Abstract
In an immune system, dendritic cells (DCs) are professional antigen-presenting cells (APCs) as well as powerful sensors of danger signals. When DCs receive signals from infection and tissue stress, they immediately activate and instruct the initiation of appropriate immune responses to T cells. However, it has remained unclear how the tissue microenvironment in a steady state shapes the function of DCs. Recent many works on thymic stromal lymphopoietin (TSLP), an epithelial cell-derived cytokine that has the strong ability to activate DCs, provide evidence that TSLP mediates crosstalk between epithelial cells and DCs, involving in DC-mediated immune homeostasis. Here, we review recent progress made on how TSLP expressed within the thymus and peripheral lymphoid and non-lymphoid tissues regulates DC-mediated T-cell development in the thymus and T-cell homeostasis in the periphery.
Keywords: DC (Dendritic cell), homeostasis, regulatory T cell, thymus, TSLP
INTRODUCTION
TSLP is an interleukin (IL)-7-like cytokine that was cloned from murine thymic stromal cell line.1-3 TSLP is expressed mainly by epithelial cells at barrier surfaces and is capable of initiating a wide variety of responses in many cell types, particularly myeloid DCs. The TSLP receptor (TSLPR) complex consists of a heterodimer of the IL-7 receptor α chain (IL-7Rα) and TSLPR.4-7 In humans, TSLPR is highly expressed by myeloid DCs (mDCs), and TSLP produced by epitherial cells strongly activates mDCs to upregulate MHC class II and co-stimulatory molecules, improve survival, and produce a variety of chemokines, such as CCL-17 (TARC) and CCL-21 (MDC). Interestingly, unlike other signals that activate mDCs such as the ligand for TLR3 or TLR4, TSLP does not induce mDCs to produce proinflammatory cytokines IL-12, IL-6, TNF-α, and IL-1.8 Epithelial cells in the tissue microenvironment appear to play a key role in instructing the tissue-resident DCs to control immune responses and homeostasis. However, it has been unclear how DCs regulate immune homeostasis at the steady state and during disease.
TSLP AND THYMIC SELECTION OF REGULATORYT (TREG) CELLS
TREG CELL DEVELOPMENT IN THYMUS
While majority of hematopoietic cells develop in the bone marrow, bone marrow-derived T cell progenitors migrate into the thymus and complete their development in the thymus. It has been generally accepted that when developing T cells express a T cell antigen receptor (TCR), they undergo two different types of selection based on the binding affinity of the TCR to a self-peptide-MHC complex presented by thymic epithelial cells or dendritic cells. First, developing T cells express a functional TCR that binds to a self-peptide-MHC class I or class II complex, which is presented by epithelial cells in the thymic cortex, then they undergo a process known as positive selection.9-11 While the developing T cells that fail positive selection die by apoptosis, the positively selected T cells survive and migrate into the medullar area of the thymus. These T cell precursors undergo a process of negative selection by which T cel1s carrying a TCR with high-affinity for a self-peptide-MHC complex expressed by APCs in the thymic medulla. However, some self-reactive T cells escape into the periphery and could cause autoinununity. It is now well established that these self-reactive T cells are controlled in the periphery by CD4+Foxp3+ Treg cells that developed in the thymus.12-14 The importance of Treg-mediated tolerance is illustrated by the observation that the acute elimination of Foxp3+ Treg cells in normal healthy animals can lead to death owing to multi-organ autoimmune disease.15,16 However, it is unclear what type of APCs positively selects Treg cells in the thymus and how these self-reactive Treg cells escape selection mediated by thymic APCs.
