Abstract
In addition to their well-known role in acute injury and chronic inflammation “innate” cytokines play an important role in health and the maintenance of normal immune homeostasis. This group includes the prototypic cytokines IL-1 and TNF as well as several other members belonging to the IL-1 and TNF family, such as IL-18, IL-33, IL-36-38, and TL1A. The dichotomous role of these cytokines has been best characterized in the intestine where innate cytokines may play both a protective and a pro-inflammatory role, depending upon the immmunological status of the host or the type and phase of the inflammatory process. This new information has produced novel pathogenetic hypotheses that have important translational implications both in regard to the prevention and treatment of chronic intestinal inflammation, including Crohn’s disease and ulcerative colitis, the two major forms of inflammatory bowel disease. This review will discuss and summarize current data regarding the role of IL-1, TNFα, and their family members in regulating gut mucosal homeostasis and chronic intestinal inflammation.
Keywords: Cytokines, TNF superfamily, Interleukins, Intestinal inflammation, inflammatory bowel disease
1. Introduction
There is increasing evidence that “innate” cytokines, such as IL-1, TNFα, and others, play an important role in health and the maintenance of homeostasis, in addition to their well-characterized role in acute injury and chronic inflammatory diseases [1]. The gastrointestinal system represents one of the best examples of cytokine-targeted organs in which these mechanisms take place [2]. In fact, cytokines, such as TNF that clearly contribute to the pathogenesis of inflammatory bowel disease (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC), have been shown to also play important roles in regulating epithelial barrier function and gut homeostasis [3, 4]. These important functions are now being reported for other cytokines, including the relatively newer cytokines IL-18, TL1A, IL-33 and others, and new insights have been described based on recent and very important accomplishments at both the experimental and clinical levels. Firstly, several animal models of acute and chronic intestinal inflammation have been developed that have allowed for the understanding of the specific role of cytokines in experimental IBD [5, 6]. Secondly, an abundance of whole-genome wide scans have identified associations between cytokine gene polymorphisms and increased susceptibility for developing CD and/or UC [7, 8]. In turn, these associations have been followed by functional studies of the translated proteins encoded by the candidate genes. These studies not only reveal potential pathogenetic mechanisms of chronic gut inflammation, but allow for a retrospective view on cytokine-dependent cellular and molecular pathways that predominate in the healthy mucosa. Herein, we will review some of the important cytokine-mediated mechanisms that have recently been shown to participate in mucosal homeostasis and chronic intestinal inflammation.
2. Homeostasis and inflammation at the intestinal mucosa
The luminal surface of the small and large intestine is a unique environment whereby an enormous population of bacteria exists in close proximity to the richest immunological structure of the human body. In practical terms, this translates to the interplay of 1012 microorganisms per gm of feces with 106 immune cells per gm of enteric tissue (considering the lymphocyte count only). From an evolutionary perspective, this interaction represents a well-developed mutual relationship [9]: on one hand, intestinal microorganisms take advantage of a nutrient-rich environment that facilitates their survival; on the other hand, the host also benefits as microbiota participate in the digestion and metabolism of intraluminal nutrients, compete against 4 pathogenic bacteria, and contribute to the shaping of the structural and functional integrity of the intestine, in particular of the gut-associated lymphoid tissue (GALT) [10, 11].
However, from a pathophysiological standpoint, the close proximity between bacteria (and their products) and the dense immunological network of intestinal mucosa risks certain consequences. Unrestricted encounter of immunocytes with microbial products can easily lead to excessive activation of the former, and the generation of effector molecules with deleterious effects for the integrity of the intestinal mucosa. For such an undesired outcome to be avoided, the intestinal mucosa is equipped with multiple preventive mechanisms that are organized into distinct, but also overlapping, defense barriers [12]. At a primary level, intraluminal microorganisms are physically separated from the immunologically-rich lamina propria by a well-formed mechanical barrier. This consists of the single layer of epithelial cells, the complex structure of interconnecting cell-cell contacts, as well as the thick mucus layer that covers the luminal mucosal surface. This physical separation is complemented by the presence of an antimicrobial barrier that mainly includes natural antimicrobial peptides and IgA. Examples of the former, which are concentrated within the mucus layer, include the α-defensins that are secreted by Paneth cells and the β-defensins and cathelicidins that are produced by intestinal epithelial cells [13, 14]. IgA is secreted by plasma cells under the instructive signals of resident, bacteria-sampling dendritic cells, and transcytosed across the epithelium [15]. Finally, cytokines, such as TNFα and IL-1β, play a critical role in recognizing and destroying intestinal pathogens through activation of intestinal innate immune responses. The combined effect of these factors is further prevention of bacterial-epithelial contact and the maintenance of normal gut homeostasis.
Nevertheless, even in the presence of such defensive mechanisms, intraluminal bacteria and their products are constantly presented at the lamina propria. This may occur in a passive manner through temporary breaches in the epithelial integrity, but can also take place actively via sampling of intraluminal constituents by dendritic cells. Excessive reactivity against these bacteria-derived factors is avoided through the existence of an additional mucosal checkpoint; that is the immunological barrier [16]. The latter initially depends upon the function of the innate immune system. In particular, lamina propria phagocytes are responsible for the rapid and effective elimination of any intruding pathogenic commensal microorganisms, without generating substantial inflammatory reactions that may be harmful for the mucosa. Concomitantly, adaptive immunological responses against bacterial antigens are initiated, which lead to the selection of CD4 lymphocytes with regulatory/anti-inflammatory properties. The adequate function of all three mucosal barriers is the prerequisite for the proper symbiosis between commensal bacteria and GALT.
In addition to maintaining a healthy relationship with the microbiota, the intestinal mucosa should also be capable of handling any invasion by pathogenic microorganisms. On such occasions, abundant, intact bacteria and their products reach the lamina propria. This evokes an immediate and potent immunological response that eliminates the offensive infectious agent through an acute inflammatory reaction, leading to a temporary structural and functional compromise. Nonetheless, this is a self-contained reaction as it is followed by a rapid and complete mucosal recovery. The latter is accomplished through gradual elimination of effector immunocytes via apoptosis, combined with full architectural restitution of the epithelial barrier, under the influence of epithelial-derived healing factors. Therefore, a healthy intestinal mucosa is characterized by a state of non-reactivity against commensal flora, along with the ability of effective immunity against pathogenic intruders. This dual functionality determines the concept of intestinal homeostasis.
The critical importance of maintaining this mucosal balance is highlighted when anatomical and/or functional deficiencies exist, and when this established balance is lost. The most profound example of such failure in maintaining mucosal homeostatic mechanisms is during the development of chronic intestinal inflammation, such as that observed in IBD [17]. Until very recently, the pathogenesis of IBD has been primarily attributed to hyper-reactivity of the adaptive arm of the immune response. This notion most probably originated from the abundance of lymphocytes in the inflammatory infiltrate of the lamina propria, commonly observed upon histological examination of diseased bowel segments. However, such an approach may miss the early events that take place during the initiation of inflammation and that may substantially differ from late disease. Nonetheless, the focus on adaptive immunity was soon followed by an effort to explain the pathogenesis of IBD via a simplified immunological model, proposing diversification of effector immune responses based on the prevalence of specific cytokine profiles. In particular, a pure Th1 adaptive response was supposed to mediate CD, whereas Th2 pathways primarily contributed to the pathogenesis of UC.
