Abstract
Background
The fractional concentration of exhaled nitric oxide (FeNO) is a noninvasive marker for airway inflammation but requires further study in pre-school children to determine its clinical relevance.
Objective
To determine whether the risk of respiratory tract illnesses (RTI), disease burden and atopic features are related to FeNO in preschool children with moderate-to-severe intermittent wheezing.
Methods
We determined FeNO using the off-line tidal breathing technique in 89 children, 12–59 months old, with moderate-severe intermittent wheezing. Risk of RTI was determined by comparing participants with baseline FeNO >75th percentile (24.4ppb) to those with FeNO ≤75th percentile using Cox regression analysis.
Results
The risk of RTI was significantly higher in children with FeNO >24.4ppb relative to those with lower FeNO values (adjusted RR= 3.8, 95% CI: 1.74–8.22; p=0.0008). FeNO levels >24ppb were associated with a greater number of positive skin tests to aeroallergens (p=0.03), but not with other atopic characteristics or historic parameters of illness burden.
Conclusion
Elevated FeNO in preschool children with moderate-to-severe intermittent wheezing was associated with an increased risk of RTI during a one-year follow-up. In addition, higher FeNO was associated with aeroallergen sensitization.
Keywords: Preschool children, exhaled nitric oxide, respiratory tract illness, wheezing
Introduction
Fractional concentration of exhaled nitric oxide (FeNO) is a noninvasive biomarker reflecting eosinophilic airway inflammation 1. The interest in adding FeNO measurement to the routine assessment of asthma patients derives from the fact that this test requires little effort from the patient, can be performed even by young children, is non-invasive and safe 2.
Levels of FeNO in school age children with mild-moderate persistent asthma correlate with features of atopy and asthma, including peripheral blood eosinophilia, IgE levels, number of positive aeroallergen skin tests and airway hyperresponsiveness 3, as well as disease severity and disease activity 4, 5. Furthermore, monitoring airway inflammation by FeNO levels may be useful in optimizing inhaled steroid dose adjustment both in adults and school age children 6–9.
Data on the utility of FeNO determination in preschool children are limited to two reports suggesting that FeNO may help to diagnose asthma 10, 11. To provide additional evidence on the role of FeNO in characterizing preschool children with moderate-severe intermittent wheezing, we determined whether FeNO levels are associated with the risk of respiratory tract illnesses (RTI), disease burden, and atopic features in this population.
Methods
Study population
The cohort described in these analyses is a subgroup of children 12–59 months of age recruited into the Acute Intervention Management Strategies (AIMS) clinical trial of the Childhood Asthma Research and Education (CARE) Network. AIMS was a multi-center, double-blind, randomized, placebo-controlled, double-dummy, parallel group comparison of three treatment regimens (inhaled corticosteroid, leukotriene receptor antagonist, or β2 agonists alone) used episodically at the early signs of RTIs in children with recurrent episodes of moderate to severe wheezing 12. After IRB approval, written informed consent was obtained from the parent of each participant.
Children 12–59 months of age were included in the trial if they had at least two episodes of moderate to severe wheezing in the context of an upper respiratory infection (URI) over the 12 months prior to screening. At least one episode had to be documented by a health care provider, and at least one episode had to be within 6 months prior to screening. These criteria were established in order to maximize the likelihood of occurrence of moderate-severe wheezing episode(s) during the trial. The term moderate-severe is an overall descriptor of the severity of prior wheezing episodes (as defined by the Inclusion Criteria). In addition, children were considered to have intermittent disease based upon a clinical history of intermittent illness in the prior year and experiencing symptoms (nocturnal cough, daytime cough, wheezing, difficulty breathing, or symptoms interfering with activities) and/or requiring albuterol use on average fewer than 4 days per week in the 2-week observation period prior to the randomization visit. Children were excluded if, during the prior 12 months, they received more than 6 courses of systemic corticosteroids, had more than two hospitalizations for wheezing illnesses, or were treated with controller medications for more than 4 months or within the 2 weeks prior to screening. Comprehensive allergy and asthma questionnaires were administered at enrollment. At the randomization visit, the following studies were performed: allergy prick skin testing to a panel of 8 aeroallergens (house dust mite mix, cat, dog, tree mix, grass mix, weed mix, mold mix, and cockroach mix) and 3 food allergens (milk, egg, and peanut), serum total IgE level, peripheral blood eosinophil counts, assessments of quality of life 13 and completion of the Juniper Pediatric Asthma Caregiver’s Quality of Life Questionnaire 14.
