Abstract
Bacterial infection elicits a range of beneficial as well as detrimental host inflammatory responses. Key among these responses are macrophage/monocyte necrosis, release of the pro-inflammatory factor high-mobility group box 1 protein (HMGB1), and induction of the cytokine IL-1. While the control of IL-1β has been well-studied, processes that control macrophage cell death and HMGB-1 release in animals are poorly understood. This study utilizes Klebsiella pneumonia as a model organism since it elicits all three responses in vivo. The regulation of these responses is studied in the context of the inflammasome components, NLRP3 and ASC, which are important for caspase-1 activation and IL-1β release. Using a pulmonary infection model that reflects human infection, we show that K. pneumonia-induced mouse macrophage necrosis, HMGB-1 and IL-1β release are dependent on NLRP3 and ASC. K. pneumoniae infection of mice lacking Nlrp3 results in decreased lung inflammation and reduced survival relative to control indicating the overall protective role of this gene. Macrophage/monocyte necrosis and HMGB1 release are controlled independently of caspase-1 suggesting that the former two responses are separable from inflammasome-associated functions. These results provide critical in vivo validation that the physiologic role of NLRP3 and ASC is not limited to inflammasome formation.
Introduction
The NLR (nucleotide binding domain, leucine rich repeats-containing) family (formerly known as CATERPILLER, NOD, NACHT-LRR, NOD-like receptor) family of genes/proteins is increasingly implicated in the regulation of immunity (1). The NLR family member NLRP3 (formerly cryopyrin, CIAS1, NALP3), which is expressed abundantly in neutrophils and macrophages, has emerged as a critical mediator of inflammation. NLRP3/CIAS1, the human gene encoding this protein, was first identified through its association with the hereditary periodic fever syndrome, CAPS (CIAS1-associated periodic syndrome), which comprises a wide range of inflammatory symptoms and severity (2–6). This condition results from gain-of-function mutations in NLRP3. Disease-associated variant forms of NLRP3 exhibit enhanced capacity to induce caspase-1 and IL-1β maturation, and a necrotic pathway of macrophage cell death that indirectly enhances inflammation (7–9).
A role for NLRP3 in caspase-1 maturation is well-studied. Following stimulation, NLRP3, the adaptor ASC (Apoptotic Speck-like protein containing a Card), Cardinal/TUCAN, and pro-caspase-1 combine to form one of several known inflammasome complexes. Within this complex, pro-caspase-1 is activated, which in turn cleaves and activates the pyrogenic cytokines IL-1β and IL-18 (9). Currently, the inflammasome protein complex has also been demonstrated for NLRP1 (NALP1), and the function of caspase-1/IL-1β release has been associated with NLRC4 (IPAF/CLAN) and NAIP5, however activation of the NLRP3 inflammasome is associated with the widest spectrum of stimuli. Among these are gram-positive and gram-negative bacteria, including Staphylococcus aureus, Listeria monocytogenes, and Shigella flexneri, toxins as well as uric acid crystals and pathogen or non-pathogen derived nucleic acid (8, 10, 11). Environmental pollutants such as asbestos and silica, as well as particulate adjuvants and β-amyloid have also been found to require NLRP3 for caspase-1 mediated cytokine secretion (12–16)
Recent work has suggested a second pro-inflammatory function for NLRP3 involving monocyte necrosis (7, 8). Although the study of this process has been previously limited to in vitro culture, this form of necrosis has been shown to occur in monocytic cells infected with intracellular bacteria or following exposure to toxins. Both microbial pathogen (S. flexneri) induced necrosis and necrotic death associated with CAPS require NLRP3, its partner protein ASC, and the lysosomal protease cathepsin B (8). However, NLRP3-dependent necrosis is entirely independent of caspase-1 and IL-1β, and other apoptosis-associated caspases and has been named pyronecrosis. One defining feature of monocytic necrosis is the loss of plasma membrane integrity and subsequent spilling of intracellular inflammatory contents, most notably the nuclear factor HMGB1, which elicits strong pro-inflammatory effects when released into the microenvironment (17). HMGB1 can activate the RAGE receptor and TLRs to elicit proinflammatory responses including the release of TNF-α and IL-1β. HMGB1 is considered a therapeutic target as antibodies against HMGB1 have been shown to effectively reduce sepsis, arthritis, and cancer in animals (18–20). However NLR-dependent inflammatory necrosis or HMGB1 release remain to be validated in a physiologic setting (21).
Klebsiella pneumoniae is among the most common gram negative bacteria encountered by clinicians worldwide and is a leading cause of community-acquired and hospital-associated respiratory infection (22). Its frequency in the latter context is particularly alarming as K. pneumoniae is responsible for up to 23% of nosocomial infections, and a mortality rate of up 50% in elderly or otherwise compromised patients (23). Moreover, the growing prevalence of antibiotic resistant strains in this species has led to increased attention and concern (24, 25). K. pneumoniae is a non-motile, non-flagellated, gram negative, rod-shaped bacterium which normally resides within the mouth, skin, and intestines. Pathogenic K. pneumoniae invades the lungs where it is capable of inducing severe bacterial pneumonia that is often complicated with bacteremia and sepsis (26). Airway infection typically leads to extensive lung injury resulting from increased inflammation, hemorrhage, and the necrotic destruction of lung tissue. This process results in thick, blood-laced mucous known as “currant jelly” sputum, which is characteristic of K. pneumoniae-induced pneumonia. In addition to cellular necrosis, this bacteria also induces HMGB1 release in humans (27) . Though recent work has started to identify innate immune mechanisms underlying these diverse host responses to bacterial infection, little work has been done to examine the regulation of these diverse inflammatory responses elicited by K. pneumoniae infection, or the contribution of these mechanisms to pathogenesis or immunity (28–30). Due to the multiple inflammatory responses that are elicited by K. pneumoniae including cytokines, necrosis and HMGB1 induction, and its potential as a public health threat caused by the rise of antibiotic-resistance strains, we elected to study the roles of NLRP3 and ASC using this bacteria in animals.
Despite the wealth of in vitro data implicating the NLR family in pathogen-induced inflammation, in vivo evidence for the importance of these proteins in inflammation has been limited to IL-1β and IL-18 release. This report shows that NRLP3 activates macrophage necrosis as well as HMGB1 release in addition to IL-1β/IL-18 secretion in response to K. pnuemoniae in infected animals. NRLP3 is an important immune protection factor during infection with this bacteria as its absence decreases the inflammatory response and the rate of survival in mice. This is the first in vivo analysis depicting an inflammasome-independent function of NRLP3 which reveals a much broader physiologic role for NRLP3 that extends beyond IL-1β/IL-18 secretion.
Materials and Methods
Experimental Animals
All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. Mice deficient in Nlrp3ASC, Nlrc4, and caspase-1 were generated as previously described (31, 32). All animals were maintained in pathogen-free facilities at The University of North Carolina at Chapel Hill.
Cell lines and reagents
THP-1 cells were obtained from American Type Culture Collection (ATCC) and cultured as described previously (33). Antibodies: anit-caspase-3 from Cell Signaling; anti-PARP, anti-Actin, and HRP-conjugated secondary antibodies from Santa Cruz Biotechnology; anti-HMGB1 from Abcam; Super Signal ECL reagent from BioRad. The preparation of retroviral vectors and THP-1 cell lines stably expressing shRNA has been described (34). The shRNA target sequences are: shASC-GCTCTTCAGTTTCACACCA, shCtrl-GCTCTTCctggcCACACCA, shNLRP3-GGATGAACCTGTTCCAAAA.
