Abstract
Background
While positive associations have consistently been reported between sleep disruption and breast cancer, less is known about its potential role in prostate cancer.
Methods
Within the prospective AGES-Reykjavik cohort study, we followed 2,102 men recruited in 2002–2006 until the end of 2009. Participants answered questions on sleep disruption. Information on the occurrence of prostate cancer was obtained through record-linkages across the Icelandic Cancer Registry. We used Cox regression models with 95% confidence intervals [CIs] to estimate hazard ratios [HR] of prostate cancer by symptoms of sleep disruption.
Results
During follow-up, 135 men (6.4%) were diagnosed with prostate cancer. Compared to men without sleep disruption, those with problems falling and staying asleep were at significantly increased risk of prostate cancer [HR, 1.7 (95% CI, 1.0–2.9) and 2.1 (95% CI, 1.2–3.7)], respectively, with increasing sleep disruption severity. When restricted to advanced prostate cancer (≥ stage T3 or lethal disease), these associations became even stronger [HRs 2.1 (95% CI, 0.7–6.2) and 3.2 (95% CI, 1.1–9.7)]. The results did not change after excluding from the analyses men who woke up during the night, indicative of nocturia, suggesting limited risk of reverse association.
Conclusions
Our data suggest that certain aspects of sleep disruption may confer an increased risk of prostate cancer and call for additional, larger studies with longer follow-up times.
Impact
Prostate cancer is one of the leading public health concerns in men; if confirmed in future studies the association between sleep disruption and prostate cancer risk may open new avenues for prevention.
Keywords: prostate cancer, circadian disruption, sleep disruption, cohort study, melatonin, light at night
Introduction
In 2007 the International Agency for Research on Cancer (IARC) designated shift work involving circadian disruption as a probable carcinogen in humans (Group 2A) (1). In addition to extensive animal and in vitro studies (2), the ruling was based primarily on data showing that breast cancer risk among women working night shifts was ~50% higher as compared to those who had not worked night shifts (3). While data examining this hypothesis for prostate cancer risk among men are more sparse (4), two Japanese cohort studies and two Canadian case-control studies have suggested an association between shift work and prostate cancer risk (5–8), although a Swedish cohort study reported no association (9).
One of the major behavioral consequences of night shift work is displacement of the sleep-wake cycle, which results in shift workers having difficulty falling asleep and staying asleep when they attempt to sleep during the day (10). Short night-time sleep has been shown to be associated with an increased risk of prostate cancer in non-shift working men (11), suggesting that sleep per se may be an important contributing risk factor. Further, current sleep problems seem to be indicative of persistent sleep disruption over time(12). We therefore examined the association between sleep disruption and prostate cancer risk in the population-based AGES-Reykjavik cohort. We hypothesized that men with disruption of sleep would have an increased risk of prostate cancer as compared to men without sleep disruption.
Materials and Methods
Study population and material
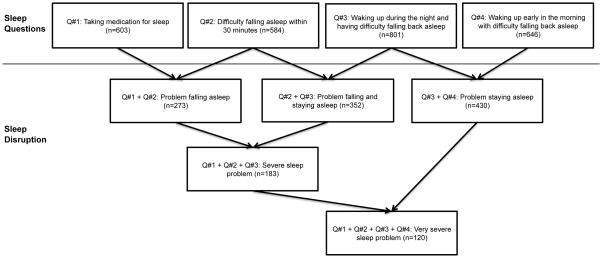
The AGES-Reykjavik study included 2,425 men aged 67 to 96 years who were randomly drawn from an established population-based cohort, the Reykjavik study, and recruited in 2002–2006. The AGES-Reykjavik study has been described in detail by Harris et al. (13). At study entry all men completed a detailed questionnaire, including the five following questions on sleep: (1) “How often do you take medicines to help you sleep?”; (2) “How often do you experience not getting to sleep within 30 minutes?”; (3) “How often do you wake up during the night having difficulty getting back to sleep?”; (4) How often do you wake up early in the morning having difficulty getting back to sleep?”, and; (5) “How often are you feeling unrested during the day no matter how many hours of sleep you had?”. We excluded Question #5 in our analysis as it did not address sleep behaviour specifically. There were 5 answer categories: “Never or almost never”, “Less than once a week”, “1–2 times per week”, “3–5 times per week”, or “6–7 times per week”. We combined the four sleep questions in various ways to group symptoms consistent with problems falling asleep, problems staying asleep, or both, and the severity of each (Figure 1). Our rationale for the combination of the sleep questions was based on the symptomology of different types of sleep problems. For example, Questions 1 and 2 are indicative of difficulty falling asleep, which might occur in sleep-onset insomnia, whereas Questions 3 and 4 denote problems staying asleep, a common compliant in sleep-maintenance insomnia. The combinations of three of more complaints was an attempt to assess severity of sleep complaints. While it is not possible to confirm a clinical sleep disorder in the current dataset, the combinations are based on logic consistent with known sleep disorders. Those with sleep problems of any type were classified as having any answer other than “Never or almost never”, which was used for comparison.