FeNO measurements
110/280 (39.3% of the entire AIMS study cohort) of the children attempted the eNO collection procedure, of which 89 (80.9%) had determinations that were determined acceptable by pre-specified standards (requirements for adequate FeNO collection are described below). The determination of FeNO in only 39.3% of the entire AIMS cohort was due to initiating FeNO collections after the commencement of the trial, as trial recruitment began in February 2004, and FeNO levels measurements began to be obtained in June 2004. FeNO was measured either at the study randomization visit (n=57) or, for subjects enrolled prior to June 2004, before any study medication was administered (n=32). Thus, eNO was only measured for subjects who had not been treated with asthma controller medication for at least 4 weeks preceding the measurement, as it is established that ICS therapy can reduce FeNO levels 5, 15, 16.
FeNO was measured according to American Thoracic Society/European Respiratory Society recommendations 1 by an offline reservoir technique using the NIOX system (Aerocrine AB, Stockholm, Sweden). Children were seated on their mother’s lap breathed through a face mask that was gently placed on the child’s face. The face mask was attached to a 2-way valve that allowed the inspiration of NO-free air to prevent contamination with ambient air. After 5 breaths of NO-free air, 5 breaths of exhaled air were collected in triplicate from each participant in a non-diffusing (polyethylene) 0.5 liter gas collection bag (Hans Rudolph, Inc. Shawnee KS) during quiet and regular tidal breathing. Nasal contamination was prevented by closing the velum by exhaling against 5 cm H2O oral pressure, achieved by a resistor between the collection bag and the expiratory side of the valve. If a child did not cooperate (crying or irregular breathing), or if a leak was detected, the collection was repeated after several minutes. If more than 3 attempts did not allow FeNO collection as specified above, the child was not included in the FeNO sub-study. The FeNO concentration in the bags was analyzed by the NIOX offline analyzer within 3 hours of collection. The final FeNO level represents the average of these triplicates.
Outcomes and statistical analysis
Parameters of demographics, atopic characterization and disease burden (number of emergency visits in past year, number of physician office visits, number days of missed school/daycare in past year, asthma controller medication use in past year, number of courses of oral corticosteroids in past year, and scores on 2 quality of life questionnaires (PACQLQ and Peds QL)) were compared between quartiles of FeNO levels using one-way ANOVA or Kruskal-Wallis tests for continuous data and Chi-square tests for categorical data. These analyses included data from all 89 subjects with acceptable FeNO measurements.
Survival analysis methods (Kaplan-Meier curves and Cox proportional hazards regression) were used to characterize risk of RTI, over one year follow-up period, according to FeNO level. An RTI describes the constellation of respiratory symptoms that, by the parents perception, would be expected to precede and signal the development of an episode of wheezing. As described above, 32 patients had FeNO measurements obtained after the randomization visit. The inclusion of these participants in the survival analysis would cause selection bias toward patients with decreased risk of RTI, since those who had an early RTI were excluded from FeNO measurements at a visit after randomization because they had used study medication. In order to eliminate this bias, these analyses included only data from the 57 subjects with FeNO measurements collected at randomization. Kaplan-Meier curves for time to first RTI were stratified according to baseline FeNO levels dichotomized at the 75th (24.4ppb) percentile. We chose these FeNO levels as the cutoff for our analysis based on the fact that similar FeNO levels were reported by Meyts et al as the upper limit of the 95% CI in preschool children with a history of recurrent wheezing17.
Cox proportional hazards regression was used to estimate the relative risk of RTI associated with high FeNO levels. This analysis was performed with and without adjustment for potential confounders, including API status 18, ≥1 positive aeroallergen skin test, number of courses of oral corticosteroids in the prior year, and number of ED visits for acute wheezing in the prior year. All statistical analyses were performed using SAS Version 9.1 statistical software (SAS Institute, Inc, Cary, NC), while graphs were created using Splus 8.0 (Insightful Corp, Seattle, WA).