Bacteria
K. pneumoniae 43816, serotype 2 was obtained from the ATCC and cultured in LB. Bacteria density was estimated by measuring the absorbance at 600 nm (1 OD600 = 3 × 108 bacteria/ml). Accurate CFUs were determined for each experiment by plating an aliquot on LB agar plates.
Bacterial induced inflammation
Cultures of K. pneumoniae were pelleted, washed twice in PBS, and resuspended in PBS. Mice were anesthetized and challenged via intratracheal (i.t.) instillation with 7.4 × 104 CFUs of K. pneumoniae in 50 µl of PBS, as previously described (28). Mock challenged mice received 50 µl of PBS. THP-1 cells were infected with Klebsiella at a MOI=50 for 6 hours. Bone marrow derived macrophages (BMDM) were prepared as previously described (8) and infected with Klebsiella (MOI=200, 6 hours) or Salmonella (MOI=50, 1 hour). Samples were centrifuged at 650*g for 10 minutes immediately following addition of bacteria. Gentamicin (50 µg/ml) was added to cultures 1 hour post infection.
Assessment of bacteria burden
Mice were euthanized via i.p. injection with 2,2,2 tribromoethanol (avertin). Whole liver, spleen and lungs were removed, wet weight assessed, homogenized in 500 µl HBSS with a Tissue Master 125 (Omni International) and centrifuged. The resulting supernatants were plated on LB agar plates.
Assessments of airway inflammation
Mice were euthanized via i.p. injection of avertin and serum was harvested via cardiac puncture. The liver, kidney, and spleen were removed, weighed, and either homogenized in 500 µl HBSS or fixed in 4% paraformaldehyde (PFA). Mice were then perfused with HBSS and a tracheal cannula was inserted below the larynx. The lungs were lavaged 5 times with 1 ml HBSS followed by centrifugation to isolate BAL cells and cell free supernatants. Red blood cells were lysed via hypotonic saline treatment and cellularity assessed with a hemacytometer. Aliquots of BALF were cytospun onto slides and Diff-Quik (Dade Behring) stained for differential cell counts. Leukocytes were identified based on morphology of ≥200 cells per sample. An aliquot of BALF was plated on LB agar to assess bacteria burden. The remaining BALF was centrifuged and the supernatant was collected. Following BALF harvest, the lungs were fixed by inflation (20-cm pressure) and immersed in 4% PFA.
Histopathologic examination
For histopathological examination, lungs were fixed by inflation (20-cm pressure) and immersion in 10% buffered formalin. Whole inflated lungs were embedded in paraffin wax. Sections (4-µm) were cut and stained with hematoxylin and eosin (H&E). Serial sections of the left lobes of the lungs that yield maximum longitudinal visualization of the intrapulmonary main axial airway were examined and inflammation was scored by one of the authors (I.C.A.) who was blinded to genotype and treatment. Histology images were evaluated and each of the following inflammatory parameters was scored between 0 (absent) and 3 (severe): mononuclear cell infiltration; polymorphonuclear cell infiltration; airway epithelial cell hyperplasia/injury; extravasation; perivascular cuffing; and percent of lung involved with inflammation, as previously described (35). The scores of the parameters were averaged for a total histology score.
Cytokine and chemokine assessment
Cell free supernatants from K. pneumoniae (MOI=200, 6 hour) or S. typhi (MOI=50, 1 hour) infected thioglycolate-elicited peritoneal macrophages were analyzed using RayBio® Cytokine Antibody Array G Series 3 (RayBiotech Inc). Axon scanner 4000B with GenePix software was used to collect fluorescence intensities. These values were normalized to the ratio of positive control values for each sample. Total normalized fluorescence values of replicate spots were averaged and expressed as fold increase over non-infected samples. “N/D” indicates cytokines where fluorescence values of replicate spots deviated more than 2 fold and were thus dismissed. If this occurred in the non-treated sample, the cytokine was removed from the data set. Cytokine concentrations were determined by RayBiotech Inc. using their Quantibody service.
ELISA assay
Samples were collected at indicated times and assayed with OptEIA Human IL-1β ELISA Set or OptEIA Mouse IL-1β or IL-6 ELISA Sets (BD).
Cell viability determination
Viability was assayed per manufacturer protocol using either CytoTox-ONE™ Homogeneous Membrane Integrity Assay (Promega), ToxiLight® BioAssay Kit (Lonza Bioscience), or 7-AAD (BD) staining as indicated. In the case of 7-AAD staining, cells were collected, washed twice in PBS, resuspended in 0.5ml PBS with 1 µl 7-AAD, and incubated for 15 minutes before analysis on a FACScan (BD).
Results
K. pneumoniae-induced cell death and IL-1β release require NLRP3 and ASC
The role of NLR proteins in host immune response to K. pneumoniae was first examined in a human macrophage cell line. We observed both cell IL-1β release (Fig. 1A) and cell death (Fig. 1B) following infection of THP-1 human monocytic cells with K. pneumoniae. Both processes were abrogated in a human macrophage cell line with reduced NLRP3 expression (Fig. 1A and 1B). Reduction of NLRP3 was achieved through stable integration of retroviruses encoding shRNAs designed to promote the targeted degradation of NLRP3 mRNA (labeled shNLRP3), as described and demonstrated previously (8, 34).
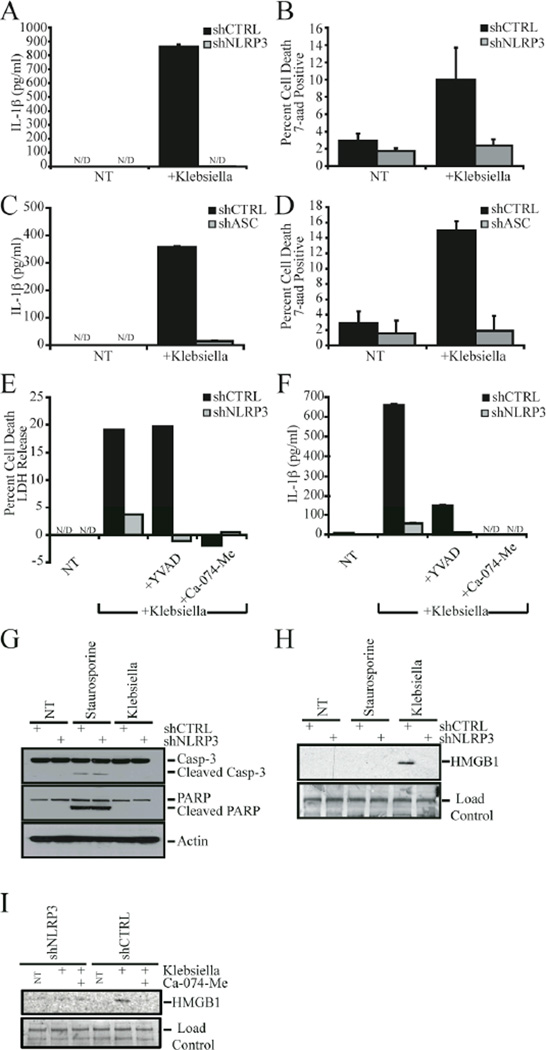
Figure 1. Klebsiella pneumoniae induced cell death and IL-1β required NLRP3 and ASC.
