Abstract
Self-reported ratings of sleep quality and symptoms of poor sleep have been linked to increased risk of coronary heart disease (CHD), Type 2 diabetes, and hypertension with recent evidence suggesting stronger associations in women. At this time, the mechanisms of action that underlie these gender-specific associations are incompletely defined. The current study examined whether gender moderates the relation of subjective sleep and sleep-related symptoms to indices of inflammation, coagulation, insulin resistance (IR), and psychosocial distress, factors associated with increased risk of cardiovascular and metabolic disorders. Subjects were 210 healthy men and women without a history of sleep disorders. The Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality and frequency of sleep symptoms. In multivariate-adjusted models, overall poor sleep quality, more frequent problems falling asleep (≥ 2 night/week), and longer periods to fall asleep (≥ 30 min) were associated with greater psychosocial distress, higher fasting insulin, fibrinogen and inflammatory biomarkers, but only for women. The data suggest that subjective ratings of poor sleep, greater frequency of sleep-related symptoms, and longer period of time to fall asleep are associated with a mosaic of biobehavioral mechanisms in women and that these gender-specific associations have direct implications to recent observations suggesting gender differences in the association between symptoms of poor sleep and cardiovascular disease.
Keywords: sleep disturbance, inflammation, coagulation, adiposity, psychosocial distress, gender differences
1. Introduction
Recent evidence from large epidemiological studies has suggested that poor sleep and sleep-related problems are more strongly associated with poor health outcomes in women than in men. For example, in one study, subjective symptoms of disturbed sleep were associated with a greater risk of cardiovascular disease (CVD) in older women than in men and these associations persisted even after adjustments for potential confounders (Newman, et al., 2000). Similar findings were observed by researchers from the Whitehall II community study who showed that short sleep duration was associated with both prevalence and incidence of hypertension with these associations found to be significant only for women (Cappuccio, et al., 2007). These novel, but important, gender differences suggest that disturbances in sleep quantity and quality may be differentially associated with putative mechanisms implicated in the onset and progression of cardiovascular and metabolic diseases in men and women. To date, no study has examined mechanisms of actions that could explain the observed gender-specific differences in the relation of sleep to disease outcomes.
Researchers have postulated a number of pathways whereby poor sleep quantity and quality can contribute to poor cardiovascular and metabolic health. One set of hypotheses posit that poor sleep quality is associated with physiological perturbations such as inflammation, coagulation and insulin resistance (IR), mechanisms that alone or in conjunction are implicated in both coronary heart disease (CHD) and Type 2 diabetes (e.g., Dandona, et al., 2003; Libby & Ridker, 2006). A second set of hypotheses suggests that the increased risk of CHD and Type 2 diabetes associated with poor sleep reflects greater psychosocial distress as indexed by elevations in hostility, anger and depression (Ford & Cooper-Patrick, 2001; Neckelmann, et al., 2007; Pilcher, et al., 1997), and lower levels of perceived social support (Akerstedt, et al., 2002; Brummett, et al., 2006). The indicators of psychosocial distress, alone or in combination, have been shown to be associated with increased risk of cardiovascular and metabolic disorders (Boyle, et al., 2007; Krantz & McCeney, 2002). Thus, evidence linking sleep quality and quantity to putative mechanisms provides a framework for understanding how sleep incurs a heightened risk for poor health.
In testing the above described hypotheses, most studies have employed clinical samples of individuals with a diagnosis of clinical sleep disorders. For example, there is a large body of evidence linking obstructive sleep apnea to increases in IR and biomarkers of inflammation. (Parati, et al., 2007; Vgontzas, et al., 2005) Considerably fewer studies, however, have employed non-clinical samples that are composed of individuals whose reported sleep disturbances do not reach clinical criteria. Although few in number, results have indicated that elevations in biomarkers of inflammation (Friedman, et al., 2005; McDade, et al., 2006) and coagulation (von Kanel, et al., 2006) as well as IR and psychosocial distress (Brummett, et al., 2006) are associated with individual differences in sleep quality and quantity.
Due to small sample size and/or the exclusion of women, statistical evaluation of the proposed hypothesis that gender moderates the relation of sleep to putative mechanisms has not been conducted. One recently published study, however, did suggests that gender is a characteristic that could modify the relation of poor sleep to inflammatory biomarkers, but due to small number of men and women in the sample, the investigators indicated that they were not able to test this association (Hong, et al., 2005). In light of the evidence from epidemiological studies for gender-specific association in sleep and healthy outcomes, and the fact that individual differences in sleep quality and quantity have been associated with putative mechanisms of cardiovascular and metabolic disease, the current observational study examined whether gender significantly moderates the relation of symptoms of disturbed sleep to markers of psychosocial distress and pathophysiological mechanisms in a large sample of men and women (Trenell, et al., 2007). Guided by previous observations, selection of biological and psychosocial markers was based on prior evidence indicating that elevations in levels of inflammatory (C-reactive protein (CRP) and interleukin (IL)-6) (Pradhan, et al., 2001; Ridker & Morrow, 2003), coagulation (fibrinogen) (Fibrinogen Studies, 2007; Temelkova-Kurktschiev, et al., 2002) and metabolic (fasting insulin, glucose and insulin resistance) (Arad, et al., 2001; Yanase, et al., 2004) biomarkers, as well as indicators of psychosocial distress (hostility, anger, depression and social support) (Krantz & McCeney, 2002; Rozanski, et al., 1999; Rozanski, et al., 2005), are associated with an increased risk of cardiovascular and metabolic disorders. It was hypothesized that self-reported poor sleep quality and severity of sleep-related symptoms would be more strongly associated with elevated levels of inflammatory (CRP and IL-6), coagulation (fibrinogen), metabolic (fasting insulin, glucose and insulin resistance) biomarkers and indicators of psychosocial distress (hostility, anger, depression and social support) in women than in men.