Results
Between June and November 2004, FeNO levels were measured successfully in 89 out of 110 children (81%). Reasons for inability to measure FeNO levels were as follows: uncooperative child (n=15), child or parent refused (n=3), or equipment failure (n=3). The sub-group of the uncooperative children included mainly younger children, as 13 out of the 15 children in this sub-group were younger than 36 months.
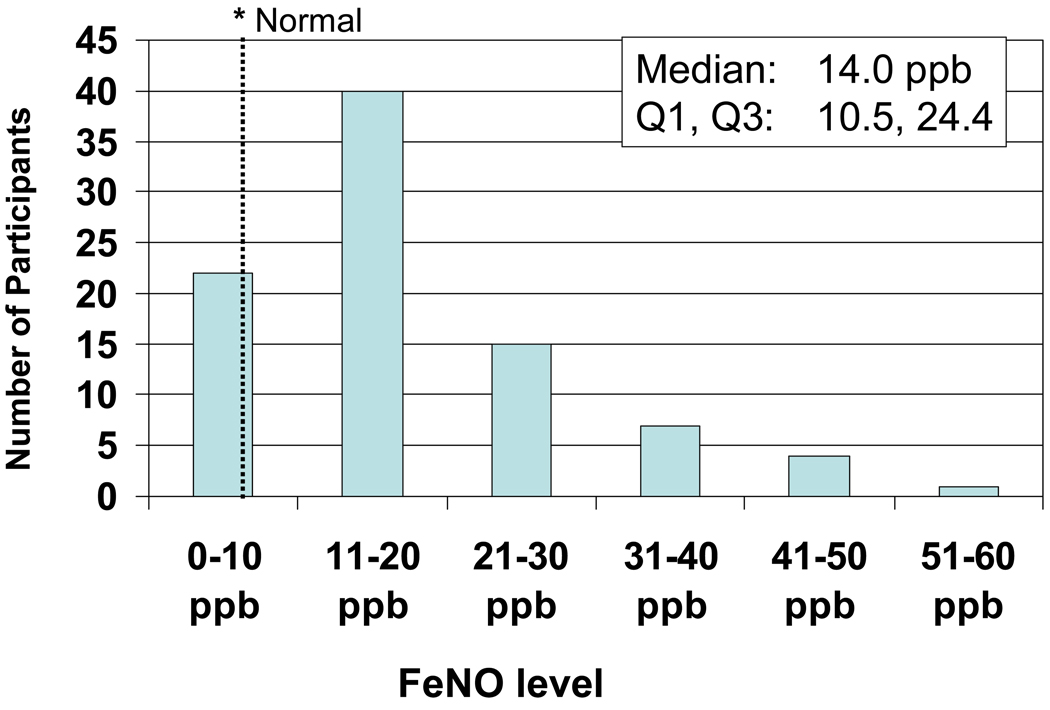
The distribution of FeNO (Fig. 1) is skewed towards the right with a median of 14.0 ppb (Q1,Q3: 10.5, 24.4). FeNO levels were measured with high intra-subject reproducibility, as evident by 86.5% of the children having at least two FeNO measurements within 5ppb or 10% of each other19. Median FeNO levels (Q1,Q3) did not differ between children who were recruited to the study in the spring (15.50 (11.17, 26.67) ppb; n=18), summer (13.03 (9.97, 21.40) ppb; n=51) or fall (17.40 (11.68, 33.48); n=20) (P=0.25). No children were recruited during the winter.
Figure 1.
Distribution of FeNO levels
FeNO levels by quartile were not associated with age or gender (Table 1). Children with FeNO levels in the highest 2 quartiles were significantly more likely to be of Hispanic or Latino ethnicity than those in the lower quartiles (p=0.004). However, FeNO levels did not differ between Caucasians and non-Caucasians. Higher FeNO levels were associated with a greater number of positive skin tests to aeroallergens (p=0.03), but not with other atopic characteristics including number of positive skin tests to food allergens, IgE levels, eosinophils counts, presence of eczema, positive modified Asthma Predictive Index (API) status 18, or parental asthma (Table 1). FeNO levels were not associated with parameters of illness burden during the year prior to enrollment including: number of emergency visits, number days of missed school/daycare, number of courses of oral corticosteroids, or asthma quality of life scores (Table 2).
Table 1.