Although it was initially reported that Treg cell development starts at the CD4+CD8+ (DP) stage,17,18 more recent studies suggest that most Treg cells develop after positively selected CD4+ thymocytes migrate from the cortex to the medulla, which is comprised of medullary thymic epithelial cells (mTECs) and hematopoietic DCs.19-21 Pioneering studies using mouse models suggest that thymic epithelial cells are crucial for the induction of non-deletional tolerance by generating Treg cells. Mouse chimeras in which bone marrow-derived APCs were deficient in MHC class II showed normal numbers of thymic Treg cells,18,22 suggesting that mTECs may be crucial for Treg cell development. However in TCR-transgenic systems, the expression of cognate antigens on either epithelial cells or DCs seemed to be able to induce Treg development.23,24 We and other groups recently found that the expression of CD80/CD86 and CD40, which are key co-stimulatory molecules for Treg cell development, on only DCs subsets was also sufficient to generate a normal percentage of Treg cells.25-27 Moreover, normal or elevated numbers of Treg cells were observed when MHC class II presentation was decreased on AIRE+ mTECs,28 supporting the notion that either mTECs or DCs are sufficient for Treg cell development in the thymic medulla. These studies conclude that both mTECs and hematopoietic DCs can facilitate Treg cell development. However, it is still unclear 1) how DCs can have a positive role in the selection of self-reactive Treg cells as well as a negative role in the deletion of high-affinity self-reactive thymocytes and, 2) why multiple APCs are involved in generating Treg cells in the thymus. Our findings may give answer to these Questions.
TSLP EXPRESSED BY HASSAL’S CORPUSCLES INSTRUCTS DCs TO SELECT TREG IN HUMAN THYMUS
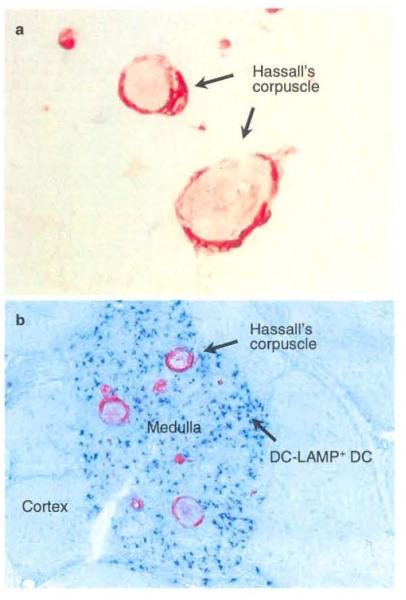
Corpuscular bodies of epithelial cells called Hassall’s corpuscles (HCs) are located in the medulla of the human thymus. After the first description by Arthur Hill Hassall in 1849, it was suggested that HCs represent the “graveyard” for dead thymocytes29,30 and the “privileged” area for the maturation of medullary thymocytes.31 Other studies have provided evidence that HCs are active in cytokine/ growth factor receptor-mediated cell signaling, transcription and metabolism.32-37 Although HCs have been proposed to act in both the removal of apoptotic thymocytes and the maturation of developing thymocytes in the thymus, their actual function has remained unclear. We found that TSLP is selectively expressed by epithelial cells of HCs within the human thymic medulla (Fig. 1a).38 TSLP appears to activate DCs isolated from human thymus, and anatomical analysis by immune histochemistry showed that TSLP expression is associated with an activated subpopulation of mDCs in the thymic medulla (Fig. 1b). Because the thymus is normally not exposed to microbial infections, this raised a question regarding the functions of TSLP and TSLP-activated DCs in the thymus. It has been proposed that TSLP-activated mDCs may play an important role in the selection of self-reactive thymocytes to differentiate into Treg cells because TSLP-mDCs have 1) high expression levels of MHC class-II and the costimulatory molecules CD80/CD86, which are ligands for CD28 and are critical for Treg cell development in the medulla of the human thymus.39,40 2) sparse production of proinflammatory cytokines such as IL-1, IL-6, and IL-12, which inhibit Treg cell development; and 3) the ability to induce robust homeostatic proliferation of naïve CD4+ T cells owing to their unique ability to form strong and prolonged conjugates with autologous CD4+ T cells.41 Therefore, TSLP-activated DCs may use the same mechanisms to provide strong and long-lasting survival signals to self-reactive thymocytes and switch negative selection to a positive selection of Treg cell development.
Fig. 1.
Hassall’s corpuscles express TSLP. a. Epithelial cells in Hassall’s corpuscles express TSLP (red). b. TSLp-expressed HCs (red) co-localize with DC-LAMP+ activated DCs (blue) in the medulla of the human thymus (Original magnifications, a: ×400, b: ×200).