Recent converging lines of evidence have, however, strongly questioned the validity of this pathogenic model in explaining “adaptive immunity” in IBD [18]. These data include genetic associations identified through whole-genome wide scans for IBD-specific polymorphisms, studies in animal models of experimental intestinal inflammation and finally, information regarding the use of novel therapeutic agents, most importantly, single-molecule neutralizing medications. From such studies, it has become evident that the original adaptive immunity-focused hypothesis does not sufficiently explain the immunobiology of IBD. Consequently, a substantial shift has occurred in regards to our understanding of the etiopathogenesis of both UC and CD, and the level(s) at which loss of homeostatic control takes place during chronic intestinal inflammation [19]. The major components of this developing new dogma for IBD pathogenesis are: a) the pivotal importance of innate immunity for the control of commensal flora and the lack of appropriate responsiveness towards bacterial signals as the definitive point of loss of tolerance; b) the discrimination between the immunological pathways that mediate the initiation of inflammation at the mucosa, and those that maintain and perpetuate tissue injury; and, c) the existence of overlapping and redundant effector mediators of chronic intestinal inflammation, which do not adequately fit into the Th1/CD vs. Th2/UC paradigm. Instead, it is now evident that a complex mucosal network of immune mediators exists, among which cytokines display the most prominent role [20].
3. Genetic associations
The number of susceptibility loci for IBD that have been identified through genome wide association studies was almost 100 by the end of 2011 [7]. A detailed review of current developments in this field is beyond the scope of the present article and can be found elsewhere [21, 22]. It is, however, worth mentioning that it was from these genetic associations that the central role of innate immunity and defective clearance of bacteria (in particular intracellular) originally emerged. Indeed, among several potential pathways, the strongest genetic associations point to intracellular bacterial sensing and autophagy as the main candidates in the pathogenesis of IBD.
Polymorphisms in the card15/nod2 gene have been constantly associated with increased risk for developing CD. This gene encodes for a protein that acts as a intracellular “pattern recognition receptor” for muramyl dipeptide (MDP), a constituent of the bacterial wall component petidoglycans [23]. CD-linked card15/nod2 mutations result in defective MDP-induced signaling, which may lead to intestinal inflammation through either decreased secretion of antimicrobial peptides by Paneth cells, or by loss of regulatory control over pro-inflammatory pathways [16]. On the other hand, autophagy refers to an intracellular process that involves the lysosomal degradation of ingested bacteria, but also self-digestion of organelles [24]. Polymorphisms in atg16l1 and irgmtwo genes that are critically involved in autophagy pathways, have been genetically linked to IBD [25]. Once again, Paneth cells appear to be major cellular targets for defective function of the ATG16L1 protein, as described in both patients with CD carrying the respective mutations and in mice rendered defective in atg16l1 expression. The end result is loss of antimicrobial peptide secretion by Paneth cells, as well as generation of pro-inflammatory responses [26].
Although the functional implications of defective CARD15- or ATG16L1-dependent mechanisms in CD have not yet been revealed, a recent study reported that these two intracellular pathways are interrelated [27]. Briefly, investigators showed that NOD2 stimulation is capable of initiating the autophagy process in dendritic cells. For effective intracellular digestion and bacterial clearance to be achieved, both intact NOD2 and ATG16L1 functions are required. In contrast, when CD-linked polymorphisms are present in either gene, autophagy in response to MDP is compromised, eventually resulting in reduced bacterial elimination. These defects affect adaptive immune responses as they compromise antigen presentation and lymphocyte priming.
Finally, a third pathway relating to the cellular sensing of external stimuli, including bacterial factors, has been identified that appears to be defective in a subset of IBD patients. This is the unfolded protein response (UPR)/endoplasmic reticulum (ER) stress pathway [28]. The association with IBD was established through the presence of genetic variants of the transcription factor XBP1 in patients with either CD or UC. XBP1 is a critical component of the ER, and when defective, leads to dysfunction of intestinal epithelial cells, including the subset of Paneth cells. In addition, hyper-reactivity to bacterial products, such as flagellin, has been reported in mice with deletion of the xbp1 gene, leading to pro-inflammatory responses and enteritis [28].
Taken together, it is clear that the development of either CD or UC cannot be explained by single-gene mutations. On the other hand, it should be noted that the majority of described genetic associations fall into few distinct pathophysiological categories, thereby pointing to a limited number of inherent defects. The most profound of these abnormalities definitely relate to the function of the innate immune system and effective recognition, intracellular manipulation, and elimination of bacterial factors. Loss of these regulatory mechanisms may lead to unrelenting chronic intestinal inflammation.
4. Innate cytokines as the critical determinants of mucosal homeostatic or inflammatory pathways
Several recent studies in animal models of acute intestinal injury, repair, and chronic inflammation have provided very important insights into the role of cytokine-driven pathways in mucosal immunity. What is intriguingly interesting about intestinal homeostasis and inflammation is that similar cellular elements and soluble mediators mediate both processes, with several cytokines exerting dichotomous roles, depending upon the specific setting. Indeed, innate cytokines such as TNFα, IL-1β, TL1A/DR3, IL-18, and signaling molecules such as NF-B and MyD88, have long been associated with pro-inflammatory properties. Therefore, it is of no surprise that the aforementioned proteins have been established as targets of anti-inflammatory strategies in clinical and experimental IBD. Nevertheless, what has been increasingly evident in recent years is that the same proteins are necessary for the maintenance of mucosal homeostasis by effectively handling microbiota, as well as by protecting and restoring the integrity of the epithelial barrier.
Studies on the role of IL-1 in the pathogenesis of formalin-immune complex colitis in rabbits provided original insight into the bi-directional effects of an innate cytokine. In this model, IL-1 displayed pro-inflammatory properties, as its neutralization by either endogenous or exogenous administration of IL-1Ra resulted in significant amelioration of the severity of colitis [29–31]. Nevertheless, administration of recombinant IL-1β had a similar beneficial effect, indicating that IL-1β is necessary for mucosal protection and maintenance of homeostasis [32]. Protection by IL-1β was only achieved with administration of a low dose, and only when given 24h, but not 30min, before induction of colitis. Such protective effects of pro-inflammatory innate cytokines have also been shown in other disease models, such as arthritis [33] and sepsis [34]. Similarly, in the DSS-colitis model, neutralization of IL-1 activity during the acute-disease phase was associated with exacerbated severity of inflammation and delayed recovery from injury [35]. No effect was observed during the chronic stage of colitis. Taken together, these temporal and dose-related effects demonstrate the complexity of immunological pathways at the intestinal mucosa and may explain why human studies with recombinant proteins or neutralizing antibodies often fail to show clinical benefit.