2. Materials and Methods
2.1 Participants
The study sample consisted of 210 healthy, nonsmoking men (n = 115) and women (n = 95) recruited from the surrounding community using community flyers and advertisements placed in local newspapers as well as online announcements on the Duke Clinical Trials website. Interested individuals were asked to contact the laboratory where they were initially screened for inclusion criteria. Individuals were included if they were non-smokers and in good health, defined as having no history of chronic medical illness, no acute medical conditions or injuries, and no current or recent (< 1 month) use of medications, whether over-the-counter or prescribed. Individuals taking low-dose aspirin on a regular basis were excluded. Premenopausal women were excluded if they reported use of oral contraceptives within the previous 6 months and postmenopausal women were excluded if the reported use of hormone replacement therapy within the previous 6 months. Women suspected of being perimenopausal (i.e., irregular menstrual cycle during the previous 6 months) were excluded. Men were excluded if they were currently or had been treated with androgen replacement therapy 6 months prior to participation. The Institutional Review Board (IRB) of Duke University Health System (DUHS) approved this study and appropriate informed consent was obtained from participants prior to their participation.
Procedures
Subjects reported to the laboratory between 8:00 and 9:00 AM after fasting for approximately 12 hours. Premenopausal women were scheduled during the follicular phase (days 5–10) of the menses to minimize the effects of menstrual hormone fluctuation. Blood samples were collected in chilled EDTA tubes, spun and stored at −80°C until assays were conducted. Blood related measures included CRP, IL-6, fibrinogen, fasting insulin and glucose. Adiposity was measured by calculating body mass index (BMI) from height and weight.
After blood draw, subjects completed the Pittsburgh Sleep Quality Index (PSQI) (Buysse, et al., 1989). The PSQI is a well established and validated paper-and-pencil questionnaire that assesses over-all sleep quality and sleep related symptoms over the previous 1-month period. The 19-items yield 7-component scores that reflect the frequency of sleep problems in the areas of: subjective sleep quality; sleep latency; sleep duration; habitual sleep efficiency; sleep disturbance; use of sleep medication and daytime dysfunction. The sum of the 7-components yields one global score that ranges from 0 to 21 with poor sleep quality associated with higher scores. A PSQI global score of 5 and above has a diagnostic sensitivity of 89.6 and specificity of 86.5 in differentiating “poor sleepers” from “good sleepers” (e.g., PSQI global score < 5) subjects (Buysse, et al., 1989). Subjects also provided the average number of minutes it takes to fall asleep and the average number of hours slept.
In addition to the PSQI, subjects completed the Beck Depression Inventory (BDI) (Beck, et al., 1988), to assess severity of depressive symptoms, the Buss-Perry Aggression Inventory (BPAI) (Buss & Perry, 1992) used to assess anger, the Cook-Medley Hostility (CMHO) Inventory (Cook & Medley, 1954) used to assess hostility, and the Interpersonal Social Evaluation List (ISEL) (Cohen, et al., 1985) used to assess perceived level of social support.
Blood Assays
Plasma glucose was measured using a hexokinase-coupled reaction and plasma insulin was measured using a solid phase radioimmunoassay procedure. IR was determined using the Homeostatic Model assessment (HOMA) method as described by Matthews et al (Matthews, et al., 1985). The HOMA-IR index was calculated as the product of fasting glucose (G0) (mmol/liter) and fasting insulin (I0) (μU/liter) values divided by the constant 22.5: HOMA = (G0 x I0)/22.5.
CRP was measured in duplicate with an ultrasensitive, enzyme-linked, immunometric latex-enhanced assay (Diagnostic Product Corporation, Los Angeles, CA) using purified protein and polyclonal anti-CRP antibodies (Macy, et al., 1997). This system has a lower detection level of < 0.10 mg/L with coefficients of variation ranging from 6.6% to 9.9%. IL-6 was assessed using an enzyme-linked immunosorbent assay (ELISA) kit (Peirce-Endogen, Rockford, IL). Samples were assayed in duplicate and IL-6 concentrations were derived from a standard curve. The detectable limit of plasma IL-6 was < 1 pg/ml and the intra- and inter-assay coefficients of variation were < 5% and < 10%, respectively. Lastly, plasma fibrinogen was quantified using a modified Clauss method and MDA coagulometer (Organon Teknika, Durham, NC).
Analyses
Data analyses were performed using multivariate and univariate techniques. To control for the number of tests, initial test of the hypothesis examined the interaction between gender and sleep variables in multivariate models. Multivariate analysis of covariance (MANCOVA) was performed on inflammatory biomarkers (CRP, IL-6), metabolic indicators (insulin, glucose, IR) and coagulation (fibrinogen) were age, BMI, exercise regularly (Y/N), alcohol use and use of sleep medication as covariates. Use of sleep medication was assessed using a single item on the PSQI (How often have you taken medication (prescribed or “over the counter”) to help you sleep?) with responses ranging from not during the past month, less than once a week, once or twice a week, and three or more times a week. For the study sample, 92% reported no use of medications during the past month, with 6% reporting ‘less than once a week’, 0.5% reporting ‘once or twice a week’ and 1.5% reporting three or more times a week. Given this distribution, this variable was dichotomized and included in the analyses as a yes/no (0/1) coded variable1.
The multivariate coefficient of the interaction was evaluated using Wilk’s lambda. If the interaction was not significant, multivariate analyses were performed using a main effects only model. As levels of inflammatory biomarkers and HOMA-IR were positively skewed, a logarithmic transformation was applied to these variables prior to analyses.
Analysis was also conducted to test the quadratic effect of sleep duration (number of hours of sleep) and its interaction with gender. This specific quadratic trend was examined in light of preliminary evidence suggesting that the relation of sleep duration to poor health is nonlinear with the greatest risk apparent among individuals with the shortest and longest sleep duration (Gangwisch, et al., 2005). To test this quadratic trend, we squared the sleep duration component of the PSQI and included that effect in the model along with the gender X (sleep duration)2 interaction. In the case where neither the two-way nor quadratic interaction is significant, we included only main effects in the multivariate analysis. Analyses controlling for age, exercise status, BMI and use of sleep medication failed to reveal a quadratic trend among males, females or the group as a whole for any of the outcome variables. No statistical results are reported given the lack of a quadratic effect
The primary variable of interest was the total score on the PSQI, which reflects the sum of all components. Analyses of components scores and other sleep variables were considered secondary analyses, and thus are more exploratory. Nevertheless, the consistency of the findings using component scores, and particularly scores on the sleep latency component, argues for reporting of the findings.