This hypothesis is supported by our experiments showing that TSLP-activated DCs, but not DCs stimulated with or without IL-7, CD40 ligands or poly (I: C), could induce the expansion and differentiation of CD4+CD8−CD25− SP thymocytes into CD4+CD8−CD25+FOXP3+ Treg cells.38 The ability of TSLP-activated DCs to induce Treg cell differentiation was dependent on IL-2 and CD28 signals. Anatomically, CD4+CD25+ thymocytes are exclusively located in the thymic medulla in close association with DC-LAMP+ CD86+-activated DCs and HCs. These results suggest that human Treg cells are generated in the thymic medulla in close association with DCs that seem to be activated by TSLP, which is produced by epithelial cells of HCs.
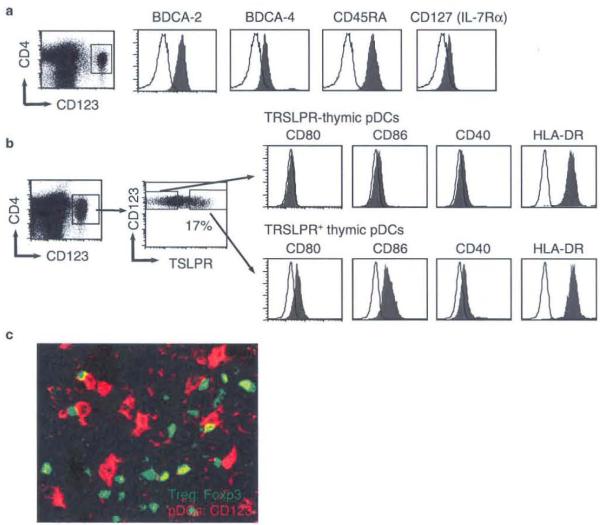
The human thymus contains three distinct populations of DCs induding plasmacytoid DCs (pDCs) and two subsets of conventional myeloid DCs (cDCs), CO11c+CD11b− and the CD11c+CD11b+.42,43 We recently found that a subpopulation of pDCs (~20%) isolated from the human thymus also expressed the TSLP receptor (TSLPR) at the steady state (Fig. 2a, b).44 This pDC subpopulation is located in close association with FOXP3+ T cells in the thymic medulla. In vitro, TSLP conditioned pDCs to produce the chemokines CCL-17 (TARC) and CCL-21 (MDC), which are important in guiding the traffic of immature thymic T cells into the medulla. Furthermore, the TSLP-conditioned pDCs could efficiently induce the generation and expansion of CD4+CD25+FOXP3+ Treg cells from CD4+CD25−FOXP3− thymocytes but not peripheral naïe CD4+ T cells in vitro. Interestingly, the TSLP-pDC-induced Treg cells were IL-10high/TGF-βlow while the TSLP-mDC-induced Treg cells were IL-10low/TGF-βhigh after activation, suggesting that multiple types of APCs in the thymus, such as TSLP-mDCs and TSLP-pDCs, are responsible for the imprinting of functionally distinct subsets of FOXP3+ Treg cells. These data are consistent with our recent observation that the human thymus, peripheral blood, and secondary lymphoid tissues contain two subsets of CD25+FOXP3+ Treg cells based on expression of ICOS and production of IL-10/TGF-β.45 Differentiation of T cells into distinct cell lineages is controlled by several key transcriptional factors that have pivotal roles in determining cell fate during the early stage of lymphoid cell development. These lineage specification factors not only promote a particular T cell fate, but they are also responsible for repressing alternative differentiation pathways. In our experiments, IL-12 and IL-4, which are critical for promoting Th1 or Th2 differentiation, respectively, and proinflammatory cytokines actively repress the fate of CD4+ thymocytes to differentiate into Treg cells, suggesting that the transcriptional factors induced by these cytokines override FOXP3-dependent generation of Treg cells. Both TSLP-mDCs and TSLP-pDCs produce low levels of Th1-promoting cytokines and prointlammatory cytokines. This sterile/aseptic way to activate DCs by TSLP may explain a unique feature that is likely to be essential for the differentiation of FOXP3+ Treg cells in the thymic microenvironment. Although the existence of a unique niche in the thymus has been proposed, its exact nature has remained unclear.22,46 Decoding the molecular signature of DCs, including surface molecules and soluble factors, such as cytokines, that positively or negatively regulate thymic selection, should be accomplished in order to determine mechanisms of Treg differentiation in the thymus. Thus, we speculate that Treg differentiation in the thymus is regulated in a very subtle way by DCs, providing a suitable niche that allows Treg cells to develop.