TNFα represents the prototypic pro-inflammatory innate cytokine for mucosal immunity and was the first to be targeted by a clinically applicable neutralizing antibody. However, it has been shown that TNF may also exert anti-inflammatory properties depending on the clinical scenario and the cellular target [36]. This may be of particular relevance in regard to mucosal homeostasis or inflammation. DSS-induced colitis is an established murine model of acute injury and repair at the intestinal mucosa. Two separate studies have clearly shown that in the absence of endogenous TNFα, or after its neutralization with monoclonal antibodies, the severity of acute colitis substantially worsens [35, 37]. Likewise, when DSS was administered in RAG KO mice that also lacked the expression of TNFR1, high mortality and impaired epithelial regeneration was observed in comparison to RAG KO with intact TNFR1 [38]. Mucosal responses were restored and mortality reversed upon transplantation of RAG KO-derived bone marrow cells (which express TNFR1). Similar results were also reported for the trinitrobenzene sulfonic acid (TNBS) colitis model. Deletion of TNFR1 resulted in more severe disease, as indicated by increased weight loss, hypothermia, and increased mortality compared to wild-type C57Bl/6 mice [39]. Finally, similar protective effects of TNFα signaling were also seen in sepsis that takes place after cecal ligation and puncture, although in this model, protective mechanisms were dependent upon intact TNFR2 expression [40]. Taken together, these studies clearly show that the role of TNFα during the initiating phases of intestinal inflammation may be opposite to its effects later on as chronicity is established. In the same line, it is of particular interest that in one of the aforementioned studies, although neutralization of TNFα resulted in more severe acute DSS colitis, it also ameliorated inflammation in the chronic model, i.e. after repeated courses of DSS administration [35].
The mechanism(s) by which TNFα protects from intestinal inflammation after an acute injury are not clear; several mechanisms, however, may be speculated upon. One explanation may be that TNFα interferes with the apoptotic elimination of effector immunocytes from the lamina propria. These cells are essential components of the immunological barrier against both intruding pathogens, but also occasional invaders from the microbiota. Nevertheless, extended survival of these populations would enrich the mucosal milieu with pro-inflammatory factors, leading to continuous damage. Therefore, it is extremely relevant that TNF has been shown to induce apoptosis of activated effector cells [41,42], a property that may explain how this cytokine may ameliorate acute inflammation. A second mechanism may involve the upregulation of endogenous corticosteroids by TNFα. This was elegantly demonstrated by a recent study that reported production of glucocorticosteroids with anti-inflammatory properties in the intestinal mucosa during acute intestinal inflammation [43]. This production was not observed in the absence of TNF and was restored after therapeutic administration of TNF. Once again, lack of TNF resulted in exacerbation of DSS-colitis, whereas treatment with TNF ameliorated intestinal inflammation induced by oxazolone [43]. A third possibility is that TNF exerts its protective effect by maintaining the integrity of the epithelial barrier. This mechanism was clearly demonstrated in a recent report on the effects of VSL#3, a mixture of six probiotic strains, in the prevention of ileitis in SAMP1/YitFc mice. Inflammation in this strain is triggered via a loss of epithelial integrity and disturbed permeability, which takes place at a very early age and before inflammation is established [3, 44]. Early administration of VSL#3 resulted in almost complete prevention of ileitis in this strain, subsequent to restoration of gut permeability [46]. Very interestingly, these beneficial effects were accompanied by a substantial induction of TNFα expression in intestinal epithelial cells. The specificity of this finding was demonstrated by the abrogation of the beneficial effect of VSL#3 by the administration of anti-TNF neutralizing antibodies [45]. Further studies from our group, utilizing transepithelial electrical resistance (TEER) measurements of ex vivo-cultured ilea, shed light on the molecular basis of the beneficial effect of TNF. This work showed that pre-treatment of ilea from pre-inflamed SAMP mice with VSL#3-conditioned media or TNF decreased ileal paracellular permeability through modulation of tight junction proteins. Addition of anti-TNF abrogated these effects. This outcome was not observed in ilea from mice with fully established ileitis (Corridoni and Pizarro, submitted). These studies provide direct evidence for the participation of TNFα in gut homeostasis. At the same time, they also emphasize the complexity of the involved mechanisms, as they clearly demonstrate that the pathways that mediate early vs. late ileitis are quite different, even if the same molecules participate in the pathogenesis of both phases.
The aforementioned studies also point out that the cellular origin of a specific cytokine may be of critical importance. For example, epithelial-derived TNF may be protective for the defense barrier and therefore may exert anti-inflammatory functions, whereas monocyte- or lymphocyte-derived TNF may be pro-inflammatory at later stages of the disease process [46]. Similarly, functional deletion of the transcription factor NF- B in epithelial cells leads to loss of homeostasis and development of inflammation [46]. This is of particular significance as NF- B is the master regulator of pro-inflammatory responses and upon its absence, there is defective production of innate cytokines. This concept is further supported by studies of the role the common TLR signaling adaptor protein, MyD88, plays in the development of chronic intestinal inflammation. One study reported that mice transgenic for an epithelial specific, dominant-negative mutant of MyD88 developed spontaneous small intestinal inflammation associated with innate and adaptive cell infiltration and upregulation of the corresponding cytokines (TNFα, IFNγ, IL-1β, and IL-17). Therefore, epithelial MyD88-mediated pathways critically participate in mucosal immunoregulation [47]. Additional studies have directly shown that cellular compartmentalization of MyD88 is critical for the fate of bacterial recognition in the gut towards homeostatic balance or the inflammatory response [48]. By utilizing several animal models of IBD, investigators demonstrated that epithelial MyD88 signaling was required for host survival, whereas MyD88-dependent activation of myeloid cells was necessary for the development of chronic intestinal inflammation. The effects of microbiota-driven MyD88 activation for the secretion of innate cytokines and the impact on mucosal homeostasis were further elaborated in recent studies by the Medzhitov Lab. They have shown that mice deficient in MyD88 suffered from more severe colitis and substantial morbidity upon administration of DSS [49]. This was associated with an inability to upregulate local expression of innate cytokines, including TNFα and IL-1β, upon mucosal injury. This impairment continued throughout the repair phase of colitis, further compromising mucosal integrity.
Other cytokines of the innate immune system have also been shown to contribute to maintaining the balance between immunoregulation and inflammation. IL-6 may facilitate mucosal healing after injury, as indicated by impaired colonic restitution in mice defective in IL-6-mediated gp130 signaling [50]. IL-18 may also display dichotomous roles in acute inflammatory events and in chronic inflammation, a diversity that may also be related to its cellular source [51]. In particular, during the initiation phase of inflammation, IL-18 derived from intestinal epithelial cells may exert a protective role, facilitating tissue repair and supporting homeostatic mechanisms. Supporting this notion is the fact that IL-18 or IL-18R KO mice are more susceptible to acute DSS-colitis than their wild-type littermates [52]. This protective effect is most probably mediated through an IL-11 dependent mechanism [51]. This epithelial-derived IL-18 is also critical for the protection from DSS colitis conferred by NLR-mediated signaling, as shown in studies in mice deficient in NLRP3 [53]. In contrast, during chronic intestinal inflammation, production of IL-18 shifts from epithelial cells to lamina propria monocytes [54]. This cellular re-distribution may be responsible for the pro-inflammatory role that IL-18 appears to play during chronic inflammatory responses in the gut mucosa.
