined their capacity to confer drug resistance. As shown in Figure 2c, patients’-derived MΦs efficiently protected MM (ARP-1) cells from melphalan-induced apoptosis in a direct coculture (P<0.01, compared with melphalan-treated ARP-1 culture alone). We also isolated CD14 + monocytes/MΦs from MM patients’ BM aspirates. These cells were cultured overnight first and then used for protection assay as described above. The results showed that MΦs from tumor bed were also efficient at protecting MM (ARP-1) cells from melphalan-induced MM cell apoptosis (Figure 2d; P<0.01, compared with melphalan-treated ARP-1 culture alone). Overall, our results showed that monocyte-derived MΦs from blood donors and MM patients’ as well as MΦs isolated from the BM of MM patients’ could render MM drug resistance in vitro.
Identification of upregulated, paired membrane proteins on MM cells and MΦs
As MΦ-mediated MM drug resistance is cell-contact dependent, we hypothesized that cell surface molecules on those cells were important. Specifically, cell contact happens and is stabilized via interactions between paired plasma membrane proteins on each type of cells, and the interaction might initiate downstream survival signaling transduction in MM cells. Therefore, we analyzed the gene expression profiles of MΦs and MM cells under two culture conditions (culture alone vs coculture), and identified differentially regulated, paired membrane protein genes on the cells. The workflow of analysis was summarized in Supplementary Figure 1. We identified top 250 upregulated, paired membrane protein genes, and a representative set of the identified genes is shown in Supplementary Table 1.
Selectins and their ligand PSGL-1 are crucial for MΦ-mediated MM drug resistance
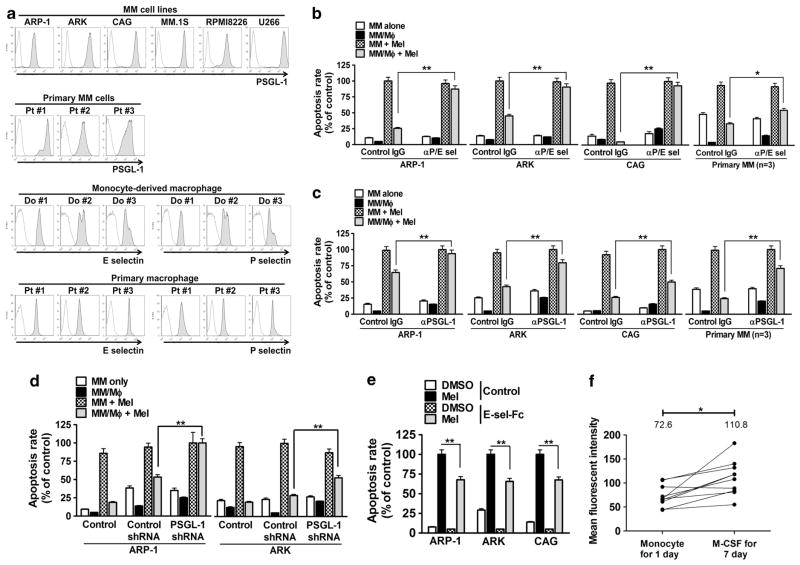
On the basis of the gene expression profile data, we chose to examine selectins (on MΦs) and their ligand PSGL-1 (also known as SELPLG, on MM cells) for their roles in MΦ-mediated MM drug resistance. Results showed that all tested MM cell lines and primary MM cells expressed PSGL-1 (Figure 3a), whereas all MΦs expressed both P-selectin and E-selectin. The expression of P/E-selectins by monocyte-derived MΦs was also confirmed by reverse transcriptase–PCR (Supplementary Figure 2A).
Figure 3.
E/P-selectins and their ligand PSGL-1 are crucial for MΦ-mediated protection of MM cells. (a) Expression of PSGL-1 on human MM cell lines and primary MM cells, and expression of E/P-selectins on monocyte-derived MΦs and primary MM–BM MΦs by flow cytometry analysis. Percentage of melphalan (15 μM)-induced apoptotic MM cells (ARP-1, ARK, CAG, or primary MM cells from three patients’s) in direct coculture with MΦs in the presence or absence of (b) P/E-selectin-blocking antibody (αP/E sel, 20 μg/ml) or (c) PSGL-1-blocking antibody (αPSGL-1, 20 μg/ml). Equal amounts of control IgG were used as controls. (d) Melphalan (15 μM)-induced apoptotic, PSGL-1-knocked down (PSGL-1 shRNA) ARP-1 or ARK cells in coculture with MΦs. Wild-type (control) and control shRNA-transfected (control shRNA) cells served as controls. (e) Melphalan-induced apoptotic MM cells (ARP-1, ARK or CAG) in the presence of E-selectin–Fc fusion protein (E-sel-Fc, 2 μg/ml). (f) Expression of E-selectin on monocytes and monocyte-derived MΦs from 10 blood donors by flow cytometry analysis. Numbers indicate the mean values of mean fluorescent intensity (MFI). Summarized results from four independent experiments are shown. *P<0.05, **P<0.01.