A) K. pneumoniae induced IL-1β release was decreased in THP-1 cells stably transduced with NLRP3-specific shRNA (shNLRP3). B) shNLRP3-THP-1 cells were resistant to K. pneumoniae-induced death. C and D) ASC was required for K. pneumonia-induced IL-1β release (C) and cell death (D) in THP-1 cells. E and F) Addition of 50 µM cathepsin B inhibitor (Ca-074-Me) substantially abrogated K. pneumoniae induced cell death (E) and IL-1β release (F) in shCTRL and shNLRP3 cells. In contrast, 100 µM caspase-1 specific (YVAD-CHO) inhibitors failed to block K. pneumoniae-induced cell death (E), but did inhibit IL-1β release (F). G) Caspase-3 and PARP were cleaved in response to an apoptotic stimulus (staurosporine), but not in THP-1 cells following K. pneumoniae infection. H) K. pneumoniae-induced HMGB1 release was abrogated in cells deficient in NLRP3 IL-1β release was determined by ELISA. I) Addition of 50 µM cathepsin B inhibitor (Ca-074-Me) substantially abrogated K. pneumoniae induced HMGB1 release. Caspase-3, PARP, and HMGB1 levels were assessed by western blot analysis. NT, no treatment; N/D, not detected.
Previously, we demonstrated that ASC, a partner protein of NLRP3, is required for S. flexneri–induced cell death in THP-1 cells (8). Therefore, we tested the ability of Klebsiella to elicit cell death and IL-1β in ASC-deficient THP-1 cells. Both IL-1β release and cell death were substantially abrogated in the shASC cells (Fig. 1C and 1D). Having established that NLRP3 is essential for Klebsiella-induced cell death, we sought to determine the nature of this phenomenon. Indicative of pyronecrosis, K. pneumoniae-induced cell death was markedly reduced in control THP-1 cells treated with the cathepsin-B inhibitor Ca-074-Me (Fig. 1E). The caspase-1 specific inhibitor YVAD-cho had no effect on cell death as measured by LDH release (Fig. 1E). The caspase-1 specific inhibitor was used at concentrations sufficient to inhibit caspase activity, as evidenced by the attenuation of Klebsiella-induced IL-1β release (Fig. 1F). Interestingly, the cathepsin B inhibitor not only blocked Klebsiella-induced cell death in THP-1 cells, but also prevented IL-1β release in response to the pathogen (Fig. 1F). This indicates that cathepsin B also controls IL-1β release.
Additional features of Klebsiella-induced cell death are also consistent with pyronecrosis. During apoptosis, caspase-3 undergoes activating cleavage. In turn, caspase-3 cleaves PARP and other downstream substrates. Neither caspase-3 nor PARP were cleaved in shCTRL or shNLRP3 cells infected with Klebsiella, though both were cleaved in staurosporine-treated cells (Fig. 1G). During macrophage necrosis, HMGB1 is released. In accordance with this, HMGB1 is released from K. pneumoniae infected shCTRL cells but not infected shNLRP3 cells as determined by a western blot (Fig. 1H). HMGB1 is not released by THP-1 cells treated with staurosporine, a well-established inducer of apoptotic cell death (Fig 1H). HMGB1 release from shCTRL cells is abrogated by a Cathepsin B inhibitor (Ca-074-Me), indicating that Klebsiella induced HMGB1 release requires cell death (Fig 1I).
Klebsiella-induced IL-1β is reduced in Nlrp3−/− and Asc−/− macrophages
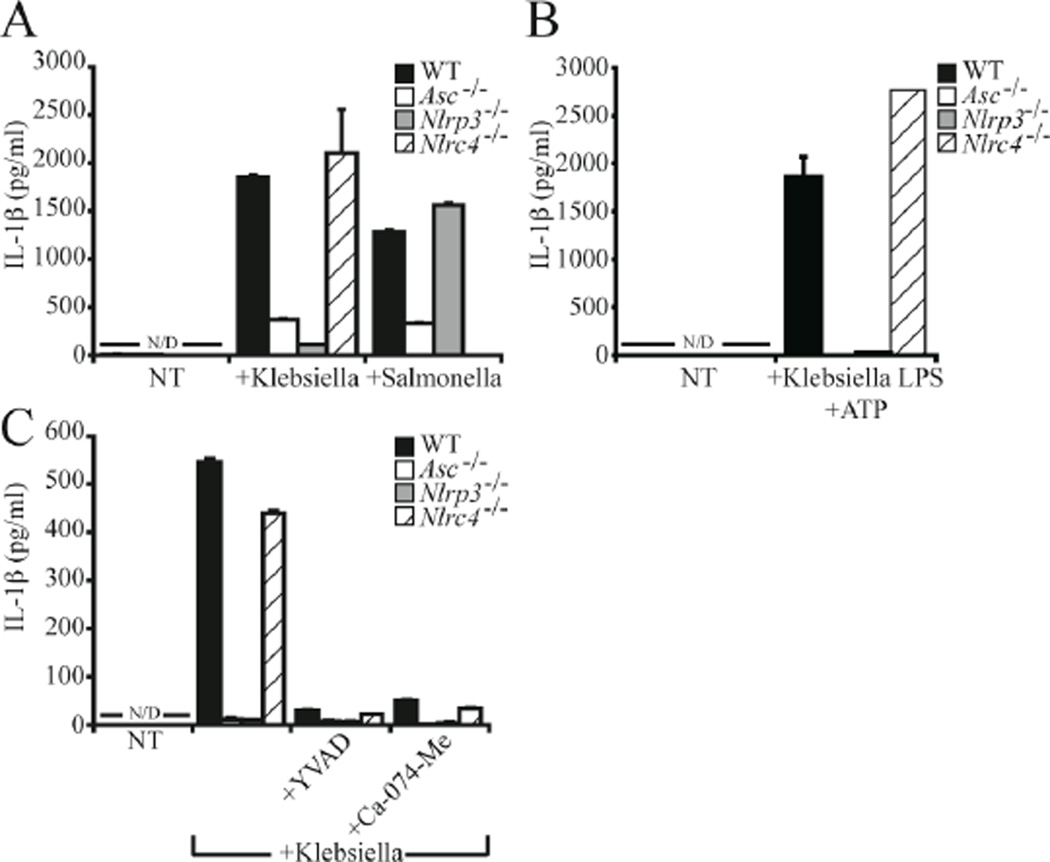
To examine the physiologic importance of these results, BMDM were isolated from WT mice and mice deficient for Nlrp3, Asc, or Nlrc4. Deletion of Nlrp3 or Asc resulted in a near complete inhibition of IL-1β induced by Klebsiella as measured by ELISA, whereas Nlrc4 null macrophages demonstrated no substantial difference from WT (Fig. 2A). Importantly, this phenomenon is not common to all pathogenic bacteria. In agreement with previous work, IL-1β release from macrophages infected with S. typhi was unaffected by Nlrp3 deletion, whereas deletion of Nlrc4 eliminated the inflammatory response (Fig. 2A). The NLRP3 inflammasome was previously reported to be activated by a combination of E. coli lipopolysaccharide (LPS) and ATP (9). To determine if the NLRP3 inflammasome is also activated by Klebsiella LPS, BMDM were challenged with 50 ng/ml LPS isolated from K. pneumoniae for 16 hours followed by stimulation with 5 mM ATP for 20 minutes. In contrast to WT and Nlrc4 deficient macrophages, deletion of Asc or Nlrp3 eliminated Klebsiella LPS-induced IL-1β release (Fig. 2B). In agreement with observations in THP-1 cells, activation of the inflammasome by K. pneumoniae was abrogated by both caspase-1 specific inhibitors (YVAD) and the cathepsin B inhibitor, CA-074-Me (Fig. 2C). Together, these results indicate that NLRP3 is the predominant NLR activated by Klebsiella and that deletion of either Nlrp3 or Asc substantially abrogates host inflammatory responses.