Figure 1.
Categorization of sleep disruption according to combination of four questions (Q) on sleep from the AGES-Reykjavik Cohort.
n=number of participants who have specified sleep problem (any other answer than “never or almost never”)
Of the 2,425 men in the cohort we excluded 104 men who did not answer the questions on sleep and 215 men who had been diagnosed with prostate cancer before study entry. Thus, none of the participants had been diagnosed with prostate cancer at study entry. Further, 4 men who were censored at diagnosis of other cancer, leaving 2,102 men to form our base population.
Covariates
We collected information on several factors that could potentially confound the association between sleep disruption and prostate cancer. From the questionnaire at study enrollment we obtained information on age at study entry; family history of prostate cancer (father/brother/son); visit to doctor during previous 12 months for any type of illness, injury or health check-up; level of education (elementary school/secondary school/college/university); smoking status (never smoked/ past smoker of at least 100 cigarettes or 20 cigars in lifetime/current smoker); alcohol use (g/week); and diagnosis of benign prostate disease (yes/no). We obtained information on body mass index (BMI, m/kg2) from the clinical examination records and presence of diabetes mellitus was based on self-report, a fasting blood glucose of ≥ 126 mg/d, or medication use.
Follow-up and ascertainment of outcome
The men were followed through December 31, 2009 for the occurrence of prostate cancer and all-cause mortality. Using unique identification numbers assigned to all Icelandic citizens, we performed record linkages across: the nationwide Icelandic Cancer Registry (14–16) to obtain information on prostate cancer diagnoses (over 95% are histologically verified) (17), and; the Statistics Iceland for Causes of Death Register (18) to obtain information on prostate cancer-specific death and all-cause mortality. The cancer registry receives infomation on TNM stage of prostate cancer from medical records; the TNM stage was available for only 68% of the cases. We did not have information on Gleason grade. Advanced prostate cancer was defined as anatomic stage T3 or T4 or N1/M1 at diagnosis according to the TNM staging system, i.e. when the cancer has spread through the prostatic capsule, invaded nearby structures, or has spread to lymph nodes or other organs. To obtain a more complete picture of advanced disease, men who died from prostate cancer were also classified as having advanced disease, regardless of the stage at diagnosis; all of the death-specific diagnoses had previously been retreived from the cancer registry (Figure 2).
Figure 2.
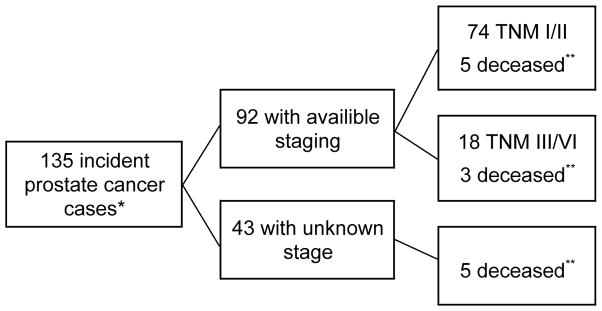
Information on TNM staging and causes of death due to prostate cancer.
*All of the incident cases were identified through record linkage with the Icelandic Cancer Registry.