Next, we interrupted selectins/PSGL-1 interaction using P/E-selectin- or PSGL-1-blocking antibodies, and examined MΦ-mediated protection. As shown in Figures 3b and c, the antibodies had little effect on melphalan-induced MM (ARP-1, ARK, CAG or primary MM) cell apoptosis in cultures without MΦs. However, both of the antibodies, but not control IgG, repressed MΦ-mediated MM drug resistance and restored, at least partially, MM cell sensitivity to melphalan in direct coculture with MΦs (P<0.05, compared with control IgG). We also tested MΦ-mediated MM chemoresistance in PSGL-1-knocked down MM cells, ARP-1 or ARK (Supplementary Figures 2B and C). Compared with controls, the mean fluorescent intensity of surface PSGL-1 expression was decreased by >50% on knocked-down cells, suggesting a significant surface protein knockdown. By knocking down PSGL-1 in MM cells, MΦs were no longer able to protect them from chemotherapy drug-induced apoptosis (Figure 3d; P<0.01, compared with control shRNA-knocked down MM cells). Alternatively, we showed that E-selectin–FC fusion protein, which binds with PSGL-1 on MM cells and is agonistic, could also protect, to a certain degree, MM cells ARP-1, ARK or CAG from melphalan-induced apoptosis (Figure 3e; P<0.01, compared with melphalan-treated MM cells).
Finally, as monocytes did not protect MM cells from melphalan-induced cell death, we measured the expression of selectins during MΦ differentiation. As shown in Figure 3f, MΦs had increased E-selectin expression as compared with monocytes (P<0.05). Overall, our findings suggested that MΦ expressed P/E-selectins, and MM cells expressed their ligand PSGL-1. Direct cell contact between MΦ and MM cells enabled PSGL-1/selectins interaction, which is crucial for MΦ-mediated drug resistance in MM cells.
ICAM-1/CD18 are critical adhesion molecules for MΦ-mediated drug resistance
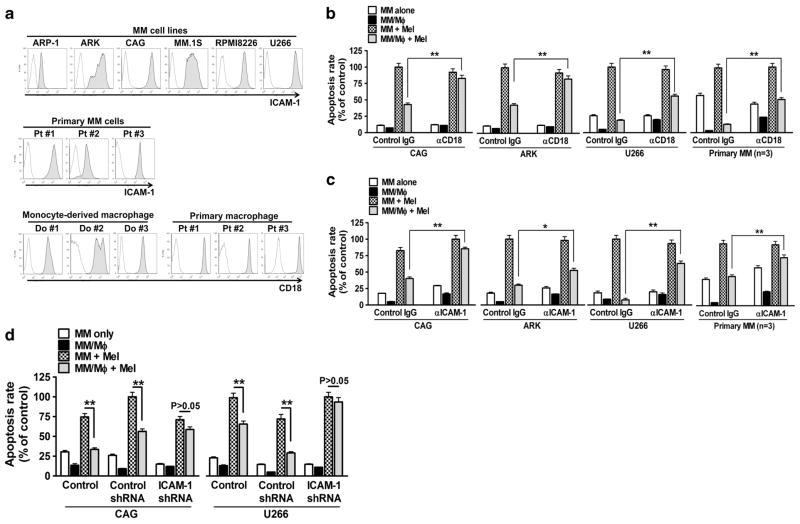
Second, we examined the role of ICAM-1/CD18 in MM drug resistance on the basis of our previous study.10 All tested MM cell lines and primary MM cells expressed ICAM-1, and monocyte-derived MΦs and purified MΦs from MM patients’ BM expressed CD18 (Figure 4a). Functional tests showed that ICAM-1 or CD18-blocking antibodies attenuated MΦ-mediated MM (CAG, ARK, U266 or primary MM) cell drug resistance (Figures 4b and c; P<0.05, compared with IgG control). Similarly, ICAM-1-knocked down MM (CAG or U266) cells (Supplementary Figures 3A and B) were not protected by MΦs against melphalan-induced apoptosis (Figure 4d). Thus, our findings suggested that cell membrane proteins CD18 (on MΦs) and ICAM-1 (on MM cells) also played an important role in MΦ-mediated MM drug resistance.