3. Results
Additional sample characteristics are presented in Table 1. Of the 210 subjects (age range: 18–65 yr.), 45% were women; 45% were minorities (i.e., African-American, Hispanic, Asian or other); 95% reported some college education or more; 30% were married or living with a significant other; 45% had annual household income of < $40,000; and 80% reported that they exercised on a regular basis. Men showed significantly higher levels of resting systolic blood pressure (SBP) and fasting glucose and higher hostility scores, whereas women had significantly higher fibrinogen and greater depression and higher levels of perceived social support.
Table 1.
Sample characteristics. Means (SD) are presented unless otherwise indicated.
| Men | Women | |
|---|---|---|
| Age, y | 28 (9.7) | 30 (9.5) |
| BMI, kg/m2 | 25.3 (4.6) | 25.1 (4.9) |
| Resting SBP, mmHg * | 116.6 (15.2) | 105.5 (12) |
| Resting DBP, mmHg | 63.4 (8.5) | 62.3 (8.1) |
| Total Cholesterol, mg/dl | 168.4 (36) | 169 (37) |
| Insulin, uIU/mL | 7.4 (4.4) | 8.5 (8.2) |
| Glucose, mg/dL* | 88 (9.2) | 85 (8.3) |
| Fibrinogen, mg/dL* | 204.3 (56) | 227.2 (51.1) |
| Interleukin-6, pg/mLa | 1.34 (.42–6.76) | 1.27 (.41–5.56) |
| C-Reactive Protein, mg/La | 1.30 (.16–6.46) | 1.35 (.15–6.69) |
| Insulin Resistance (units)a | 1.64 (.50–5.53) | 1.84 (.47–5.12) |
| Psychosocial scales | ||
| Pittsburgh Sleep Quality Index | 4.5 (2.4) | 3.9 (2.3) |
| Cook Medley Hostility* | 20 (7.4) | 17.6 (8.3) |
| Buss-Perry Anger | 15 (5.3) | 14.1 (4.9) |
| Beck Depression Inventory* | 3.5 (3.5) | 5.1 (6.0) |
| Interpersonal Social Evaluation List* | 37.3 (6.4) | 39.5 (7.1) |
p < .05 for test of gender differences;
median with 95% confidence interval
Men and women did not significantly differ in their subjective ratings of over-all sleep quality or PSQI components with the exception of daytime disturbance [F(1, 208) = 4.64, p = .03] with more men (19%) reporting greater frequency of daytime disturbance (e.g., 2 days/week or more) than women (6.3%). Age was not associated with either total PSQI score or with any sleep symptoms except daytime disturbance, whereby increasing age was associated with less frequent problems with daytime sleepiness [r = −0.26, P < .001]. Self-reported sleep quality and symptoms of sleep problems did not differ by ethnicity.
3. 1. Psychosocial Distress
Results indicated that the relation of PSQI to indicators of psychosocial distress was moderated by gender, Gender by PSQI interaction [Wilk’s λ = .94, F(4, 192) = 3.19, p = .015]. Post-hoc analyses revealed gender-related differences in the relation of PSQI total score to hostility [F(1, 201) = 4.08, p < .05], depression [F(1, 201) = 8.59, p < .01] and anger [F(1, 201) = 3.81, p < .05] but not social support [F(1, 201) = .04, ns]. Women with poor sleep quality reported higher levels of hostility [b = 1.19, p = .003], anger [b = .74, p = .002] and severity of depressive symptoms [b = 1.20, p < .001]. In men, self-reported sleep quality was similarly and significantly associated with greater severity of depressive symptoms [b = .50, p < .001] but the strength of this association was significantly weaker than that observed in women. Although we failed to detect gender-related differences in the relation of sleep quality to perceived social support, lower levels of perceived social support was significantly associated with poor sleep quality in men [b = −0.66, P = .009] and not in women [b = −0.42, ns], an observation that has been previously reported in men (Akerstedt, et al., 2002).
To explore whether the above associations were due to specific sleep symptoms, analyses were conducted to examine the interaction of gender with components of the PSQI. Results revealed a significant sleep latency by Gender interaction [Wilk’s λ = .88, F(8, 380) = 3.80, p = .002] with significant gender differences in the relation of sleep latency to anger [F(2, 199) = 3.80, p = .02] and depression [F(2, 199) = 8.44, p = .0002]. For women, greater frequencies of episodes of difficulty falling asleep were associated with greater levels of anger and severity of depressive symptoms. For men, sleep latency was not associated with any of the psychosocial factors. No other PSQI components effects were detected.
We also explored whether reported number of hours slept per night (duration) and number of minutes it took to fall asleep (MTFA) predicted psychosocial distress. Neither the main effect for sleep duration nor its interaction with gender predicted psychosocial distress. In contrast, the MTFA by Gender interaction [Wilk’s λ = .93, F(4, 195) = 3.40, p = .01] and the main effect of time to fall asleep [Wilk’s λ = .91, F(4, 196) = 5.02, p < .001] were significant. For women, longer MTFA was associated with greater hostility [b = .12, t = 2.17, p = .04], depression [b = .11, t = 3.02, p < .01] and anger [b = .07, t = 2.26, p = .03] but not social support. For men, lower social support was associated with longer MTFA [b = −.12, t = −2.43, p = .03]1.
Adiposity
Results indicated a significant PSQI by Gender interaction [F(1, 201) = 4.08, p = .03] with poor sleep quality associated with higher BMI in women [b = .50, t = .2.15, p = .03) but not men [b = −0.14, t = −0.73, ns]. Additional analyses showed that gender-related differences in the relation of BMI to daytime dysfunction, with women who reported more frequent episodes of daytime dysfunction being heavier. For men, BMI and daytime dysfunction was not associated.