Figure 2. Klebisella pneumoniae induced NLRP3 and cathepsin B dependent IL-1β release in primary macrophages.
A) Nlrp3 and Asc deficient BMDM exhibited decreased levels of IL-1β release in response to K. pneumoniae but not S. typhi. Macrophages lacking Nlrc4 demonstrated no defect in K. pneumoniae induced IL-1β release, but IL-1β release was attenuated in response to S. typhi. B) K. pneumoniae LPS (50 ng/ml, 16 hours) in combination with ATP (5 mM, 20 min.) stimulated NLRP3 and ASC-dependent, but NLRC4-independent, IL-1β release from BMDM. C) Addition of either 100 µM caspase-1 inhibitor (YVAD) or 50 µM cathepsin B inhibitor (Ca-074-Me) substantially abrogated K. pneumoniae induced IL-1β release from BMDM. IL-1β was measured by ELISA. NT, no treatment; N/D, none detected.
K. pneumoniae induces chemotactic and inflammatory cytokine production in primary mouse macrophages
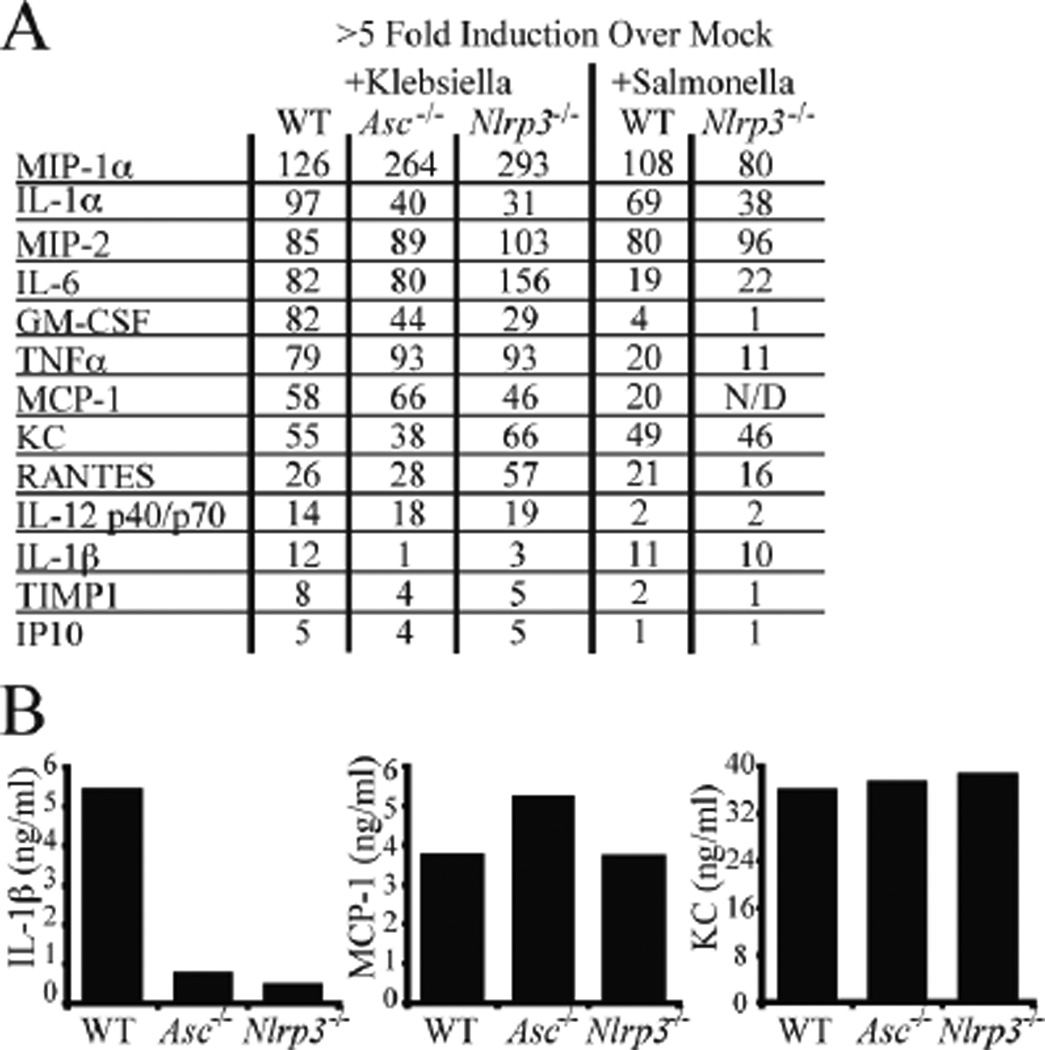
The processing and release of proinflammatory cytokines and chemokines is fundamental to proper innate immune response to pathogens. To more broadly assess the effect of NRLP3 and ASC on host inflammatory responses, cell free supernatants prepared from Klebsiella- or Salmonella-infected macrophages were analyzed on anti-cytokine antibody arrays containing antibodies to 62 inflammatory mediators. Production of IL-1β was markedly decreased in both Asc and Nlrp3 deficient macrophages as expected, but GM-CSF and IL-1α were also reduced indicating that these cytokines are coordinately controlled by ASC and NRLP3 either directly or indirectly (Fig 3A). In contrast, Mip-1 was coordinately enhanced in Nlrp3 and Asc-deficient macrophages when compared to control cells, perhaps to compensate for the loss of IL-1β. IL-6 and RANTES were increased in the Nlrp3−/− but not Asc−/− cells. As a pathogen-specificity control, IL-1β was not decreased in Nlrp3 deficient macrophages treated with Salmonella, which activates the Nlrc4 inflammasome. To confirm the results obtained by the antibody array, a subset of inflammatory mediators from K. pneumoniae challenged ex vivo macrophages were measured using quantitative multiplexed anti-cytokine arrays. Cytokine measurements of IL-1β, Mcp-1, and KC in the absence of Asc and Nlrp3 are consistent with the data obtained by the antibody arrays (Fig. 3B, complete data set shown in Table S1).
Figure 3. Klebsiella pneumoniae induced inflammatory cytokines and chemokines in primary macrophages.
A) Thioglycolate-elicted peritoneal macrophages of indicated genotypes were infected with K. pneumoniae (MOI=200, 6 hours) or S. typhi (MOI=50, 1 hour). Cell free supernatants were analyzed on RayBiotech G Series 3 cytokine antibody arrays. Cytokines and chemokines induced ≥5 fold over the non-treated control of each genotype are shown. B) Quantification of IL-1β, MCP-1, and KC support trends observed on G Series 3 cytokine arrays. Cytokine levels were determined using RayBiotech Quantibody service.