The TNF-like cytokine, TL1A and its functional receptor, DR3 are novel members of the TNF/TNFR superfamily of proteins that have been recently identified as important mediators of intestinal inflammation [55–59]. TL1A is expressed on cells of the innate immune system, most prominently in dendritic cells [60]. TL1A expression on antigen-presenting cells is induced by the FcγR signaling pathway [61]. In addition, various bacterial-derived signals may also affect TL1A expression in either a stimulatory or inhibitory fashion [62, 63]. In particular TLR1, 2, 4, and 9 exerted positive signals on TL1A expression, whereas TLR7, and 8 inhibited TL1A production. This tight regulation of TL1A activation in innate cells raises the possibility that, in addition to the established pro-inflammatory properties, TL1A/DR3 signaling may also participate in gut homeostasis. Indeed, recent studies from our group and others have utilized knockout and transgenic models to elucidate the dichotomous roles that TL1A and DR3 appear to play in mucosal immunity. When DR3 KO mice were treated with DSS, they showed significantly more severe colitis as compared with their wild-type littermates, indicated by greater weight loss and fecal bleeding and higher mortality, in addition to more severe histological injury (F. Cominelli, manuscript in preparation). Similar results were obtained when TL1A KO mice were used. Therefore, it appears that during an acute mucosal injury (but also during the recovery phase), intact TL1A/DR3 is beneficial for the maintenance of mucosal homeostasis. In contrast, chronic upregulation of TL1A in dendritic cells or lymphocytes may result in chronic intestinal inflammation, as recently demonstrated in TL1A Tg mice [64–66].
An additional pathway that has been shown to play a major proinflammatory role in chronic intestinal inflammation is the IL-23/IL-17 pathway, most prominently in CD [67]. However, IL-17A also appears to have protective effects since CD4+ cells deficient in IL-17A induced a more serve colitis in the CD45RBhigh adoptive transfer model, once again indicating that mechanisms of acute injury and repair are quite different from those of chronic inflammation [68]. Moreover, IL-23 is a strong inducer of IL-22, derived from epithelial cells. Interestingly, IL-22 administration ameliorated colitis severity in the DSS model and also was beneficial in the T-cell receptor-α KO mouse that develops colitis [69].
Finally, novel members of the IL-1 cytokine family are emerging as important regulators of gut homeostasis and chronic intestinal inflammation. In 2010, four independent groups reported the association of IL-33 (also known as IL-1F11), the 11th and newest identified member of the IL-1 family, with IBD, specifically, UC [70–74]. IL-33 is known to potently induce Th2 cytokine production and potentiate both Th1 and Th2 immune responses [75, 76], with more recent evidence also suggesting activation of Th17 immune responses in animal models of rheumatoid arthritis (75, 77), as well as IBD [78]. Although its biological effects suggest a pathogenic role, two studies published earlier this year report a protective role for IL-33 in chemically-induced colitis models, implicating an IL-33-dependent switch from a predominant Th1 to Th2 cytokine profile, as well as the promotion of Foxp3+ Treg development [79, 80]. In addition, within the last year, other IL-1 family members, including IL-36α, IL-36β, and IL-36γ (previously designated IL-1F6, IL-1F8, and IL-1F9, respectively), as well as their antagonist, IL-36Ra (IL-1F5), have been further characterized and reports have been somewhat ambiguous as to whether IL-36 has the ability to induce either Th1 or Th2 immune responses, or both [81]. Interestingly, IL-38 (IL-1F10) has recently been shown to inhibit the Th17 cytokines, IL-17 and IL-22, from memory T cells by binding to the IL-36R and thereby sharing similar biological effects to IL-36Ra [82]. Although the potential contributions of IL-36, IL-36Ra, and IL-38 in the pathogenesis of IBD have not yet been reported, IL-37 (IL-1F7), another IL-1 family member, is emerging as a potent anti-inflammatory cytokine with the ability to downregulate DSS-induced colitis [83].
A careful interpretation of the aforementioned studies on the mucosal functions of innate cytokines points to a common pattern of dual effect. During an acute insult, it appears that the initial reaction of GALT is to augment local innate immune functions. This is most likely induced by an influx of luminal antigens to the mucosa, which transiently shifts the mucosal balance towards inflammation. At this point, intact innate immunity is necessary for effectively eliminating the plethora of bacterial-driven and potentially harmful stimuli and directing adaptive immune responses towards regulatory pathways, thus resetting the mucosal immunostat into homeostatic function. It follows that any defects in innate cytokine production or signaling will interfere with this healing and repair process and set the background for perpetual inflammation. The validity of this hypothesis is supported by the exaggerated colitis after DSS administration when single or multiple innate cytokines become faulty through genetic manipulation. After chronic inflammation is established, innate cytokines are not only unable to perform their protecting roles, but most probably contribute to mucosal damage, either via direct toxicity or indirectly by shaping the adaptive arm of immunity towards pro-inflammatory, effector responses. This “paradoxical” and dichotomous functionality of innate cytokines is most probably explained by the specifics of each mucosal situation. Different cellular sources of cytokines and/or their receptors (epithelial vs. monocytic), diversity in the mucosal immunological environment, dissimilarities in antigenic loads and specificities, as well as temporal and spatial associations, may be the decisive factors for the dominance of homeostasis or inflammation. In line with this concept are converging lines of evidence that intestinal inflammation develops through distinct immunological phases that substantially differ from each other in terms of the dominant immunophenotype, including differential responses to treatment [84–86].
5. Translational implications
The vast majority of studies proposing that innate immunity is central to mucosal homeostasis and IBD pathogenesis were performed in animal models of inflammation, as described in the previous section. Nevertheless, ample evidence has now accumulated to support the hypothesis that defects in innate pathways is at the core of the pathogenesis of CD, as well as UC. Similar to the experimental data, such mechanisms are particularly relevant to events that occur in patients, during initiation of IBD.
Among the most compelling lines of evidence for a primary innate-immunodeficiency occurring in IBD is the development of IBD-like enteric disease in a substantial proportion of individuals with genetic disorders with neutrophil dysfunction. Intestinal inflammation has been described in almost every syndrome; however, the most prominent associations are with chronic granulomatous disease (CGD) [87, 88] and glycogen storage disease 1b [89–90]. Patients with CGD suffer from a genetic defect in the NADPH oxidase enzyme, which results in impaired phagocytic activity against microorganisms. The GI tract is severely affected in many patients with clinicopathological characteristics indistinguishable from those of CD. In patients with CGD, high levels of circulating anti-microbial antibodies have been detected [91]. This finding is consistent with the genetic defect in innate immunity leading to weakened clearance of commensal bacteria and initiation of IBD-like inflammation. Glycogen storage disease is associated with the development of intestinal inflammation in up to 77% of patients [92]. Similarly to CGD, patients with glycogen storage disease 1b and neutropenia demonstrate almost universal reactivity against bacterial flagellin (anti-CBir1 antibodies) [93]. More importantly, boosting innate immune responses with granulocyte-colony stimulating factor (G-CSF) has been shown to induce remission of IBD-like inflammation in these patients [94].
