Summary
The C-terminal domain (CTD) of τ subunit of the clamp loader (τc) binds to both the DnaB helicase and the DNA polymerase III α subunit (PolIIIα), and determines their relative positions and orientations on the leading and lagging strands. Here we present a 3.2 Å resolution structure of Thermus aquaticus PolIIIα in complex with τc and a DNA substrate. The structure reveals that the CTD of τc interacts with the CTD of PolIIIα through its C-terminal helix and the adjacent loop. Additionally, in this complex PolIIIα displays an open conformation including the reorientations of the oligonucleotide-binding fold and the thumb domain, which may be an indirect result of crystal packing due to the presence of τc. Nevertheless, the position of the τc on the PolIIIα allows us to suggest an approximate model for how the PolIIIα is oriented and positioned on the DnaB helicase.
Introduction
The replisome is a multi-protein machine that replicates chromosomal DNA. The essential components of the replisome are conserved and include the replicative hexameric helicase that encircles the lagging DNA strand and unwinds the duplex DNA, the primase that synthesizes short RNA primers (10-12 nucleotide), and the DNA polymerase III holoenzyme (Yao and O’Donnell, 2009, 2010). In eubacteria, the replicative DNA PolIII core, the β-sliding clamp, and the clamp loader complex assemble to form the main replicase, PolIII holoenzyme, that is responsible for DNA synthesis (Johnson and O’Donnell, 2005; McHenry, 1988). The PolIII holoenzyme in E. coli contains two PolIII cores, which are composed of the catalytic α subunit (Maki and Kornberg, 1985), the 3′-5′ proofreading exonuclease ε subunit (Scheuermann and Echols, 1984), and the θ subunit that is believed to stabilize the ε subunit and slightly stimulate its proofreading capabilities (Johnson and O’Donnell, 2005; Taft-Benz and Schaaper, 2004). The β-sliding clamp, which is assembled onto the RNA primed initiation sites by the clamp loader complex, is a homodimer that forms a ring surrounding the DNA substrates and ensures the high processivity of the PolIII holoenzyme (Georgescu et al., 2008a; Kong et al., 1992). The clamp loader complex is a multisubunit ATPase (τ3δδ′χψ) (Georgescu et al., 2011; Pritchard et al., 2000), and links the PolIII to the helicase via its τ subunits (Studwell-Vaughan and O’Donnell, 1991). The CTD of E. coli τ (Ecoτ) binds to both the PolIII core and DnaB helicase, holding them together (Gao and McHenry, 2001a, b; Kim et al., 1996).
The interaction of PolIIIα with τ has been well studied in the E. coli system. Nuclear magnetic resonance (NMR) and surface plasmon resonance (SPR) experiments have shown that the last 18 residues of τ are critical for the interaction with PolIIIα (Jergic et al., 2007; Su et al., 2007). Mutagenesis studies on the EcoPolIIIα have revealed that deletion and mutations of the C-terminal region of the polymerase severely diminish its interaction with τ, indicating a role of CTD in the interaction (Dohrmann and McHenry, 2005). Recent structural studies on PolIIIα-Thermus aquaticus PolIIIα (TaqPolIIIα), E. coli PolIIIα (EcoPolIIIα) and Geobacillus kaustophilus PolC (GkaPolC) - have provided structural insights into the mechanism of DNA replication and this interaction (Bailey et al., 2006; Evans et al., 2008; Lamers et al., 2006). PolIIIα contains six domains with different functions: the N-terminal Zn2+-dependent 3′-5′ co-proofreading exonuclease polymerase and histidinol phosphatase (PHP) domain (Stano et al., 2006; Wing et al., 2008), the catalytic palm domain, the incoming nucleotide-interacting fingers domain (Brautigam and Steitz, 1998), the nascent DNA gripping thumb domain (Steitz, 1999), the β-sliding clamp binding domain, and the CTD, which contains an oligonucleotide-binding (OB) fold and a possible external clamp binding site at the extreme C-terminus (Lopez de Saro et al., 2003). The solution structure of the domain V of the Ecoτ which lies at its C-terminus contains six α-helices interspersed with the strands of a three β-sheet fold. However, the structure of the most C-terminal portion of PolIIIα that was believed to interact with τ is absent in the apo enzyme structure. Also, the sequence of the E. coli τc is not homologous to that of a few species, such as Thermus aquaticus or Thermus thermophilus. In order to provide structural insights into the replisome architecture in T. aquaticus, we have determined the structure of TaqPolIIIα in complex with τc and a DNA substrate in the presence of deoxynucleotide triphosphates at 3.2 Å resolution through molecular replacement (MR) combined with single anomalous dispersion (SAD) using heavy atom derivatives. The structure shows that the CTD of τc interacts with the CTD of PolIIIα through its C-terminal helix and the adjacent loop, which provides a basis for constructing an atomic model of the replisome structure. Interestingly, the structure of PolIIIα in this complex displays an open conformation including the movements in the CTD and thumb domains, which is distinct from the previous apo structure (Bailey et al., 2006) and the one with only DNA bound (Wing et al., 2008); this open conformation could be an indirect effect of τc on crystal packing.