Relationships between FeNO levels and demographic and atopic characteristics*
| FeNO Quartiles | ||||||
|---|---|---|---|---|---|---|
| Parameter | ≤ 10.5ppb (1st Quartile) N=22 |
10.6ppb to 14.0ppb (2nd Quartile) N=23 |
14.1ppb to 24.4ppb (3rd Quartile) N=21 |
> 24.4ppb (4th Quartile) N=23 |
P-value | |
| Demographics: | ||||||
| Age (y) (mean ± SD) | 3.2 +/− 0.8 | 3.4 +/− 1.1 | 3.4 +/− 0.9 | 3.3 +/− 1.2 | 0.6 | |
| Gender: Male n (%) | 16 (72.7%) | 17 (73.9%) | 13 (61.9%) | 15 (65.2%) | 0.44 | |
| Race: white n (%) | 17 (77.3%) | 16 (69.6%) | 14 (66.7%) | 18 (78.3%) | 0.98 | |
| Minority: Non-white n (%) | 5 (22.7%) | 7 (30.4%) | 7 (33.3%) | 5 (21.7%) | 0.98 | |
| Ethnicity: Hispanic or Latino n (%) | 2 (9.1%) | 5 (21.7%) | 9 (42.9%) | 10 (43.5%) | 0.004 | |
|
Season of Recruitment: |
Spring n (%) | 4 (18.2%) | 3 (13%) | 5 (23.8%) | 6 (26.1%) | |
| Summer n (%) | 13 (59.1%) | 18 (78.3%) | 10 (47.6%) | 10 (43.5%) | 0.12 | |
| Fall n (%) | 5 (22.7%) | 2 (8.7%) | 6 (28.6%) | 7 (30.4%) | ||
| Atopic Characteristics: | ||||||
|
Number of positive skin tests, per child, to aeroallergens (maximum 8) (mean ± SD) |
0.7 +/− 0.9 | 0.7 +/− 1.1 | 1.5 +/− 1.8 | 1.4 +/− 1.6 | 0.03 | |
|
Number of positive skin tests, per child, to food allergens (maximum 3) (mean ± SD) |
0.6 +/− 0.9 | 0.2 +/− 0.5 | 0.6 +/− 0.9 | 0.3 +/− 0.8 | 0.62 | |
|
IgE (kU/L) (geometric means and coefficients of variation) |
44.8 +/− 4.0 | 52.7 +/− 5.1 | 89.9 +/− 4.0 | 55.9 +/− 6.6 | 0.44 | |
|
Eosinophils (%) (geometric means and coefficients of variation) |
3.8 +/− 2.0 | 3.2 +/− 1.9 | 3.8 +/− 1.8 | 5.0 +/− 2.0 | 0.12 | |
| Presence of eczema n (%) | 1 (4.5%) | 2 (8.7%) | 1 (4.8%) | 3 (13%) | 0.39 | |
| Parental asthma n (%) | 7 (31.8%) | 10 (43.5%) | 7 (35%) | 9 (39.1%) | 0.26 | |
| Positive API n (%) | 16 (72.7%) | 13 (56.5%) | 11 (52.4%) | 13 (56.5%) | 0.27 | |
Data are expressed as number of children (% of children in each quartile) except at noted.
Table 2.