A recent study by Marks et al. has elegantly demonstrated that, similar to individuals with genetic deficiencies in neutrophils, patients with CD may also carry a primary defect in phagocytic function [95]. In this study, investigators inflicted an acute trauma to either the rectum, ileum, or skin of healthy controls and patients with UC or CD and compared acute inflammatory responses between the various groups by measuring parameters, such as the immediate recruitment of neutrophils and local cytokine induction They showed that patients with CD had significantly lower accumulation of neutrophils and suppressed pro-inflammatory (IL-8 and IL-1) cytokine expression, 6h post-trauma. Cultured macrophages from CD patients displayed diminished IL-8 secretion after stimulation with various inflammatory stimuli. Finally, inoculation with E coli induced attenuated local responses in patients with CD. These findings strongly support the concept of impaired innate immunity in CD, which renders patients unable to mount an effective inflammatory response to acute injury. Interestingly, these responses were independent of the CARD15 polymorphism status, indicating that patients with an intact genotype may still have defective NOD2 signaling. Further support of this hypothesis is derived from recent studies in SAMP1/YitFc mice with CD-like ileitis (but intact CARD15 genotype), that demonstrate defective MDP-mediated responses, including innate cytokine secretion (D. Corridoni and F. Cominelli, manuscript in preparation). Work by Smith et al. [96] further elucidated the potential mechanism(s) of impaired acute inflammatory responses in patients with CD. The authors focused on macrophages and showed that they secrete significantly lower quantities of pro-inflammatory cytokines upon challenge with heat-killed E. coli or TLR agonists. More importantly, macrophages from CD patients displayed lower levels of intracellular TNFα compared to healthy controls and patients with UC. Once again, these results point to defective intracellular handling of bacterial components, which results in a failure to eliminate them during an acute episode. In all, it may be possible that the critical abnormality in a subset of CD patients is an inherent lack of response in innate immunity, leading to a progressive accumulation of luminal-derived factors in the lamina propria. This in turn results in the attraction of chronic inflammatory elements, including the formation of granulomas. As mentioned earlier, focusing on this later stage of disease carries the risk of missing the earlier pathogenetic events, which are quite diverse, but also gaining more insight into unique therapeutic possibilities, such as haemopoietic growth factors with the ability to enhance innate immunity and correct the aforementioned defects in phagocytosis, which has shown some efficacy in clinical trials [97, 98].
Additional information on the role of innate immunity in gut homeostasis and inflammation is derived from analysis of the effects of currently utilized anti-inflammatory treatment with biological agents. The most commonly utilized agents neutralize TNFα and, in addition to IBD, are currently used for the treatment of rheumatoid arthritis, ankylosing spoldylarthropathy, and psoriasis. Although administration of these therapies target TNF-mediated pro-inflammatory pathways, they also offer the unique opportunity to examine how blocking this pivotal innate cytokine affects the homeostasis of tissues that are not affected from the primary disorder. From this perspective, it is of significance that new-onset of IBD has been reported in patients treated with anti-TNF agents for other conditions [99, 100]. Although the specifics of this association need further elucidation, it may be speculated that blockade of mucosal TNF-driven pathways are rendered defective during treatment and may trigger new-onset IBD or reveal latent inflammation in the gut mucosa. Such a scenario is also supported by the fact that reverse associations have also been described, i.e. the development of skin or arthritic disease in patients receiving anti-TNF therapy for gut disease [101, 102]. Similarly, “paradoxical” de novo development of IBD, despite aggressive immunosuppression, has been reported in transplant patients [103, 104]. Taken together, these studies further emphasize the notion that, depending on the temporal and spatial parameters, the same molecules may exert pro- or anti-inflammatory properties. This may also be influenced by inter-individual variation and the specifics of each case. In support of such diversity is the fact that only a minority of patients with IBD achieves full, long term clinical remission upon treatment with anti-TNF treatment. In fact, in all clinical trials, the group of non-responders includes individuals that actually worsen under anti-inflammatory treatment. Elucidation of the pathogenetic mechanisms of this deterioration will greatly contribute to our understanding on the fine-tuning of mucosal homeostasis and its failure in IBD.
6. Conclusions
Converging lines of evidence have accumulated in recent years to support the dichotomous role of innate cytokines in gut homeostasis and chronic intestinal inflammation. This concept has generated novel hypotheses for the pathogenesis of IBD. The “new dogma” for IBD summarizes information obtained from genetic association studies, functional data from animal models of inflammation, as well as clinical studies and observations. Some of the basic constituents of this new hypothesis were described in the present manuscript. A major breakthrough has been the substitution of the immune hyper-reactivity hypothesis with the concept of a defective innate response. Consequently, CD is increasingly considered a state of immunodeficiency of the innate arm of immunity, underlined by defective bacterial recognition, autophagy and/or antigen presentation. As such, IBD may belong to a group of disorders associated with bacterial processing, in which the far end of the spectrum is represented by diseases such as CGD or glycogen storage disease 1b. A second advance is the understanding that chronic intestinal inflammation develops through distinct phases. Early disease refers to the initial events that take place when homeostatic mechanisms initially fail and acute inflammatory responses cannot be resolved. In contrast, late disease refers to the period when adaptive immunity has been irreversibly primed towards a specific effector phenotype. During these separate stages of disease evolution, innate cytokines play diverse, and oftentimes, dichotomous roles. In murine models of intestinal inflammation, TNFα, IL-1β, IL-18 and TL1A/DR3 appear to be protective during acute inflammation, but exert pure pro-inflammatory functions in later stages. Finally, the therapeutic implications of the new concepts are obvious. While late disease will continue to be treated with anti-inflammatory approaches, patients with early disease may benefit more from interventions aimed boosting innate immune responses. Recent data on the mechanisms of probiotic effects in murine ileitis substantiate the validity and applicability of this approach. In all, translational research on these exciting novel pathways will eventually lead to novel, targeted, and more effective, therapeutic options for patients with IBD.
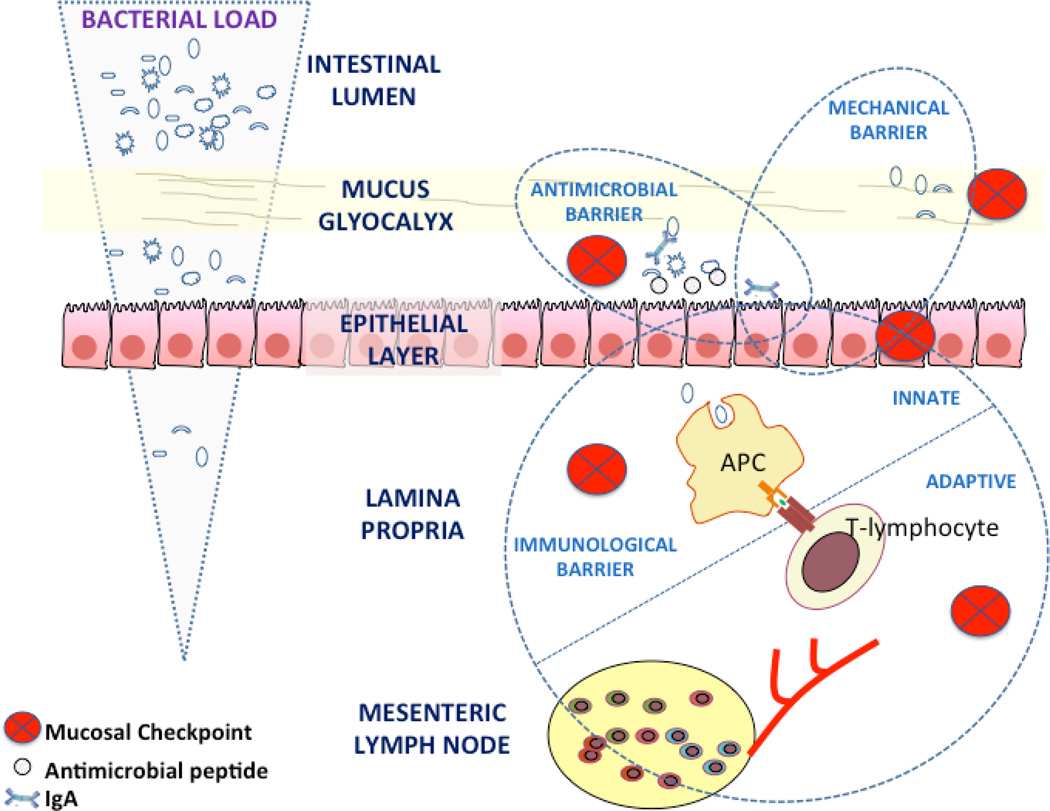
Figure 1.