Inflammatory Biomarkers
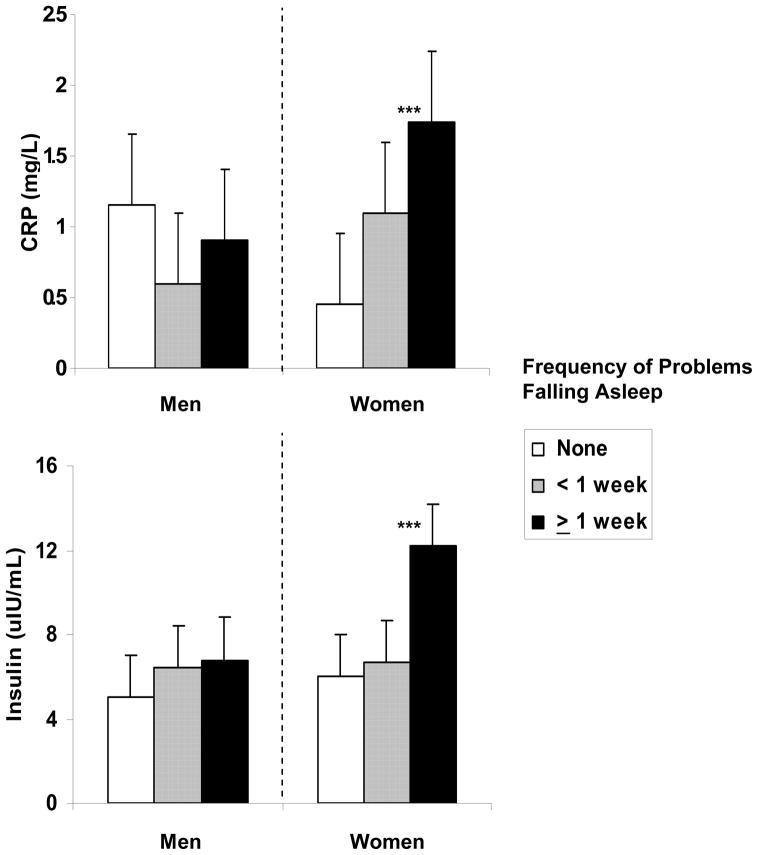
A significant PSQI by Gender interaction [Wilk’s λ = .95, F(2, 184) = 4.40, p = .01] was observed for biomarkers of inflammation with the interaction significantly predicting both CRP [F(1, 185) = 6.68, p = .01] and IL-6 [F(1, 185) = 4.32, p = .04]. The PSQI score was significantly and positively associated with CRP [b = .11, t = 2.23, p = .03) and IL-6 [b = .07, t = 2.25, p = .03] in women but not men [CRP: b = −0.05, ns; IL-6: b = −0.16, ns).
Additional analyses examined whether CRP and IL-6 were more closely associated with specific symptoms of poor sleep. CRP and IL-6 were significantly predicted by the sleep latency component by Gender [Wilk’s λ = .94, F(4, 364) = 2.52, p = .035] and sleep disturbance component by Gender [Wilk’s λ = .95, F(4, 364) = 2.56, p = .038] such that more frequent bouts of difficulty falling asleep and greater frequency of sleep disturbances were associated with higher levels of CRP and IL-6 in women, but not men.
Average number of hours slept, either as a main effect or interaction with gender, was not associated with inflammatory biomarkers. The time to fall asleep (TFA) by Gender interaction, however, was significant [Wilk’s λ = .97, F(2, 187) = 3.12, p < .05] such that among women, longer periods of TFA were associated with IL-6 [b = .001, t = 2.40, p = .02] and CRP [b = .01, t = 1.97, p = .05]. TFA was not associated with inflammatory biomarkers in men.
The total PSQI [F(1, 193) = 1.17, ns] or the PSQI by Gender interaction [F(1, 192) = 2.40, ns] did not predict fibrinogen levels. Results did indicate a significant sleep latency by Gender interaction [F(3, 192) = 3.53, p = .01] with women who reported more frequent occurrences of difficulty falling asleep having higher fibrinogen [adjusted mean (SEM) = 231.0 (15) mg/dl] than those reporting no problems falling asleep [adjusted mean (SEM) = 210.8 (15) mg/dl)]. For men, frequency of difficulty falling asleep was not related to fibrinogen [211.6 (16) vs. 196.3 (15), adjusted means (SEM) for men with and without problems falling asleep, respectively]. Analyses indicated a significant main effect for estimated hours of sleep, with higher fibrinogen levels associated with longer sleep periods [b = 6.83, t = 2.23, p = .02].
Insulin, Glucose and Insulin Resistance
Results failed to indicate a multivariate main effect for PSQI total [Wilk’s λ = .98, F(3, 199) = 1.09, ns] or its interaction with gender [Wilk’s λ = .98, F(3, 198) = 1.13, ns]. Analyses using the PSQI components, however, revealed a significant multivariate effect for the interaction between sleep latency and Gender [Wilk’s λ = .87, F(9, 472) = 3.11, p = .001] with gender moderating the relation of sleep latency to fasting insulin [F(3, 196) = 6.42, p < .001] but not to glucose or HOMA-IR. Relative to women who reported either no problems during the past month or less than once a week, women with more frequent problems falling asleep had higher fasting insulin. For men, fasting insulin was not associated with frequency of difficulties falling asleep.
There was also a significant multivariate main effect for sleep latency [Wilk’s λ = .90, F(9, 476) = 2.39, p = .01] with a significant univariate effect for HOMA-IR [F(3, 199) = 4.79, p = .004]. Individuals reporting problems falling asleep once or more times a week showed greater IR [mean (SEM) = 1.96 (.25)] relative to those with no problems or less frequent (< 1 /week) occurrences of difficulty falling asleep [mean (sem): 1.10 ± .25 and 1.30 ± .27, respectively]. No other components of the PSQI were associated with IR measures.