Results and Discussion
Overall structure
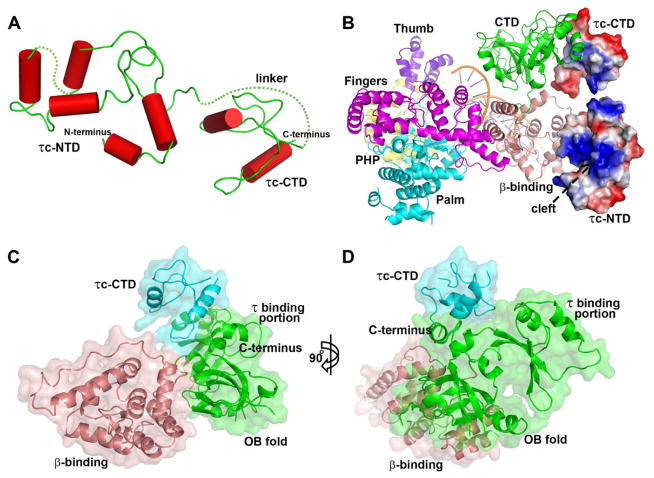
There are four copies of the complex of TaqPolIIIα with τc and DNA per asymmetric unit, with each PolIIIα bound to one τc and one DNA. In each copy, six domains of TaqPolIIIα (PHP, palm, fingers, thumb, β binding, and CTD) are clearly organized as an irregular pyramid around the central active site cavity with an open gate composed of the OB fold of CTD and the thumb domain (Figure 1 and Figures S1–S3). The relative orientations of the domains in the four copies are not identical to each other. The structures of the PHP, palm, fingers and β binding domains of the four copies in the asymmetric unit are similar (Figure S4), suggesting that these structural units are conformationally rigid. However, the relative positions and structures of the CTD, thumb domain, τc, and DNA substrates in the complex show some variations among the four copies, indicating their structural flexibilities (Figure S4). Moreover, the relative positions between the OB fold and the remaining portion of the CTD exhibited slight differences as well among the different copies.
Figure 1.
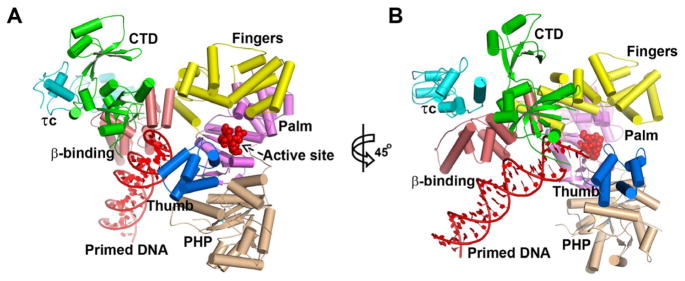
Cartoon views of the PolIIIα-τc-DNA complex. The PHP, palm, thumb, fingers, β-binding, and CTD of PolIIIα, the primed DNA, and τc are labeled with wheat, violet, marine, yellow, salmon, green, red, and cyan colors respectively. The active site metal binding residues (D463, D465 and D618) in the palm domain are shown using red spheres. The right figure (B) is achieved by rotating the left one (A) by 45° along the y axis of the figure plane. See also Figures S1–S3.
The larger number of interactions among different subunits and copies resulted in more regions of PolIIIα being visible in the electron density map compared to the map of the apo structure (Bailey et al., 2006); these include the extreme C-terminus of PolIIIα (residues 1206-1220) that now clearly shows a helical conformation close to the C-terminal portion of τc, additional portions of the CTD (residues 1081-1093 and 1055-1066), and the whole loop (residues 282-305) linking the PHP and palm domain. The third metal atom in the cluster site of the PHP domain, which was predicted to be zinc, but replaced by a water molecule in the apo structure, was built with a zinc ion (Figure S5). Interestingly, the orientation of the three zinc ions and their coordinating residues are quite similar to those of co-catalytic zinc motifs containing nucleases, especially nuclease P1 (Romier et al., 1998).
The DNA substrate is positioned in a distinct state as compared to its orientation and location in the complex with only DNA bound (Wing et al., 2008). None of the DNA substrates in the four copies are positioned in the active site cavity, and each one exhibits a slightly different binding orientation (Figure S4). They are located far from the catalytic site on the palm domain and are not bent. The incorporation of 2′,3′-dideoxycytidine-5′-triphosphate (ddCTP) into the DNA from one copy indicates that DNA synthesis should have happened in solution. The duplex portions are approximately parallel to the long axis of the β binding domain, and are all visible in the electron density. However, they end at the gate formed by the OB fold and the thumb domain and do not enter the active site cavity (Figure 1). The 5′ ssDNA overhang portions, which lie in the cavity, could not be completely built and are quite different among the four copies. The DNA substrates interact with PolIIIα with a contact area of around 500 Å2 per copy, which is less than that of their intermolecular interactions (~610 Å2 per copy). All of these observations, therefore, suggest that the DNA orientation is mainly a result of their intermolecular crystal packing interactions.