Mechanisms of intestinal homeostasis. Protection against the enormous load of luminal bacterial content is accomplished through the application of sequential mucosal checkpoints (indicated with the red-cross circles). These checkpoints are organized into three distinct but highly overlapping barriers. The mechanical barrier sustains the physical separation between intraluminal microorganisms and the gut epithelial lining. The antimicrobial barrier consists of nature peptides which act as physical antibiotics against those bacteria that achieve a close contact to the epithelium. Finally, the immunological barrier handles the occasional microbial intruders by efficient intracellular recognition and processing and by regulating the presentation of intraluminal antigens in a manner that leads to the generation of anti-inflammatory/regulatory responses. Both the innate and adaptive arms of immunity are involved in the regulation of this mucosal “immunostat” function. Dashed lines enclose the elements participating in each barrier and demonstrate the overlap between them. APC: antigen-presenting cell.
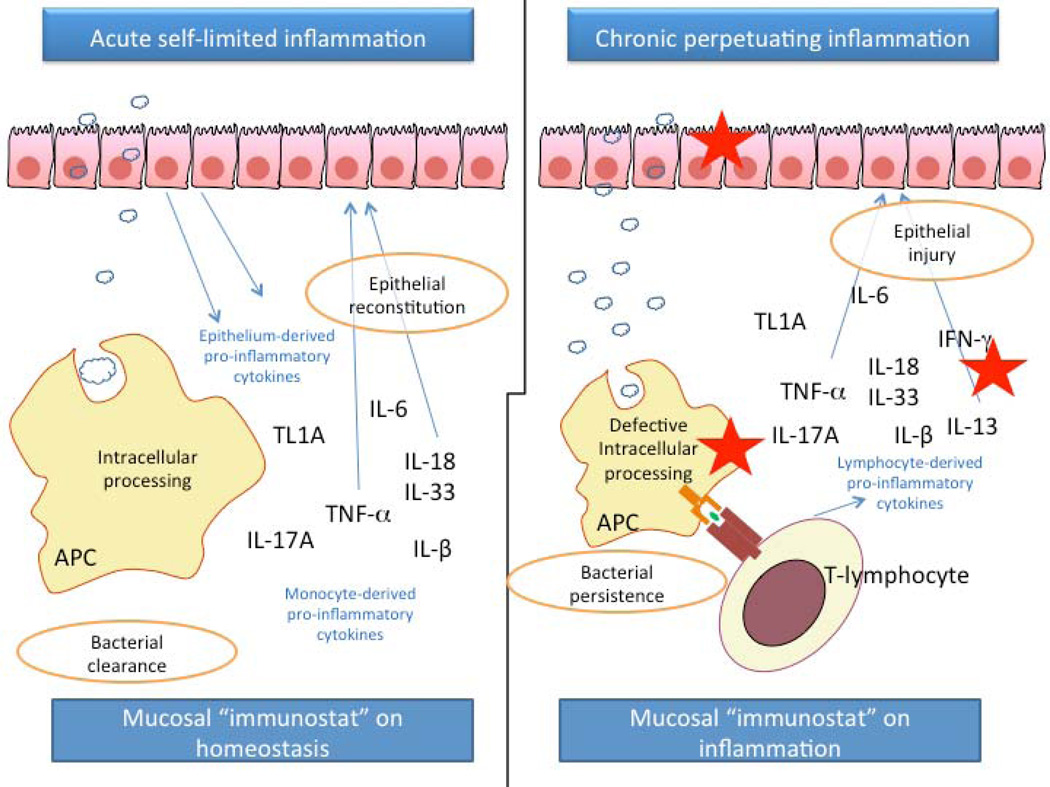
Figure 2.
Dichotomous effects of innate cytokines during homeostatic conditions and IBD. Lamina propria bacterial load may increase either as part of the normal process of intraluminal antigen sampling of commensal microorganisms or following a transient breach in barrier function. The latter may occur as a result of pathogenic infection, external injury (i.e. administration of drugs with intestinal toxicity) or concomitant systemic morbidity. Under such conditions, cells of the innate immune system respond quickly to eliminate the intruding microorganisms by phagocytosis. During this process a vast array of pro-inflammatory cytokines is induced. The cell origins for these cytokines include both monocytes involved in the phagocytosis of bacteria as well as the epithelial cells. These molecules generate an acute inflammatory response which results in the elimination of excessive numbers of bacteria. At the same time certain cytokines, such as TNFα, IL-18 and IL-33 facilitate the repair process, which re-establishes the integrity of the epithelial monolayer. In contrast, during IBD one or more of the homeostatic mechanisms are dysfunctional and several deficiencies may occur. The antimicrobial and epithelial barriers may be inadequate to hold the commensal bacteria separated from the gut-associated immune system of the lamina propria and/or the intracellular processing of bacteria may be impaired and/or the secretion of pro-inflammatory factors may be dysregulated. These points of failure are indicated in the above by the red asterisks. The end result of these defects is the setting of the mucosal immunostat on “inflammation”, which results in the continuous release of pro-inflammatory factors. In this case, several of these factors are secreted by activated lymphocytes and their different cellular origin may impact on their function. For example IL-18 and TNF produced by lymphocytes demonstrate toxicity against the epithelium, further deteriorating the integrity of the epithelial barrier.
Table 1.
Human studies supporting the role of a deficiency in innate immunity as the initiating event in Crohn’s disease.
| Genetic data | ▪ Most reliable genetic associations for CD have been established for genes involved in bacterial recognition and clearance at the lamina propria by innate immune cells (card15, atg16l1) |
| ▪ CD-related polymorphisms affect the secretion of natural antimicrobial peptides from Paneth cells | |
| Functional data | ▪ Patients with CD demonstrate diminished acute inflammatory responses at the intestinal mucosa following acute trauma or inoculation with E coli, as indicated by suppressed neutrophil recruitment and defective local innate cytokine (IL-8 and IL-1) induction. |
| ▪ Cultured macrophages from CD patients display diminished IL-8 secretion after stimulation with various inflammatory stimuli. | |
| Clinical data | ▪ Patients with various genetically determined immunodeficiencies which affect the function of phagocytes develop typical IBD-like intestinal inflammation. |
| ▪ Patients with acquired immunodeficiencies (post-transplantation status, HIV) develop de novo IBD despite aggressive immunosuppression. | |
| ▪ Therapeutic administration of granulocyte-colony stimulating factor (G-CSF) induces remission in a subgroup of patients with CD. | |
| ▪ Patients treated with anti-TNF agents for non-IBD conditions may develop intestinal inflammation that resembles IBD. | |
| ▪ A significant portion of patients with CD do not respond to anti-TNF treatment. |
Innate cytokines possess both protective and pro-inflammatory roles during gut inflammation
Early and late phases of intestinal inflammation display different cytokine profiles
GWAs have identified genes associated with innate immunity as predisposing factors to IBD
A deficit of innate immunity may cause Crohn’s disease
“Boosting” innate immunity and their associated-cytokines is a new therapy for IBD
Acknowledgments
The study was funded by the National Institutes of Health, DK055812 and DK042191 to Fabio Cominelli, DK091222 to Fabio Cominelli and Theresa Pizarro, and DK056762 to Theresa Pizarro.