Fig. 2.
TSLPR+ pDCs co-localize with FOXP3+ Treg in human thymus. a. Phenotype of pDCs enriched from total human thymocytes. b. Subpopulation of thymic pDCs expresses TSLPR at the steady state. c. Double immunofluorescence staining of FOXP3 (green) and CD123 (red) was performed on human thymus. Thymic pDCs co-localize with FOXP3+ cells in the thymic medulla (Magnification: ×40)
In mice, several studies showed that mouse TSLP strongly promotes the differentiation and expansion of Foxp3+ Treg cells in the thymus and periphery.47-49 In fetal thymus organ culture (FTOC) model, mouse TSLP increases expression of Foxp3 as well as Treg. The expression of Foxp3 is inhibited by blocking TSLP.48 A striking reduction of Treg is observed in IL-7Rα−/− mice. However, mice deficient in IL-7 or TSLPR do not exhibit any differences in Treg development, and combined deletion of IL-7 and TSLPR greatly reduced Treg development in the thymus, suggesting Treg cel1s require signals from the IL-7R, but require partially overlapping actions of both IL-7 and TSLP for development of Treg cells in mice.50 The difference in human and mice may be due to species differences, as Hassall’s corpuscle is well developed in the human thymus but poorly in mice.
TSLP IN PERIPHERAL CD4+ T CELL HOMEOSTASIS
T cell homeostasis is a self-regulating process for maintaining the overall size and TCR repertoire of the pool of mature peripheral T cells. This process is essential for enabling the adaptive immune system to respond to a variety of new pathogens and for maintaining immunological memory to previously encountered pathogens.51 T cell homeostasis contributes to the recovery of the peripheral T cell pool after T cell deletion caused by certain viral infections, chemotherapy and radiation treatment.52 It can also maintain peripheral T cell numbers throughout life when production of new naïve T cells has been reduced because of an atrophic thymus. In mice, T cell homeostasis is maintained by T cell survival and expansion triggered by self-antigen and cytokines. Survival and homeostatic expansion of naïve CD4+ and CD8+ T cells requires the interaction between the TCR and self-peptide-MHC complex along with IL-7.51-55 Memory CD4+ T cell homeostasis is regulated by IL-7 and TCR signals, whereas memory CD8+ T cell homeostasis depends on IL-15 and/or IL-7.51-56 In humans, the memory CD4+ T cell population consists of central memory T cells and an effector memory T cell subset.57 Central memory CD4+ T cells express CCR7 and CD62L, which are homing receptors to secondary lymphoid organs, and lack immediate effector function. Effector memory T cells express receptors that permit migration into inflamed tissues and show immediate effector function. Effector memory CD4+ T cells proliferate in response to the combination of IL-7 and IL-15.58 In contrast, naïve CD4+ T cells and central memory CD4+ T cells do not proliferate in response to IL-7 and IL-15. Although monocyte-derived mature DCs endow naïve CD4+ T cells with the capacity to respond to IL-7 and IL-15, this combination facilitates differentiation of central memory CD4+ T cells into effector memory T cells.58
Thus, requirements for homeostatic proliferation of human naïve and central memory CD4+ T cells are still unclear. Because human TSLP-DCs induce very strong antigen-specific expansion of naïve CD4+ T cells,8 we hypothesize that human TSLP-DCs support T cell homeostasis by promoting autologous CD4+ T cell proliferation in the absence of foreign antigens.
