Abstract
Current approaches to the management of immunosuppression are largely empiric and reactive rather than proactive due to our inability to predict accurately how the recipient immune system will respond to a given organ allograft. The validation of simple, reliable, non-invasive assays exploring allogeneic anti donor responsiveness or donor specific non-responsiveness are of interest for several reasons: (i) it would allow for early and non-invasive detection of acute or chronic allograft rejection such that intervention could be initiated before effector mechanisms and organ destruction occur, (ii) it would allow for individual immunosuppressive drug therapy thereby avoiding the unwanted consequences of over immunosuppression, and (iii) the identification of the immunological phenotype related to operational tolerance could allow for the complete cessation of immunosuppressants. This review will summarize in vitro assays of T cell reactivity that reflect allo-antigen-specific responses.
Keywords: immune monitoring, limiting dilution assays, alloreactivity, carboxyfluorescein succinimidyl ester, enzyme linked immunosorbent spot
With the success of solid organ transplantation has come the realization of the post-transplant morbidity and mortality related to the use of immunosuppressive medications (1–3). Induction of tolerance to the transplanted organ would likely prevent these complications, however, the use of tolerance induction protocols in solid organ transplantation will require assays that reflect the level of immune alloreactivity (allosensitization) of the recipient towards the donor.
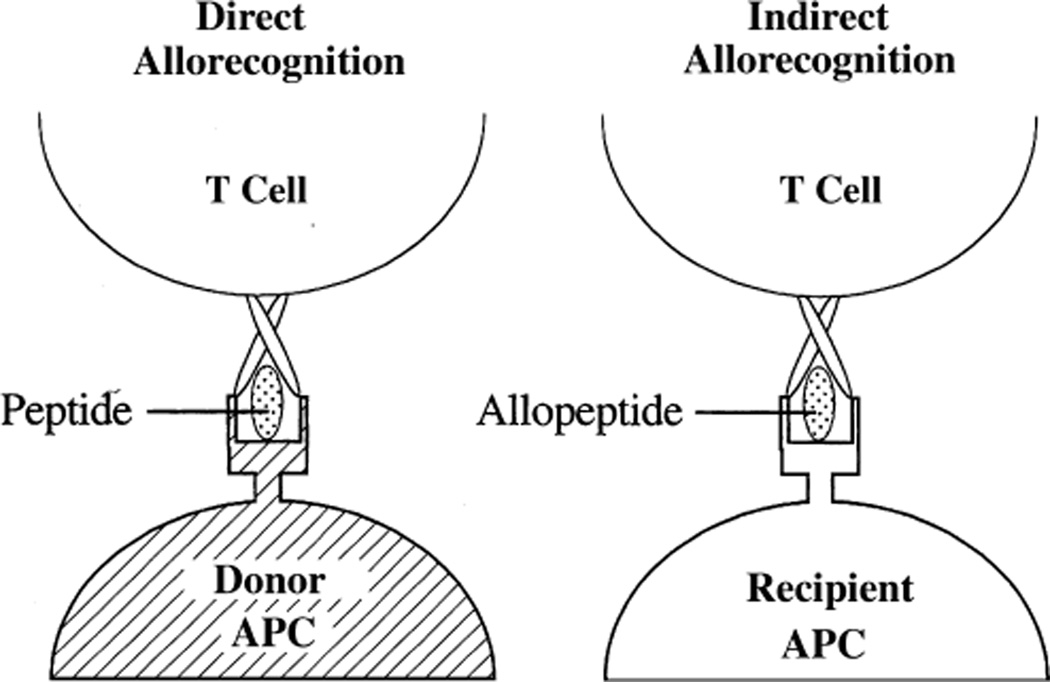
In order to understand the basis of in vitro assays of allosensitization, several concepts have to be considered. The dominant form of alloantigen is the non-self HLA molecule complexed with a variety of different peptides in its antigen binding groove. The frequency of precursor cells for an alloresponse is thought to be 100–1000 times as strong as the response to standard non-self antigens (4). Additionally, the alloresponse, unlike the response to most other antigens, is not dependent on previous exposure for an initial immune response (4). Any strategies to prevent allorecognition must take into account these cardinal observations. For the immune system to mount a response against a foreign antigen, it must be aware of the antigen’s presence. T cells recognize alloantigen via both direct and indirect pathways (Fig. 1) (5). In the direct pathway, recipient T cells recognize intact donor MHC alloantigens on the surface of donor-derived APC. Acute allograft rejection is thought to be mediated predominantly via this pathway (6). In the indirect pathway, allogeneic MHC molecules shed from the graft are processed by the recipient APC and then presented as peptides within the peptide binding sites of the recipient’s own MHC molecules. There is ample evidence that indirect allorecognition is an important driver of organ transplant rejection in humans (7–10). One of the challenges with measuring responses mediated by the indirect pathway is the low precursor frequency of indirectly primed T cells (6) compared to directly primed cells.
Fig. 1.
Two mechanisms for allorecognition. Direct allorecognition involves recognition of foreign HLA with a variety of potential peptides present in the antigen-presentation groove. In contrast, indirect allorecognition involves recognition of specific foreign HLA sequences as peptides in the context of self-HLA. APC, antigen presenting cell.
This review will summarize in vitro assays of T cell reactivity that reflect alloantigen-specific responses. Humoral issues, proteomics, and genomics will not be covered in this review.
Measurement of T cell alloreactivity
LDA
The LDA is a quantitative assay for measuring CTLpf. It correlates T-cell number from a functional activity.