Abbreviations used
- IBD
inflammatory bowel disease
- CD
Crohn’s disease
- UC
ulcerative colitis
- GALT
gut-associated lymphoid tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: There are no conflicts of interest with regard to this paper.
References
- 1.Flavell R. Cytokines in Health and Disease. J Med Sci. 2008;1(1):26–28. [Google Scholar]
- 2.Pizarro TT, Cominelli F. Cytokine therapy for Crohn's disease: advances in translational research. Annu Rev Med. 2007;58:433–444. doi: 10.1146/annurev.med.58.121205.100607. [DOI] [PubMed] [Google Scholar]
- 3.Olson TS, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203(3):541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagnini C, et al. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A. 2010;107(1):454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pizarro TT, et al. Mouse models for the study of Crohn's disease. Trends Mol Med. 2003;9(5):218–222. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 6.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 7.Lees CW, et al. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 8.Shih DQ, Targan SR, McGovern D. Recent advances in IBD pathogenesis: genetics and immunobiology. Curr Gastroenterol Rep. 2008;10(6):568–575. doi: 10.1007/s11894-008-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemente JC, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Round JL, O'Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2010;34(3):J220–J225. doi: 10.1016/j.jaut.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 12.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 13.Oppenheim JJ, et al. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62(Suppl 2):ii17–ii21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouellette AJ. Paneth cell alpha-defensins in enteric innate immunity. Cell Mol Life Sci. 2011;68(13):2215–2229. doi: 10.1007/s00018-011-0714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macpherson AJ, et al. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1(1):11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 16.Kelsall BL. Innate and adaptive mechanisms to control [corrected] pathological intestinal inflammation. J Pathol. 2008;214(2):242–259. doi: 10.1002/path.2286. [DOI] [PubMed] [Google Scholar]
- 17.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 18.Cominelli F. Cytokine-based therapies for Crohn's disease--new paradigms. N Engl J Med. 2004;351(20):2045–2048. doi: 10.1056/NEJMp048253. [DOI] [PubMed] [Google Scholar]
- 19.Arseneau KO, et al. Innate and adaptive immune responses related to IBD pathogenesis. Curr Gastroenterol Rep. 2007;9(6):508–512. doi: 10.1007/s11894-007-0067-3. [DOI] [PubMed] [Google Scholar]
- 20.Bamias G, Kaltsa G, Ladas SD. Cytokines in the pathogenesis of ulcerative colitis. Discov Med. 2011;11(60):459–467. [PubMed] [Google Scholar]
- 21.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140(6):1704–1712. doi: 10.1053/j.gastro.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werts C, et al. Nod-like receptors in intestinal homeostasis, inflammation, and cancer. J Leukoc Biol. 2011;90(3):471–482. doi: 10.1189/jlb.0411183. [DOI] [PubMed] [Google Scholar]
- 24.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cadwell K, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooney R, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16(1):90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 28.Kaser A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134(5):743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferretti M, et al. Neutralization of endogenous IL-1 receptor antagonist exacerbates and prolongs inflammation in rabbit immune colitis. J Clin Invest. 1994;94(1):449–453. doi: 10.1172/JCI117345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cominelli F, et al. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology. 1992;103(1):65–71. doi: 10.1016/0016-5085(92)91096-m. [DOI] [PubMed] [Google Scholar]
- 31.Cominelli F, et al. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990;86(3):972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cominelli F, et al. Interleukin 1 suppresses inflammation in rabbit colitis. Mediation by endogenous prostaglandins. J Clin Invest. 1990;85(2):582–586. doi: 10.1172/JCI114476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs C, et al. In vivo treatment with IL-1 reduces the severity and duration of antigen-induced arthritis in rats. J Immunol. 1988;141(9):2967–2974. [PubMed] [Google Scholar]
- 34.van der Meer JW, et al. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988;85(5):1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojouharoff G, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107(2):353–358. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohan A, et al. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem. 2003;278(38):36688–36698. doi: 10.1074/jbc.M304011200. [DOI] [PubMed] [Google Scholar]
- 37.Naito Y, et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18(5):560–569. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 38.Mizoguchi E, et al. TNF receptor type I-dependent activation of innate responses to reduce intestinal damage-associated mortality. Gastroenterology. 2008;134(2):470–480. doi: 10.1053/j.gastro.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 39.Ebach DR, Newberry R, Stenson WF. Differential role of tumor necrosis factor receptors in TNBS colitis. Inflamm Bowel Dis. 2005;11(6):533–540. doi: 10.1097/01.mib.0000163698.34592.30. [DOI] [PubMed] [Google Scholar]
- 40.Ebach DR, Riehl TE, Stenson WF. Opposing effects of tumor necrosis factor receptor 1 and 2 in sepsis due to cecal ligation and puncture. Shock. 2005;23(4):311–318. doi: 10.1097/01.shk.0000157301.87051.77. [DOI] [PubMed] [Google Scholar]
- 41.Zheng L, et al. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377(6547):348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 42.Zhou T, et al. Greatly accelerated lymphadenopathy and autoimmune disease in lpr mice lacking tumor necrosis factor receptor I. J Immunol. 1996;156(8):2661–2665. [PubMed] [Google Scholar]
- 43.Noti M, et al. TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. J Exp Med. 2010;207(5):1057–1066. doi: 10.1084/jem.20090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pizarro TT, et al. SAMP1/YitFc mouse strain: a spontaneous model of Crohn's disease-like ileitis. Inflamm Bowel Dis. 2011;17(12):2566–2584. doi: 10.1002/ibd.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pagnini C, et al. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A. 2010;107(1):454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446(7135):557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 47.Gong J, et al. Epithelial-specific blockade of MyD88-dependent pathway causes spontaneous small intestinal inflammation. Clin Immunol. 2010;136(2):245–256. doi: 10.1016/j.clim.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Asquith MJ, et al. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 2010;139(2):519–529. doi: 10.1053/j.gastro.2010.04.045. 529 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rakoff-Nahoum S, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Tebbutt NC, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8(10):1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 51.Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol. 2004;34(9):2347–2355. doi: 10.1002/eji.200425351. [DOI] [PubMed] [Google Scholar]
- 52.Takagi H, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38(8):837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 53.Zaki MH, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32(3):379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pizarro TT, et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162(11):6829–6835. [PubMed] [Google Scholar]
- 55.Bamias G, et al. Differential expression of the TL1A/DcR3 system of TNF/TNFR-like proteins in large vs. small intestinal Crohn's disease. Dig Liver Dis. 2012;44(1):30–36. doi: 10.1016/j.dld.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Bamias G, et al. High intestinal and systemic levels of decoy receptor 3 (DcR3) and its ligand TL1A in active ulcerative colitis. Clin Immunol. 2010;137(2):242–249. doi: 10.1016/j.clim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Bamias G, et al. Expression, localization, and functional activity of TL1A, a novel Th1- polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171(9):4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 58.Hsu H, Viney JL. The tale of TL1A in inflammation. Mucosal Immunol. 2011;4(4):368–370. doi: 10.1038/mi.2011.20. [DOI] [PubMed] [Google Scholar]