τc structure and the PolIIIα-τc interaction
Sequence alignments show that Taqτc and Tthτc share a 54.3% sequence identity and 61.3% homology, however, Taqτc and Ecoτc (mainly composed of domains IV and V) only share a 13.4% sequence identity and 20.3% homology, which means that Taqτc is homologous to Tthτc, but not to Ecoτc (Figure S6). This information is consistent with the structural differences between Taqτc and Ecoτc observed here. The NMR structure of Ecoτ domain V contains six α-helices inter-mixed with a three-stranded β-sheet (Su et al., 2007). However, the structure of Taqτc does not exhibit a similar fold, but rather has two domains that have only α-helices and are linked by a proline-rich loop (Figure 2A). The linker loop (residues 460-486) is disordered and thus invisible in the map. The NTD of Taqτc corresponds to Ecoτ domain IV that binds to both DnaB helicase and DNA (Jergic et al., 2007; Johnson and O’Donnell, 2005). In this structure, the NTD (involving residues 371-376) makes contact with the β binding domain of PolIIIα, which may be a consequence of crystal packing interactions. However, the possibility for the structure revealing a possible weak transient functional interaction would exist as well. Interestingly, there is a cleft on the NTD that exhibits positive surface electrostatic potential (Figure 2B), and may be the region that has been proposed to bind DNA (Jergic et al., 2007). The reason why the interactions between the ssDNA and τc are not observed here may be due to the length of the 5′ overhang being too short for the DNA to bind the N-terminal portion of τc and/or the binding orientation of the DNA seen here not being the catalytically relevant one.
Figure 2.
τc structure and the interactions of the CTD of τc and the CTD of PolIIIα. (A) The structure of τc is composed of two domains that contain only α-helices and are linked by a proline-rich linker. The dotted lines represent the disordered regions. (B) The primed DNA and the six domains of PolIIIα are shown in a schematic representation with different colors, whereas τc is shown in an electrostatic surface representation using PyMOL. The positively charged cleft that may bind ssDNA on the NTD of τc is labeled. (C and D) Surface representation shows the interaction regions of τc CTD and PolIIIα and their relative positions. The NTD of τc is omitted here. The right figure (D) is obtained by rotating the left one (C) by 90° along the y axis of the figure plane. See also Figure S4.
The CTD of Taqτc interacts with the CTD of PolIIIα through its C-terminal helix (residues 531-543) and the following loop (residues 525-530) (Figures 2C and 2D), which is consistent with previous work predicting that the C-terminal portion of Ecoτc forms a helix-loop-helix structure to interact with PolIIIα (Jergic et al., 2007), and that the last 18 residues of Ecoτc are critical for the PolIIIα-τc interaction (Su et al., 2007). These interactions do not appear to support the previous proposal that τ sequesters the polymerase tail from the β-sliding clamp (Lopez de Saro et al., 2003). In T. aquaticus, the CTD of PolIIIα comprises an OB fold (residues 1012-1119), a putative τ binding portion (residues 1128-1220), and an α-helix linker between them. The regions of PolIIIα that were observed binding to τc include the previously proposed portion, and also the linker helix as well as the OB fold. The contact area between the CTD of τc and PolIIIα constitutes 52% of all the τc-PolIIIα interface areas (~650 Å2).
The specific side chain interactions made by the C-terminal helix of τc with the τ binding portion of PolIIIα vary among the four copies, which may be due to the flexibility of the τ binding portion (average RMSD of Cα atoms: 2.17 Å) and/or the perturbation by crystallization; however, the contacts between the loop of τc that follows the C-terminal helix and the CTD of PolIIIα show slight changes, involving hydrophobic interactions and hydrogen bonds. Previous studies have revealed that no single mutation in the region of the predicted loop and C-terminal helix of the Ecoτc could completely disrupt the PolIIIα-τc interaction, and that the mutations in the beginning part of the helix and the possible loop region had larger effects on the binding of PolIIIα (Jergic et al., 2007). Also, the region of EcoPolIIIα that may bind to Ecoτc was identified using mutagenesis studies and appears to be located in its unstructured extreme C-terminus (Dohrmann and McHenry, 2005) that shows to be a α-helix in this complex. Therefore, all of these data suggest that the loop and the beginning part of the helix might be close to or contact the polymerase tail and play a more important role in stabilizing τc-PolIIIα interface in the E. coli system. One region of Taqτc (residues 525-534) makes similar interactions in the four copies and constitutes 83.2% of all the PolIIIα-the CTD of τc interface areas. The average RMSD of their Cα atoms is 0.95 Å.