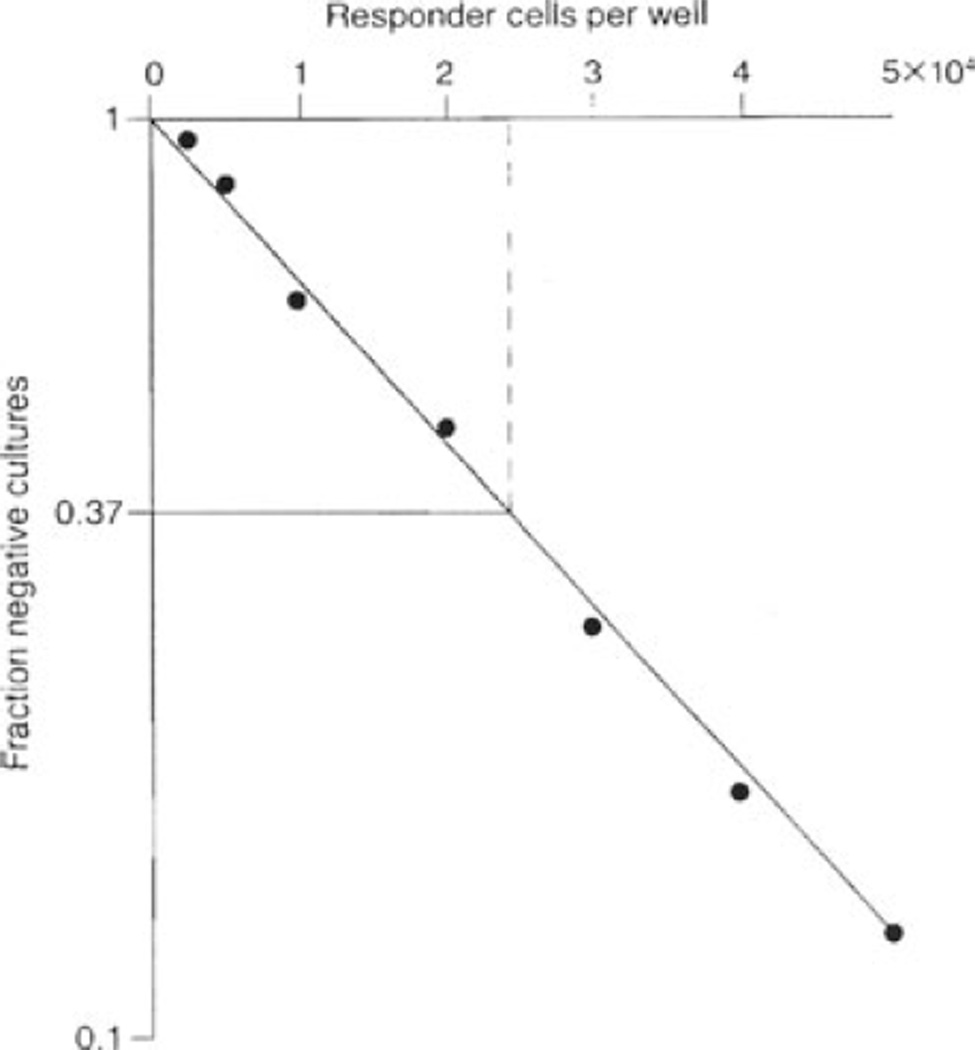
LDA involves the serial dilution of T cells in a large number of wells, followed by an in vitro stimulation phase and target lysis phase. The activity of alloreactive cytotoxic precursor cells can be assessed in-vitro by measuring the release of 51Cr from labeled target cells. An arbitrary level is set to facilitate the discrimination of positive wells from negative wells. This is usually set at 3 standard deviations above background (background being the mean level of chromium release by cultures set up without responder cells) (11). Determination of the number of cultures that are negative in the experiment gives an estimate of the frequency of the antigen-specific precursor. Poisson distribution analysis is applied to the results to determine the proportion of wells at a particular T-cell dilution that have ≥1 antigen-specific precursor at the start of the stimulation. For example, as shown in Fig. 2 (11), the fraction of negative cultures is converted to its negative logarithm and plotted graphically in a linear form. Using the zero term of the Poisson equation which predicts that when 37% of the test cultures are negative, there is an average of one precursor cell per well, the frequency of precursors can be extrapolated directly from the graph by drawing a line from 0.37 to the point where it intersects with the fitted line (11).
Fig. 2.
Limiting dilution assay: an example of a graphical representation of LDA data. The fraction of negative cultures is converted to its negative logarithm, and plotted on the ordinate, and the cell concentration is plotted on the abscissa to give a straight line. Using the Poisson equation, when 37% of test cultures are negative, there is an average of one precursor cell per well. The frequency of precursors can therefore be extrapolated directly from the graph by drawing a line from 0.37 to the point where it intersects with the fitted line.
Many protocols for LDA have been described as shown in Table 1. Briefly, irradiated stimulator cells are added to a minimum of 24 replicate cultures containing responder lymphocytes at different titrations. The cultures are incubated for 10 days at 37 °C, in 10% CO2. Recombinant IL-2 is added on days 3 and 6 and the wells are assayed on day 10. (The incubation period of LDA cultures must be long enough to allow functional maturation and clonal expansion of the populations being assayed.)
Table 1.
Summary of frequencies of alloreactive CTL-p obtained using different LDA protocols (11)
| Responder cells | Stimulator cells | Filler cells | Maximum frequency | Target cells | Culture period (days) | IL-2 source |
|---|---|---|---|---|---|---|
| PBMC | PBMC | + | 1:5000 | PHA blasts | 7 | TCGF |
| PBMC/spleen | PBMC | + | 1:870 | PHA blasts | 6 | TCGF |
| PBMC | PBMC | + | 1:733 | PHA blasts | 10 | S |
| PBMC | PBMC | − | 1:2000 | PHA blasts | 10 | 5 U/mL rIL-2 |
| PBMC | PBMC/spleen | − | 1:3322 | PHA blasts | 6 | TCGF |
| PBMC | PBMC | − | 1:646 | B-LCL | 10 | 2.5 U/mL riL-2 |
| PBMC | B-LCL | − | 1:900 | B-LCL | 10 | TCGF |
| PBMC | T-cell clones | − | 1:500 | T-cell clones | 10 | 5 ng/mL rIL-2 |
| T cells | PBMC/spleen | − | 1:240 | ConA blasts | 10 | 25 U/mL riL-2 + S |
| CD4+ T cells | PBMC | − | 1:441 | B-LCL | 10 | 2.5 U/mL riL-2 |
| CD4+ T cells | PBMC | − | 1:1620 | ConA blasts | 10 | 25 U/mL riL-2 + S |
| CD4+ T cells | Spleen | − | 1:11600 | PHA blasts | 14–18 | TCGF |
| CD8+ T cells | Spleen | − | 1:710 | PHA blasts | 14–18 | TCGF |
| CD8+ T cells | PBMC | − | 1:190 | ConA blasts | 10 | 25 U/mL riL-2 + S |
| T cells | Spleen | − | 1:2010 | PHA blasts | 14–18 | TCGF |
| T cells | B-LCL | − | 1:66 | B-LCL | 10–12 | 25 U/mL riL-2 + S |
| T cells | B-LCL | − | 1:535 | ConA blasts | 10–12 | 25 U/mL riL-2 + S |
CTL-P, cytotoxic T-lymphocyte precursor; LDA, limiting dilution assays; PHA, phytohemagglutinin; TCGF, T-cell growth factor; S, MLC supernatant; B-LCL, EBV-transformed B-cell line; PBMC, peripheral blood mononuclear cells.