Figure 4.
CD18 and its ligand ICAM-1 are crucial for MΦ-mediated protection of MM cells. (a) Expression of ICAM-1 on human MM cell and primary MM cells, and expression of CD18 on monocyte-derived MΦs and primary MM-BM-MΦs by flow cytometry analysis. Percentage melphalan (15 μM)-induced apoptotic MM cells (CAG, ARK, U266 or primary MM cells from three patients’s) in direct coculture with MΦs, in presence or absence of (b) CD18-blocking antibody (αCD18, 20 μg/ml) or (c) ICAM-1-blocking antibody (αICAM-1, 20 μg/ml). Equal amounts control IgG were used as controls. (d) Percentage of melphalan (15 μM)-induced apoptotic, ICAM-1-knocked down (ICAM-1 shRNA) CAG U266 cells in coculture with MΦs. Wild-type (control) and control shRNA-transfected (control shRNA) cells served as controls. Summarized results from four independent experiments are shown. *P<0.05, **P<0.01.
MΦ-mediated MM drug resistance is associated with survival signaling in MM cells
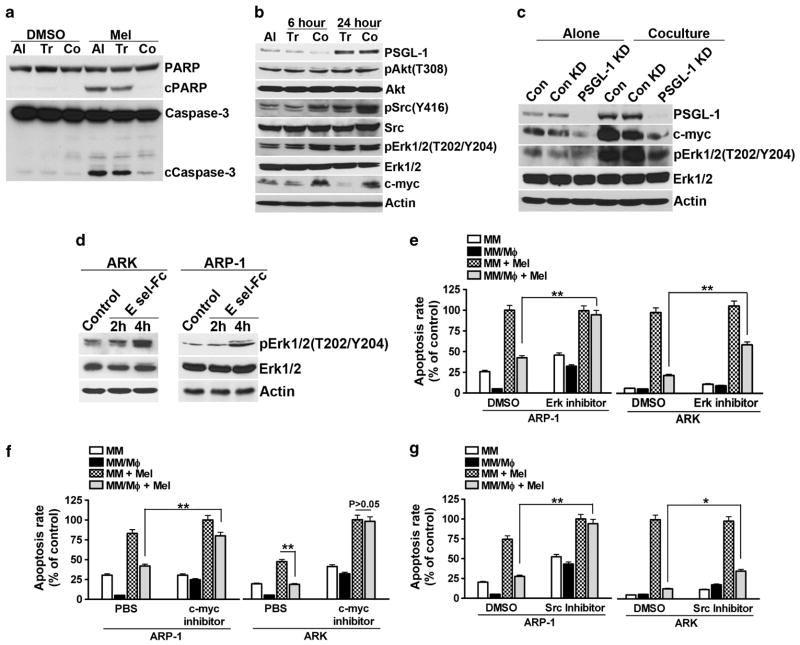
We hypothesized that MΦ/MM cocultures activated cell survival signaling in MM cells, which conferred MM multidrug resistance. First, we examined MM cell apoptosis signaling under (co)culture conditions. As shown in Figure 5a, melphalan treatment stimulated PARP and caspase-3 cleavage, both of which are characteristics of apoptotic cells. However, in MM (ARP-1) cells cocultured with MΦs, melphalan-induced PARP and caspase-3 cleavages were highly repressed. Next, we studied the signaling molecules in MM cells. We found that proto-oncoprotein c-myc and phosphorylated Src and Erk1/2 kinases were upregulated in MΦ-cocultured ARP-1 cells, but not in ARP-1 cells cultured alone or cocultured in transwell inserts (Figure 5b). Similar findings were also observed from other MM cell lines (Supplementary Figure 4A).
Figure 5.