**Information on cause-specific death was obtained through record linkage with the Statistics Iceland.
Statistics
We present the distribution of potential covariates according to categories of sleep disruption. We used Cox regression models to estimate age-adjusted hazard ratios [HRs] with 95% confidence intervals [CIs] for total and advanced incident prostate cancer, as well as added potential covariates in two additional multivariable models. The covariates selected were based on potential confounding effects or factors other than circadian disruption that may be related to sleep and prostate cancer. The second model was further adjusted for family history of prostate cancer, education, visit to a doctor in previous 12 months, diagnosis of benign prostate disease, BMI and diabetes mellitus; the third model additionally controlled for smoking and alcohol consumption. As age- and multivariate-adjusted results were similar and power was limited in the analyses, we present age-adjusted HRs as our main results. We imputed missing values of BMI and alcohol use using the mean. For ordinal variables, we used the missing indicator method for handling missing data by creating a separate category for missing data and a new indicator variable to designate missingness. The category with the most missing data was education with 55 missing values (2.6% of all men). We used SPSS Software version 19.0 (SPSS Inc., 2010, IBM Chicago, IL, www.spss.com) for all statistical analysis.
To assess potential reverse association bias, whereby undiagnosed prostate cancer might cause sleep disturbance, we performed several sensitivity analyses. First, we repeated our analyses after excluding cases diagnosed within two years after study entry. Second, we excluded men who reported waking up during the night (Question #3) since men with nocturia related to undiagnosed prostate cancer may be more likely to wake up during the night, and hence report sleep disruption. Men reporting taking medication for sleep (Question #1) were also excluded in this sensitivity analysis. Therefore, in this secondary analysis, we limited sleep disruption to difficulties falling asleep (Question #2) and early morning awakening (Question #4).
Ethical approval
The study protocol was approved by the Icelandic Ethical Review Board and the Icelandic Data Protection Authority.
Results
Baseline characteristics
During the mean 5.0 years of follow-up, 135 of 2,102 eligible men (6.4%) were diagnosed with prostate cancer. Information on disease staging was available for 92 men (68%) of whom 16 (17%) had advanced TNM stage. In addition to the 16 men with advanced disease, 10 men who died from prostate cancer but had localized disease or unknown stage at diagnosis were classified as having advanced disease: leaving us with 26 men (19%) with advanced prostate cancer.
The characteristics of the participants are presented in Table 1, according to presence or absence of sleep disruption. Between 5.7 and 20.5 percent of the men were classified with sleep disruption, depending on the type of sleep problem. The comparison group consisted of 755 men (36% of total) who did not report any sleep disturbances for any of the four questions. The mean age of participants at baseline was 76.4 years and mean BMI 26.9 m/kg2. Men with and without sleep problems were similar with respect to age, education, family history of prostate cancer, smoking status, and BMI but those with sleep disruption were more likely to have visited a doctor in the previous 12 months and to have been diagnosed with diabetes mellitus. The men with problems getting to sleep and staying asleep (see Figure 1 for definitions) were more likely to have benign prostatic disease. Only the men with very severe sleep problems were more likely to consume more alcohol.
Table 1.
Characteristics of the Male Participants in the AGES-Reykjavik Cohort by Sleep Disruption (four sleep questions), Iceland, 2002–2009.
| Category | Characteristic | Total (N= 2,102) No. (%) or Mean (SD) | Problem Falling Asleepa No. (%) or Mean (SD) | Problem Staying Asleepa No. (%) or Mean (SD) | Problem Falling and Staying Asleepa No. (%) or Mean (SD) | Severe Sleep Problema No. (%) or Mean (SD) | Very Severe Sleep Problema No. (%) or Mean (SD) | No Sleep Problemb (n=755) No. (%) or Mean (SD) |