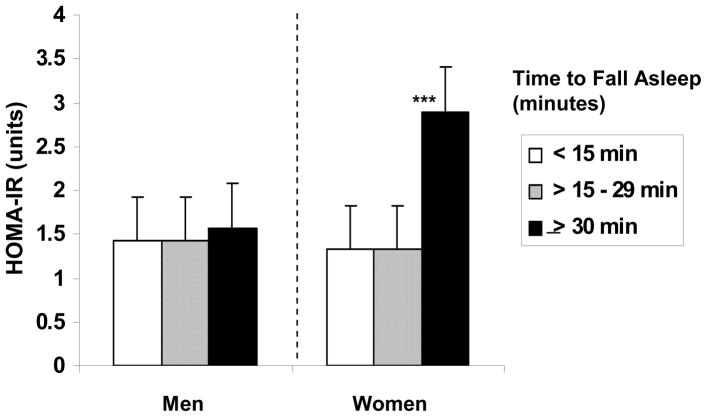
Lastly, the TFA by Gender interaction was also significant [Wilk’s λ = .86, F(3, 201) = 10.90, p < .001] with women who took 30 min or longer to fall asleep showing significantly higher fasting insulin [b = .26, t = 5.49, P < .0001] and greater IR [b = .01, t = 3.24, p < .01] than those who fell asleep within 30 minutes (see Figure 1). In men, the time it took to fall asleep was not associated with either fasting insulin or IR.
Figure 1.
C-reactive protein (CRP), and fasting insulin in men (n = 110) and women (n = 95) as a function of frequency of problems falling asleep. Means are adjusted for age, body mass index, race, exercise, and use of sleep medication in the prior month.
4. Discussion
In a multiethnic sample of adult men and women, poor sleep quality, more frequent occurrences of sleep-related symptoms, and a longer period of time to fall asleep (≥ 30 min) predicted a mosaic of putative psychosocial and physiological factors associated with increased risk of Type 2 diabetes and CVD. These associations, however, were significantly moderated by gender such that poor sleep quality and prolonged sleep latency incurred a greater psychological and physiological toll on women relative to men. Thus, among male and female subjects classified as poor sleepers by the PSQI a priori criteria (Buysse, et al., 1989), 51% of the women and 34% of the men were either overweight (BMI ≥ 25.0 kg/m2) or obese (BMI ≥ 30.0 kg/m2); 31% of the women and 21% of men showed CRP levels at or above 3.0 mg/l, and 27% showed HOMA values above 1.96, a level suggestive of IR in nondiabetic adults (Nakai, et al., 2002).Similarly, among individuals who reported taking 30 minutes or longer to fall asleep, 53% of the women were overweight or obese, 35% exhibiting CRP levels at or above 3.0 mg/l, and 32% had IR values above 1.96. These observations were statistically independent of age1, BMI, race, severity of depressive symptoms, alcohol use, use of sleep medications during the last month, and exercise participation as well as other potential confounders that were controlled by methodological entry criteria, such as smoking, exogenous hormone use, and menstrual cycle phase (for premenopausal women).
The observed gender disparity has direct relevance to observations from two large epidemiological studies suggesting that symptoms of sleep disturbances incur a greater risk of CVD and hypertension in women than in men (Cappuccio, et al., 2007; Newman, et al., 2000). To date, no study has examined potential mechanisms that could explain these gender specific observations. This is due to the fact that investigators have focused their efforts on examining the relation of sleep disturbances to pathophysiological factors and indicators of psychosocial distress without testing the moderating effects of gender. Therefore, the current findings are the first to suggest that gender significantly moderates the relation of poor sleep to putative mechanisms implicated in a number of chronic diseases. Therefore, an increased risk of CVD and hypertension associated with disturbed sleep in women could be due to psychosocial distress and pathophysiological mechanisms.
In minimizing the influence of various potential confounders, the current study employed strict methodological procedures. This methodology included inclusion/exclusion criteria that focused on negative history of chronic medical and psychiatric conditions including diagnosis of clinical sleep disorders, lifestyle factors associated with poor health such as smoking, use of medications, hormone therapy/oral contraceptive and holding time of data collection constant. Furthermore, for premenopausal women, data were collected only during the follicular phase of the menstrual cycle. These methodological constraints were coupled with multivariate techniques that were employed to minimize the influence of those factors that were not controlled methodologically, such as adiposity, age, race/ethnicity, exercise or use of sleep medications. Combined, methodological procedures and the statistical approached implemented in this study contribute to a high level of confidence that the observed gender differences were not due to the above mentioned confounding factors. What may explain the current observations, therefore, is opened to speculation.
One possible explanation is that these data reflect gender-related differences in the actions of the amino acid tryptophan (TRP), the neurotransmitter serotonin (5-HT), and the neurohormone melatonin (ML). Evidence suggests both direct and indirect effects of TRP, 5-HT and ML not only on sleep and sleep onset (Voderholzer, et al., 1998) but also on mood regulation (Booij, et al., 2002; Jans, et al., 2006), inflammation (Kubera, et al., 2005; Suarez, et al., 2000), thrombogenesis (Dale, et al., 2002), eating (Waldbillig, et al., 1981), and insulin regulation (Virkkunen & Naervaenen, 1987) with some studies suggesting gender-differences. For example, acute tryptophan depletion, which reduces 5-HT synthesis (Delgado, et al., 1989), has a greater mood altering effects on women than men (Booij, et al., 2002). In this laboratory, we have observed gender-related differences in the relation of TRP to various psychological factors associated with an increased risk of CHD and Type 2 diabetes (Suarez & Krishnan, 2006). Gender differences have also been reported in response to pharmacological treatments for depression with women showing more favorable responses to selective serotonin reuptake inhibitors (SSRI), such as fluoxetine, relative to men (Sloan & Kornstein, 2003). Thus, it may be the case that dysregulation of the serotonergic system, implicated in the regulation of sleep and sleep maintenance (Voderholzer, et al., 1998), is a potential mechanism that underlies the observed gender-specific relationship between sleep symptoms and putative risk factors.
Gender differences in the peroxisome proliferators-activated receptor (PPAR)-α may also explain the current observations (Barbier, et al., 2002; Daynes & Jones, 2002). Growing evidence points to a causative relationship between PPAR activity and the metabolic syndrome, IR, glucose intolerance, Type 2 diabetes, obesity, dyslipidemia, hypertension, atherosclerosis, and albuminuria (Zambon, et al., 2006). Increased PPARα expression is associated with decreased nuclear factor (NF)-κB and c-jun, transcription factors implicated in inflammation (Barnes & Karin, 1997). Particularly relevant to these observations, PPARα is expressed at higher levels in males than in females with higher testosterone associated with greater expression (Dunn, et al., 2007). While PPARα was not assessed in this study, poor sleep quality in men was associated with higher testosterone (partial r = .19, P = .05). Thus, in men, testosterone-related increases in the expression of PPARα leading to a decreased expression of NF-κB and c-jun may account for the lack of associations between poor sleep quality, inflammatory and metabolic factors.