Coculture with MΦs activates survival signaling in MM cells. Western blotting analyses showing: (a) melphalan (15 μM)-induced cleavage and activation of PARP and caspase-3 in ARP-1 cells in different culture conditions; cultured alone (Al) or cocultured in transwell inserts (Tr) or in direct contact (Co) with MΦs; (b) expression of PSGL-1, pAkt(T308), Akt, pSrc(Y416), Src, pErk1/2(T202/Y204), Erk1/2, c-myc and actin in culture of ARP-1 cells alone or after transwell (Tr) or direct coculture (Co) with MΦs; (c) expression of PSGL-1, c-myc, pErk1/2(T202/204), Erk1/2 and actin in culture of PSGL-1-knocked down ARP-1 cells (PSGL-1 KD) alone or after coculture with MΦs for 24 h. Wild-type (con) and control shRNA-transfected (con KD) ARP-1 were used as controls; or (d) expression of pErk1/2(T202/Y204), Erk1/2 and actin in ARP-1 or ARK cells treated with 2 μg/ml of E-selectin-Fc fusion protein for 2 or 4 h. Percentage of melphalan-induced apoptotic ARP-1 or ARK cells cultured alone or in direct cocultures with MΦs in the presence or absence of (e) Erk1/2 inhibitor (U0126, 5 μM), (f) c-myc inhibitor (1 μM), or (g) Src inhibitor (PP2, 5 μg/ml). DMSO or phosphate-buffered saline served as vesicle controls. Summarized results from three independent experiments are shown. *P<0.05, **P<0.01.
Next we studied the downstream signaling of PSGL-1 using PSGL-1-knocked down ARP-1 cells. As shown in Figure 5c, coculture with MΦs did not stimulate Erk1/2 phosphorylation or c-myc overexpression in PSGL-1-knocked down ARP-1 cells. Similar findings were also observed in PSGL-1-knocked down ARK cells (Supplementary Figures 4B and C). Such findings suggested that PSGL-1-mediated MM drug resistance may be through Erk1/2 pathway activation and c-myc upregulation. In line with this finding, activation of PSGL-1 by soluble E-selectin–Fc fusion protein also induced phosphorylation of Erk1/2 (Figure 5d). Conversely, MΦ-mediated MM drug resistance was highly repressed by inhibitors to Erk, Src or c-myc (Figures 5e–g; P<0.01, compared with control).
Our results that PSGL-1 expression in ARP-1 was upregulated after coculture (Figure 5b) suggested a feedback loop of PSGL-1 in MΦ-mediated MM drug resistance. As transwell-cocultured ARP-1 also had increased PSGL-1, it was likely that some soluble factors contributed to PSGL-1 upregulation. Further test showed that PSGL-1 overexpression was regulated by IFN-α. IFN-α-blocking antibody repressed MΦ coculture-induced PSGL-1 overexpression (Supplementary Figure 4D). However, IFN-α-blocking antibody did not affect MΦ-mediated MM drug resistance (Supplementary Figure 4E).
In vivo effects of MΦ-mediated MM drug resistance
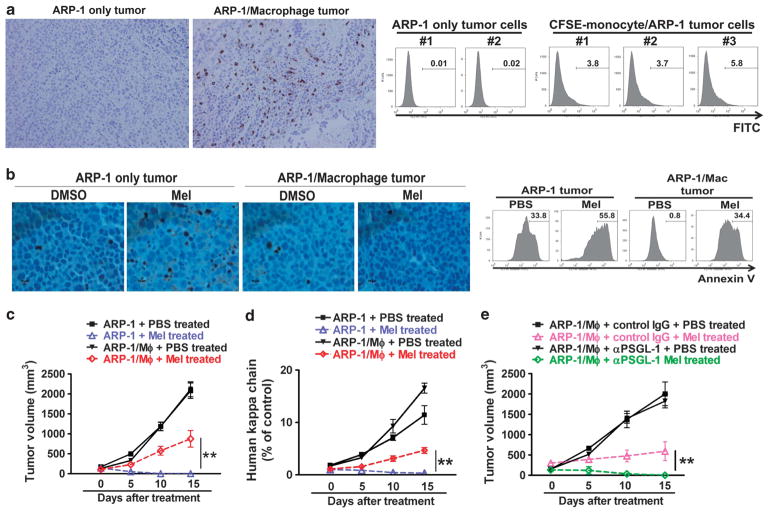
The human MM-SCID mouse model15 was used to examine whether MΦs could protect MM cells from drug-induced apoptosis in vivo. Human myeloma cells and (CFSE-labeled, in some experiments) monocytes were mixed and injected into SCID mice as detailed in Materials and Methods. After tumors were developed, CFSE-labeled human MΦs in tumors could be observed and enumerated by immunohistochemistry staining using anti-human CD68 antibody or flow cytometry analyses for CFSE-labeled cells (Figure 6a). To measure melphalan-induced cell death in vivo, mice bearing ARP-1 tumor with human MΦs or ARP-1 tumor alone were treated with the drug daily for three consecutive days. After the treatment, tumors were harvested and subjected for immunohistochemistry analysis with anti-cPARP antibody (Figure 6b; left panel) and apoptosis assay (Figure 