|---|---|---|---|---|---|---|---|---|
| Prevalence of sleep problem | 662 (31.5) | 273 (13.0) | 430 (20.5) | 352 (16.7) | 183 (8.7) | 120 (5.7) | 0 | |
| Number of cases | 135 | 27 | 34 | 29 | 21 | 16 | 49 | |
| Agec, years | 76.4 (5.3) | 77.3 (5.1) | 76.8 ( 5.3) | 76.9 (5.4) | 77.0 (5.0) | 77.2 (5.1) | 76.0 (5.2) | |
| Education | Elementary | 339 (16.5) | 44 (16.6) | 70 (16.9) | 60 (17.4) | 29 (16.3) | 18 (15.7) | 125 (16.9) |
| Secondary | 1091 (53.2) | 146 (55.1) | 218 (52.5) | 191 (55.4) | 97 (54.5) | 59 (51.3) | 391 (52.8) | |
| College | 255 (12.4) | 33 (12.5) | 47 (11.3) | 36 (10.4) | 21 (11.8) | 15 (13.0) | 101 (13.6) | |
| University | 367 (17.9) | 42 (15.8) | 80 (19.3) | 58 (16.8) | 31 (17.4) | 23 (20.0) | 123 (16.6) | |
| Family history of prostate cancer | 194 (9.2) | 25 (9.2) | 33 (7.7) | 33 (9.4) | 16 (8.7) | 6 (5.0) | 70 (9.3) | |
| Visit to doctor in previous 12 months | 1714 (81.7) | 237 (86.8) | 375 (87.2) | 302 (85.8) | 159 (86.9) | 105 (87.5) | 569 (75.4) | |
| Diagnosed as diabetic | 365 (15.7) | 57 (20.9) | 76 (17.7) | 68 (19.3) | 39 (21.3) | 21 (17.5) | 115 (15.2) | |
| Smoking status | Never | 581 (28.2) | 61 (23.0) | 108 (26.0) | 84 (24.3) | 40 (22.5) | 33 (28.7) | 239 (32.3) |
| Previously | 1234 (60.0) | 177 (66.8) | 268 (64.4) | 218 (63.2) | 121 (68.0) | 75 (65.2) | 428 (57.8) | |
| Current | 242 (11.8) | 27 (10.2) | 40 (9.6) | 43 (12.5) | 17 (9.6) | 7 (6.1) | 74 (10.0) | |
| Benign prostate disease | 723 (34.4) | 116 (42.5) | 157 (36.5) | 133 (37.8) | 77 (42.1) | 47 (39.2) | 231 (30.6) | |
| Alcoholc, g/week | 22.4 (42.7) | 24.2 (45.8) | 24.3 (44.7) | 24.7 (48.6) | 26.5 (51.3) | 29.4 (56.6) | 20.8 (42.6) | |
| Body mass indexc, m/kg2 | 26.9 (3.8) | 27.0 (3.8) | 26.8 (3.8) | 27.0 (3.8) | 27.0 (3.6) | 26.8 (3.6) | 27.0 (3.9) |
Less than once per week up to 6 times per week;
Never or almost never;
Mean (Standard deviation)
Sleep disruption and risk of prostate cancer
Compared to men who did not report any sleep problems, in age-adjusted analyses, those who reported problems falling and staying asleep (Figure 1) were significantly at increased risk of prostate cancer with a hazard ratio of 1.6 (95% CI, 1.0–2.5), 1.7 (95% CI, 1.0–2.9), and 2.1 (95% CI, 1.2–3.7), respectively for increase in severity of problems falling or staying asleep (Table 2). The association did not change materially after adjustment for potential confounding factors. The association was stronger for advanced prostate cancer than for overall prostate cancer for all types of sleep problems, especially for very severe sleep problems (HR, 3.2; 95% CI, 1.1–9.7), when compared to men without sleep problems.
Table 2.
Estimated Risk of Prostate Cancer by Sleep Disruptiona among Males in the AGES-Reykjavik Cohort.
| Category | Characteristic | No. of Cases | Person years | Hazard Ratiob (95% CI) | Hazard Ratioc (95% CI) | Hazard Ratiod (95% CI) |
|---|---|---|---|---|---|---|
| Problem falling asleep | Total prostate cancer | |||||
| Q#1 + Q#2 | No sleep disruption | 49 | 3809 | Ref | Ref | Ref |
| Sleep disruption | 27 | 1385 | 1.6 (1.0–2.5) | 1.6 (1.0–2.6) | 1.6 (1.0–2.6) | |
| Advanced disease | ||||||
| No sleep disruption | 9 | 3809 | Ref | Ref | Ref | |
| Sleep disruption | 6 | 1385 | 1.7 (0.6–4.8) | 1.9 (0.7–5.4) | 1.8 (0.6–5.3) | |
| Problem staying asleep | Total prostate cancer | |||||
| Q#3 + Q#4 | No sleep disruption | 49 | 3809 | Ref | Ref | Ref |
| Sleep disruption | 34 | 2211 | 1.2 (0.8–1.9) | 1.2 (0.8–1.9) | 1.2 (0.8–1.9) | |
| Advanced disease | ||||||
| No sleep disruption | 9 | 3809 | Ref | Ref | Ref | |
| Sleep disruption | 9 | 2211 | 1.6 (0.6–4.1) | 1.7 (0.7–4.4) | 1.7 (0.7–4.3) | |
| Problem falling and staying asleep | Total prostate cancer | |||||
| Q#2 + Q#3 | No sleep disruption | 49 | 3809 | Ref | Ref | Ref |
| Sleep disruption | 29 | 1807 | 1.3 (0.8–2.0) | 1.3 (0.8–2.0) | 1.3 (0.8–2.0) | |
| Advanced disease | ||||||
| No sleep disruption | 9 | 3809 | Ref | Ref | Ref | |
| Sleep disruption | 8 | 1807 | 1.7 (0.6–4.4) | 1.8 (0.7–4.8) | 1.7 (0.7–4.6) | |