We found that TSLP is expressed by crypt epitherial cells of human tonsils, and that its expression is closely associated with DC-LAMP+-activated DCs under normal physiological conditions (Fig. 3).41 Because TSLP-activated DCs have the capacity to induce very strong expansion of naïve CD4+ T cells, we hypothesize that human TSLP expressed by the epithelial cells of peripheral mucosa lymphoid tissues may playa critical role in DC-mediated homeostatic proliferation of naïve and memory T cells. Indeed, we found that only TSLP-activated mDCs, but not resting or IL-7, CD40L, LPS, or poly (I:C)-activated mDCs, could induce a robust and sustained expansion of autologous naïve CD4+ T cells without any exogenous antigens, cytokines, or fetal bovine serum. This unique ability of TSLP-activated DCs correlated with their strong capacity to form prolonged conjugates with the autologous naïve CD4+ T cells, thus providing sustained proliferation and survival signals. The expansion of autologous naïve CD4+ T cells induced by TSLP-activated mDCs displayed features of homeostatic expansion mediated by self-peptide-MHC complexes, 1) it was dependent on MHC class II and the costimulatory molecules CDB0/CDB6, but not on IL-7 or IL-15, 2) it was a polyc1onal expansion as indicated by TCR Vβ repertoire analysis and CFSE-labeling experiments, and 3) the expanded T cells displayed a central memory T cell phenotype (CD45R0+CCR7+CD27+CD62L+) and had the potential to further expand and differentiate into either Th1 or Th2 effector cells.
Fig. 3.
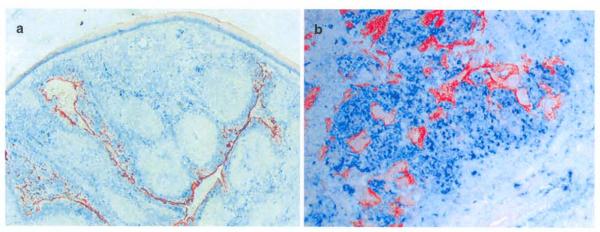
Expression of TSLP in human tonsillar epitherial cells and its association with activated Des. a-b. Double staining of TSLP (red) and DC-LAMP (blue) shows expression of TSLP by crypt epitherial cells. which are in close association with DC-LAMP+ activated DCs (Original magnifications, a: ×100, b: ×200).
Homeostatic proliferation of CRTH2+CD4+ memory T cell is also supported by TSLP-activated autologous DCs. After multiple rounds of stimulation with TSLP-DCs, they maintain the central memory T cell phenotypes and Th2 commitment.59 Under this condition, OX40/OX40L interaction between TSLP-DCs and CRTH2+CD4+T cells, which sustained DC-T cell conjugate formation, contributes to longevity of both DCs and T cells. The survival of T cells by OX40/OX40L was associated with the induction of Bcl-xL and 8c1-2 expression.59-61
A recent study suggests that TSLP constitutively produced by epithelial cell in the gut is critical to the conditioning of mucosal DCs to have a non-inflammatory phenotype and maintain mucosal homeostasis.62-66 In support of this model, decreased TSLP production was found to be associated with Crohn’s disease.63 Further evidence for the involvement of TSLP in maintaining gut homeostasis is the finding that TSLPR-deficient mice showed a more rapid onset and severity of disease in a commensal bacteria-dependent mouse model of inflammatory bowel disease,64 suggesting that one of the signals that induces TSLP expression by epithelial cells may be triggered by commensal bacteria.
SUMMARY
The major cell type responsive to TSLP is mDCs. TSLP represents the only factor that activates mDCs without inducing them to produce Th1- and proinfiammatory cytokines. This sterile/aseptic way of activating mDCs is in contrast to the way TLR ligands activate DCs, and may explain the uniqueness of TSLP-DC function. Under normal physiological conditions, human TSLP appears to play a critical role in CD4+T cell homeostasis in the peripheral mucosa-associated lymphoid tissues41,63 and in the positive selection of Tregs in the thymus.38,44
Footnotes
Conflict of interest: No potential conflict of interest was disclosed.