The effector function of cytotoxic precursors is then monitored by measuring the release of 51Cr from labeled target cells. Target cells used in the assay include PHA blasts, EBV-transformed B-cell lines and murine L-cell transfectants. The relation between the number of negative wells and the mean number of precursors is plotted and a frequency obtained. There are various statistical methods used to estimate the effector frequency from the experimental data and these include: least squares, weighted means, minimum c-square and maximum likelihood (11).
Although LDA have been shown to be specific and reproducible as a measurement of alloreactivity (11), conflicting data regarding the usefulness of measurement of cytotoxic T-cell precursors for diagnosis/prediction of rejection in the context of solid organ transplantation have been reported (12, 13). CTLpf in blood samples from 10 heart transplant recipients was followed over a five yr period. Donor-specific CTLpf in samples taken before transplantation were positively correlated with acute rejection episodes in the transplanted hearts. Although recipients with a higher CTLpf against the donor usually had a higher incidence of rejections, multiple rejections also occurred in some recipients with low donor-reactive CTLpf. Therefore measurement of CTLpf against donor spleen cells in the peripheral blood is not a test that provides reliable evidence for prediction of acute rejection in heart transplant recipients.
Kidney transplant recipients with stable renal function showed a decrease in donor-reactive CTLpf three months post-transplant, however, the inverse was not true as some recipients showed a sharp increase in anti-donor CTLpf at six months without any alteration of renal function. Additionally, the reduction in antidonor CTLpf was paralleled by a reduction in third-party CTLpf in some recipients indicating the lack of specificity of the immune hyporesponsiveness in such cases that may be related to the effect of high doses of immunosuppressants received during the first weeks post-transplant.
More recently, the donor-specific cytotoxic T-lymphocyte limiting dilution precursor enumeration assay was used to identify patients in whom immunosuppression might be tapered following renal transplantation (14). Of 81 renal transplant recipients over two yr post-transplant and on steroids and an antimetabolite, 50 demonstrated no donor-specific CTLpf compared to CTLpf against third-party lymphocytes. Of those recipients with no or low donor-specific CTLpf, immunosuppression minimization was attempted and successful in 49 patients, one patient developing rejection during a median observation period of 12 months. The data suggests that measures of donor-specific cytotoxicity of recipient T-cells following transplantation may be useful in guiding decisions about immunosuppressive drug management, however, the assay typically assesses CD8+ T-cell-mediated activity and does not give a comprehensive picture of alloreactivity.
LD tests for precursor frequency enumeration require only small numbers of responder cells, however, they are considerably more time consuming, labor intensive, expensive, and require complex data analyses than conventional assays of immune function They may therefore be unsuitable for studies which require the simultaneous comparison of several sets of experimental data.
MLR
Assessment of proliferative responses of human lymphocytes is a fundamental technique for the assessment of their biological responses to various stimuli. Most simply, measurement of proliferation involves the measurement of the number of cells present in culture before and after the addition of a stimulating agent; however, this can be both labor intensive and difficult. The most common assessment of proliferation is performed by measuring new DNA synthesis, an essential process in cell division. The amount of new DNA synthesized can be assessed by measuring the incorporation of tritiated thymidine into DNA, a process which is closely related to underlying changes in cell number (15). In vitro induced proliferation assays have been used in the clinical setting for the assessment of an individual’s response to mitogens and specific antigens, including foreign alloantigens (15).
Briefly, responder/recipient cells are incubated with irradiated or mitomycin-C treated donor cells acting as stimulators for a period of 5–7 days. In the last 18 h of incubation, the cells are pulsed with tritiated thymidine. Following incubation, the cells are then harvested and lysed. The lysate including DNA is subsequently transferred to a scintillation counter for counting. The mean cpm, (a direct correlate of newly synthesized DNA and an indirect measure of the amount of cellular proliferation), are determined for background cultures and for each experimental condition. Typically all cultures are performed in triplicates and there should be less than 20% variation in replicate cultures. To correct for differences in background proliferation, the data may be presented as ∇ cpm (i.e., experimental – background) or as the stimulation index, (i.e., experimental cpm/background cpm). One of the drawbacks of MLR using tritiated thymidine incorporation is that it provides information on the entire cell population, not on individual cells or subset of cells. It is not possible to establish specific functional characterization of proliferating cells.
Previous attempts to characterize the immune systems of tolerant transplant recipients using the MLR assay with tritiated thymidine incorporation have been inconsistent and have failed to reliably distinguish tolerant from non-tolerant patients (16–18). Recently, Koshiba et al. (19) have been able to demonstrate in operationally tolerant liver transplant recipients (i.e., individuals off of all immunosuppression), using MLR, increased proliferation of recipient T cells following stimulation with both irradiated donor APC and third party APC in the presence of recipient CD4+ CD25− cells, however, suppression of proliferation following donor stimulation compared to third party stimulation, in the presence of recipient CD4+ CD25+ cells. This suggests the presence in the recipient of potentially reactive T cells to donor antigens which are suppressed by regulatory T cells.