PolIIIα in complex with τc and DNA displays an open form
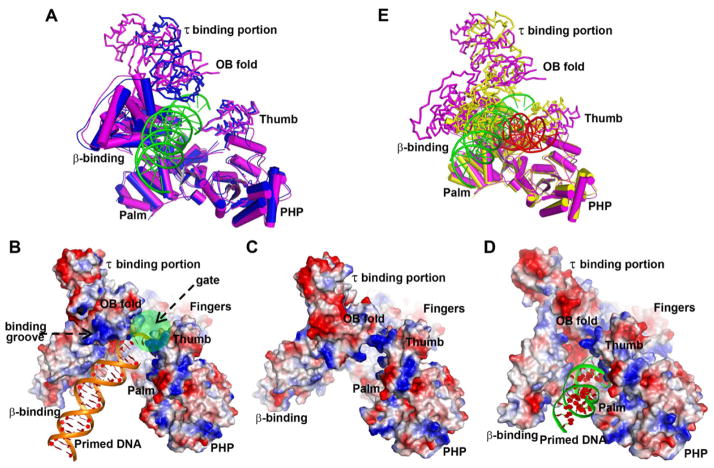
The conformation of PolIIIα in this complex is different from that of the apo enzyme, with the most notable differences being the orientations of the CTD and the thumb domain (Figure 3A) (Bailey et al., 2006). The new orientation of the CTD results in part from a rigid body movement of ~12 Å along y axis of the plane of Figure 3, as well as a 15° rotation of the τ binding portion and a 5° rotation of the OB fold along the x axis; this leads to the increased angle between the OB fold and the τ binding portion. These movements position the ssDNA binding groove on the OB fold facing away from the active site cavity, instead of facing towards the DNA binding site on the thumb domain as is observed in the apo structure (Figures 3B and 3C). In addition, this displacement, together with the movement of the thumb domain (a rotation of 5° along the x axis), results in the formation of an open gate conformation that would enable the entrance or exit of the DNA substrate from the active site cavity. The gate is closed in both the apo structure and the structure of the complex with only DNA bound (Figures 3C and 3D). Since τc makes extensive contacts with all the three portions of the CTD (Figure 2), these conformational changes might result from the binding of τc, however, the influence of crystal packing may be a larger factor. Because the complex assumes a less compact structure, we have called it the “open form”. Furthermore, superimposition of this open form on the previous structure of PolIIIα in complex with DNA which we call the closed form (Figure 3E) (Wing et al., 2008), shows that the binding of the DNA substrate to the complex of PolIIIα with τc will induce significant conformational changes to form the closed conformation of the complex that is used during the leading and lagging strand DNA replication (Evans et al., 2008; McHenry, 2011). While the structure of the complex presented here is influenced by crystal packing, nevertheless, the structure of this complex shows the position of τc on the polymerase thereby providing the first step in understanding how PolIIIα is positioned on DnaB helicase.
Figure 3.
Comparison among the PolIIIα-τc-DNA complex (the open form), the apo-PolIIIα, and the closed form (PolIIIα-DNA complex). τc is omitted here. (A) Superimposition of the structure of the open form (magenta) and that of the apo (blue) on their PHP, palm, fingers, and β-binding domains shows the differences in the positions of the CTD and the thumb domain. (B–D) Electrostatic surface representations of the polymerase molecules in these forms are displayed by PyMOL. (B) The gate, labeled with a green circle, of the PolIIIα-τc-DNA complex is open and the potential DNA binding groove faces away from the active site cavity. The gates in the apo (C) and the PolIIIα-DNA complex (D) are closed. (E) The significant structural differences between the open form (magenta) and the closed form (yellow) are seen in the orientations of the CTD, the thumb domain, the β-binding domain, and DNA by superimposing their PHP, palm and fingers domains. The DNA substrates in them are green and red respectively.
An atomic model of the replisome structure at the replication fork
PolIIIα, β clamp, clamp loader, primase, τc and helicase are important components of replisome. The structure of the complex in this study, together with recent crystal structures of different replisome complexes, allows us to construct a structural model of these components assembled at the replication fork (Figure 4). The closed form of the polymerase (Wing et al., 2008) was chosen for the model of PolIIIα at the replication fork since it is the conformation of the enzyme during the DNA replication. Then, the structure of E. coli β clamp in complex with DNA (PDB code: 3BEP) (Georgescu et al., 2008a) was mapped onto the PolIIIα-DNA complex by superimposing their DNA substrates. The putative internal β binding region (Dohrmann and McHenry, 2005) contacts the hydrophobic groove on the β clamp (Georgescu et al., 2008b) in the model perfectly. The length of DNA substrate from the active site in the palm domain to the β clamp ring is around 24 base pairs (~80 Å). τc was subsequently positioned onto the PolIIIα-β clamp-DNA model by superimposing the CTDs of the two forms of PolIIIα. Since the CTD of the helicase ring (Haroniti et al., 2004; Martinez-Jimenez et al., 2002) has been shown to interact with the NTD of τc (Gao and McHenry, 2001a), the helicase ring was positioned in our model to contact the NTD of τc as proposed in the previous atomic force microscopy model (Haroniti et al., 2004). The structure of the DnaB helicase in complex with ssDNA was utilized here (Itsathitphaisarn et al., 2012). Meanwhile, it appears that the proposed interaction of helicase and τ in this model could also accommodate the observed τ-β binding domain contacts in the structure of the complex. However, since high resolution structural data on the interactions between the helicase and τc have not yet been obtained, the orientations and conformations of the helicase ring and τc are currently only a guess. The primase (PDB code: 2AU3) (Corn et al., 2005) and the clamp loader complex (PDB code: 3GLH) (Simonetta et al., 2009) were then docked into the model according to the previously proposed models (Bailey et al., 2007; Haroniti et al., 2004). In addition, the model presented here only contains two polymerases, but recent studies have shown that there may have the third polymerase involved during the DNA replication (Georgescu et al., 2011; Reyes-Lamothe et al., 2010).