| Severe sleep problem | Total prostate cancer | |||||
| Q#1 + Q#2 + Q#3 | No sleep disruption | 49 | 3809 | Ref | Ref | Ref |
| Sleep disruption | 21 | 952 | 1.7 (1.0–2.9) | 1.7 (1.0–2.9) | 1.7 (1.0–2.9) | |
| Advanced disease | ||||||
| No sleep disruption | 9 | 3809 | Ref | Ref | Ref | |
| Sleep disruption | 5 | 952 | 2.1 (0.7–6.2) | 2.2 (0.7–6.8) | 2.2 (0.7–6.9) | |
| Very severe sleep problem | Total prostate cancer | |||||
| Q#1 + Q#2 + Q#3 + Q#4 | No sleep disruption | 49 | 3809 | Ref | Ref | Ref |
| Sleep disruption | 16 | 606 | 2.1 (1.2–3.7) | 2.1 (1.2–3.8) | 2.2 (1.2–3.9) | |
| Advanced disease | ||||||
| No sleep disruption | 9 | 3809 | Ref | Ref | Ref | |
| Sleep disruption | 5 | 606 | 3.2 (1.1–9.7) | 3.5 (1.1–10.7) | 3.8 (1.2–11.7) | |
Four questions on sleep (Q#1, Q#2, Q#3, and Q#4) combined in different categories. See figure 1 for the definitions.
Age-adjusted HR
Additional adjustment for family history of prostate cancer, benign prostate disease, education, visit to a doctor in previous 12 months, BMI, and diabetes mellitus
Additional adjustment for smoking and alcohol
Sensitivity analyses
After excluding men who were diagnosed with prostate cancer within two years from study entry, too few advanced cases remained to conduct the 2-years lagged analyses. However, the association between sleep disruption and prostate cancer remained after excluding men with potential symptoms of nocturia (men who reported waking up during the night), with an age-adjusted HR of 2.2 (95% CI, 1.3–3.7) for overall prostate cancer (68 cases) and and 3.3 (95% CI, 1.2–9.3) for advanced disease (15 cases).
Discussion
In this prospective cohort study we found that men with sleep disruption were at increased risk of prostate cancer, particularly advanced prostate cancer, when compared to men who did not report any sleep problems.
The association between sleep disruption and prostate cancer was stronger for advanced disease than for overall prostate cancer. This may be a chance finding due to limited number of cases in the analyses for advanced cases. It is also possible that underlying mechanisms of sleep disturbance, such as circadian disruption and reduced melatonin levels, affect prostate cancer progression to a greater extent than prostate cancer initiation (19). Nonetheless, our data support the hypothesis that some aspect related to sleep disruption may confer an increased risk of prostate cancer.
Most epidemiological studies to date on the effect of sleep or circadian rhythm disruption have focused on the impact of shift work on cancer risk. Consistent with the hypotheses for sleep disruption, four studies found an increased risk of prostate cancer among night shift workers (5–8), although one did not (9).
To our knowledge the role of sleep disruption per se, separate from the impact of shift work, has only been assessed in one study on prostate cancer risk. Kakizaki et al. reported that men who slept for 6 hours or less were at non-significant increased risk of prostate cancer (HR, 1.34; 95% CI, 0.83–2.17) and those who slept for 9 hours or more at lower risk (HR, 0.48; 95% CI, 0.29–0.79) when compared to men who slept for 7–8 hours (11). Our data are consistent with this finding and suggest that impairment of sleep, either through reduced sleep duration or greater sleep disruption, increases the risk of prostate cancer. Limited data are indeed available on the direct role of melatonin on prostate cancer risk. Shorter sleep duration and greater sleep disruption may be viewed as a proxy for increased melatonin suppression, given that individuals are likely to be exposed to light when not asleep at night. Bartsch et al. have reported that men with prostate cancer have lower melatonin levels when compared to men with benign prostate hyperplasia (BPH) and young men (20, 21). Interestingly blind men, who may also have reduced exposure to light, have lower prostate cancer incidence when compared to the general population (22, 23), similar to lower breast cancer risk in blind compared to sighted women (24). Further work to establish causality is required, however.