Mice lacking Nlrp3 and Asc demonstrate significantly increased mortality following K. pneumoniae airway infection
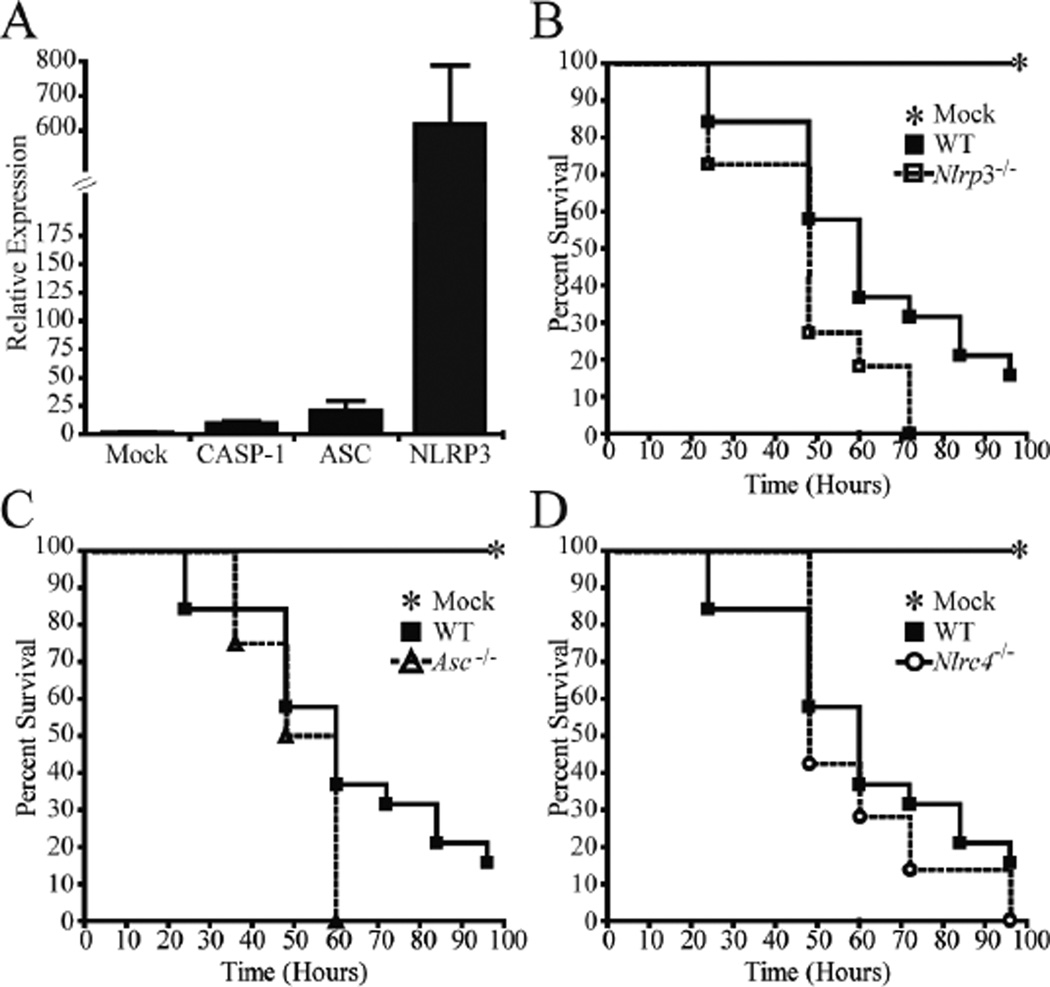
Our in vitro results suggested that ASC and NRLP3 are critical regulators of K. pneumoniae induced inflammation and cell death. To determine if inflammation was also reduced in vivoAsc and Nlrp3 deficient mice were challenged with (7.4×104) CFUs of K. pneumoniae delivered through airway infection. Previous reports show that E. coli LPS enhances NLRP3 expression in vitro (36). To assess this issue in animals in the context of K. pneumoniae, we show that in vivo NRLP3 expression is greatly induced (600×) by this bacteria while ASC expression is increased by 25× (Fig. 4A). To determine whether NRLP3 and ASC are involved in mediating the overall host response to K. pneumoniae in vivo, animals were challenged via intratracheal instillation and survival was assessed over the course of 4 days. Mice lacking the Nlrp3 gene demonstrated moderate but statistically significant increased mortality compared with WT mice (p < 0.05, Logrank Test) (Fig. 4B). Asc deficient mice demonstrated similar increases in mortality (Fig. 4C). No significant difference in survival was observed between Nlrc4-deficient mice and the WT controls (Fig. 4D). This moderate effect of Nlrp3 on animal survival may be explained by other NLRs that can mediate inflammasome formation in the lung, as well as by the compensatory increase in other inflammatory cytokines, such as IL-6, which might mask the effect of Nlrp3-deficiency in animals.
Figure 4. Nlrp3 and Asc deficient mice demonstrated significantly increased mortality following K. pneumoniae airway infection.
Mice were intratracheally challenged with (7.4×104) CFUs of K. pneumoniae and survival was assessed over the course of 4 days. A) Total RNA was extracted from whole, homogenized lungs and expression was determined via real time PCR. NLRP3 expression is dramatically increased 48 hours post K. pneumoniae infection. B and C) Mice lacking Nlrp3 (B) or Asc C) demonstrated significantly increased mortality compared with wild type (WT) mice (p < 0.05, Logrank Test). D) No significant difference in survival was observed between WT mice and animals lacking Nlrc4 following K. pneumoniae challenge. Mock: n = 7; Nlrp3−/−: n = 11; Asc−/−: n = 4; Nlrc4−/−: n = 7; WT: n = 19
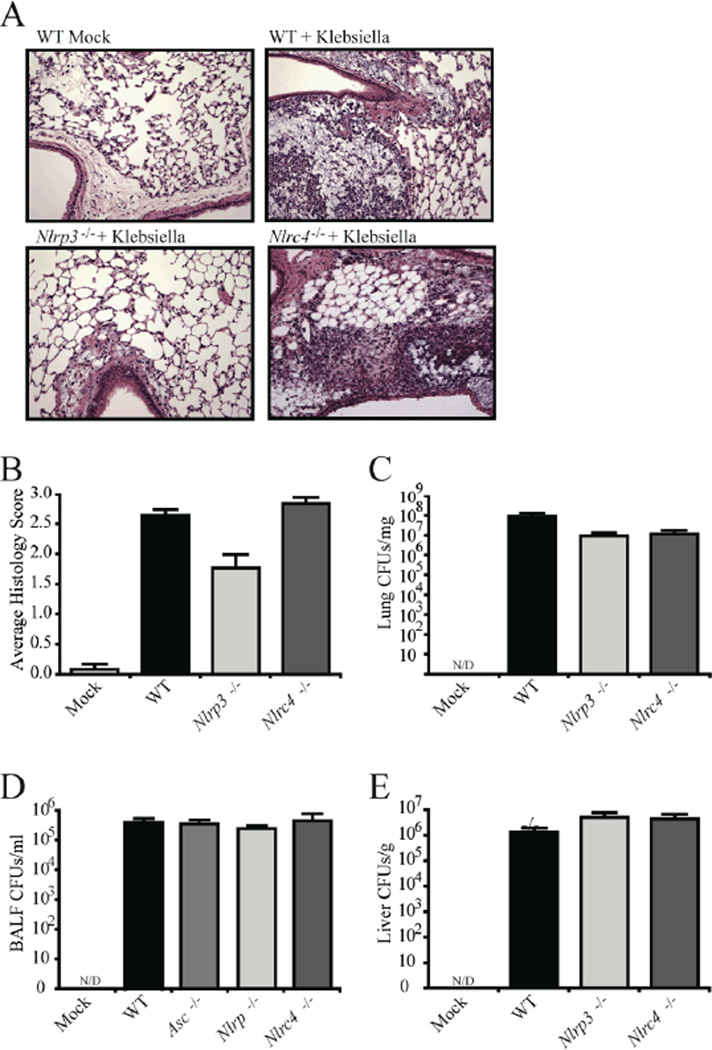
Nlrp3 deficient mice demonstrate significantly attenuated airway inflammation following K. pneumoniae infection
The above results suggested that NRLP3 is an important mediator of several inflammatory cytokines and is essential for mouse survival in an in vivo model of K. pneumoniae infection. To determine if airway inflammation was attenuated, Nlrp3 deficient mice were challenged via intra-tracheal instillation with K. pneumoniae and the lungs were harvested 48 hours post inoculation for histology analysis. Lung sections were prepared to reveal the main bronchi of the left lobe from the indicated genotypes and representative sections were examined (10× and 20× magnification) (Fig. 5A). In comparison to WT and Nlrc4−/− mice, Nlrp3−/− mice show decreased inflammatory cell recruitment and less occlusion of the alveolar spaces (Fig. 5A). These findings are consistent with a significant attenuation in airway inflammation observed in mice lacking Nlrp3, but not Nlrc4, following pulmonary challenge with K. pneumoniae (Fig. 5B).