REFERENCES
- 1.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–8. [PubMed] [Google Scholar]
- 2.Levin SD, Koelling RM, Friend SL, et al. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999;162:677–83. [PubMed] [Google Scholar]
- 3.Sims JE, Williams DE, Morrissey PJ, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. 2000;192:671–80. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujio K, Nosaka T, Kojima T, et al. Molecular cloning of a novel type 1 cytokine receptor similar to the common gamma chain. Blood. 2000;95:2204–10. [PubMed] [Google Scholar]
- 5.Hiroyama T, Iwama A, Morita Y, Nakamura Y, Shibuya A, Nakauchi H. Molecular cloning and characterization of CRLM-2, a novel type I cytokine receptor preferentially expressed in hematopoietic cells. Biochem Biophys Res Commun. 2000;272:224–9. doi: 10.1006/bbrc.2000.2764. [DOI] [PubMed] [Google Scholar]
- 6.Pandey A, Ozaki K, Baumann H, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 7.Park LS, Martin U, Garka K, et al. Cloning of the murine thymic stromal iymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–70. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 9.von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–28. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 10.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–82. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 11.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 12.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–11. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–25. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 15.Chinen T, Volchkov PY, Chervonsky AV, Rudellsky AY. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J Exp Med. 2010;207:2323–30. doi: 10.1084/jem.20101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 17.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J Exp Med. 2001;194:427–38. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liston A, Nutsch KM, Farr AG, et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci U S A. 2008;105:11903–8. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 20.Lee HM, Hsieh CS. Rare development of Foxp3+ thymocytes in the CD4+CD8+ subset. J Immunol. 2009;183:2261–6. doi: 10.4049/jimmunol.0901304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodewald HR, Paul S, Haller C, Bluethmann H, Blum C. Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature. 2001;414:763–8. doi: 10.1038/414763a. [DOI] [PubMed] [Google Scholar]
- 22.Aschenbrenner K, D’Cruz LM, Vollmann EH, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–8. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 23.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–63. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 24.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 25.Proietto AI, Lahoud MH, Wu L. Distinct functional capacities of mouse thymic and splenic dendritic cell populations. Immunol Cell Biol. 2008;86:700–8. doi: 10.1038/icb.2008.63. [DOI] [PubMed] [Google Scholar]
- 26.Román E, Shino H, Qin FX, Liu YJ. Cutting edge: Hematopoietic-derived APCs select regulatory T cells in thymus. J Immunol. 2010;185:3819–23. doi: 10.4049/jimmunol.0900665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spence PJ, Green EA. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci U S A. 2008;105:973–8. doi: 10.1073/pnas.0709071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nat Immunol. 2010;11:512–9. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 29.Blau JN. The dynamic behaviour of Hassall’s corpuscles and the transport of particulate matter in the thymus of the guinea-pig. Immunology. 1967;13:281–92. [PMC free article] [PubMed] [Google Scholar]
- 30.Blau JN, Veall N. The uptake and localization of proteins, Evans Blue and carbon black in the normal and pathological thymus of the guinea-pig. Immunology. 1967;12:363–72. [PMC free article] [PubMed] [Google Scholar]
- 31.Senelar R, Escola MJ, Escola R, Serrou B, Serre A. Relationship between Hassall’s corpuscles and thymocytes fate in guinea-pig foetus. Biomedicine. 1976;24:112–22. [PubMed] [Google Scholar]
- 32.Nishio H, Matsui K, Tsuji H, Tamura A, Suzuki K. Immunolocalization of the mitogen-activated protein kinase signaling pathway in Hassall’s corpuscles of the human thymus. Acta Histochem. 2001;103:89–98. doi: 10.1078/0065-1281-00581. [DOI] [PubMed] [Google Scholar]
- 33.He W, Zhang Y, Deng Y, Kabelitz D. Induction of TCR-gamma delta expression on triple-negative (CD3-4-B-) human thymocytes. Comparative analysis of the effects of IL-4 and IL-7. J Immunol. 1995;154:3726–31. [PubMed] [Google Scholar]
- 34.Le PT, Lazorick S, Whichard LP, Haynes BF, Singer KH. Regulation of cytokine production in the human thymus: epidermal growth factor and transforming growth factor alpha regulate mRNA levels of interleukin 1 alpha (IL-1 alpha), IL-1 beta, and IL-6 in human thymic epithelial cells at a post-transcriptional level. J Exp Med. 1991;174:1147–57. doi: 10.1084/jem.174.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romagnani P, Annunziato F, Manetti R, et al. High CD30 ligand expression by epithelial cells and Hassal’s corpuscles in the medulla of human thymus. Blood. 1998;91:3323–32. [PubMed] [Google Scholar]