Sleep disruption induced by shift work induces a number of physiological changes that have been proposed as possible mechanisms underlying the observed increase in cancer risk. The endogenous circadian pacemaker, located in the suprachiasmatic nuclei (SCN) of the hypothalamus, is a major determinant of the timing, duration and structure of sleep (25) such that sleep propensity and consolidation are maximized when sleep occurs during the night. Further, disruption of the molecular components of circadian clocks, particularly expression of the Period2 gene (Per2) is thought to have tumor-suppressive properties (26, 27). Notably, expression levels of Per2 were significantly lower in all proliferative prostate diseases compared with normal prostate tissue (28). Also, a major consequence of shift work is light-induced inhibition of pineal melatonin secretion, which is acutely suppressed by the electric light required to enable night-shift work. Melatonin is produced only during the biological night and is the biochemical correlate of darkness; light exposure during the night inhibits melatonin production (29). The presence of melatonin has been shown to inhibit or slow down tumor growth both in vitro and in vivo, including prostate cancer (30–35), whereas suppression of melatonin via constant light exposure or pinealectomy increases tumor growth in a dose-dependent manner in experimental models (36). Melatonin is also a potent free radical scavenger (37) and may facilitate reduction of oxidative stress implicated in prostate cancer progression (19).
The prospective design, complete follow-up and detailed information on a variety of potential confounders, constitute important strengths of our study. Nevertheless, several potential limitations should be considered. First, our definition of sleep disruption rests on the four questions included in the AGES entry questionnaire on problem falling asleep, staying asleep, early morning awakening (with difficulty falling back asleep) and use of sleep medication. These questions have not been validated against objective measures of sleep disruption. Morover, we have no information on the timing or duration of sleep, which can be important additonal factors when assessing sleep disruption. Second, we had limited clinical information at diagnosis, with stage information for only two-thirds of the cases. Our analyses showed that the association was particularly strong for advanced disease, but the small number of cases with advanced disease limited our statistical power and yielded wide confidence intervals. Third, despite inclusion of a wide variety of potential confounding factors in our models, we cannot exclude the possiblity that residual confounding unknown to us may account for these associations. Lastly, and importantly, observation time in our study was short (5 years) and the men only provided information on sleep problems during the prior few months, whereas the time from prostate cancer onset to clinical detection has been estimated to be a decade or more (38, 39). If the carcinogenic effect of sleep disruption on tumour progression was mediated through melatonin suppression, laboratory studies suggest that the impact of reduced melatonin could be quite rapid (36), although there is no parallel clinical evidence in humans. It is also plausible that reports about current sleep problems are indicative of persistent sleep disruption over time (12) that may underlie a longer-term disease process. Nevertheless, the short observation time in our study may raise concerns of reverse association bias; for example, that men with undiagnosed prostate cancer may have symptoms such as nocturia before diagnosis that consequently lead to sleep disturbances. Men with urinary symptoms (hence sleep disruption) related to prostate cancer, especially advanced cancer, often suffer from nocturia (waking up during the night). To address this concern, we conducted sensitivity analyses in which we exluded men with symptoms of sleep disturbance that might be indicative of nocturia. Notably, the point estimates remained essentially unchanged, to some extent alleviating these concerns, although the number of cases were few.
These data lend support to the hypothesis that sleep disruption may affect prostate carcinogenesis. Sleep disruption and light-induced melatonin suppression represent plausible biological explanations underlying cancer risk, although prospective studies are needed to substantiate their respective roles. Large cohort studies entailing longer observation times, allowing for closer investigatons of the temporality of the association between sleep disruption and prostate cancer, will be needed to address this hypothesis further.
Acknowledgments
The authors thank Orn Olafsson and Johanna E. Torfadottir for assistance with statistical analyses and David Havelick for his work.