6b; right panel). Our results showed that melphalan-induced tumor-cell apoptosis was significantly repressed when human MΦs were present, suggesting that MΦs protected MM cells from melphalan-induced apoptosis in vivo. Consistently, co-injection of human MΦs resulted in significantly compromised therapeutic effects of melphalan (a 15-day treatment schedule) on the tumors, measured as tumor volume (Figure 6c; P<0.05, compared with melphalan treatment) or circulating human kappa chain (Figure 6d; P<0.01, compared with melphalan treatment). To confirm that selectin/PSGL-1 pathway was involved in vivo, mice bearing ARP-1/MΦ tumors were treated with PSGL-1-neutralizing antibody together with melphalan as described above. The result showed that PSGL-1-neutralizing antibody significantly repressed MΦ-mediated drug resistance in vivo (Figure 6e; P<0.01, compared with IgG treatment).
Figure 6.
In vivo effect of MΦ-mediated MM drug resistance in myeloma SCID mouse model. (a) Staining for CD68+ human MΦs in tumors from myeloma-bearing mice. MM cells ARP-1 (ARP-1 only tumor) or ARP-1/CFSE-labeled monocytes (ARP-1/Macrophage tumor) were subcutaneously inoculated into SCID mice. After tumor size reached 5 × 5 mm2, tumors were harvested for immunohistochemistry staining of CD68 (left panel) and flow cytometry analysis of CFSE+ human MΦs (right panel). (b) Detection of apoptotic cells in tumors from myeloma-bearing mice. Myeloma-bearing SCID mice were treated with intraperitoneal injections of melphalan (100 μg per day for 3 consecutive days). After the treatment, tumors were removed and examined by immunohistochemistry of cleaved PARP+ (left panel) and flow cytometry analysis for Annexin V+ cells (right panel). Myeloma-bearing mice treated with DMSO served as controls. Also shown are tumor burdens, detected as (c) tumor volume or (d) level of circulating human κ chain in myeloma-bearing SCID mice treated with melphalan (100 μg per treatment, intraperitoneal injection every 3 days) for 15 days, and (e) tumor volume of myeloma/MΦ-bearing SCID mice (n = 5 per group) treated with melphalan (100 μg per treatment, intraperitoneal injection every 3 days) and PSGL-1-neutralizing antibody (100 μg per treatment, intraperitoneal injection every 3 days). *P<0.05, **P<0.01.
Interestingly, we also found that co-injection of MΦs led to a faster tumor development as compared with injection of MM (ARP-1) cell alone (Supplementary Figure 5A; P<0.01). Although palpable tumors in each group grew at a similar rate (Figure 6c), MΦ-ARP-1 tumors developed earlier than ARP-1-alone tumors did (Supplementary Figure 5A). We investigated whether MΦs could affect MM proliferation, and showed that MΦ coculture promoted slightly but significantly myeloma cell proliferation (Supplementary Figure 5B; P<0.05 to P<0.01, compared with ARP-1 cell alone). Moreover, using colony-formation assay, we showed that MΦ coculture significantly promoted MM cell colony formation on soft agar (Supplementary Figure 5C; P<0.01, compared with ARP-1 cells alone), indicating that MΦ may enhance MM proliferation and tumorigenicity in vivo.
DISCUSSION
It has been well documented that MΦs infiltrate into the tumor bed of various solid tumors and those MΦs are termed tumor-associated MΦs tumor-associated MΦs.19 Tumor-associated MΦs exhibit tumor promoting activities by increasing tumor angiogenesis, metastasis and suppressing anti-tumor immunity.20,21 In this study, we demonstrated a novel function, that is, promoting multidrug resistance in MM, for these cells.