CFSE – MLR
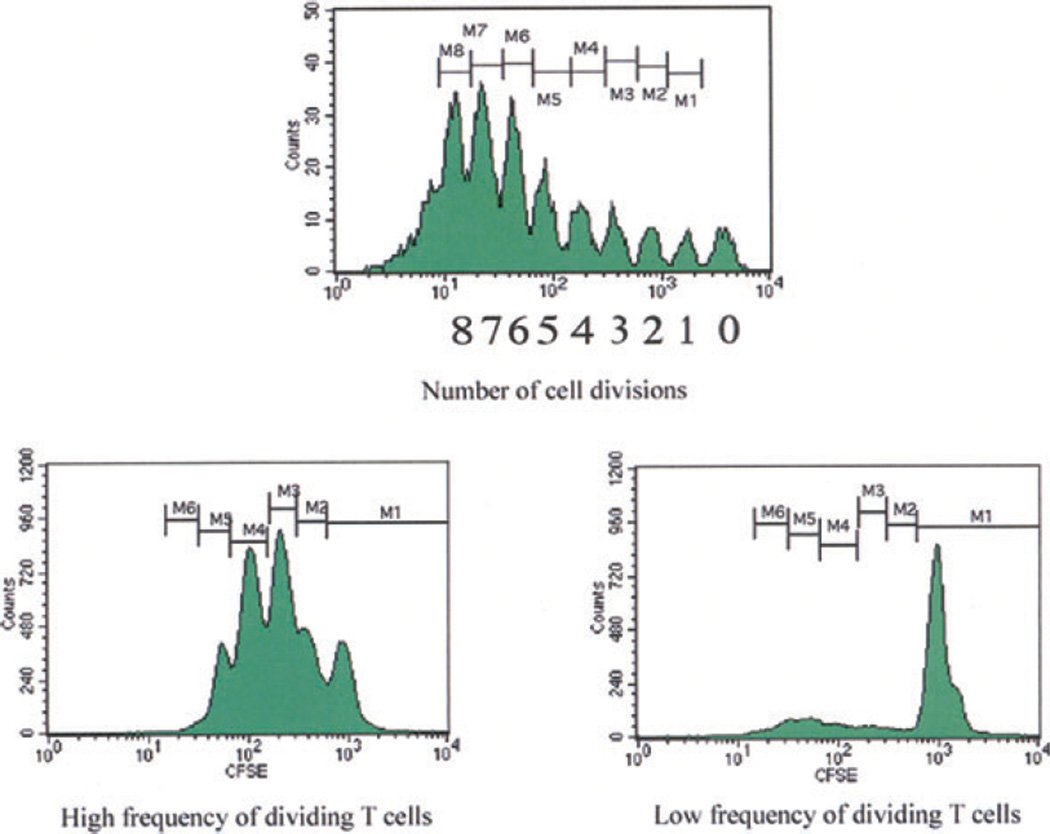
The MLR assay was initially used to determine the proliferation of host T cells in response to donor antigens (20, 21). However, a drawback with traditional MLR using tritiated thymidine incorporation is inability to perform phenotypic or functional analysis of the proliferating cells. In order to measure specific populations of proliferating cells responding to donor antigen, a dye dilution method of responding cells has been established using FCM analysis of lymphocyte division by serial halving of the fluorescence intensity of the intracellular dye CFSE, in MLR (22–24). Briefly, CFSE stably stains intracellular proteins without toxicity, and the fluorescence of each stained cell segregates equally to daughter cells upon cell division, resulting in sequential halving of cellular fluorescence intensity with each successive generation (25). This sequential halving of fluorescence is seen as distinct peaks using flow cytometry and is used to track cell division in proliferating cells. In T-lymphocytes, up to eight cell divisions have been accurately distinguished (Fig. 3) (5). Using multi-color FCM analysis, this method offers the advantage of being able to characterize the phenotype of the dividing cells as well as determining the number of cells that undergo division.
Fig. 3.
Flow cytometry analysis of cell proliferation by CFSE labeling: Following lymphocyte activation and proliferation, each cell division results in a halving of the fluorescence intensity of the intracellular fluorescent dye CFSE. This sequential halving of cellular fluorescence intensity is visualized as distinct peaks or populations of cells and can be used to track cell division in populations of proliferating cells. In T lymphocytes, up to eight cell divisions can be accurately distinguished. CFSE, carboxyfluorescein succinimidyl ester.
The use of CFSE labeling to assess allogeneic responses in vivo has been widely studied in murine systems (26, 27). Tanaka et al. (28) reported on 12 of 29 living donor liver transplant recipients with abnormal liver function tests, of whom four diagnosed as having acute cellular rejection histologically were also found to have an increase in proliferation of CD8+ T-cells in association with CD25 expression on anti-donor CFSE-MLR compared to third party. The other eight patients who demonstrated an absence of significant anti-donor responses of CD8+ T-cells had diagnoses ranging from drug-induced hepatotoxicity, congestion of the anterior segment of the liver, hepatitis C recurrence and autoimmune hepatitis. There are, however, no human studies to date where CFSE-MLR has been used to predict ability to wean immunosuppression or the development of tolerance.
ELISPOT assay
The ELISPOT assay quantifies the frequency of memory T cells that respond to donor antigens by producing a selected cytokine. It therefore allows for the direct enumeration of antigen-specific T cells. In order to assess donor alloreactivity in an ELISPOT assay, recipient T cells are cultured with inactivated donor or third party cells in tissue culture plates that are coated with an antibody specific for the cytokine of interest. Once the alloantigen is recognized, T cells respond by releasing the cytokine of interest, which is bound by the well-coated antibody. Following a period of incubation and plate washing, the bound cytokine is detected using a secondary antibody conjugated with an enzyme, usually horse radish peroxidase, against a different epitope of the same cytokine. A substrate is added which interacts with the secondary antibody to develop a spot on the plate where reactive cells had secreted the specific cytokine. Each spot therefore corresponds to a single antigen-experienced T-cell (Fig. 4) (5).
Fig. 4.
ELISPOT assay: Cells are incubated in plates coated by a capture antibody specific for the cytokine of interest. Following a period of incubation, the cells are washed away and the cytokine remains bound to the antibody. The bound cytokine is detected using labeled secondary antibodies. In the final step, a substrate is added that precipitates where the secondary antibody was bound, forming spots that correspond to cells producing the cytokine.
An advantage of the ELISPOT assay is that cytokines are captured as soon as the cell secretes them; therefore they are not subject to breakdown, dilution or being used up by other cells. It is also able to differentiate between donor antigens presented by the direct pathway vs. antigen presentation via the indirect pathway (29). The ELISPOT is less labor-intensive than the LDA with results being analyzed in 48 h (vs. 7–10 days for LDA). However, there is a chance of error due to subjectivity in the interpretation of results, as the threshold for size, intensity, and gradient of the spots is user-defined.
Salama et al. (30) have reported on the potential use of the ELISPOT assay to demonstrate possible suppression by antigen-specific regulatory cells in 40% of stable renal transplant recipients, who demonstrated hyporesponsiveness to a panel of synthetic peptides corresponding to sequences from donor HLADR molecules. In stable renal transplant recipients, the frequency of IFN-γ-producing cells detected by ELISPOT has been reported to correlate with the incidence of acute rejection, chronic rejection, and chronic allograft nephropathy (31–33). There are, however, no published reports of the ELISPOT assay being used to identify functionally tolerant transplant recipients.