Grant support
This study was supported byRANNIS (the Icelandic Research Fund) and the Harvard Catalyst Award (CTSA; awards UL1 RR 025758 and KL2 RR 025757) from the National Center for Research Resources, a part of the National Institutes of Health. This study was also funded in part by the US National Institute on Aging contract N01-AG-1-2100, the Intramural Research Program of the National Institute on Aging, the Icelandic Cancer Society, and the Icelandic Heart Association.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007 Dec;8(12):1065–6. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 2.Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005 Jul;27(2):179–88. doi: 10.1385/ENDO:27:2:179. [DOI] [PubMed] [Google Scholar]
- 3.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: World Health Organization, International Agency for Research on Cancer; 2010. [Google Scholar]
- 4.Sigurdardottir LG, Valdimarsdottir UA, Fall K, Rider JR, Lockley SW, Schernhammer E, et al. Circadian Disruption, Sleep Loss, and Prostate Cancer Risk: A Systematic Review of Epidemiologic Studies. Cancer Epidemiol Biomarkers Prev. 2012 May 31; doi: 10.1158/1055-9965.EPI-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubo T, Oyama I, Nakamura T, Kunimoto M, Kadowaki K, Otomo H, et al. Industry-based retrospective cohort study of the risk of prostate cancer among rotating-shift workers. Int J Urol. 2011 Mar;18(3):206–11. doi: 10.1111/j.1442-2042.2010.02714.x. [DOI] [PubMed] [Google Scholar]
- 6.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007 Jan;18(1):182–3. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 7.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006 Sep 15;164(6):549–55. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 8.Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012 Nov 1;176(9):751–9. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 9.Schwartzbaum J, Ahlbom A, Feychting M. Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health. 2007 Oct;33(5):336–43. doi: 10.5271/sjweh.1150. [DOI] [PubMed] [Google Scholar]
- 10.Akerstedt T. Shift work and disturbed sleep/wakefulness. Occup Med (Lond) 2003 Mar;53(2):89–94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]
- 11.Kakizaki M, Inoue K, Kuriyama S, Sone T, Matsuda-Ohmori K, Nakaya N, et al. Sleep duration and the risk of prostate cancer: the Ohsaki Cohort Study. Br J Cancer. 2008 Jul 8;99(1):176–8. doi: 10.1038/sj.bjc.6604425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007 Mar;30(3):274–80. [PubMed] [Google Scholar]
- 13.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007 May 1;165(9):1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Icelandic Cancer Registry. ICR: Reykjavik. Available from: www.cancerregistry.is [cited 2012April 23]
- 15.Jonasson JG, Tryggvadottir L, editors. Cancer incidence in five continents. Iceland: 2007. [Google Scholar]
- 16.Moller B, Fekjaer H, Hakulinen T, Tryggvadottir L, Storm HH, Talback M, et al. Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev. 2002 Jun;11( Suppl 1):S1–96. [PubMed] [Google Scholar]
- 17.Sigurdardottir LG, Jonasson JG, Stefansdottir S, Jonsdottir A, Olafsdottir GH, Olafsdottir EJ, et al. Data quality at the Icelandic Cancer Registry: Comparability, validity, timeliness and completeness. Acta Oncol. 2012 Sep;51(7):880–9. doi: 10.3109/0284186X.2012.698751. [DOI] [PubMed] [Google Scholar]
- 18.Statistics Iceland. SI: Reykjavik. Available from: www.statice.is [cited 2012 April 23]
- 19.Nguyen HL, Zucker S, Zarrabi K, Kadam P, Schmidt C, Cao J. Oxidative stress and prostate cancer progression are elicited by membrane-type 1 matrix metalloproteinase. Mol Cancer Res. 2011 Oct;9(10):1305–18. doi: 10.1158/1541-7786.MCR-11-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartsch C, Bartsch H. Melatonin in cancer patients and in tumor-bearing animals. Adv Exp Med Biol. 1999;467:247–64. doi: 10.1007/978-1-4615-4709-9_32. [DOI] [PubMed] [Google Scholar]