Figure 5. Nlrp3 deficient mice demonstrated significantly attenuated airway inflammation following Klebsiella pneumoniae infection.
A) In comparison to WT and Nlrc4 deficient mice, mice lacking Nlrp3 showed decreased inflammatory cell recruitment and less occlusion of the alveolar spaces following K. pneumoniae infection. Mice were challenged with 7.4×104 CFUs of K. pneumoniae. Whole lungs were harvested 48 hrs post-infection and each lobe was assessed and scored at specific locations along the main bronchi. Representative histology sections from the apical region of the main bronchi of the large lobe (10× magnification) are shown. B) Histology images were evaluated for a variety of inflammatory parameters and scored between 0 (absent) and 3 (severe). Significant attenuation in airway inflammation was observed in K. pneumoniae challenged mice lacking Nlrp3 p < 0.05). Mock, n=3; Nlrp3−/−, n=6; Nlrc4−/−, n=6; WT, n=12.
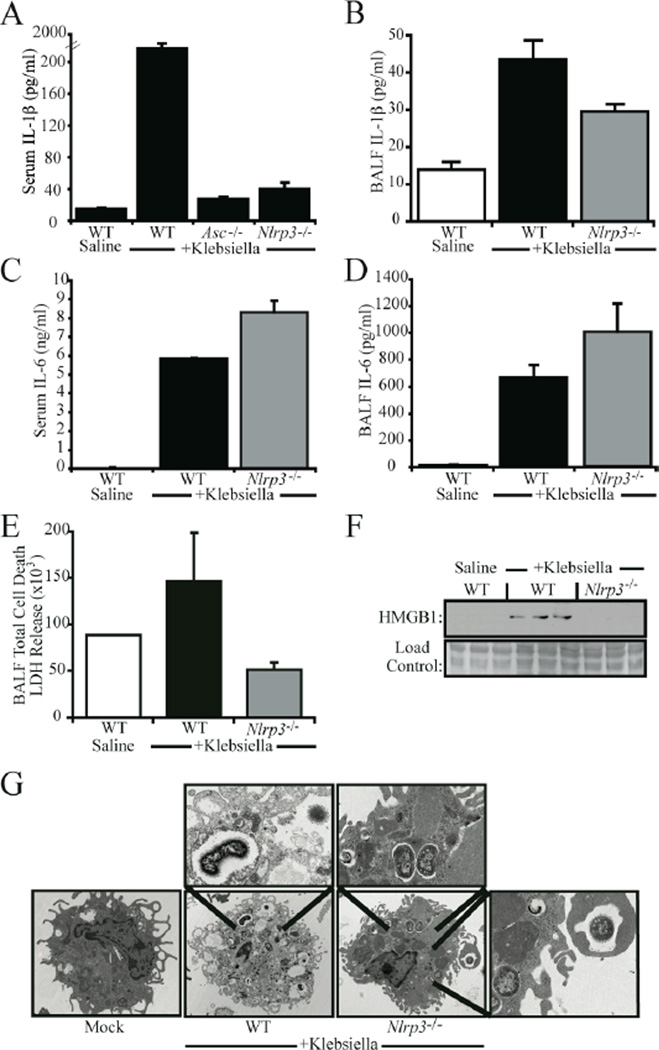
In vivo levels of IL-1β and cell death are reduced in K. pneumonia challenged Nlrp3−/− mice
We have demonstrated that NLRP3 is required for necrosis in both human and mouse cell cultures challenged with Klebsiella. We further demonstrate that NRLP3 mediates pulmonary inflammation and protects against the bacterial infection. We next sought to assess the in vivo relevance of this gene in cytokine production, bacterial-induced necrosis and HMGB1 release. Processes that regulate the latter two have not been extensively studied in an in vivo setting. To determine the physiologic effects of NRLP3, we first measured IL-1β levels in the BALF and serum of K. pneumoniae infected mice. Deletion of Nlrp3 caused a near-abrogation of serum IL-1β, which is consistent with the important role NRLP3 plays in IL-1β production by monocytes (Fig. 6A). Deletion of Asc also resulted in marked decreases in circulating levels of IL-1β (Fig. 6A). IL-1β in the BALF was modestly reduced in the Nlrp3−/− mice, a finding that is similar to other in vivo analyses (13). The modest effect is likely due to redundant NLR proteins in the lung stroma that remained intact in these mice. This decrease in IL-1β was accompanied by a modest increase of IL-6 observed between Nlrp3−/− and WT mice in serum and BALF samples (Fig. 6C–D), which is consistent with the cytokine array analysis of in vitro cultured cells shown in Fig. 3. No significant differences were observed in the cellular composition of the BALF between genotypes (Table S2). Deletion of Nlrp3 also decreased overall levels of cell death in BALF samples as determined by LDH release (Fig. 6E). To examine whether NRLP3 is responsible for the induction of HMGB1, HMGB1 levels were measured in serum samples of K.pneumoniae challenged mice. As measured by western blot analysis, Klebsiella-induced HMGB1 release was substantially abrogated in Nlrp3−/− mice (Fig. 6F). Having determined that NLRP3 was involved in the initiation of cell death in response to K. pneumoniae in vivo, we sought to determine if this cell death was morphologically consistent with necrosis. Cells collected from BALF samples obtained from K. pneumoniae infected animals were subjected to electron microscopy analysis. Cells harvested from WT animals demonstrate several morphological features consistent with necrosis, including loss of plasma membrane integrity and lack of chromatin condensation (Fig. 6G). In contrast, cells obtained from Nlrp3 deficient animals exhibit no signs of cell death, despite the presence of several intracellular K. pneumoniae bacteria (Fig 6G). These results indicate that Nlrp3 mediates K. pneumoniae induced inflammation and cell death in vivo, and that this cell death is morphologically and biochemically consistent with necrosis.
Figure 6. NRLP3 regulated Klebsiella pneumoniae induced IL-1β and necrotic cell death in vivo.
A) Serum levels of IL-1β were significantly reduced in both Nlrp3 and Asc deficient mice challenged with K. pneumoniae p<0.05). B) A modest, yet significant, decrease in K. pneumoniae induced IL-1β was observed in the BALF of Nlrp3−/− mice compared to WT (p<0.05). In contrast, IL-6 levels were elevated in the serum (C) and BALF (D) of Nlrp3−/− mice following K pneumoniae infection. IL-1β and IL-6 were measured by ELISA. E) Decreased levels of cell death were detected in the BALF of K. pneumoniae infected Nlrp3 deficient mice as determined by LDH release. F) Serum levels of HMGB1 were dramatically reduced in mice lacking Nlrp3 as determined by western blot analysis. G) Cells obtained by BALF were collected from K. pneumoniae challenged WT and Nlrp3 deficient animals and were subjected to electron microscopy analysis. Cells from WT mice exhibited cell death features morphologically consistent with necrosis. In contrast, cells obtained from Nlrp3 deficient animals did not exhibit a morphology indicative of cell death. Magnified intracellular K. pneumoniae are shown in insets.