Perturbations in physiological mechanisms and increases in psychosocial distress may underlie the observed gender differences in the association between poor sleep and BMI. From the current data, we observed that sleep disturbances were more strongly associated with depression in women than in men. This replicates findings from a recent study showing that depression was associated with higher BMI and this association was stronger in women (Schieman, et al., 2007). Given this, psychosocial distress is one possible pathway that could explain the gender differences in the association of sleep to BMI. Another possibility is that sleep alters regulatory hormones associated with appetite and food intake. Evidence suggests that sleep disturbances are associated with neuroendocrine regulation of appetite with levels of the anorexigenic hormone leptin being decreased and levels of the orexigenic hormone ghrelin increases (Spiegel, et al., 2008). Consistent with this possibility, gender differences in the levels of leptin and brain sensitivity to leptin have been reported (Casabiell, et al., 1998; Clegg, et al., 2003; Licinio, et al., 1998). Insulin may also play an important part in explaining the observed differences in the association of BMI to sleep. As reported, the current study showed that greater sleep disturbances are associated with insulin but only in women. These gender specific sleep related effects, , alone or in combination, could explain the stronger association between BMI and poor sleep in women.
For men, higher levels of testosterone provide a biologically plausible explanation for the lack of influence of disturbed sleep on putative mechanisms. Higher testosterone is associated with lower CRP and IL-6, greater insulin sensitivity and lower BMI (Liverman & Blazer, 2004). It is reasonable to assume, therefore, that higher testosterone levels blunt the health damaging consequences of poor sleep in men. Consistent with this hypothesis, one recent study showed that higher testosterone levels reduced the risk of developing Type 2 diabetes associated with sleep duration, and most dramatically among those with short sleep duration (Yaggi, et al., 2006).
There current study has various limitations. The aim of the study was to detect gender-specific differences in the relation of poor sleep to mechanism contributing to cardiovascular and metabolic disease onset and progression. The study was not designed to test directionality. While there is an abundance of data suggesting that psychological and medical conditions promote sleep disturbances, there is emerging evidence to suggest that sleep disturbances can evoke changes in psychological distress and physiological markers of health. In one large population, poor sleep, and particularly those sleep symptoms that are chronic, significantly increased the future risk for the development of anxiety and new episodes of major depression (Ford & Cooper-Patrick, 2001; Neckelmann, et al., 2007) as well as increased risk for hypertension (Cappuccio, et al., 2007). Experimental studies have shown that laboratory sleep restriction leads to greater IR in healthy young adults (Spiegel, et al., 2005). A parallel line of evidence suggests that inflammatory proteins, and specifically some proinflammatory cytokines, can contribute to sleep dysregulation (Opp, et al., 1992). This association has been most clearly illustrated in studies that have employed cytokine therapy, such as interferon (INF)-alpha, in treatment of clinical disease. In those studies sleep disturbances has been frequently noted (Capuron & Miller, 2004). Another limitation is use of a self-report scale in the assessment of sleep problems. Although the PSQI is a well-validated and recognized assessment tool to assess sleep quality, it cannot assess aspects of disturbed sleep that could potentially explain these findings, specifically, sleep disordered breathing (SDB). In a number of clinical studies, SDB has been linked with adverse health outcomes (e.g., Bassetti, et al., 2006; Mooe, et al., 2001; Peppard, et al., 2000; Reichmuth, et al., 2005) as well as potential pathophysiological mechanisms (Vgontzas, et al., 2000). Thus, it will be necessary for future studies to examine whether the observed gender differences emerge when assessment of sleep disturbances include measures of SDB.
Another important caveat is the fact that recruitment was not targeted at individuals with a history of sleep disorders. It is likely, however, that recruitment of individuals with severe sleep pathology would have resulted in even stronger associations given previous reports of the relationships between clinical sleep disorders and putative mechanisms (Burgos, et al., 2006; Hall, et al., 2000).
There current investigation also has notable strengths one of which is the large sample size, and particularly the sufficient number of women that allowed for the statistical evaluation of the hypothesized gender-specific interaction. As noted, few studies have included sufficient number of women to allow for appropriate statistical evaluation of the moderating effect of gender. Other studies have only included men. Inclusion of women, however, can present interpretational and statistical challenges due to hormonal variations throughout the menstrual cycle (Tworoger, et al., 2005). To reduce the influence of hormonal fluctuations associated with the menstrual cycle, data were collected only during the late follicular phase (days 5–10) in women who were premenopausal. Employing this approach minimized the possibility that differences in levels of ovarian hormones could account for the observed gender differences.
In conclusion, in women but not men, poor sleep and sleep-related symptoms are associated by a mosaic of plasma biomarkers and psychosocial distress associated with increased risk of Type 2 diabetes, CVD and other chronic medical conditions. The rationale underlying for the proposed study stemmed from epidemiological findings of gender-specific associations between sleep and risk of CVD. While limited by the cross-sectional nature of the design, the strength of the current data lies in the extent to which poor sleep quality and prolonged sleep latency were associated with a cluster of risk markers that are inter-related and, in combination, incur a heightened risk of most major chronic diseases. This is particularly true when it comes to the relation of sleep symptoms to both CRP and fibrinogen, a combination that has been shown to identify individuals who are at risk of becoming diabetic due to IR and not poor insulin secretion (Festa, et al., 2003), as well as the combination of hostility-anger-depression, which my laboratory has shown to be the best predictor of incident CHD in a 20-year follow-up of initially healthy adults (Boyle, et al., 2007). That sleep is a modifiable factor suggests its usefulness as a target for clinical intervention programs aimed at reducing primary disease risk. As a result, improvements in sleep as a mean of reducing risk for CVD may prove particularly effective in women. What may pose a greater challenge, however, will be in developing specific interventions particularly effective for women.
Figure 2.
Homeostasis Model Assessment (HOMA) estimated insulin resistance (IR) as a function of length of time to fall asleep. Means are adjusted for age, body mass index, race, exercise, and use of sleep medication in the prior month.