MΦ-mediated MM multidrug resistance required direct cell contact between MΦs and MM cells. MΦs conferred strong drug resistance to MM cells, which might be mediated by paired cell surface protein interactions. In this study, we demonstrated that two of those paired membrane proteins, selectins/PSGL-1 and CD18/ICAM-1, were important for MΦ/MM cell interaction to confer drug resistance to MM cells. Selecins/PSGL-1 proteins have been well characterized in leukocyte migration.22 Selectin expression on mouse MΦs has been reported.23,24 Both P-selectins and E-selectins have a common ligand, PSGL-1. One recent publication showed that MM cells had increasing PSGL-1 expression during disease progression, and PSGL-1 was important for MM homing to the BM and MM drug resistance.25 In our studies, we found that PSGL-1 expression had limited effect on MM spontaneous apoptosis, but was critical for MΦ-mediated MM drug resistance. The other identified paired membrane proteins are CD18/ICAM-1. Previous studies have shown that MM cells with high ICAM-1 expression have increased cell survival.26 As an adhesion molecule, ICAM-1 interacts with CD18 on the counterpart cells. Specifically, CD18 may associate with CD11a, CD11b or CD11c and form complexes known as LFA-1, Mac-1 or p150/95, respectively.27 However, none of the blocking antibodies to these molecules (CD11a, CD11b, and CD11c) were able to affect MΦ-mediated MM drug resistance. This may indicate that CD11 molecules had no direct interaction with ICAM-1 or MM cells. However, it is likely that more membrane proteins are involved and we are actively working to identify them.
Although our data clearly showed that cell–cell contact was required for MΦ-mediated protection of MM drug resistance, a role for soluble factors in MΦ-mediated MM drug resistance cannot be ruled out. As shown in Figure 1a, culture of MΦs and MM cells separately by transwell inserts failed to protect MM cells, but MΦ-MM cell direct coculture resulted in a protection of bystander MM cells in the transwell inserts. These observations indicated that direct coculture of MΦs and MM cells led to release of soluble factors that protected the by-stander MM cells. Our previous study showed that IL-6 was upregulated in the coculture but IL-6-neutralizing antibody did not compromise the protective effect.10 Therefore, further studies are required to define the protective soluble factors.
Our data also showed that MΦs mediated the multidrug resistance of MM cells to both conventional chemotherapy drugs and novel agents such as bortezomib. As those drugs have different tumoricidal mechanisms, our results suggested that MΦ-mediated drug resistance may be associated with activation of canonical survival signaling pathways in MM cells.28,29 Indeed, we observed Src and Erk1/2 kinase activation and c-myc accumulation in MM cells following coculture with MΦs, all of which have been shown to promote MM cell survival and drug resistance.30,31 We also identified that Erk kinase activation and c-myc overexpression were downstream of PSGL-1 engagement. The contact-dependent intra-MM survival signaling activation may indicate an epigenetic alteration of MM after MΦ infiltration. On the basis of our findings, it is highly possible that MΦ infiltration in tumor bed renders MM cells, especially MM cells directly interacting with surrounding MΦs, with pro-survival and drug resistance capacity. Furthermore, soluble factors derived from the interaction of MM cells and MΦs also support bystander MM cells to develop drug resistance. Thus, it is plausible that these MΦ-interacting and surrounding MM cells are better protected against chemotherapy and become relapsed/refractory tumors after chemotherapy. Further studies are warranted to investigate MΦ infiltration of BM and MM prognosis to further evaluate and confirm the clinical relevance of MΦs in the disease progression.