Association of FeNO levels with pre-trial illness burden*
| FeNO Quartiles | ||||||
|---|---|---|---|---|---|---|
| Parameter | ≤ 10.5ppb (1st Quartile) N=22 |
10.6ppb to 14.0ppb (2nd Quartile) N=23 |
14.1ppb to 24.4ppb (3rd Quartile) N=21 |
> 24.4ppb (4th Quartile) N=23 |
P-value | |
| Disease burden: | ||||||
| Number of ED visits in past year | 0.8 +/− 1.2 | 1.0 +/− 2.0 | 2.0 +/− 5.2 | 1.1 +/− 1.6 | 0.53 | |
| Number of office visits in past year | 3.1 +/− 1.5 | 4.4 +/− 3.7 | 4.6 +/− 5.1 | 3.6 +/− 3.0 | 0.63 | |
|
Number days of missed school/daycare in past year |
8.9 +/− 21.4 | 4.5 +/− 5.8 | 4.1 +/− 6.6 | 2.9 +/− 4.0 | 0.11 | |
| Medication use in past year: Number (% of children in each quartile) | ||||||
| Inhaled corticosteroid | 10 (58.8%) | 7 (53.8%) | 6 (35.3%) | 8 (57.1%) | 0.62 | |
| Montelukast | 2 (11.8%) | 2 (15.4%) | 1 (5.9%) | 3 (21.4%) | 0.65 | |
|
Any controller (Including oral corticosteroids) |
17 (77.3%) | 13 (56.5%) | 17 (81%) | 14 (60.9%) | 0.56 | |
|
Number of courses of oral corticosteroids in past year: |
0 | 10 (45.5%) | 10 (43.5%) | 6 (28.6%) | 14 (60.9%) | |
| 1 | 5 (22.7%) | 3 (13%) | 5 (23.8%) | 5 (21.7%) | ||
| 2 | 6 (27.3%) | 4 (17.4%) | 6 (28.6%) | 3 (13%) | 0.4 | |
| 3 | 1 (4.5%) | 2 (8.7%) | 4 (19%) | 0 (0%) | ||
| 4+ | 0 (0%) | 4 (17.4%) | 0 (0%) | 1 (4.3%) | ||
| Quality of life Scores: | ||||||
|
PACQLQ overall score (maximum =7) |
6.62 +/− 0.66 | 6.6 +/− 0.6 | 6.5 +/− 0.8 | 6.5 +/− 0.8 | 0.44 | |
|
Peds QL total score (maximum=100) |
0.29 +/− 0.25 | 0.4 +/− 0.4 | 0.4 +/− 0.22 | 0.5 +/− 0.3 | 0.17 | |
Data are expressed as means ± standard deviation, except at noted.
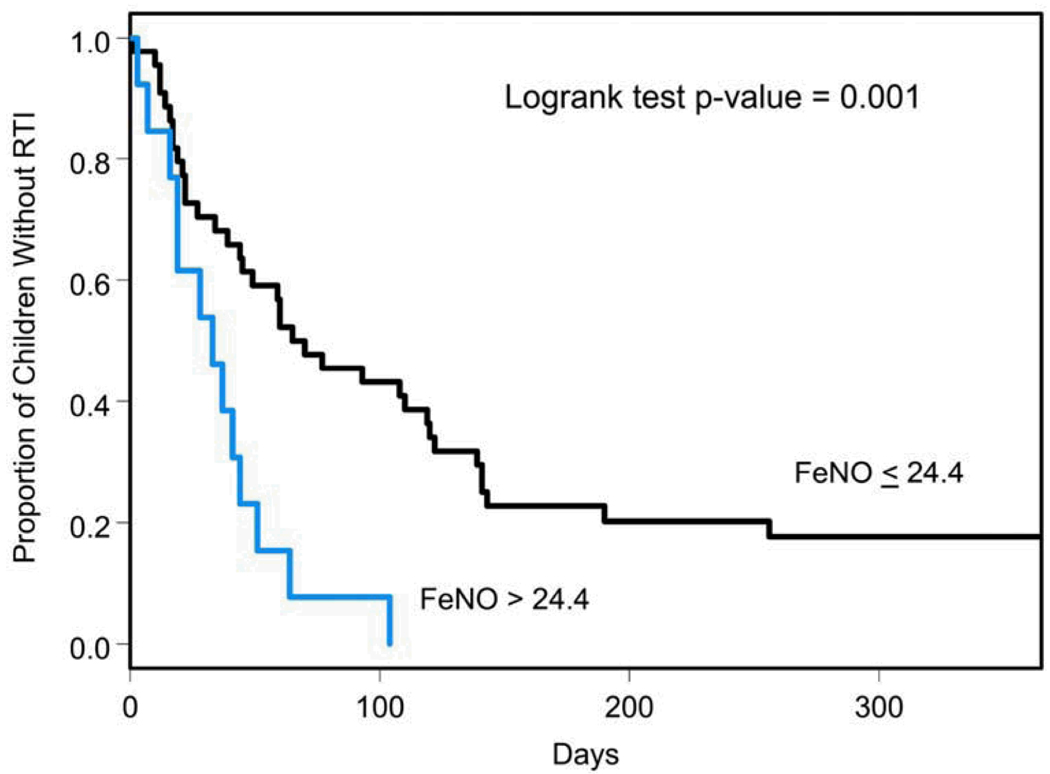
The risk of experiencing an RTI was evaluated in the 57 subjects from whom FeNO was collected at the randomization visit. This subgroup was comparable to the 32 children who had FeNO measurement following randomization except for having a significantly higher proportion of Caucasians (86.0% vs. 50.0% p<0.001). Risk of RTI was significantly higher in children with FeNO levels at or above the 75th percentile (24.4 ppb) relative to those with FeNO levels below 24.4 ppb (RR= 3.0, 95% CI:1.5–6.01; p=0.002) (Figure 2). This elevated risk of RTI was not associated with any other baseline characteristics as described in Tables 1 and 2. Furthermore, after adjustment for potential confounders including API status, ≥1 positive aeroallergen skin test, number of courses of oral corticosteroids in the prior year and number of ED visits for acute wheezing in the prior year, the risk for RTI remained significantly elevated (RR= 3.8, 95% CI:1.74–8.22; p=0.0008). In order to further examine possible confounding between API status and baseline FeNO levels, we repeated this analysis including the interaction between FeNO and API status. The interaction was not significant (p=0.95) and the FeNO effect remained significant within both API strata (RR= 3.56, 95% CI:1.45–8.76 for API positive and RR= 3.74, 95% CI:1.26–11.1 for API negative).