- 21.Bartsch C, Bartsch H, Schmidt A, Ilg S, Bichler KH, Fluchter SH. Melatonin and 6-sulfatoxymelatonin circadian rhythms in serum and urine of primary prostate cancer patients: evidence for reduced pineal activity and relevance of urinary determinations. Clin Chim Acta. 1992 Aug 31;209(3):153–67. doi: 10.1016/0009-8981(92)90164-l. [DOI] [PubMed] [Google Scholar]
- 22.Pukkala E, Ojamo M, Rudanko SL, Stevens RG, Verkasalo PK. Does incidence of breast cancer and prostate cancer decrease with increasing degree of visual impairment. Cancer Causes Control. 2006 May;17(4):573–6. doi: 10.1007/s10552-005-9005-6. [DOI] [PubMed] [Google Scholar]
- 23.Feychting M, Osterlund B, Ahlbom A. Reduced cancer incidence among the blind. Epidemiology. 1998 Sep;9(5):490–4. [PubMed] [Google Scholar]
- 24.Flynn-Evans EE, Stevens RG, Tabandeh H, Schernhammer ES, Lockley SW. Total visual blindness is protective against breast cancer. Cancer Causes Control. 2009 Nov;20(9):1753–6. doi: 10.1007/s10552-009-9405-0. [DOI] [PubMed] [Google Scholar]
- 25.Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980 Dec 12;210(4475):1264–7. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 26.Hua H, Wang Y, Wan C, Liu Y, Zhu B, Yang C, et al. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci. 2006 Jul;97(7):589–96. doi: 10.1111/j.1349-7006.2006.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen-Goodspeed M, Lee CC. Tumor suppression and circadian function. J Biol Rhythms. 2007 Aug;22(4):291–8. doi: 10.1177/0748730407303387. [DOI] [PubMed] [Google Scholar]
- 28.Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res. 2010 Aug;49(1):60–8. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod. 1998 Jan;3(1):13–22. doi: 10.1530/ror.0.0030013. [DOI] [PubMed] [Google Scholar]
- 30.Anisimov VN, Baturin DA, Popovich IG, Zabezhinski MA, Manton KG, Semenchenko AV, et al. Effect of exposure to light-at-night on life span and spontaneous carcinogenesis in female CBA mice. Int J Cancer. 2004 Sep 10;111(4):475–9. doi: 10.1002/ijc.20298. [DOI] [PubMed] [Google Scholar]
- 31.Shiu SY, Law IC, Lau KW, Tam PC, Yip AW, Ng WT. Melatonin slowed the early biochemical progression of hormone-refractory prostate cancer in a patient whose prostate tumor tissue expressed MT1 receptor subtype. J Pineal Res. 2003 Oct;35(3):177–82. doi: 10.1034/j.1600-079x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 32.Xi SC, Siu SW, Fong SW, Shiu SY. Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: association of antiproliferative action of the pineal hormone with mt1 receptor protein expression. Prostate. 2001 Jan 1;46(1):52–61. doi: 10.1002/1097-0045(200101)46:1<52::aid-pros1008>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 33.Shiu SY, Xi SC, Xu JN, Mei L, Pang SF, Yao KM, et al. Inhibition ofmalignant trophoblastic cell proliferation in vitro and in vivo by melatonin. Life Sci. 2000 Sep 15;67(17):2059–74. doi: 10.1016/s0024-3205(00)00792-x. [DOI] [PubMed] [Google Scholar]
- 34.Moretti RM, Marelli MM, Maggi R, Dondi D, Motta M, Limonta P. Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol Rep. 2000 Mar-Apr;7(2):347–51. [PubMed] [Google Scholar]
- 35.Marelli MM, Limonta P, Maggi R, Motta M, Moretti RM. Growth-inhibitory activity of melatonin on human androgen-independent DU 145 prostate cancer cells. Prostate. 2000 Nov 1;45(3):238–44. doi: 10.1002/1097-0045(20001101)45:3<238::aid-pros6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 36.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009 Aug;13(4):257–64. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011 Aug;51(1):1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 38.Savage CJ, Lilja H, Cronin AM, Ulmert D, Vickers AJ. Empirical estimates of the lead time distribution for prostate cancer based on two independent representative cohorts of men not subject to prostate-specific antigen screening. Cancer Epidemiol Biomarkers Prev. 2010 May;19(5):1201–7. doi: 10.1158/1055-9965.EPI-09-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finne P, Fallah M, Hakama M, Ciatto S, Hugosson J, de Koning H, et al. Lead-time in the European Randomised Study of Screening for Prostate Cancer. Eur J Cancer. 2010 Nov;46(17):3102–8. doi: 10.1016/j.ejca.2010.09.034. [DOI] [PubMed] [Google Scholar]