FCM detection of intracellular cytokines
Intracellular cytokine staining was pioneered by the Anderssons in Stockholm in the 1980s, initially to immunostain tissue sections. After the introduction of methods to fix and permeabilize lymphocytes, the next development was the use of secretion inhibitors to accumulate cytokines intracellularly, allowing improvement of the signal/noise ratio. Finally, screening of large panels of monoclonal antibodies to select those that bind cytokines in their fixed form allowed practical methods to be developed (34).
Briefly, some form of activation is required. This may be a polyclonal stimulus such as PMA and ionophore or PHA or anti-CD3 and anti-CD28 or, if the specificity is known, specific antigen presented by APC. For alloantigen specific responses, following a period of incubation of responder/recipient cells with donor cells, blockade of cytokine secretion and intracellular accumulation is achieved by treatment with brefeldin A (10 µg/mL) or monensin (2–10 µM). The cells are then fixed and permeabilized prior to cell surface and intracellular cytokine staining, with subsequent analysis of cytokine production by different cell populations using flow cytometry. FCM analysis for intracellular cytokines allows individual characterization of large numbers of cells and can fully display the heterogeneity of cell populations. The advantage of intracellular cytokine staining is that multicolor staining can demonstrate exclusive or mutual coexpression of different cytokines in individual cells (e.g., CD4 vs. CD8), thus allowing the characterization of T cell subsets on the basis of cytokine production rather than just surface markers.
FCM detection of intracellular cytokines was used to investigate intracellular cytokine patterns in renal transplant recipients with and without chronic allograft nephropathy vs. healthy controls. The assay was sensitive enough to detect differences between transplant recipients and healthy controls, i.e., the percentage of CD3+ cells positive for IFN-γ, IL-5, and IL-10 were greater in transplanted patients compared with healthy controls, however, the investigators were unable to detect significant differences between recipients with and without chronic allograft nephropathy (35). The investigators however, used a non-physiologic method for activation of recipient peripheral blood mononuclear cells (PMA/ionomycin delivers a non-physiologic stimulus to T cells, bypassing the CD3-TCR complex on the surface of the T cell). Activation of lymphocytes ex vivo with a physiological stimulus such as donor cells or allopeptides may be a more sensitive method to detect differences between transplanted patients. Intracellular cytokine flow cytometry has also been applied to detect the capacity of CD4+ and CD8+ T cells to produce IL-2 as a means of measuring the effects of immunosuppressive drugs (36).
Further intensive investigation in mice has led to the discovery of a new subset of CD4+ T-cells, Th17 cells, which produce IL-17 (37). IL-17 is a pro-inflammatory cytokine with many targets and downstream mediators (38); however, its role in transplantation immunity is unclear. Data in mice suggest that T-cells receiving a TGF-β signal can acquire the potential to develop into either the T-regulatory or the Th17 lineage (39). Foxp3 (the transcription factor for T-regulatory cells) induction restrains the differentiation of inflammatory Th17 cells in response to TGF-β in the absence of other pro-inflammatory cytokines by inhibiting the activity of the transcription factor for Th17 cells, RORγt. In the presence of pro-inflammatory cytokines, the suppression of Foxp3 expression and inhibitory function, together with the concurrent upregulation or stabilization of RORγt, leads to full progression towards the Th17 lineage (39). However, the factors that determine the expression of IL-17 in human CD4+ T-cells are completely different from mice. Human studies have demonstrated that IL-6 and IL-21 are unable to induce IL-17 expression in naıve CD4+ T-cells, and TGF-β actually inhibits IL-17 expression (40). Th17 induction is further increased by blockade of IFN-γ signaling via the suppressive effects of T-regulatory cells on Th1 cells (40).
Other groups have demonstrated that differentiation of human naıve CD4+ T cells into Th17 cells is promoted by IL-1β and IL-6 (41, 42).
Studies of acute rejection in humans demonstrate that IL-17 protein is elevated in renal allografts during subclinical rejection together with detectable IL-17 mRNA in the urinary mononuclear cell sediment of these patients; conversely, IL-17 was not detectable in both biopsy specimen and urinary sediment of non-rejecting patients (43, 44). Increased IL-17 protein levels have also been detected in bronchoalveolar lavage during acute lung allograft rejection (45).
The role of Th17 cells in human disease is not yet firmly established; however, understanding the pathways that regulate the Th17 subset in humans, particularly where this differs from those operative in mice is clearly critical for the rational translation of these findings into safe and effective human therapeutics (40).
Tetramer staining
In the last several years, it has become possible to visualize antigen-specific T-cells under flow cytometry using soluble, fluorescently labeled, multimeric MHC peptide complexes that bind stably, specifically and avidly to antigen-specific T-cells (46).
The class I MHC transmembrane protein consists of a single heavy chain that contains the complete peptide binding groove of the MHC molecule, and is stable in soluble form when complexed with its essentially invariant light chain, β2-microglobulin (46). Peptide loaded class I MHC monomers are connected by the addition of fluorochrome-labeled streptavidin; its four biotin binding sites result in the formation of tetrameric complexes. For the enumeration of class I MHC restricted antigen-specific T-cells, phycoerythrin is the commonly preferred fluorochrome for its high signal to noise ratio; alternatively, fluorescein isothiocyanate or allophycocyanin may be used (47). Tetramers therefore consist of four MHC-peptide complexes covalently linked to a flurochrome. The binding of the MHC-peptide complex to the TCR of T-cells that are specific for the given peptide-MHC molecule complex or allo-MHC allows for the identification of antigen-specific T-cells in vivo by flow cytometry, regardless of their ability to produce cytokines.
The construction of class II MHC peptide tetramers has been technically more demanding than construction of class I MHC peptide tetramers. Additionally, the use of class II MHC peptide tetramers to detect and monitor antigen-specific CD4+ T-cells is less straight forward than that of class I tetramers for CD8+ (48). CD4+ T-cells specific for particular antigens typically circulate at very low frequencies, which are below the detection limit of flow cytometry. In order to detect these low frequency CD4+ T-cells, an in vitro amplification step involving stimulation of the CD4+ T-cells of interest with the study antigen is necessary (49).