- 36.Zaitseva M, Kawamura T, Loomis R, Goldstein H, Blauvelt A, Golding H. Stromal-derived factor 1 expression in the human thymus. J Immunol. 2002;168:2609–17. doi: 10.4049/jimmunol.168.6.2609. [DOI] [PubMed] [Google Scholar]
- 37.Annunziato F, Romagnani P, Cosmi L, et al. Macrophage-derived chemokine and EBI1-ligand chemokine attract human thymocytes in different stage of development and are produced by distinct subsets of medullary epithelial cells: possible implications for negative selection. J Immunol. 2000;165:238–46. doi: 10.4049/jimmunol.165.1.238. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe N, Wang YH, Lee HK, et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 39.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 40.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat immunol. 2005;6:152–62. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe N, Hanabuchi S, Soumelis V, et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5:426–34. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- 42.Bendriss-Vennare N, Barthélémy C, Durand I, et al. Human thymus contains IFN-alpha-producing CD11c-, myeloid CD11c+, and mature interdigitating dendritic cells. J Clin Invest. 2001;107:835–44. doi: 10.1172/JCI11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semi Immunol. 2005;17:304–12. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Hanabuchi S, Ito T, Park WR, et al. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184:2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito T, Hanabuchi S, Wang YH, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–80. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–6. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Besin G, Gaudreau S, Ménard M, Guindi C, Dupuis G, Amrani A. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes. 2008;57:2107–17. doi: 10.2337/db08-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Q, Su H, Knudsen G, Helms W, Su L. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol. 2006;7:6. doi: 10.1186/1471-2172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JY, Lim YM, Park MJ, et al. Murine thymic stromal lymphopoietin promotes the differentiation of regulatory T cells from thymic CD4+CD8−CD25− naive cells in a dendritic cell-independent manner. Immunol Cell Biol. 2008;86:206–13. doi: 10.1038/sj.icb.7100127. [DOI] [PubMed] [Google Scholar]
- 50.Mazzucchelli R, Hixon JA, Spolski R, et al. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112:3283–92. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–56. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 52.Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Semin Immunol. 1997;9:339–46. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 53.Goldrath AW. Maintaining the status quo: T-cell homeostasis. Microbes Infect. 2002;4:539–45. doi: 10.1016/s1286-4579(02)01570-8. [DOI] [PubMed] [Google Scholar]
- 54.Marrack P, Bender J, Hildeman D, et al. Homeostasis of alpha beta TCR+ T cells. Nat Immunol. 2000;1:107–11. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 55.Prlic M, Lefrancois L, Jameson SC. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J Exp Med. 2002;195:F49–52. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seddon B, Mason D. The third function of the thymus. Immunol Today. 2000;21:95–9. doi: 10.1016/s0167-5699(99)01559-5. [DOI] [PubMed] [Google Scholar]
- 57.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 58.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med. 2001;194:1711–9. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YH, Ito T, Wang YH, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–38. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 60.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bc1-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 61.Soroosh P, Ine S, Sugamura K, Ishii N. OX40-0X40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J Immunol. 2006;176:5975–87. doi: 10.4049/jimmunol.176.10.5975. [DOI] [PubMed] [Google Scholar]
- 62.Iliev ID, Spadoni I, Mileti E, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–9. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 63.Rimoldi M, Chieppa M, Salucci V, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–14. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 64.Taylor BC, Zaph C, Troy AE, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–67. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cel1-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–6. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 66.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogellic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]