Acknowledgments
Funding for this work was provided by a grant from the National Institute of Heart, Lung and Blood (NHLBI) (HL67459) to ECS. The NHLBI had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Over-the-counter (OTC) sleep medications may have influence on a number of outcome variables. Thus, all analyses were also performed on the 92% (n = 196) of subjects who reported no use of medicines during the past month. Without exception, the results were the same as those reported. Thus, analyses retained the small number of subjects (8%) reporting some use of OTC sleep medications and sleep medication use was entered as a covariate. In addition, the item to assess medication use did not ask for additional details (type/amount). What can be said is that, for the week prior to participating, all subjects reported no use of medications, whether prescribed or over-the-counter.
Recent evidence has suggested that age may significantly moderate the relation of sleep disturbances to adverse health and putative mechanisms. Although the study sample included a wide age range (18–65 yr), only 5% of the sample was over the age of 50. Thus, it was not possible to examine the moderating effect of age due to lack of power. All analyses, however, used age as a covariate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours: a cross-sectional study. J Psychosom Res. 2002;53:741–748. doi: 10.1016/s0022-3999(02)00333-1. [DOI] [PubMed] [Google Scholar]
- Arad Y, Newstein D, Cadet F, Roth M, Guerci AD. Association of multiple risk factors and insulin resistance with increased prevalence of asymptomatic coronary artery disease by an electron-beam computed tomographic study. Arterioscler Thromb Vasc Biol. 2001;21:2051–2058. doi: 10.1161/hq1201.100257. [DOI] [PubMed] [Google Scholar]
- Barbier O, Torra IP, Duguay C, Blanquart C, Fruchart JC, Glineur C, Staels B. Pleiotropic actions of peroxisome proliferators-activated receptors in lipid metabolism and atherosclerosis. Arterioscler, Thromb and Vasc Biol. 2002;22:717–726. doi: 10.1161/01.atv.0000015598.86369.04. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Mechanisms of disease: Nuclear factor-(kappa)B -- a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Booij L, Van der Does W, Benkelfat C, Bremner JD, Cowen PJ, Fava M, Gillin C, Leyton M, Moore P, Smith KA, Van der Kloot WA. Predictors of mood response to acute tryptophan depletion. A reanalysis. Neuropsychopharmacology. 2002;27:852–861. doi: 10.1016/S0893-133X(02)00361-5. [DOI] [PubMed] [Google Scholar]
- Boyle SH, Michalek JE, Suarez EC. Covariation of psychological attributes and incident coronary heart disease in US Air Force veterans of the Vietnam War. Psychosom Med. 2007;68:844–850. doi: 10.1097/01.psy.0000240779.55022.ff. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Seigler IC, Vitaliano PP, Ballard EL, Gwyther LP, Williams RB. Associations among perceptions of social support, negative affect, and quality of sleep in caregivers and noncaregivers. Health Psychol. 2006:25. doi: 10.1037/0278-6133.25.2.220. In Press. [DOI] [PubMed] [Google Scholar]
- Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, Voderholzer U, Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20:246–253. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Stranges S, Kandala N-B, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension. The Whitehall II Study. Hypertension, HYPERTENSIONAHA.107.095471. 2007 doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Casabiell X, Pineiro V, Peino R, Lage M, Camina J, Gallego R, Vallejo LG, Dieguez C, Casanueva FF. Gender differences in both spontaneous and stimulated leptin secretion by human omental adipose tissue in vitro: dexamethasone and estradiol stimulate leptin release in women, but not in men 10.1210/jc. 83.6.2149. J Clin Endocrinol Metab. 1998;83:2149–2155. doi: 10.1210/jcem.83.6.4849. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck TW, Hoberman H. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social Support: Theory, Research and Application. The Hague: Martinus Nijhoff; 1985. pp. 73–94. [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–418. [Google Scholar]
- Dale GL, Friese P, Batar P, Hamilton SF, Reed GL, Jackson KW, Clemetson KJ, Alberio L. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002;415:175–179. doi: 10.1038/415175a. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Chaudhuri A, Bandyopadhyay A. The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis-related complications in Type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2003;88:2422–2429. doi: 10.1210/jc.2003-030178. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Charney DS, Price LH, Landis H, Heninger GR. Neuroendocrine and behavioral effects of dietary tryptophan restriction in healthy subjects. Life Sci. 1989;45:2323–2332. doi: 10.1016/0024-3205(89)90114-8. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, Crowell A, Loh J, Oksenberg J, Steinman L. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med. 2007;204:321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa A, Hanley AJ, Tracy RP, D’Agostino R, Jr, Haffner SM. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003;108:1822–1830. doi: 10.1161/01.CIR.0000091339.70120.53. [DOI] [PubMed] [Google Scholar]
- Fibrinogen Studies C. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am J Epidemiol. 2007;166:867–879. doi: 10.1093/aje/kwm191. [DOI] [PubMed] [Google Scholar]
- Ford DE, Cooper-Patrick L. Sleep disturbances and mood disorders: an epidemiologic perspective. Depress Anxiety. 2001;14:3–6. doi: 10.1002/da.1041. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Singer BH, Ryff CD. Social relationships, sleep quality, and interleukin-6 in aging women. PNAS. 2005;102:18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, III, Kupfer DJ. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–230. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- Hong S, Mills PJ, Loredo JS, Adler KA, Dimsdale JE. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun. 2005;19:165–172. doi: 10.1016/j.bbi.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Jans LAW, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2006:1–22. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Krantz DS, McCeney MK. Effects of psychological and social factors on organic disease: A critical assessment of research on coronary heart disease. Annu Rev Psychol. 2002;53:341–369. doi: 10.1146/annurev.psych.53.100901.135208. [DOI] [PubMed] [Google Scholar]