Figure 4.
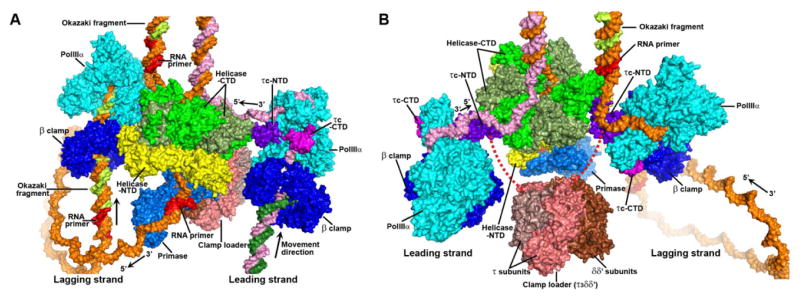
An atomic model of the replisome structure at the replication fork. (A and B) The surface representations of these replisome components are displayed along two different directions. PolIIIα, β clamp, the NTD of τc, the CTD of τc, primase, and the NTD of helicase are labeled with cyan, blue, purple, magenta, marine and yellow respectively. The CTDs of helicase are labeled with green and darkgreen. The five subunits of clamp loader are labeled with salmon, deepsalmon, and brown. The red dash lines represent the loop linking the two domains of the τ subunit. The modeled DNA strands are labeled with orange and pink for the mother strands, and with lime and forest for the daughter ones. The RNA primers are labeled with red. See also Movie S1.
Experimental Procedures
Cloning, Expression, and Purification
See the Supplemental Information for details of vector construction. Thermus aquaticus PolIIIα was expressed and purified as described previously (Bailey et al., 2006). Purification of the τc fragment was achieved by heat treatment and Co2+ affinity chromatography (see Supplemental Experimental Procedures for further details).
Formation of the PolIIIα-τc-DNA complex
The purified PolIIIα was mixed with an excess of τc and purified through gel filtration chromatography (see Supplemental Information for the details). The PolIIIα-τc-DNA complex was formed by directly mixing the PolIIIα-τc complex with 10 mM MgCl2, ddCTP (1 mM), dATP (1 mM) and a 2-fold molar excess of the pre-formed DNA substrate (primer sequence: 5′-cgaaacgacggccagtgcca-3′; template sequence: 5′-tttttttgtggcactggccgtcgtttcg-3′) at room temperature.
Crystallization, Data Collection and Processing
Crystals of the ternary complex were grown in 2–3 days after setting up the drop with 1:1 ratio of the complex sample to the initial well solution: 0.1 M TRIS pH 8.8, 18% (w/v) polyethylene glycol 4000, 0.2 M MgCl2, by using the sitting drop vapor diffusion method at 12 °C. Crystal diffraction was improved to around 3–3.5 Å by optimizing conditions and utilizing a dehydration procedure (see Supplemental Information for further details). Derivatives were prepared by directly soaking dehydrated crystals in the drops containing 10 mM K2PtCl4 or 10 mM HgCl2 for 30 minutes. Data sets were collected at 100K using beamline 8.2.2 at the Advanced Light Source of Lawrence Berkeley National Laboratory and beamline X-25 at the National Synchrotron Light Source of Brookhaven National Laboratory. All data were integrated and scaled using both the HKL2000 suite of programs (Otwinowski and Minor, 1997) and IMOSFLM (Leslie, 1999) plus SCALA (Bailey, 1994). H test in the processing showed that datasets are pseudomerohedrally twinned with a twinning operator: -h, -k, l.