The use of MHC-peptide tetramer staining has been exploited in illnesses with limited and defined antigenic spectra such as autoimmune diseases (50) and viral diseases (49). Here, addition of MHC-peptide tetramers to CD4+ T-cells can elicit partial activation responses that may be useful as markers for detection of specific cells. These activated CD4+ T-cells have been reportedly found frequently among islet antigen-stimulated peripheral blood lymphyocytes of patients with recent onset insulin-dependent diabetes mellitus. Tetramer analysis of these CD4high activated cells identifies and enumerates the antigen-specific high-affinity cells, which are not present in peripheral blood lymphocytes from normal controls. In autoimmune diabetes, these activated, tetramer-positive phenotype has also reportedly correlated with recent onset of disease. This finding suggests that this approach could be useful in diseases in which an autoantigen has been identified.
Soluble tetramerized class II MHC molecules, loaded with an immunodominant peptide from hemagglutinin and labeled with fluorescent dyes have been used to directly identify antigen-specific T-cells from influenza-immune individuals. The identification of antigen-specific T helper cells in the peripheral blood provides a means for tracking the immune response against infectious agents.
Although peptide MHC tetramers are powerful tools, they have certain limitations. They can only be used to detect immune responses to known antigens, because the peptide of interest must be loaded into the peptide MHC tetramer and thus must already be known and synthesized. Within the field of solid organ transplantation, the difficulty in using this technique for immune monitoring is that unique tetramers will be needed for each donor/recipient combination and for direct and indirect immune responses.
Immune cell function assay
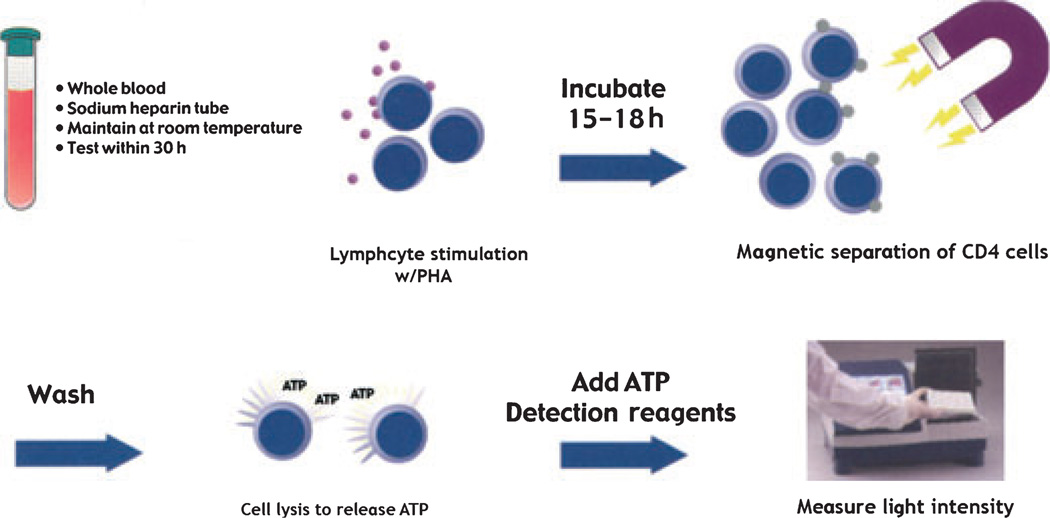
This non-invasive assay was developed to measure global immune response in transplant patients receiving immunosuppressive therapy. The assay uses the plant lectin PHA to stimulate lymphocyte activation. As most of the effector functions of immune cells depend upon cellular energy supply, the assay measures increases in intracellular ATP within CD4+ cells following activation by mitogen (51). Immune responses are categorized as strong (≥ 525), moderate (226–524), and low (≤ 225). A strong immune response implying less immunosuppression and a low immune response implying heavy immunosuppression.
Briefly, as shown in Fig. 5, whole blood is incubated over night with PHA in a 5% CO2 incubator at 37 °C. CD4+ cells are magnetically selected with a monoclonal antibody to the CD4 epitope. The cells are then lysed to release intracellular ATP. The amount of ATP is measured using luminescence reagents and a luminometer. (51).
Fig. 5.
Immune cells are stimulated overnight with a mitogen. Intracellular ATP production is measured in immunoselected CD4+ cells using luminescence reagents and a luminometer. ATP, adenosine triphosphate.
Clinical validity of this assay as an objective tool for assessing immune function was demonstrated in a retrospective study with multivisceral transplant recipients at the University of Pittsburgh (52). Moderate to strong immune function levels measured prior to weaning of tacrolimus indicated a higher risk for rejection. However, if a patient’s immune function was tightly clustered near the boundary between moderate and low immune responses (approximately 200 ng/mL ATP) weaning was more successful and recipients remained clinically stable. (52).
A meta-analysis of 504 solid organ transplant recipients from 10 US centers was performed using the above assay (53). Odds ratio analysis of infection or rejection based on the strength of immune function indicated an intersection of risk curves at 280 ng/mL of ATP with a negative predictive value of 96%. The authors therefore surmised that the high negative predictive value of adverse events at the intersection of the relative risk curves for infection and rejection at 280 ng/mL of ATP is useful in providing a target for individualizing a recipient’s immunosuppressive therapy. This value is thought to give the highest probability of maintaining clinical stability with the lowest amount of immunosuppression (53).
It is important to mention, however, that as a measure of immune function, the above assay is not designed to be diagnostic for rejection or as a replacement for biopsy. Similarly, it is not designed to be diagnostic for infection or as a replacement for viral load or culture.
Along similar lines, the residual expression of NFAT-regulated genes was measured and used as a molecular pharmacodynamic marker to assess cyclosporine effects on lymphocytes, and correlated with the frequency of recurrent infections and malignancies in renal transplant recipients (54). Patients with strong suppression of NFAT-regulated genes (as judged by a residual level of transcription of less than 15% after drug intake) developed significantly more infections and malignancies.
Conclusions
The assays described relate to studying T lymphocyte responses, as these are thought to orchestrate the allogeneic immune response. With regards to solid organ transplantation, it could be argued that the responses of cells from the peripheral blood may not necessarily reflect what is going on in the transplanted organ, however, published data would suggest that it is sufficiently sensitive (55). It is unlikely that a single assay will provide us with a broad picture of T cell alloreactivity after transplantation; rather a combination of sequentially performed assays should allow the determination of the fingerprint of the immune response at any given time in a given individual.