Discussion
Several recent reports have established a role for NRLP3 in mediating pathogen-induced inflammation in vitro, but the majority of evidence has been provided using ex vivo cultures obtained from gene-deletion mice. Here, we identify NRLP3 as a critical in vivo effector of the host immune response to K. pneumonia, a major cause of community-acquired bacterial pneumonia. K. pneumoniae was selected for analysis because it induces a flagrant cytokine and inflammatory response, including the release of HMGB1, and significant necrosis in the lung, thus allowing us to assess the effect of NRLP3 and ASC on all three processes. This is the first combined in vitro and in vivo demonstration that an NLR molecule is a key regulator of not only IL-1β maturation, but also HMGB1 release and necrotic cell death in response to pathogen exposure. Furthermore, these results demonstrate the in vivo consequences of NRLP3 activity on host survival and inflammation. Despite substantial decreases in lung inflammation and tissue destruction (as evaluated by cytokine analysis and criteria specified in Materials and Methods), mice lacking Nlrp3 demonstrate increased susceptibility to Klebsiella-induced lethality. This finding confirms that NRLP3 activity contributes to protective host responses to bacterial pathogens via both inflammasome-dependent and independent processes. This is similar to recent findings regarding the role of NRLP3 during infection by influenza virus where the Nlrp3 gene is found to be important for increased pulmonary inflammation, cellular infiltrate and IL-1β/IL-18 production, all of which coincide with increased animal survival and decreased viral load (37, 38).
After S. flexneriK. pneumoniae is the second gram negative bacterial pathogen identified which activates the NLRP3-dependent cell death program termed pyronecrosis (8). This pathway of cell death has morphological features characteristic of necrosis, and similar to necrosis is inherently pro-inflammatory. Cellular components spill out from the pyronecrotic cell into the microenvironment. Among these components is HMGB1, a nuclear protein which takes on the role of a powerful pro-inflammatory cytokine when released from the cell (17, 39). HMGB1 stimulates the RAGE, TLR2, and TLR4 receptors on neighboring monocytes and macrophages and results in the induction of several inflammatory cytokines, including TNFα and IL-1β (40–42). K. pneumoniae induces a significant increase in the systemic levels of HMGB1 in the WT mice, while no HMGB1 is observed in the serum from Nlrp3−/− animals. This NLRP3-dependent release of HMGB1 is also observed in human THP-1 monocytic cells challenged with Klebsiella. Caspase-1 inhibitors failed to abrogate NLRP3-mediated HMGB1 release, suggesting that this phenomenon does not require inflammasome activity. It should be noted that HMGB1 levels are significantly increased in human septic patients, including those with K. pneumoniae sepsis (27). Neutralization of HMGB1 is currently under investigation as a therapeutic target for the intervention of sepsis, bacteremia, and induced acute respiratory distress syndrome (43, 44). Though a broad inhibition of NLRP3-dependent responses may be detrimental to host survival, neutralization of HMGB1 may provide an opportunity to abrogate NLRP3-mediated sepsis or bacteremia without increasing host mortality.
Pyronecrosis serves as an interesting contrast to pyroptosis, another form of pathogen-induced cell death (45). While pyronecrosis requires cathepsin B but not caspase activity, pyroptosis requires the activity of caspase-1 (21). These two pathways also appear to be induced by different stimuli and involve different NLRs. Pyronecrosis has been observed in monocytic cells infected with 50 MOI of Shigella flexneri, an MOI previously shown to cause necrosis (8). Pyroptosis has been observed in monocytic cells infected with Salmonella (31, 46), Pseudomonas aeruginosa and low dose (<10 MOI) S. flexneri (31, 46–48). Interestingly, though ASC is required for activation of caspase-1, deletion of ASC does not abrogate caspase-1 dependent pyroptosis initiated by P. aeruginosa (47).
Our in vitro and in vivo results indicate that NLRP3-dependent pyronecrosis is the predominant cell death and inflammation pathway induced by Klebsiella. In contrast to the Salmonella-induced pyroptosis pathway which is dependent on caspase-1 and NLRC4, ablation of the Nlrc4 or caspase-1 gene has minimal effect on inflammation or cell death induced by K. pneumoniae. Interestingly, cathepsin B inhibitors not only block NRLP3-mediated pathogen induced cell death, but also block the induction of IL-1β maturation by K. pneumoniae. A similar requirement for cathepsin B in NRLP3 mediated IL-1β maturation in response to silica crystals, aluminum salts, and lysosomal permeabilization has also been recently reported (16, 49). These finding suggests that a significant portion of K. pneumoniae-induced IL-1β release might lie downstream of pyronecrosis, which is not surprising given the strong pro-inflammatory activity of HMGB1.
Previously, we demonstrated that NLRP3 and ASC were required for the release of IL-1β and HMGB1 from a human macrophage cell line, THP-1, infected with Shigella flexneri. Here we have shown that the release of IL-1β and HMGB1 is also markedly decreased from Nlrp3 and Asc deficient macrophages infected with K. pneumoniae and extended this finding to a study of the whole animal. Furthermore, our results reveal additional cytokines and chemokines, such as GM-CSF and IL-1α, which are also reduced in the absence of Nlrp3 or Asc. Perhaps to compensate for the lack of these inflammatory mediators, the levels of Mip-1α, IL-6, and RANTES were increased in Nlrp3−/− or Asc−/− cells. The mechanisms by which NRLP3 and ASC regulate the production of these cytokines and chemokines are currently under investigation, however these results demonstrate that NRLP3 and ASC may directly or indirectly influence the inflammatory response to K. pneumoniae, through both inflammasome dependent and independent pathways.
In summary, our results indicate that NLRP3 and ASC are key regulators of necrosis and inflammation associated with K. pneumoniae infections in human and mouse cells. Most important, this body of work demonstrates that altering specific NLRs can dramatically affect the in vivo host immune response in a common and clinically relevant model of Gram-negative bacterial pneumonia. Given the clear phenotype observed in the Nlrp3 and Asc deficient mice, it will be interesting to further define the mechanisms underlying the attenuation of inflammation and increased mortality, with special emphasis on the role of HMGB1. Our findings suggest the cumulative effect of NRLP3 on cytokines and chemokines, cell death, and HMGB1 protects the host from pathogen induced mortality. These findings exemplify the complex interactions associated with NLRP3 in innate immune signaling and potential limitations of therapeutic intervention of NLRP3 activity.
Supplementary Material
Acknowledgements
We thank Drs. John Bertin and Charles Gray (Millenium Pharmaceuticals), Vishva M. Dixit Genentech, Inc), Richard Flavell (Yale University) and Fayyaz Sutterwala (University of Iowa) for supplying the Nlrp3AscNlrc4 and caspase-1 deficient mice. We thank Dr. Beverly Koller (University of North Carolina at Chapel Hill) for her thoughtful direction and technical guidance. We are grateful to everyone in the UNC Microscopy Services Laboratory, especially Victoria Madden, for experimental direction and outstanding technical support.