Figure 2.
Kaplan-Meier Estimates of the Time to a First Episode of RTI by FeNO level at randomization
Discussion
We have demonstrated that FeNO levels can be measured effectively and with high reproducibility in the majority of preschool children who participated in this clinical trial. This study investigated the association between baseline FeNO levels and parameters of disease burden, atopic characteristics, and the risk for RTI, in the largest cohort published to date of preschool age children with moderate-severe intermittent wheezing. Although their disease was intermittent in nature, as evidenced by experiencing asthma-like symptoms on approximately 25% of days (or an average of 1.75 days/week) during the trial 20, these children exhibited a high level of pre-trial disease morbidity, reflected by high rates of health care utilization and oral corticosteroid use in the year prior to enrollment 12. High FeNO levels (>24.4 ppb) observed in these pre-school children was associated with a 3-fold greater risk of RTI over the subsequent 12 months. This finding was not due to different patterns or degree of aeroallergen sensitivity, nor was it related to baseline characteristics. To the best of our knowledge, this is the first report on the association between elevated FeNO levels and the constellation of respiratory symptoms that, by the parents perception, would be expected to precede and signal the development of an episode of wheezing.
Among this cohort, the median FeNO level at baseline was 14.0 ppb, a level higher than seen in similarly aged non-atopic healthy controls by the off-line technique (5.9 ±0.7 ppb 21 and 3.9 ppb (3.5–4.2) 22). The differences in FeNO levels between these cohorts might be explained by higher prevalence of atopy and history of recurrent wheezing (one of the major inclusion criteria) in our cohort. While the elevated median FeNO levels measured in our study reflects the high prevalence of atopy in this population, it was not associated with the pre-trial disease activity of these children. The relationship between FeNO levels and markers of atopy 23, 24, or with the diagnosis of atopic dermatitis 25 have been noted previously, and may be partially explained by polymorphisms in the NO synthase gene that were found in association with both elevated FeNO and IgE levels 23.
In addition, a recent study26 supports the proposed association between elevated FeNo levels and history of recurrent wheezing episodes, as pre-school children with recurrent wheezing episodes and stringent asthma predictive indices had significantly higher FeNO when compared to children with chronic persistent or recurrent cough but no history of wheeze. These authors suggest that FeNO is not only a marker for allergic inflammation, but may have a role in the pathogenesis of respiratory disease. Their suggestion could be supported by the finding that higher FeNO levels, measured when asymptomatic and during the first month of life among infants born to atopic and/or smoking mothers, were associated with a significantly increased risk of subsequent respiratory symptoms during the first year of life 27.
The only demographic characteristic that was associated with higher baseline FeNO was Hispanic/Latino ethnicity a finding which has not been reported previously. How this relates to the higher prevalence of asthma among Hispanics in the US 28, 29 is not clear. However, we are cautious in the interpretation of this finding due to the small number of Hispanic children in our cohort; more studies are needed for confirmation. Ethnic differences in FeNO levels for ”normal” children have been reported previously: Asian children, especially boys, have significantly higher FeNO levels compared to Caucasians 30. These differences may be related to genetic variations in the neuronal nitric oxide synthases gene 31, 32. It was also reported by Kovesi et al, that African American children have higher FeNO levels then Caucasian children 31, but the small sample size of African Americans in Kovesi’s study results in wide confidence intervals which make definitive conclusion inappropriate.