Abbreviations
- APC
antigen presenting cell
- ATP
adenosine triphosphate
- CFSE
carboxyfluorescein succinimidyl ester
- CPM
counts per minute
- CTLpf
cytotoxic T-lymphocyte precursor frequency
- DR
major histocompatibility complex, class II
- EBV
Epstein–Barr virus
- ELISPOT
enzyme linked immunosorbent spot
- FCM
flow cytometric
- HLA
human leukocyte antigen
- IFN
interferon
- IL
interleukin
- LDA
limiting dilution assays
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte reaction
- NFAT
nuclear factor of activated T-cells
- PHA
phytohemagglutinin
- PMA
phorbol 12-myristate 13-acetate
- ROR
retinoic acid receptor-related orphan receptor gammet
- TCR
T cell receptor
- TGF
transforming growth factor
References
- 1.Wallot MA, Mathot M, Janssen M, et al. Long-term survival and late graft loss in pediatric liver transplant recipients – a 15-year single-center experience. Liver Transplant. 2002;8:615–622. doi: 10.1053/jlts.2002.34149. [DOI] [PubMed] [Google Scholar]
- 2.Fridell JA, Jain A, Reyes J, et al. Causes of mortality beyond 1 year after primary pediatric liver transplant under tacrolimus. Transplantation. 2002;74:1721–1724. doi: 10.1097/00007890-200212270-00014. [DOI] [PubMed] [Google Scholar]
- 3.Mcdiarmid SV. Management of the pediatric liver transplant patient. Liver Transplant. 2001;7:S77–S86. doi: 10.1053/jlts.2001.28643. [DOI] [PubMed] [Google Scholar]
- 4.Krensky A. Primer on Transplantation. New Jersey: American Society of Transplantation; 2001. [Google Scholar]
- 5.Hernandez-Fuentes MP, Salama A. In vitro assays for immune monitoring in transplantation. Methods Mol Biol. 2006;333:269–290. doi: 10.1385/1-59745-049-9:269. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Sun YK, Xi YP, et al. Contribution of direct and indirect recognition pathways to T cell alloreactivity. J Exp Med. 1993;177:1643–1650. doi: 10.1084/jem.177.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SiveSai KS, Smith MA, Poindexter NJ, et al. Indirect recognition of donor HLA class I peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Transplantation. 1999;67:1094–1098. doi: 10.1097/00007890-199904270-00002. [DOI] [PubMed] [Google Scholar]
- 8.Vella JP, Spadafora-Ferreira M, Murphy B, et al. Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Transplantation. 1997;64:795–800. doi: 10.1097/00007890-199709270-00001. [DOI] [PubMed] [Google Scholar]
- 9.Ciubotariu R, Liu Z, Colovai AI, et al. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest. 1998;101:398–405. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornick PI, Mason PD, Baker RJ, et al. Significant frequencies of T cells with indirect anti-donor specificity in heart graft recipients with chronic rejection. Circulation. 2000;101:2405–2410. doi: 10.1161/01.cir.101.20.2405. [DOI] [PubMed] [Google Scholar]
- 11.Sharrock CE, Kaminski E, Man S. Limiting dilution analysis of human T cells: A useful clinical tool. Immunol Today. 1990;11:281–286. doi: 10.1016/0167-5699(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 12.Hu H, Robertus M, De Jonge N, et al. Reduction of donor-specific cytotoxic T lymphocyte precursors in peripheral blood of allografted heart recipients. Transplantation. 1994;58:1263–1268. [PubMed] [Google Scholar]
- 13.Mestre M, Massip E, Bas J, et al. Longitudinal study of the frequency of cytotoxic T cell precursors in kidney allograft recipients. Clin Exp Immunol. 1996;104:108–114. doi: 10.1046/j.1365-2249.1996.d01-657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weimar W, Rischen-Vos J, De Kuiper P, et al. Tapering immunosuppression in recipients of living donor kidney transplants. Nephrol Dial Transplant. 2004;19(Suppl. 4):v61–iv63. doi: 10.1093/ndt/gfh1044. [DOI] [PubMed] [Google Scholar]
- 15.James SP. Measurement of Basic Immunologic Characteristics of Human Mononuclear Cells. New York: John Wiley & Sons, Inc.; 2004. [Google Scholar]
- 16.Strober S, Benike C, Krishnaswamy S, Engleman EG, Grumet FC. Clinical transplantation tolerance twelve years after prospective withdrawal of immunosuppressive drugs: Studies of chimerism and anti-donor reactivity. Transplantation. 2000;69:1549–1554. doi: 10.1097/00007890-200004270-00005. [DOI] [PubMed] [Google Scholar]
- 17.Ishido N, Matsuoka J, Fujiwara T, Tanaka S, Matsuno T, Tanaka N. Donor specific hyporesponsiveness in a clinically tolerant renal transplant recipient. Transplant Proc. 1999;31:2880–2882. doi: 10.1016/s0041-1345(99)00600-4. [DOI] [PubMed] [Google Scholar]
- 18.Strober S, Dhillon M, Schubert M, et al. Acquired immune tolerance to cadaveric renal allografts. A study of three patients treated with total lymphoid irradiation. N Engl J Med. 1989;321:28–33. doi: 10.1056/NEJM198907063210106. [DOI] [PubMed] [Google Scholar]
- 19.Koshiba T, Li Y, Takemura M, et al. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transplant Immunol. 2007;17:94–97. doi: 10.1016/j.trim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Bain B, Vas MR, Lowenstein L. The development of large immature mononuclear cells in mixed leukocyte cultures. Blood. 1964;23:108–116. [PubMed] [Google Scholar]
- 21.Bach F, Hirschhorn K. Lymphocyte Interaction: A potential histocompatibility test in vitro. Science. 1964;143:813–814. doi: 10.1126/science.143.3608.813. [DOI] [PubMed] [Google Scholar]
- 22.Popma SH, Krasinskas AM, McLean AD, et al. Immune monitoring in xenotransplantation: The multiparameter flow cytometric mixed lymphocyte culture assay. Cytometry. 2000;42:277–283. doi: 10.1002/1097-0320(20001015)42:5<277::aid-cyto4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Nitta Y, Nelson K, Andrews RG, Thomas R, Gaur LK, Allen MD. CFSE dye dilution mixed lymphocyte reactions quantify donor-specific alloreactive precursors in non-human primate cardiac graft rejection. Transplant Proc. 2001;33:326–329. doi: 10.1016/s0041-1345(00)02032-7. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y, Ohdan H, Onoe T, Asahara T. Multiparameter flow cytometric approach for simultaneous evaluation of proliferation and cytokine-secreting activity in T cells responding to allo-stimulation. Immunol Invest. 2004;33:309–324. doi: 10.1081/imm-120038079. [DOI] [PubMed] [Google Scholar]