Structure Determination and Refinement
The twinned data were used to solve the structure. Initially, individual domains of the apo TaqPolIIIα (PDB code: 2HPI) were utilized as the searching models with PHASER (McCoy et al., 2007). However, only the PHP, palm, β-binding, and fingers domains could be located using native data sets, which might be due to the presence of pseudomerohedral twinning with an operator (-h, -k, l). Therefore, the SAD phases were then combined with the partial phases obtained by MR using PHENIX programs (Adams et al., 2010). Difference Fourier maps calculated using the combined phases located 24 platinum atoms or 8 mercury atoms. The platinum atoms are bound to the surface methionine residues on the PHP, palm, fingers and β binding domains (Figure S1A), whereas the mercury atoms are observed to bind to the cysteine on the CTD of PolIIIα and one tryptophan on the τc (Figure S1B). The thumb domains were clearly placed using combined phase with platinum derivative using COOT (Emsley and Cowtan, 2004). The combined phase with mercury derivative gave better density maps for the CTD and τc. Phases were improved through density modification, including multi-domain averaging using NCSMASK (Bailey, 1994) and DM (Cowtan, 1999) (Figures S1C and S1D). Cross-averaging among different crystals could not be performed in this study since the twinning fractions of different crystals vary. Then the model was transferred to the higher resolution native dataset through MR using PHASER. Further multi-domain averaging was also performed and the whole PolIIIα was then rebuilt using COOT. The τc model was initially built using the combined phases and then improved using the native dataset to continue refinement, side chain assignment, and residue location (Figure S2). The final electron density map allowed building the N-terminal and C-terminal portions of τc, but the linker loop is disordered. Subsequent to rebuilding the whole PolIIIα, the Fo−Fc difference maps clearly showed the density for the DNA substrates (Figure S3). Initial rigid body refinements were performed by assigning 40 rigid domains and including the amplitude based twin refinement using REFMAC 5 (Murshudov et al., 1997) with the twinned data. Further restrained refinements were performed by including TLS (translation, liberation, screw-rotation displacement) refinement, twin refinement, and non-crystallographic symmetry (NCS) restraints with the twinned data. Structure validation was performed using PROCHECK (Laskowski et al., 1993). The data collection and refinement statistics are shown in Table 1. All figures were created using PyMOL (DeLano, 2002). The interfaces of the complex were analyzed using AREAIMOL (Lee and Richards, 1971) and PISA service at European Bioinformatics Institute (http://www.ebi.ac.uk/pdbe/prot_int/pistart.html) (Krissinel and Henrick, 2007).
Table 1.
Data collection and refinement statistics
| Dataset | Native | Pt-derivative | Hg-derivative |
|---|---|---|---|
| Data collection | |||
| Wavelength (Å) | 0.9999 | 1.0715 | 1.0093 |
| Resolution (Å)a | 50-3.20 (3.37-3.20) | 50-3.80 (3.94-3.80) | 50-3.60 (3.73-3.60) |
| Space group | P21 | P21 | P21 |
| Cell dimension | |||
| a, b, c (Å) | 188.53, 94.97, 204.08 | 190.14, 94.86, 204.86 | 187.45, 95.81, 204.17 |
| α, β, γ (°) | 90.00, 89.97, 90.00 | 90.00, 90.01, 90.00 | 90.00, 90.12, 90.00 |
| Completeness (%)a | 93.8 (93.8) | 99.9 (100) | 99.8 (99.4) |
| Unique reflectionsa | 112427 (14848) | 71832 (7161) | 84784 (8354) |
| Total reflections | 265336 | 478720 | 620862 |
| <I/σI>a | 11.8 (1.8) | 19.9 (1.1) | 21.2 (2.1) |
| Rsym (%)a,b | 4.4 (53.9) | 9.2 (100) | 13.5 (100) |
| Redundancya | 2.4 (2.3) | 6.7 (6.4) | 7.3 (7.1) |
| Copies in AU | 4 | 4 | 4 |
| Twin fractionc | 0.30 | 0.43 | 0.29 |
| Refinement | |||
| Resolution (Å) | 20-3.20 | ||
| No. of reflections | 108754 | ||
| Rfactor/Rfree (%) | 26.63/30.47 | ||
| R.m.s deviation | |||
| Rmsd bond (Å) | 0.010 | ||
| Rmsd angle (°) | 1.272 | ||
| Ramachandran plot (%) | |||
| Preferred regions | 97.9 | ||
| Allowed regions | 2.1 | ||
| Access code | 4IQJ | ||
Numbers in parentheses correspond to the highest resolution shell.
Rsym = Σ|I − <I>|/ΣI, where I is the observed intensity and <I> is the averaged intensity of multiple observation of symmetry- related reflections.
Twin fractions were estimated by the H test for twinning in SCALA.
Supplementary Material
Highlights.