In summary, in this cohort of preschool children with moderate-severe intermittent wheezing, high FeNO levels at randomization were associated with an increased likelihood of a subsequent RTI. Furthermore, in this cohort of children with moderate-severe intermittent wheezing, FeNO levels were elevated compared to reported norms in healthy preschool children. FeNO levels in asymptomatic children were related to the degree of aeroallergen sensitization and Hispanic ethnicity, but not with other atopic or demographic characteristics. Our results suggest that FeNO levels might be used as an additional tool in the assessment and development of treatment strategies in young children with moderate-severe intermittent wheezing. The detection of a high FeNO level may serve as a clinical indicator for a child being at greater risk for an RTI over the next 12 months. Whether the use of asthma controller medications or other strategies are effective in the prevention of wheezing episodes which are commonly triggered by RTI in children with elevated FeNO levels, requires further study.
Acknowledgments
Supported by: Grants 5U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064307, 5U10HL064305, 5U10HL064313 from the National Heart, Lung, and Blood Institute.
Footnotes
Authors’ contribution to the manuscript:
AB, LBB: Design of study, interpretation of data, writing of the manuscript
DTM, BRP: Design of study, statistical analysis, interpretation of data, revision of manuscript
RSZ, LMT, RCS: Design of study, interpretation of data, revision of manuscript
References
- 1.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 2.Buchvald F, Bisgaard H. FeNO measured at fixed exhalation flow rate during controlled tidal breathing in children from the age of 2 yr. Am J Respir Crit Care Med. 2001;163:699–704. doi: 10.1164/ajrccm.163.3.2004233. [DOI] [PubMed] [Google Scholar]
- 3.Strunk RC, Szefler SJ, Phillips BR, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112:883–892. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–1225. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covar RA, Szefler SJ, Martin RJ, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J Pediatr. 2003;142:469–475. doi: 10.1067/mpd.2003.187. [DOI] [PubMed] [Google Scholar]
- 6.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 7.Zacharasiewicz A, Wilson N, Lex C, et al. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med. 2005;171:1077–1082. doi: 10.1164/rccm.200409-1242OC. [DOI] [PubMed] [Google Scholar]
- 8.Pijnenburg MW, Bakker EM, Hop WC, De Jongste JC. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2005;172:831–836. doi: 10.1164/rccm.200503-458OC. [DOI] [PubMed] [Google Scholar]
- 9.Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231–237. doi: 10.1164/rccm.200610-1427OC. [DOI] [PubMed] [Google Scholar]
- 10.Malmberg LP, Pelkonen AS, Haahtela T, Turpeinen M. Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax. 2003;58:494–499. doi: 10.1136/thorax.58.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avital A, Uwyyed K, Berkman N, Godfrey S, Bar-Yishay E, Springer C. Exhaled nitric oxide and asthma in young children. Pediatr Pulmonol. 2001;32:308–313. doi: 10.1002/ppul.1124. [DOI] [PubMed] [Google Scholar]
- 12.Bacharier LB, Phillips BR, Bloomberg GR, et al. Severe intermittent wheezing in preschool children: a distinct phenotype. J Allergy Clin Immunol. 2007;119:604–610. doi: 10.1016/j.jaci.2006.12.607. [DOI] [PubMed] [Google Scholar]
- 13.Varni JW, Burwinkle TM, Rapoff MA, Kamps JL, Olson N. The PedsQL in pediatric asthma: reliability and validity of the Pediatric Quality of Life Inventory generic core scales and asthma module. J Behav Med. 2004;27:297–318. doi: 10.1023/b:jobm.0000028500.53608.2c. [DOI] [PubMed] [Google Scholar]
- 14.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Qual Life Res. 1996;5:27–34. doi: 10.1007/BF00435966. [DOI] [PubMed] [Google Scholar]