- 25.Paramore CG, Turner DA, Madison RD. Fluorescent labeling of dissociated fetal cells for tissue culture. J Neurosci Methods. 1992;44:7–17. doi: 10.1016/0165-0270(92)90108-p. [DOI] [PubMed] [Google Scholar]
- 26.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: New answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Fueyo A, Weber M, Domenig C, Strom TB, Zheng XX. Tracking the immunoregulatory mechanisms active during allograft tolerance. J Immunol. 2002;168:2274–2281. doi: 10.4049/jimmunol.168.5.2274. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka Y, Ohdan H, Onoe T, et al. Low incidence of acute rejection after living-donor liver transplantation: Immunologic analyses by mixed lymphocyte reaction using a carboxyfluorescein diacetate succinimidyl ester labeling technique. Transplantation. 2005;79:1262–1267. doi: 10.1097/01.tp.0000161667.99145.20. [DOI] [PubMed] [Google Scholar]
- 29.Najafian N, Salama AD, Fedoseyeva EV, Benichou G, Sayegh MH. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLADR peptides: Potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol. 2002;13:252–259. doi: 10.1681/ASN.V131252. [DOI] [PubMed] [Google Scholar]
- 30.Salama AD, Najafian N, Clarkson MR, Harmon WE, Sayegh MH. Regulatory CD25+ T cells in human kidney transplant recipients. J Am Soc Nephrol. 2003;14:1643–1651. doi: 10.1097/01.asn.0000057540.98231.c1. [DOI] [PubMed] [Google Scholar]
- 31.Gebauer BS, Hricik DE, Atallah A, et al. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. Am J Transplant. 2002;2:857–866. doi: 10.1034/j.1600-6143.2002.20908.x. [DOI] [PubMed] [Google Scholar]
- 32.Hricik DE, Rodriguez V, Riley J, et al. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am J Transplant. 2003;3:878–884. doi: 10.1034/j.1600-6143.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 33.Poggio ED, Clemente M, Riley J, et al. Alloreactivity in renal transplant recipients with and without chronic allograft nephropathy. J Am Soc Nephrol. 2004;15:1952–1960. doi: 10.1097/01.asn.0000129980.83334.79. [DOI] [PubMed] [Google Scholar]
- 34.Pala P, Hussell T, Openshaw PJ. Flow cytometric measurement of intracellular cytokines. J Immunol Methods. 2000;243:107–124. doi: 10.1016/s0022-1759(00)00230-1. [DOI] [PubMed] [Google Scholar]
- 35.Magee CC, Denton MD, Womer KL, Khoury SJ, Sayegh MH. Assessment by flow cytometry of intracellular cytokine production in the peripheral blood cells of renal transplant recipients. Clin Transplant. 2004;18:395–401. doi: 10.1111/j.1399-0012.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 36.van den Berg AP, Twilhaar WN, Mesander G, et al. Quantitation of immunosuppression by flow cytometric measurement of the capacity of T cells for interleukin-2 production. Transplantation. 1998;65:1066–1071. doi: 10.1097/00007890-199804270-00010. [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Wood KJ. Interleukin-23 and TH17 cells in transplantation immunity: Does 23+17 equal rejection? Transplantation. 2007;84:1071–1074. doi: 10.1097/01.tp.0000287126.12083.48. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 42.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 43.Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol. 2002;197:322–332. doi: 10.1002/path.1117. [DOI] [PubMed] [Google Scholar]
- 44.Van Kooten C, Boonstra JG, Paape ME, et al. Interleukin-17 activates human renal epithelial cells in vitro and is expressed during renal allograft rejection. J Am Soc Nephrol. 1998;9:1526–1534. doi: 10.1681/ASN.V981526. [DOI] [PubMed] [Google Scholar]
- 45.Vanaudenaerde BM, Dupont LJ, Wuyts WA, et al. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27:779–787. doi: 10.1183/09031936.06.00019405. [DOI] [PubMed] [Google Scholar]
- 46.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 47.Gratama JW, Cornelissen JJ. Clinical utility of tetramer-based immune monitoring in allogeneic stem cell transplantation. BioDrugs. 2003;17:325–338. doi: 10.2165/00063030-200317050-00003. [DOI] [PubMed] [Google Scholar]
- 48.Kwok WW. Challenges in staining T cells using HLA class II tetramers. Clin Immunol. 2003;106:23–28. doi: 10.1016/s1521-6616(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 49.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nepom GT, Buckner JH, Novak EJ, et al. HLA class II tetramers: Tools for direct analysis of antigen-specific CD4+ T cells. Arthritis Rheum. 2002;46:5–12. doi: 10.1002/1529-0131(200201)46:1<5::AID-ART10063>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 51.Kowalski R, Post D, Schneider MC, et al. Immune cell function testing: An adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant. 2003;17:77–88. doi: 10.1034/j.1399-0012.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 52.Zeevi A, Britz JA, Bentlejewski CA, et al. Monitoring immune function during tacrolimus tapering in small bowel transplant recipients. Transplant Immunol. 2005;15:17–24. doi: 10.1016/j.trim.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Kowalski RJ, Post DR, Mannon RB, et al. Assessing relative risks of infection and rejection: A meta-analysis using an immune function assay. Transplantation. 2006;82:663–668. doi: 10.1097/01.tp.0000234837.02126.70. [DOI] [PubMed] [Google Scholar]
- 54.Sommerer C, Konstandin M, Dengler T, et al. Pharmacodynamic monitoring of cyclosporine a in renal allograft recipients shows a quantitative relationship between immunosuppression and the occurrence of recurrent infections and malignancies. Transplantation. 2006;82:1280–1285. doi: 10.1097/01.tp.0000243358.75863.57. [DOI] [PubMed] [Google Scholar]
- 55.Orosz CG, Zinn NE, Sirinek L, Ferguson RM. In vivo mechanisms of alloreactivity I Frequency of donor-reactive cytotoxic T lymphocytes in sponge matrix allografts. Transplantation. 1986;41:75–83. [PubMed] [Google Scholar]