- 59.Meylan F, Richard AC, Siegel RM. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunol Rev. 2011;244(1):188–196. doi: 10.1111/j.1600-065X.2011.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bamias G, et al. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006;103(22):8441–8446. doi: 10.1073/pnas.0510903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prehn JL, et al. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007;178(7):4033–4038. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 62.Saruta M, et al. TLR8-mediated activation of human monocytes inhibits TL1A expression. Eur J Immunol. 2009;39(8):2195–2202. doi: 10.1002/eji.200939216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shih DQ, et al. Microbial induction of inflammatory bowel disease associated gene TL1A (TNFSF15) in antigen presenting cells. Eur J Immunol. 2009;39(11):3239–3250. doi: 10.1002/eji.200839087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shih DQ, et al. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS One. 2011;6(1):e16090. doi: 10.1371/journal.pone.0016090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taraban VY, et al. Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol. 2011;4(2):186–196. doi: 10.1038/mi.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meylan F, et al. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4(2):172–185. doi: 10.1038/mi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarra M, et al. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16(10):1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- 68.O'Connor W, Jr, et al. A protective function for interleukin-17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolk K, et al. Biology of interleukin-22. Semin Immunopathol. 2010;32(1):17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 70.Seidelin JB, et al. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett. 2010;128(1):80–85. doi: 10.1016/j.imlet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 71.Beltrán CJ, et al. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(7):1097–1107. doi: 10.1002/ibd.21175. [DOI] [PubMed] [Google Scholar]
- 72.Pastorelli L, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A. 2010;107(17):8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kobori A, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastronterol. 2010;45(10):999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 74.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Smithgall MD, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20(8):1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 76.Xu D, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008;105(31):10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palmer G, et al. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60(3):738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- 78.Delaney P, et al. 2012 unpublished results. [Google Scholar]
- 79.Duan L, et al. IL-33 ameliorates experimental colitis through promoting Th2/Foxp3(+) Treg responses in mice. Mol Med. 2012 doi: 10.2119/molmed.2011.00428. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grob P, et al. IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.22900. [DOI] [PubMed] [Google Scholar]
- 81.Vigne S, et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood. 2011;118(22):5813–5823. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- 82.van de Veerdonk FL, et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci U S A. 2012;109(8):3001–3005. doi: 10.1073/pnas.1121534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McNamee EN, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A. 2012;108(40):16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bamias G, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128(3):654–666. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 85.Kugathasan S, et al. Mucosal T-cell immunoregulation varies in early and late inflammatory bowel disease. Gut. 2007;56(12):1696–1705. doi: 10.1136/gut.2006.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spencer DM, et al. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology. 2002;122(1):94–105. doi: 10.1053/gast.2002.30308. [DOI] [PubMed] [Google Scholar]
- 87.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38(1):3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 88.Marks DJ, et al. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn's disease. Am J Gastroenterol. 2009;104(1):117–124. doi: 10.1038/ajg.2008.72. [DOI] [PubMed] [Google Scholar]
- 89.Dieckgraefe BK, et al. Association of glycogen storage disease 1b and Crohn disease: results of a North American survey. Eur J Pediatr. 2002;161(Suppl 1):S88–S92. doi: 10.1007/s00431-002-1011-z. [DOI] [PubMed] [Google Scholar]
- 90.Visser G, et al. Granulocyte colony-stimulating factor in glycogen storage disease type 1b. Results of the European Study on Glycogen Storage Disease Type 1. Eur J Pediatr. 2002;161(Suppl 1):S83–S87. doi: 10.1007/s00431-002-1010-0. [DOI] [PubMed] [Google Scholar]
- 91.Yu JE, et al. High levels of Crohn's disease-associated anti-microbial antibodies are present and independent of colitis in chronic granulomatous disease. Clin Immunol. 2011;138(1):14–22. doi: 10.1016/j.clim.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Visser G, et al. Intestinal function in glycogen storage disease type I. J Inherit Metab Dis. 2002;25(4):261–267. doi: 10.1023/a:1016572706488. [DOI] [PubMed] [Google Scholar]
- 93.Davis MK, et al. Antibodies to CBir1 are associated with glycogen storage disease type Ib. J Pediatr Gastroenterol Nutr. 2010;51(1):14–18. doi: 10.1097/MPG.0b013e3181c15f78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alsultan A, et al. Long term G-CSF-induced remission of ulcerative colitis-like inflammatory bowel disease in a patient with glycogen storage disease Ib and evaluation of associated neutrophil function. Pediatr Blood Cancer. 2010;55(7):1410–1413. doi: 10.1002/pbc.22706. [DOI] [PubMed] [Google Scholar]
- 95.Marks DJ, et al. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet. 2006;367(9511):668–678. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 96.Smith AM, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J Exp Med. 2009;206(9):1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valentine JF, et al. Steroid-sparing properties of sargramostim in patients with corticosteroid-dependent Crohn's disease: a randomised, double-blind, placebo-controlled, phase 2 study. Gut. 2009;58(10):1354–1362. doi: 10.1136/gut.2008.165738. [DOI] [PubMed] [Google Scholar]
- 98.Korzenik JR, et al. Sargramostim for active Crohn's disease. N Engl J Med. 2005;352(21):2193–2201. doi: 10.1056/NEJMoa041109. [DOI] [PubMed] [Google Scholar]
- 99.van Dijken TD, et al. Development of inflammatory bowel disease in patients with juvenile idiopathic arthritis treated with etanercept. J Rheumatol. 2011;38(7):1441–1446. doi: 10.3899/jrheum.100809. [DOI] [PubMed] [Google Scholar]
- 100.Toussirot E, et al. Development of inflammatory bowel disease during anti-TNF-alpha therapy for inflammatory rheumatic disease. A nationwide series. Joint Bone Spine. 2011 doi: 10.1016/j.jbspin.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Guerra I, et al. Induction of psoriasis with anti-TNF agents in patients with inflammatory bowel disease: A report of 21 cases. J Crohns Colitis. 2011 doi: 10.1016/j.crohns.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 102.Cullen G, et al. Psoriasis associated with anti-tumour necrosis factor therapy in inflammatory bowel disease: a new series and a review of 120 cases from the literature. Aliment Pharmacol Ther. 2011;34(11-12):1318–1327. doi: 10.1111/j.1365-2036.2011.04866.x. [DOI] [PubMed] [Google Scholar]
- 103.Ramji A, et al. Post-liver transplant Crohn's disease: graft tolerance but not self-tolerance? Dig Dis Sci. 2002;47(3):522–527. doi: 10.1023/a:1017951632444. [DOI] [PubMed] [Google Scholar]
- 104.Halim MA, et al. De novo Crohn's disease in a renal transplant recipient. Transplant Proc. 2007;39(4):1278–1279. doi: 10.1016/j.transproceed.2007.03.045. [DOI] [PubMed] [Google Scholar]