Crystal structure of the DNA polymerase IIIα in complex with τc and a DNA substrate
τc interacts with the C-terminal domain of PolIIIα through its C-terminal helix and the loop that follows it
PolIIIα in complex with τc and DNA displays an open form
The structure of this complex suggests an atomic model of the bacterial replisome
Acknowledgments
The authors declare no competing financial interests. We thank the staff at ALS beamline 8.2.2 and at NSLS beamline X-25. We also thank R.C. Wilmouth and L.S. Lai for helpful discussion and the staff of the CSB core at Yale. B.L. performed experiments and solved the structure. J.Z.L. provided assistance in phase determination. B.L. and T.A.S. wrote the manuscript. This work was supported by both HHMI funding and NIH grant GM57510 to T.A.S..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst. 2010;D66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S. The CCP4 suite: Programs for protein crystallography. Acta Cryst. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Bailey S, Eliason WK, Steitz TA. Structure of hexameric DnaB helicase and its complex with a domain of DnaG primase. Science. 2007;318:459–463. doi: 10.1126/science.1147353. [DOI] [PubMed] [Google Scholar]
- Bailey S, Wing RA, Steitz TA. The structure of T. aquaticus DNA polymerase III is distinct from eukaryotic replicative DNA polymerases. Cell. 2006;126:893–904. doi: 10.1016/j.cell.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Brautigam CA, Steitz TA. Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes. Curr Opin Struct Biol. 1998;8:54–63. doi: 10.1016/s0959-440x(98)80010-9. [DOI] [PubMed] [Google Scholar]
- Corn JE, Pease PJ, Hura GL, Berger JM. Crosstalk between primase subunits can act to regulate primer synthesis in trans. Mol Cell. 2005;20:391–401. doi: 10.1016/j.molcel.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Cowtan K. Error estimation and bias correction in phase-improvement calculations. Acta Cryst. 1999;D55:1555–1567. doi: 10.1107/s0907444999007416. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular Graphics System. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
- Dohrmann PR, McHenry CS. A bipartite polymerase-processivity factor interaction: only the internal beta binding site of the alpha subunit is required for processive replication by the DNA polymerase III holoenzyme. J Mol Biol. 2005;350:228–239. doi: 10.1016/j.jmb.2005.04.065. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Cryst. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Davies DR, Bullard JM, Christensen J, Green LS, Guiles JW, Pata JD, Ribble WK, Janjic N, Jarvis TC. Structure of PolC reveals unique DNA binding and fidelity determinants. Proc Natl Acad Sci U S A. 2008;105:20695–20700. doi: 10.1073/pnas.0809989106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, McHenry CS. tau binds and organizes Escherichia coli replication proteins through distinct domains. Domain IV, located within the unique C terminus of tau, binds the replication fork, helicase, DnaB. J Biol Chem. 2001a;276:4441–4446. doi: 10.1074/jbc.M009830200. [DOI] [PubMed] [Google Scholar]
- Gao D, McHenry CS. tau binds and organizes Escherichia coli replication through distinct domains. Partial proteolysis of terminally tagged tau to determine candidate domains and to assign domain V as the alpha binding domain. J Biol Chem. 2001b;276:4433–4440. doi: 10.1074/jbc.M009828200. [DOI] [PubMed] [Google Scholar]
- Georgescu RE, Kim SS, Yurieva O, Kuriyan J, Kong XP, O’Donnell M. Structure of a sliding clamp on DNA. Cell. 2008a;132:43–54. doi: 10.1016/j.cell.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu RE, Kurth I, O’Donnell ME. Single-molecule studies reveal the function of a third polymerase in the replisome. Nat Struct Mol Biol. 2011;19:113–116. doi: 10.1038/nsmb.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu RE, Yurieva O, Kim SS, Kuriyan J, Kong XP, O’Donnell M. Structure of a small-molecule inhibitor of a DNA polymerase sliding clamp. Proc Natl Acad Sci U S A. 2008b;105:11116–11121. doi: 10.1073/pnas.0804754105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroniti A, Anderson C, Doddridge Z, Gardiner L, Roberts CJ, Allen S, Soultanas P. The clamp-loader-helicase interaction in Bacillus. Atomic force microscopy reveals the structural organisation of the DnaB-tau complex in Bacillus. J Mol Biol. 2004;336:381–393. doi: 10.1016/j.jmb.2003.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsathitphaisarn O, Wing RA, Eliason WK, Wang JM, Steitz TA. The hexameric helicase DnaB adopts a nonplanar conformation during translocation. Cell. 2012;151:267–277. doi: 10.1016/j.cell.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergic S, Ozawa K, Williams NK, Su XC, Scott DD, Hamdan SM, Crowther JA, Otting G, Dixon NE. The unstructured C-terminus of the tau subunit of Escherichia coli DNA polymerase III holoenzyme is the site of interaction with the alpha subunit. Nucleic Acids Res. 2007;35:2813–2824. doi: 10.1093/nar/gkm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, O’Donnell M. Cellular DNA replicases: components and dynamics at the replication fork. Annu Rev Biochem. 2005;74:283–315. doi: 10.1146/annurev.biochem.73.011303.073859. [DOI] [PubMed] [Google Scholar]