Footnotes
This work is supported by National Institutes of Health grants AI63031, AI67798 and AI077437. I.C.A. has been supported by a Lineberger Comprehensive Cancer Center Basic Science Research Program Fellowship T32CA009156 and a Thurston Arthritis Center fellowship T32AR007416. He is the recipient of a NIH NRSA postdoctoral fellowship. J.A.D. was supported by the UNC Clinical Translation Science Award-K12 Scholars Program, NIH KL2RR025746 and the Burroughs Wellcome Fund Career Award for Medical Scientists. J.B. was supported by an NIAID award R21-AI061059.
References
- 1.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 3.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, Stein L, Russo R, Goldsmith D, Dent P, Rosenberg HF, Austin F, Remmers EF, Jr. JEB, Rosenzweig S, Komarow H, Shoham NG, Wood G, Jones J, Mangra N, Carrero H, Adams BS, Moore TL, Schikler K, Hoffman H, Lovell DJ, Lipnick R, Barron K, O'Shea JJ, Kastner DL, Goldbach-Mansky Raphaela. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): A new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis & Rheumatism. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, de Saint Basile G. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman HM, Wanderer AA, Broide DH. Familial cold autoinflammatory syndrome: Phenotype and genotype of an autosomal dominant periodic fever. Journal of Allergy and Clinical Immunology. 2001;108:615–620. doi: 10.1067/mai.2001.118790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott MF, Aksentijevich I. The autoinflammatory syndromes. Curr. Opin. Allergy Clin. Immunol. 2002;2:511–516. doi: 10.1097/00130832-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Fujisawa A, Kambe N, Saito M, Nishikomori R, Tanizaki H, Kanazawa N, Adachi S, Heike T, Sagara J, Suda T, Nakahata T, Miyachi Y. Disease-associated mutations in CIAS1 induce cathepsin B-dependent rapid cell death of human THP-1 monocytic cells. Blood. 2007;109:2903–2911. doi: 10.1182/blood-2006-07-033597. [DOI] [PubMed] [Google Scholar]
- 8.Willingham SB, Bergstralh DT, O'Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell RA, Deshmukh M, Hoffman HM, Ting JP. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007;2:147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 Forms an IL-1b-Processing Inflammasome with Increased Activity in Muckle-Wells Autoinflammatory Disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 10.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 12.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008 doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate Immune Activation Through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Willingham SB, Ting JPY, Re F. Cutting Edge: Inflammasome Activation by Alum and Alum's Adjuvant Effect Are Mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to beta-amyloid. Nat Immunol. 2008 doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin Protects against Experimental Sepsis by Inhibiting High-Mobility Group Box 1 Release and by Killing Bacteria. J Immunol. 2008;180:8369–8377. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]
- 20.Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT. Masquerader: High Mobility Group Box-1 and Cancer. Clin Cancer Res. 2007;13:2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 21.Ting JPY, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8:372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 22.Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, McCormack JG, Yu VL. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis. 2002;8:160–166. doi: 10.3201/eid0802.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman C, Ross S, Mahomed AG, Omar J, Smith C. The aetiology of severe community-acquired pneumonia and its impact on initial, empiric, antimicrobial chemotherapy. Respiratory Medicine. 1995;89:187–192. doi: 10.1016/0954-6111(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 24.Keynan Y, Rubinstein E. The changing face of Klebsiella pneumoniae infections in the community. International Journal of Antimicrobial Agents. 2007;30:385–389. doi: 10.1016/j.ijantimicag.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39:31–37. doi: 10.1086/420816. [DOI] [PubMed] [Google Scholar]
- 26.Sahly H, Podschun R. Clinical, bacteriological, serological aspects of Klebsiella infections and their spondylarthropathic sequelae. Clin. Diagn. Lab. Immunol. 1997;4:393–399. doi: 10.1128/cdli.4.4.393-399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a Late Mediator of Endotoxin Lethality in Mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 28.Ledford JG, Kovarova M, Koller BH. Impaired Host Defense in Mice Lacking ONZIN. J Immunol. 2007;178:5132–5143. doi: 10.4049/jimmunol.178.8.5132. [DOI] [PubMed] [Google Scholar]
- 29.Jeyaseelan S, Young SK, Yamamoto M, Arndt PG, Akira S, Kolls JK, Worthen GS. Toll/IL-1R Domain-Containing Adaptor Protein (TIRAP) Is a Critical Mediator of Antibacterial Defense in the Lung against Klebsiella pneumoniae but Not Pseudomonas aeruginosa. J Immunol. 2006;177:538–547. doi: 10.4049/jimmunol.177.1.538. [DOI] [PubMed] [Google Scholar]
- 30.Kostina E, Ofek I, Crouch E, Friedman R, Sirota L, Klinger G, Sahly H, Keisari Y. Noncapsulated Klebsiella pneumoniae Bearing Mannose-Containing O Antigens Is Rapidly Eradicated from Mouse Lung and Triggers Cytokine Production by Macrophages following Opsonization with Surfactant Protein D. Infect. Immun. 2005;73:8282–8290. doi: 10.1128/IAI.73.12.8282-8290.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 32.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, Vogel SN, Braunstein M, Ting JP-Y. The CATERPILLER Protein Monarch-1 Is an Antagonist of Toll-like Receptor-, Tumor Necrosis Factor α-, Mycobacterium tuberculosis-induced Pro-inflammatory Signals. J. Biol. Chem. 2005;280:39914–39924. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taxman DJ, Livingstone LR, Zhang J, Conti BJ, Iocca HA, Williams KL, Lich JD, Ting JP, Reed W. Criteria for effective design, construction, and gene knockdown by shRNA vectors. BMC Biotechnol. 2006;6:7. doi: 10.1186/1472-6750-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly-Welch AE, Melo ME, Smith E, Ford AQ, Haudenschild C, Noben-Trauth N, Keegan AD. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. J Immunol. 2004;172:4545–4555. doi: 10.4049/jimmunol.172.7.4545. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor W, Jr, Harton JA, Zhu X, Linhoff MW, Ting JP-Y. Cutting Edge: CIAS1/Cryopyrin/PYPAF1/NALP3/ CATERPILLER 1.1 Is an Inducible Inflammatory Mediator with NF-kB Suppressive Properties. J. Immunol. 2003;171:6329–6333. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 37.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas PG, Dash P, Aldridge, Jr. JR, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunden-Cullberg J, Norrby-Teglund A, Treutiger CJ. The role of high mobility group box-1 protein in severe sepsis. Curr. Opin. Infect. Dis. 2006;19:231–236. doi: 10.1097/01.qco.0000224816.96986.67. [DOI] [PubMed] [Google Scholar]
- 40.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, Morser J, Stern D, Schmidt AM. The Receptor for Advanced Glycation End Products (RAGE) Is a Cellular Binding Site for Amphoterin. J. Biol. Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 41.Andersson U, Wang H, Palmblad A-C, Aveberger K, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High Mobility Group 1 Protein (HMG-1) Stimulates Proinflammatory Cytokine Synthesis in Human Monocytes. J. Exp. Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JS, Svetkauskaite D, He J-Y, Kim Q, Strassheim D, Ishizaka A, Abraham E. Involvement of Toll-like Receptors 2 and 4 in Cellular Activation by High Mobility Group Box 1 Protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 43.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. Cutting Edge: HMG-1 as a Mediator of Acute Lung Inflammation. J. Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 44.Mantell LL, Parrish WR, Ulloa L. Hmgb-1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25:4–11. doi: 10.1097/01.shk.0000188710.04777.9e. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes-Alnemri T, Wu J, Yu J-W, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Molecular Microbiology. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 47.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 49.Willingham SB, Ting JPY. NLRs and the dangers of pollution and aging. Nat Immunol. 2008;9:831–833. doi: 10.1038/ni0808-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.