- 15.Beck-Ripp J, Griese M, Arenz S, Koring C, Pasqualoni B, Bufler P. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. Eur Respir J. 2002;19:1015–1019. doi: 10.1183/09031936.02.01582001. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YG, Lee MY, Yang KD, Chu DM, Yuh YS, Hung CH. A single dose of nebulized budesonide decreases exhaled nitric oxide in children with acute asthma. J Pediatr. 2001;139:433–437. doi: 10.1067/mpd.2001.116295. [DOI] [PubMed] [Google Scholar]
- 17.Meyts I, Proesmans M, Van Gerven V, Hoppenbrouwers K, De Boeck K. Tidal off-line exhaled nitric oxide measurements in a pre-school population. Eur J Pediatr. 2003;162:506–510. doi: 10.1007/s00431-003-1215-x. [DOI] [PubMed] [Google Scholar]
- 18.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 19.Nelson KA, Lee P, Yan Y, Hodgdon K, Schootman M, Strunk RC. Effect of peak expiratory flow rate measurement on exhaled nitric oxide levels in children with asthma. J Allergy Clin Immunol. 2007;119:245–246. doi: 10.1016/j.jaci.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Bacharier LB, Phillips BR, Zeiger RS, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008 doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baraldi E, Dario C, Ongaro R, et al. Exhaled nitric oxide concentrations during treatment of wheezing exacerbation in infants and young children. Am J Respir Crit Care Med. 1999;159:1284–1288. doi: 10.1164/ajrccm.159.4.9807084. [DOI] [PubMed] [Google Scholar]
- 22.Daniel PF, Klug B, Valerius NH. Exhaled nitric oxide in healthy young children during tidal breathing through a facemask. Pediatr Allergy Immunol. 2007;18:42–46. doi: 10.1111/j.1399-3038.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 23.Malmberg LP, Petays T, Haahtela T, et al. Exhaled nitric oxide in healthy nonatopic school-age children: determinants and height-adjusted reference values. Pediatr Pulmonol. 2006;41:635–642. doi: 10.1002/ppul.20417. [DOI] [PubMed] [Google Scholar]
- 24.Jouaville LF, Annesi-Maesano I, Nguyen LT, Bocage AS, Bedu M, Caillaud D. Interrelationships among asthma, atopy, rhinitis and exhaled nitric oxide in a population-based sample of children. Clin Exp Allergy. 2003;33:1506–1511. doi: 10.1046/j.1365-2222.2003.01800.x. [DOI] [PubMed] [Google Scholar]
- 25.Dinakar C, Craff M, Laskowski D. Infants and toddlers without asthma with eczema have elevated exhaled nitric oxide levels. J Allergy Clin Immunol. 2006;117:212–213. doi: 10.1016/j.jaci.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 26.Moeller A, Diefenbacher C, Lehmann A, et al. Exhaled nitric oxide distinguishes between subgroups of preschool children with respiratory symptoms. J Allergy Clin Immunol. 2008;121:705–709. doi: 10.1016/j.jaci.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Latzin P, Kuehni CE, Baldwin DN, Roiha HL, Casaulta C, Frey U. Elevated exhaled nitric oxide in newborns of atopic mothers precedes respiratory symptoms. Am J Respir Crit Care Med. 2006;174:1292–1298. doi: 10.1164/rccm.200606-782OC. [DOI] [PubMed] [Google Scholar]
- 28.Hunninghake GM, Weiss ST, Celedon JC. Asthma in Hispanics. Am J Respir Crit Care Med. 2006;173:143–163. doi: 10.1164/rccm.200508-1232SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115:1254–1260. doi: 10.1542/peds.2004-0897. [DOI] [PubMed] [Google Scholar]
- 30.Wong GW, Liu EK, Leung TF, et al. High levels and gender difference of exhaled nitric oxide in Chinese schoolchildren. Clin Exp Allergy. 2005;35:889–893. doi: 10.1111/j.1365-2222.2005.02263.x. [DOI] [PubMed] [Google Scholar]
- 31.Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest. 2008;133:169–175. doi: 10.1378/chest.07-1177. [DOI] [PubMed] [Google Scholar]
- 32.Leung TF, Liu EK, Tang NL, et al. Nitric oxide synthase polymorphisms and asthma phenotypes in Chinese children. Clin Exp Allergy. 2005;35:1288–1294. doi: 10.1111/j.1365-2222.2005.02342.x. [DOI] [PubMed] [Google Scholar]