- Kim S, Dallmann HG, McHenry CS, Marians KJ. Coupling of a replicative polymerase and helicase: a tau-DnaB interaction mediates rapid replication fork movement. Cell. 1996;84:643–650. doi: 10.1016/s0092-8674(00)81039-9. [DOI] [PubMed] [Google Scholar]
- Kong XP, Onrust R, O’Donnell M, Kuriyan J. Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell. 1992;69:425–437. doi: 10.1016/0092-8674(92)90445-i. [DOI] [PubMed] [Google Scholar]
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Lamers MH, Georgescu RE, Lee SG, O’Donnell M, Kuriyan J. Crystal structure of the catalytic alpha subunit of E. coli replicative DNA polymerase III. Cell. 2006;126:881–892. doi: 10.1016/j.cell.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: A program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- Lee B, Richards FM. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971;55:379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Leslie AGW. Integration of macromolecular diffraction data. Acta Cryst. 1999;D55:1696–1702. doi: 10.1107/s090744499900846x. [DOI] [PubMed] [Google Scholar]
- Lopez de Saro FJ, Georgescu RE, O’Donnell M. A peptide switch regulates DNA polymerase processivity. Proc Natl Acad Sci U S A. 2003;100:14689–14694. doi: 10.1073/pnas.2435454100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki H, Kornberg A. The polymerase subunit of DNA polymerase III of Escherichia coli. II. Purification of the alpha subunit, devoid of nuclease activities. J Biol Chem. 1985;260:12987–12992. [PubMed] [Google Scholar]
- Martinez-Jimenez MI, Mesa P, Alonso JC. Bacillus subtilis τ subunit of DNA polymerase III interacts with bacteriophage SPP1 replicative DNA helicase G40P. Nucleic Acids Res. 2002;30:5056–5064. doi: 10.1093/nar/gkf650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry CS. DNA polymerase III holoenzyme of Escherichia coli. Annu Rev Biochem. 1988;57:519–550. doi: 10.1146/annurev.bi.57.070188.002511. [DOI] [PubMed] [Google Scholar]
- McHenry CS. DNA replicases from a bacterial perspective. Annu Rev Biochem. 2011;80:403–436. doi: 10.1146/annurev-biochem-061208-091655. [DOI] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Cryst. 1997;D53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Carter CW, Sweet RM, editors. Methods in Enzymology. New York: Academic Press; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Pritchard AE, Dallmann HG, Glover BP, McHenry CS. A novel assembly mechanism for the DNA polymerase III holoenzyme DnaX complex: association of deltadelta′ with DnaX(4) forms DnaX(3)deltadelta′. Embo J. 2000;19:6536–6545. doi: 10.1093/emboj/19.23.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Lamothe R, Sherratt DJ, Leake MC. Stoichiometry and architecture of active DNA replication machinery in Escherichia coli. Science. 2010;328:498–501. doi: 10.1126/science.1185757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romier C, Dominguez R, Lahm A, Dahl O, Suck D. Recognition of single-stranded DNA by nuclease P1: high resolution crystal structures of complexes with substrate analogs. Proteins. 1998;32:414–424. [PubMed] [Google Scholar]
- Scheuermann RH, Echols H. A separate editing exonuclease for DNA replication: the epsilon subunit of Escherichia coli DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1984;81:7747–7751. doi: 10.1073/pnas.81.24.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetta KR, Kazmirski SL, Goedken ER, Cantor AJ, Kelch BA, McNally R, Seyedin SN, Makino DL, O’Donnell M, Kuriyan J. The mechanism of ATP-dependent primer-template recognition by a clamp loader complex. Cell. 2009;137:659–671. doi: 10.1016/j.cell.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stano NM, Chen J, McHenry CS. A coproofreading Zn2+-dependent exonuclease within a bacterial replicase. Nat Struct Mol Biol. 2006;13:458–459. doi: 10.1038/nsmb1078. [DOI] [PubMed] [Google Scholar]
- Steitz TA. DNA polymerases: structural diversity and common mechanisms. J Biol Chem. 1999;274:17395–17398. doi: 10.1074/jbc.274.25.17395. [DOI] [PubMed] [Google Scholar]
- Studwell-Vaughan PS, O’Donnell M. Constitution of the twin polymerase of DNA polymerase III holoenzyme. J Biol Chem. 1991;266:19833–19841. [PubMed] [Google Scholar]
- Su XC, Jergic S, Keniry MA, Dixon NE, Otting G. Solution structure of Domains IVa and V of the tau subunit of Escherichia coli DNA polymerase III and interaction with the alpha subunit. Nucleic Acids Res. 2007;35:2825–2832. doi: 10.1093/nar/gkm080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft-Benz SA, Schaaper RM. The theta subunit of Escherichia coli DNA polymerase III: a role in stabilizing the epsilon proofreading subunit. J Bacteriol. 2004;186:2774–2780. doi: 10.1128/JB.186.9.2774-2780.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RA, Bailey S, Steitz TA. Insights into the replisome from the structure of a ternary complex of the DNA polymerase III alpha-subunit. J Mol Biol. 2008;382:859–869. doi: 10.1016/j.jmb.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao NY, O’Donnell M. Replisome structure and conformational dynamics underlie fork progression past obstacles. Curr Opin Cell Biol. 2009;21:336–343. doi: 10.1016/j.ceb.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao NY, O’Donnell M. SnapShot: The replisome. Cell. 2010;141:1088, 1088 e1081. doi: 10.1016/j.cell.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.