- Kubera M, Maes M, Kenis G, Kim YK, Lason W. Effects of serotonin and serotonergic agonists and antagonists on the production of tumor necrosis factor α and interleukin-6. Psychiatry Research. 2005;134:251–258. doi: 10.1016/j.psychres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM. Inflammation and Atherothrombosis: From Population Biology and Bench Research to Clinical Practice 10.1016/j.jacc.2006.08.011. J Am Coll Cardiol. 2006;48:A33–46. [Google Scholar]
- Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Negro PP, Mulla A, Veldhuis JD, Cearnal L, Flier JS, Gold PW. Sex differences in circulating human leptin pulse amplitude: clinical implications. Journal of Clinical Endocrinology & Metabolism. 1998;83:4140–4147. doi: 10.1210/jcem.83.11.5291. [DOI] [PubMed] [Google Scholar]
- Liverman CT, Blazer DG. Testosterone and Aging: Clinical Research Directions. National Academies Press; Washington, DC: 2004. [PubMed] [Google Scholar]
- Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: Implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The Chicago Health, Aging, and Social Relations Study. Psychosom Med. 2006;68 doi: 10.1097/01.psy.0000221371.43607.64. In Press. [DOI] [PubMed] [Google Scholar]
- Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. American Journal of Respiratory & Critical Care Medicine. 2001;164:1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Fukushima M, Nakaishi S, Kishimoto H, Seino Y, Nagasaka S, Sakai M, Taniguchi A. The threshold value for insulin resistance on homeostasis model assessment of insulin sensitivity. Diabet Med. 2002;19:346–347. doi: 10.1046/j.1464-5491.2002.00712_3.x. [DOI] [PubMed] [Google Scholar]
- Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–880. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, Robbins J. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- Opp MR, Kapas L, Toth LA. Cytokine involvement in the regulation of sleep. Proc Soc Exp Biol Med. 1992;201:16–27. doi: 10.3181/00379727-201-43474. [DOI] [PubMed] [Google Scholar]
- Parati G, Lombardi C, Narkiewicz K. Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1671–1683. doi: 10.1152/ajpregu.00400.2007. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being and sleepiness in college students [see comment] J Psychosom Res. 1997;42:583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. American Journal of Respiratory & Critical Care Medicine. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Morrow DA. C-reactive protein, inflammation, and coronary risk. Cardiol Clin. 2003;21:315–325. doi: 10.1016/s0733-8651(03)00079-1. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan JR. Impact of psychological factors on the pathogenesis of cardiovascular disease and implication for therapy. Circulation. 1999;99:2129–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Schieman S, McMullen T, Swan M. Relative body weight and psychological distress in late life: observations of gender and race comparisons. Journal of Aging & Health. 2007;19:286–312. doi: 10.1177/0898264307299300. [DOI] [PubMed] [Google Scholar]
- Sloan DME, Kornstein SG. Gender differences in depression and response to antidepressant treatment. Psychiatr Clin N Am. 2003;26:581–594. doi: 10.1016/s0193-953x(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2008;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Krishnan KRR. The relation of free plasma tryptophan to anger, hostility, and aggression in a nonpatient sample of adult men and women. Ann Behav Med. 2006;31:254–260. doi: 10.1207/s15324796abm3103_7. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Barefoot JC, Gadde KM, Helms MJ, Grichnick K, Kuhn C, Lewis CE, Schanberg S, Siegler IC, Stafford-Smit M, Williams RB. CSF 5-HIAA, stress and cytokine and adhesion molecule expression on blood monocytes. Psychosom Med. 2000;62:150. [Google Scholar]
- Temelkova-Kurktschiev T, Siegert G, Bergmann S, Henkel E, Koehler C, Jaross W, Hanefeld M. Subclinical inflammation is strongly related to insulin resistance but not to impaired insulin secretion in a high risk population for diabetes [see comment] Metabolism: Clinical & Experimental. 2002;51:743–749. doi: 10.1053/meta.2002.32804. [DOI] [PubMed] [Google Scholar]
- Trenell MI, Marshall NS, Rogers NL. Sleep and metabolic control: waking to a problem? Clinical & Experimental Pharmacology & Physiology. 2007;34:1–9. doi: 10.1111/j.1440-1681.2007.04541.x. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Davis S, Vitiello MV, Lentz MJ, McTiernan A. Factors associated with objective (actigraphic) and subjective sleep quality in young adult women. J Psychosom Res. 2005;59:11–19. doi: 10.1016/j.jpsychores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Medicine Reviews. 2005;9:211–224. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: Relation to visceral obesity, insulin resistance, and hypercytokinemia. Journal of Clinical Endocrinology & Metabolism. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- Virkkunen ME, Naervaenen S. Plasma insulin, tryptophan and serotonin levels during the glucose tolerance test among habitually violent and impulsive offenders. Neuropsychobiology. 1987;17:19–23. doi: 10.1159/000118335. [DOI] [PubMed] [Google Scholar]
- Voderholzer U, Hornyak M, Thiel B, Huwig-Poppe C, Kiemen A, Konig A, Backhaus J, Riemann D, Berger M, Hohagen F. Impact of experimentally induced serotonin deficiency by tryptophan depletion on sleep EEC in healthy subjects. Neuropsychopharmacology. 1998;18:112–124. doi: 10.1016/S0893-133X(97)00094-8. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Archuleta C, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s disease. J Am Geriatr Soc. 2006;54:431–437. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Waldbillig RJ, Bartness TJ, Stanley BG. Increased food intake, body weight, and adiposity in rates after regional neurochemical depletion of serotonin. J Comp Physiol Psychol. 1981;95:391–405. doi: 10.1037/h0077790. [DOI] [PubMed] [Google Scholar]
- Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of Type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- Yanase M, Takatsu F, Tagawa T, Kato T, Arai K, Koyasu M, Horibe H, Nomoto S, Takemoto K, Shimizu S, Watarai M. Insulin resistance and fasting hyperinsulinemia are risk factors for new cardiovascular events in patients with prior coronary artery disease and normal glucose tolerance. Circulation Journal. 2004;68:47–52. doi: 10.1253/circj.68.47. [DOI] [PubMed] [Google Scholar]
- Zambon A, Gervois P, Pauletto P, Fruchart JC, Staels B. Modulation of hepatic inflammatory risk markers of cardiovascular fiseases by PPAR-{alpha} activators: clinical and experimental evidence. Arterioscler Thromb Vasc Biol. 2006;26:977–986. doi: 10.1161/01.ATV.0000204327.96431.9a. [DOI] [PubMed] [Google Scholar]