Supplementary Material
Acknowledgments
This work was supported by National Cancer Institute grants R01 CA138402, R01 CA138398, R01 CA163881, and P50 CA142509; Leukemia & Lymphoma Society translational research grants; the Multiple Myeloma Research Foundation, the Commonwealth Foundation for Cancer Research; the Center for Targeted Therapy of The University of Texas MD. Anderson Cancer Center; and a grant for International Cooperation of the National Natural Science Foundation of China (81120108018). We thank our departmental Myeloma Tissue Bank for providing patients’ samples.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 3.Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- 4.Knobloch J, Reimann K, Klotz LO, Ruther U. Thalidomide resistance is based on the capacity of the glutathione-dependent antioxidant defense. Mol Pharm. 2008;5:1138–1144. doi: 10.1021/mp8001232. [DOI] [PubMed] [Google Scholar]
- 5.Laubach JP, Mitsiades CS, Roccaro AM, Ghobrial IM, Anderson KC, Richardson PG. Clinical challenges associated with bortezomib therapy in multiple myeloma and Waldenstroms macroglobulinemia. Leuk Lymphoma. 2009;50:694–702. doi: 10.1080/10428190902866732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastritis E, Palumbo A, Dimopoulos MA. Treatment of relapsed/refractory multiple myeloma. Semin Hematol. 2009;46:143–157. doi: 10.1053/j.seminhematol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia. 26:73–85. doi: 10.1038/leu.2011.310. [DOI] [PubMed] [Google Scholar]
- 8.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 9.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23:10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 12.Strobl H, Scheinecker C, Csmarits B, Majdic O, Knapp W. Flow cytometric analysis of intracellular CD68 molecule expression in normal and malignant haemopoiesis. Br J Haematol. 1995;90:774–782. doi: 10.1111/j.1365-2141.1995.tb05195.x. [DOI] [PubMed] [Google Scholar]
- 13.Dobroff AS, Wang H, Melnikova VO, Villares GJ, Zigler M, Huang L, et al. Silencing cAMP-response element-binding protein (CREB) identifies CYR61 as a tumor suppressor gene in melanoma. J Biol Chem. 2009;284:26194–26206. doi: 10.1074/jbc.M109.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Cunha JP, Galante PA, de Souza JE, de Souza RF, Carvalho PM, Ohara DT, et al. Bioinformatics construction of the human cell surfaceome. Proc Natl Acad Sci USA. 2009;106:16752–16757. doi: 10.1073/pnas.0907939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Wezeman M, Zhang X, Lin P, Wang M, Qian J, et al. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007;12:252–265. doi: 10.1016/j.ccr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–323. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, et al. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res. 2004;64:2016–2023. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- 18.Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R, et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas T, Abraham D, Aharinejad S. Modulation of tumor associated macrophages in solid tumors. Front Biosci. 2008;13:5580–5588. doi: 10.2741/3101. [DOI] [PubMed] [Google Scholar]
- 20.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 21.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 118:6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchernychev B, Furie B, Furie BC. Peritoneal macrophages express both P-selectin and PSGL-1. J Cell Biol. 2003;163:1145–1155. doi: 10.1083/jcb.200310079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Sanders JM, Phan ET, Ley K, Sarembock IJ. Arterial macrophages and regenerating endothelial cells express P-selectin in atherosclerosis-prone apolipoprotein E-deficient mice. Am J Pathol. 2005;167:1511–1518. doi: 10.1016/S0002-9440(10)61237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azab AK, Quang P, Azab F, Pitsillides C, Thompson B, Chonghaile T, et al. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood. 2012;119:1468–1478. doi: 10.1182/blood-2011-07-368050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iqbal MS, Otsuyama K, Shamsasenjan K, Asaoku H, Mahmoud MS, Gondo T, et al. Constitutively lower expressions of CD54 on primary myeloma cells and their different localizations in bone marrow. Eur J Haematol. 2009;83:302–312. doi: 10.1111/j.1600-0609.2009.01284.x. [DOI] [PubMed] [Google Scholar]
- 27.Patarroyo M, Prieto J, Rincon J, Timonen T, Lundberg C, Lindbom L, et al. Leukocyte-cell adhesion: a molecular process fundamental in leukocyte physiology. Immunol Rev. 1990;114:67–108. doi: 10.1111/j.1600-065x.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 28.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 29.Gertz MA. New targets and treatments in multiple myeloma: Src family kinases as central regulators of disease progression. Leuk Lymphoma. 2008;49:2240–2245. doi: 10.1080/10428190802475311. [DOI] [PubMed] [Google Scholar]
- 30.Kfir-Erenfeld S, Sionov RV, Spokoini R, Cohen O, Yefenof E. Protein kinase networks regulating glucocorticoid-induced apoptosis of hematopoietic cancer cells: fundamental aspects and practical considerations. Leuk Lymphoma. 2010;51:1968–2005. doi: 10.3109/10428194.2010.506570. [DOI] [PubMed] [Google Scholar]
- 31.Podar K, Anderson KC. A therapeutic role for targeting c-Myc/Hif-1-dependent signaling pathways. Cell Cycle. 2010;9:1722–1728. doi: 10.4161/cc.9.9.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.